Sleep Health
Abstract
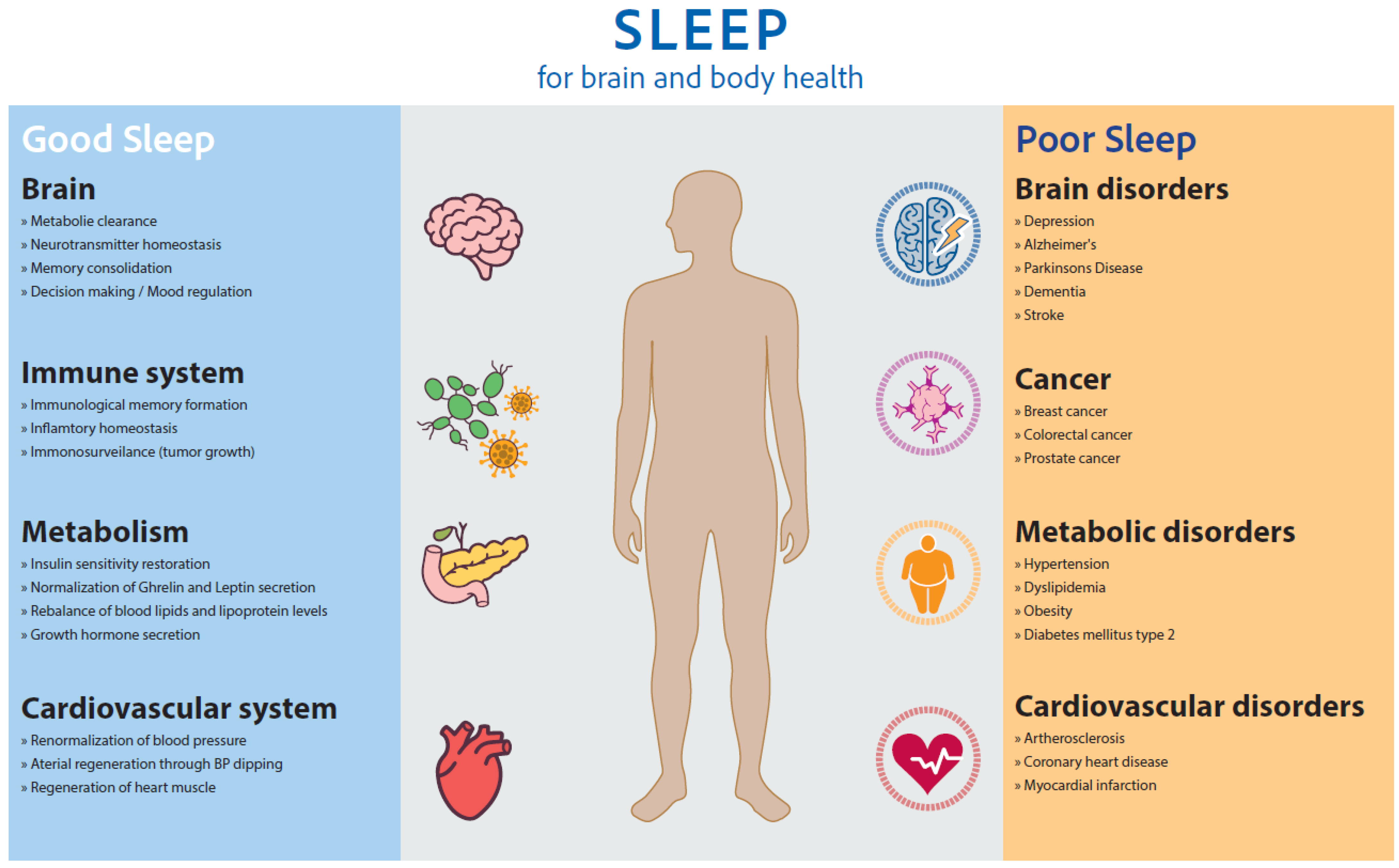
1. Sleep for Brain, Mental, Body, Occupational, and Social Health
1.1. Sleep for Learning, Memory, and Cognitive Function
1.2. Sleep for Synaptic Homeostasis
1.3. Sleep for Waste Clearance of the Brain
1.4. Sleep for Mental and Social Health
1.5. Sleep for Endocrine Functions and Immune Response
1.6. Sleep and Body Metabolism
1.7. Sleep for Cardiovascular Health
2. Sleep Health
2.1. Getting the Right Sleep
2.2. Defining Sleep Health
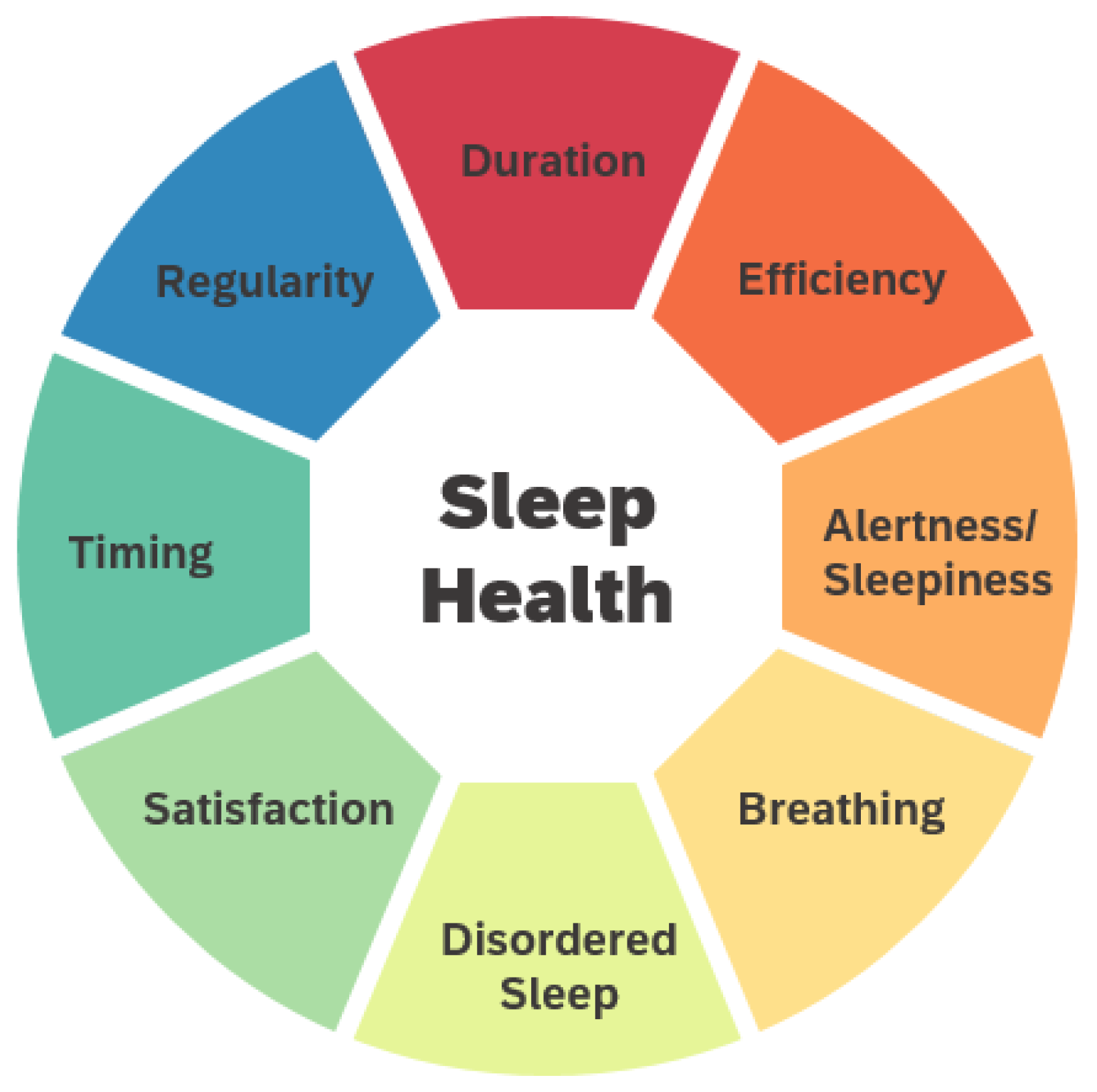
2.3. Multidimensional Factors of Sleep Health Based on Empirical Findings
2.3.1. Duration
2.3.2. Efficiency
2.3.3. Regularity and Circadian Rhythm Entrainment
2.3.4. Timing
2.3.5. Alertness/Daytime Sleepiness
2.3.6. Satisfaction/Perceived Sleep Quality
2.3.7. Good Breathing
2.3.8. Disordered Sleep
2.4. Evidence for the Sleep Health Concept
2.5. No Linear Combination of Dimensions
2.6. Measurement of Sleep Health
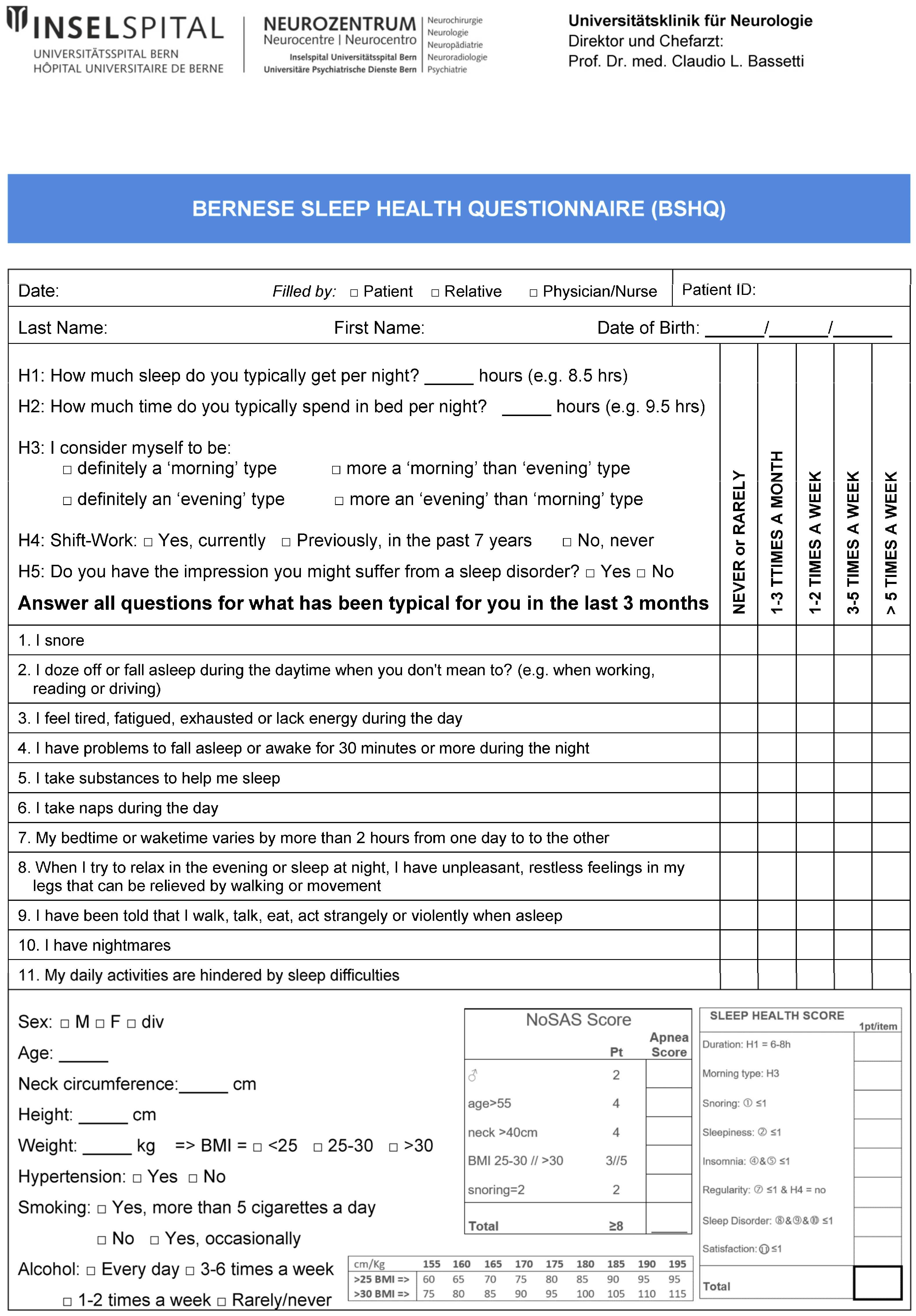
2.7. The Bernese Sleep Health Questionnaire
2.8. Recommendations to Improve Sleep Health
- Duration: Get 6–8 h of sleep daily.
- Efficiency: Restrict time in bed to the average sleep duration and leave bed during the night if awake for more than 30 min.
- Regularity: Go to bed and get up at the same time every day (±30 min) and keep a bedtime routine.
- Timing: Sleep at night according to your chronotype (early bird/night owl). Go to bed only when sleepy. Go to bed later when sleep latency is >30 min.
- Alertness: Get enough sleep at night and seek professional help to treat causes of fatigue and daytime sleepiness (e.g., sleep apnea, sleep insufficiency).
- Satisfaction: Seek professional help if you do not feel refreshed after sleep.
- Breathing: Treat snoring and sleep-related breathing disorders.
- Disordered Sleep: Seek professional help for sleep–wake disorders. Explore the triggers of parasomnias and secure the bedroom accordingly if needed.
3. Conclusions
Key Summary Points
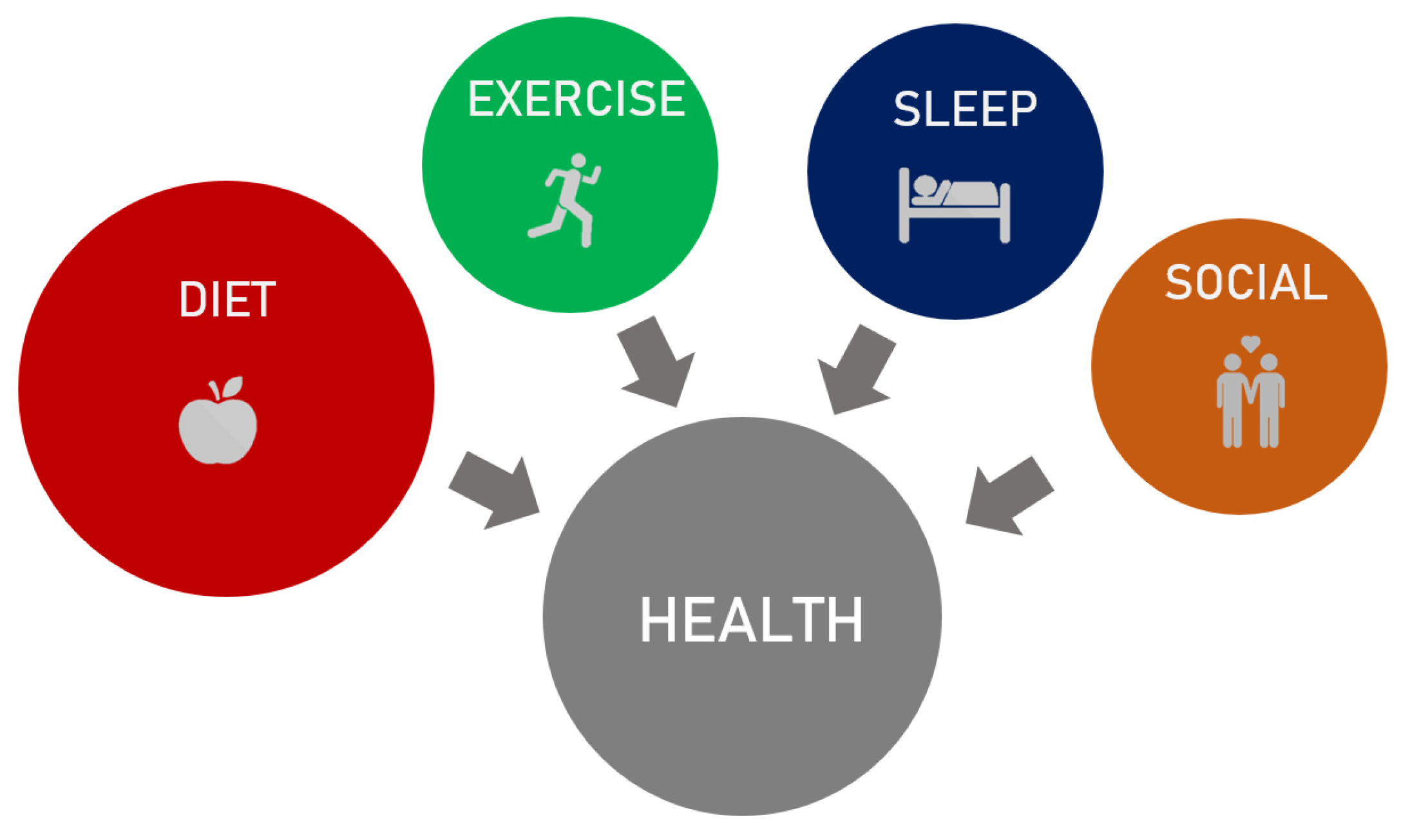
- Sleep is as central as exercise, diet, and social interactions for brain, mental, and bodily health. Good sleep quality is related to sleep duration, sleep continuity, adequate timing, and night-by-night regularity of bedtime and wake-up times.
- Sleep–wake disorders including insomnia and sleep apnea are known to be risk factors for body, brain, and mental disorders such as depression, dementia, stroke, hypertension, obesity, diabetes, and cancer. Most sleep–wake disorders can be screened using accurate short questionnaires.
- Sleep health involves various dimensions like duration, efficiency, regularity, timing, alertness, satisfaction, sleep breathing, and disordered sleep, and can be assessed using questionnaires, sleep diaries, actigraphy, or polysomnography and other sleep–wake tests.
- Measuring and enhancing sleep health should become an important strategy for disease prevention and improving overall health.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Constitution of the World Health Organization; WHO: Geneva, Switzerland, 1946; Available online: https://www.who.int/about/accountability/governance/constitution (accessed on 22 December 2023).
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Lollgen, H.; Bockenhoff, A.; Knapp, G. Physical activity and all-cause mortality: An updated meta-analysis with different intensity categories. Int. J. Sports Med. 2009, 30, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Y.; Xue, Q.; Li, X.; Wang, M.; Ma, H.; Heianza, Y.; Qi, L. Adherence to a healthy sleep pattern is associated with lower risks of all-cause, cardiovascular and cancer-specific mortality. J. Intern. Med. 2021, 291, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Endres, M.; Sander, A.; Crean, M.; Subramaniam, S.; Carvalho, V.; Di Liberto, G.; Franco, O.H.; Pijnenburg, Y.; Leonardi, M.; et al. The European Academy of Neurology Brain Health Strategy: One brain, one life, one approach. Eur. J. Neurol. 2022, 29, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Heldner, M.R.; Adorjan, K.; Albanese, E.; Allali, G.; Arnold, M.; Bègue, I.; Bochud, M.; Chan, A.; Cuénod, K.Q.D.; et al. The Swiss Brain Health Plan. Clin. Transl. Neurosci. 2023, 7, 38. [Google Scholar] [CrossRef]
- Lim, D.C.; Najafi, A.; Afifi, L.; Bassetti, C.; Buysse, D.J.; Han, F.; Hogl, B.; Melaku, Y.A.; Morin, C.M.; Pack, A.I.; et al. The need to promote sleep health in public health agendas across the globe. Lancet Public Health 2023, 8, e820–e826. [Google Scholar] [CrossRef] [PubMed]
- Klinzing, J.G.; Niethard, N.; Born, J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 2019, 22, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Vorster, A.P.; Born, J. Sleep and Memory in Mammals, Birds and Invertebrates. Neurosci. Biobehav. Rev. 2015, 50, 103–119. [Google Scholar] [CrossRef]
- Brodt, S.; Inostroza, M.; Niethard, N.; Born, J. Sleep—A brain-state serving systems memory consolidation. Neuron 2023, 111, 1050–1075. [Google Scholar] [CrossRef]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef]
- Marquie, J.C.; Tucker, P.; Folkard, S.; Gentil, C.; Ansiau, D. Chronic effects of shift work on cognition: Findings from the VISAT longitudinal study. Occup. Environ. Med. 2015, 72, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, I.; Chauvette, S. Sleep slow oscillation and plasticity. Curr. Opin. Neurobiol. 2017, 44, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.; Marshall, W.; Tononi, G.; Cirelli, C. Net decrease in spine-surface GluA1-containing AMPA receptors after post-learning sleep in the adult mouse cortex. Nat. Commun. 2021, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, L.; Yang, G.; Gan, W.B. REM sleep selectivelyprunes and maintains new synapses in development and learning. Nat. Neurosci. 2017, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Hayama, T.; Noguchi, J.; Watanabe, S.; Takahashi, N.; Hayashi-Takagi, A.; Ellis-Davies, G.C.; Matsuzaki, M.; Kasai, H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat. Neurosci. 2013, 16, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Si, Q.; Xiaoyi, Z. Obstructive sleep apnoea in patients with epilepsy: A meta-analysis. Sleep Breath 2017, 21, 263–270. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2018, 65, 106–119. [Google Scholar] [CrossRef]
- Hoshi, A.; Tsunoda, A.; Tada, M.; Nishizawa, M.; Ugawa, Y.; Kakita, A. Expression of Aquaporin 1 and Aquaporin 4 in the Temporal Neocortex of Patients with Parkinson’s Disease. Brain Pathol. 2017, 27, 160–168. [Google Scholar] [CrossRef]
- Gottlieb, E.; Landau, E.; Baxter, H.; Werden, E.; Howard, M.E.; Brodtmann, A. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med. Rev. 2019, 45, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.Z.; St Louis, E.K.; Knopman, D.S.; Boeve, B.F.; Lowe, V.J.; Roberts, R.O.; Mielke, M.M.; Przybelski, S.A.; Machulda, M.M.; Petersen, R.C.; et al. Association of Excessive Daytime Sleepiness with Longitudinal beta-Amyloid Accumulation in Elderly Persons Without Dementia. JAMA Neurol. 2018, 75, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xu, W.; Cai, Y.; Hu, Y.; Wu, C. Sleep Duration and the Risk of Dementia: A Systematic Review and Meta-analysis of Prospective Cohort Studies. J. Am. Med. Dir. Assoc. 2019, 20, 1480–1487.e5. [Google Scholar] [CrossRef] [PubMed]
- Jaussent, I.; Bouyer, J.; Ancelin, M.L.; Berr, C.; Foubert-Samier, A.; Ritchie, K.; Ohayon, M.M.; Besset, A.; Dauvilliers, Y. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep 2012, 35, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Tanner, C.M.; Masaki, K.H.; Nelson, J.S.; Curb, J.D.; Petrovitch, H. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005, 65, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Dauvilliers, Y.; Schenck, C.H.; Postuma, R.B.; Iranzo, A.; Luppi, P.H.; Plazzi, G.; Montplaisir, J.; Boeve, B. REM sleep behaviour disorder. Nat. Rev. Dis. Primers 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; John-Henderson, N.A.; Bawden, H.; Massey, A.; Powell, S.L.; Hilton, A.; Carter, J.R. Sleep restriction reduces positive social emotions and desire to connect with others. Sleep 2023, 46, zsac265. [Google Scholar] [CrossRef]
- Ben Simon, E.; Walker, M.P. Sleep loss causes social withdrawal and loneliness. Nat. Commun. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Baglioni, C.; Spiegelhalder, K.; Nissen, C.; Riemann, D. Clinical implications of the causal relationship between insomnia and depression: How individually tailored treatment of sleeping difficulties could prevent the onset of depression. EPMA J. 2011, 2, 287–293. [Google Scholar] [CrossRef][Green Version]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, insomnia and mental health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.E.A.; Shapiro, C.M. Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. J. Psychosom. Res. 2018, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. Am. J. Psychiatry 2008, 165, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, F.; Paiva, T.; Matos-Pires, A.; Cavaglia, F.; Lara, E.; Bastos, L. The sleep of non-depressed patients with panic disorder: A comparison with normal controls. Acta Psychiatr. Scand. 1996, 93, 191–194. [Google Scholar] [CrossRef]
- Fuller, K.H.; Waters, W.F.; Binks, P.G.; Anderson, T. Generalized anxiety and sleep architecture: A polysomnographic investigation. Sleep 1997, 20, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Tranter, R.; O’Donovan, C.; Chandarana, P.; Kennedy, S. Prevalence and outcome of partial remission in depression. J. Psychiatry Neurosci. 2002, 27, 241–247. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12174733 (accessed on 22 December 2023). [PubMed]
- Holl, R.W.; Hartman, M.L.; Veldhuis, J.D.; Taylor, W.M.; Thorner, M.O. Thirty-second sampling of plasma growth hormone in man: Correlation with sleep stages. J. Clin. Endocrinol. Metab. 1991, 72, 854–861. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Borniger, J.C. Molecular Mechanisms of Cancer-Induced Sleep Disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Hernandez-Jimenez, E.; Avendano-Ortiz, J.; Toledano, V.; Varela-Serrano, A.; Fernandez-Navarro, I.; Casitas, R.; Carpio, C.; Aguirre, L.A.; Garcia-Rio, F.; et al. Obstructive Sleep Apnea Monocytes Exhibit High Levels of Vascular Endothelial Growth Factor Secretion, Augmenting Tumor Progression. Mediat. Inflamm. 2018, 2018, 7373921. [Google Scholar] [CrossRef]
- Hernandez-Jimenez, E.; Cubillos-Zapata, C.; Toledano, V.; de Diego, R.P.; Fernandez-Navarro, I.; Casitas, R.; Carpio, C.; Casas-Martin, J.; Valentin, J.; Varela-Serrano, A.; et al. Monocytes inhibit NK activity via TGF-beta in patients with obstructive sleep apnoea. Eur. Respir. J. 2017, 49, 1602456. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Papadoudis, A.; Kiagia, M.; Syrigos, K. Nonpharmacologic Interventions for Improving Sleep Disturbances in Patients With Lung Cancer: A Systematic Revie and Meta-analysis. J. Pain Symptom Manag. 2018, 55, 1364–1381.e5. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Deliens, G.; Gilson, M.; Peigneux, P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 2015, 38, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ceron, E.; Fernandez-Navarro, I.; Garcia-Rio, F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med. Rev. 2016, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kaneita, Y.; Uchiyama, M.; Yoshiike, N.; Ohida, T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep 2008, 31, 645–652. [Google Scholar] [CrossRef]
- Kwok, C.S.; Kontopantelis, E.; Kuligowski, G.; Gray, M.; Muhyaldeen, A.; Gale, C.P.; Peat, G.M.; Cleator, J.; Chew-Graham, C.; Loke, Y.K.; et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008552. [Google Scholar] [CrossRef]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef]
- Portaluppi, F.; Tiseo, R.; Smolensky, M.H.; Hermida, R.C.; Ayala, D.E.; Fabbian, F. Circadian rhythms and cardiovascular health. Sleep Med. Rev. 2012, 16, 151–166. [Google Scholar] [CrossRef]
- Schussler, P.; Yassouridis, A.; Uhr, M.; Kluge, M.; Bleninger, P.; Holsboer, F.; Steiger, A. Sleep and active renin levels--interaction with age, gender, growth hormone and cortisol. Neuropsychobiology 2010, 61, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Serrador, J.; Cohen, D.; Simpson, N.; Meier-Ewert, H.; Mullington, J.M. Increasing sleep duration to lower beat-to-beat blood pressure: A pilot study. J. Sleep Res. 2013, 22, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK biobank participants. Eur. Heart J. 2019, 41, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, C.S.; Kiss, M.G.; Rattik, S.; He, S.; Vassalli, A.; Valet, C.; Anzai, A.; Chan, C.T.; Mindur, J.E.; Kahles, F.; et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019, 566, 383–387. [Google Scholar] [CrossRef]
- Vallat, R.; Shah, V.D.; Redline, S.; Attia, P.; Walker, M.P. Broken sleep predicts hardened blood vessels. PLoS Biol. 2020, 18, e3000726. [Google Scholar] [CrossRef]
- Li, X.; Zhou, T.; Ma, H.; Huang, T.; Gao, X.; Manson, J.E.; Qi, L. Healthy Sleep Patterns and Risk of Incident Arrhythmias. J. Am. Coll. Cardiol. 2021, 78, 1197–1207. [Google Scholar] [CrossRef]
- Hayter, E.A.; Wehrens, S.M.T.; Van Dongen, H.P.A.; Stangherlin, A.; Gaddameedhi, S.; Crooks, E.; Barron, N.J.; Venetucci, L.A.; O’Neill, J.S.; Brown, T.M.; et al. Distinct circadian mechanisms govern cardiac rhythms and susceptibility to arrhythmia. Nat. Commun. 2021, 12, 2472. [Google Scholar] [CrossRef]
- Cribb, L.; Sha, R.; Yiallourou, S.; Grima, N.A.; Cavuoto, M.; Baril, A.A.; Pase, M.P. Sleep regularity and mortality: A prospective analysis in the UK Biobank. eLife 2023, 12, RP88359. [Google Scholar] [CrossRef]
- Schmid, S.M.; Hallschmid, M.; Schultes, B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015, 3, 52–62. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; Tan, Y. A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep Breath. 2018, 22, 805–814. [Google Scholar] [CrossRef]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Phelan, J.; Paskow, M.J.; Roach, A.; Whiton, K.; Langer, G.; Hillygus, D.S.; Mokrzycki, M.; Broughton, W.A.; Chokroverty, S.; et al. The National Sleep Foundation’s Sleep Health Index. Sleep Health 2017, 3, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.L.; Coleman, T.S.; Mentch, L.K.; Buysse, D.J.; Graves, J.L.; Hagen, E.W.; Hall, M.H.; Stone, K.L.; Redline, S.; Peppard, P.E. Physiological sleep measures predict time to 15-year mortality in community adults: Application of a novel machine learning framework. J. Sleep Res. 2021, 30, e13386. [Google Scholar] [CrossRef]
- Wallace, M.L.; Yu, L.; Buysse, D.J.; Stone, K.L.; Redline, S.; Smagula, S.F.; Stefanick, M.L.; Kritz-Silverstein, D.; Hall, M.H. Multidimensional sleep health domains in older men and women: An actigraphy factor analysis. Sleep 2021, 44, zsaa181. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wu, Y.; Zhang, D. Nighttime sleep duration, 24-hour sleep duration and risk of all-cause mortality among adults: A meta-analysis of prospective cohort studies. Sci. Rep. 2016, 6, 21480. [Google Scholar] [CrossRef] [PubMed]
- Brindle, R.C.; Yu, L.; Buysse, D.J.; Hall, M.H. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: Results from the Midlife in the United States (MIDUS) study. Sleep 2019, 42, zsz116. [Google Scholar] [CrossRef]
- Wallace, M.L.; Lee, S.; Hall, M.H.; Stone, K.L.; Langsetmo, L.; Redline, S.; Schousboe, J.T.; Ensrud, K.; LeBlanc, E.S.; Buysse, D.J.; et al. Heightened sleep propensity: A novel and high-risk sleep health phenotype in older adults. Sleep Health 2019, 5, 630–638. [Google Scholar] [CrossRef]
- Wassing, R.; Lakbila-Kamal, O.; Ramautar, J.R.; Stoffers, D.; Schalkwijk, F.; Van Someren, E.J.W. Restless REM Sleep Impedes Overnight Amygdala Adaptation. Curr. Biol. 2019, 29, 2351–2358.e4. [Google Scholar] [CrossRef]
- Blanken, T.F.; Borsboom, D.; Penninx, B.W.; Van Someren, E.J. Network outcome analysis identifies difficulty initiating sleep as a primary target for prevention of depression: A 6-year prospective study. Sleep 2020, 43, zsz288. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Bhimasani, M.; Zangrilli, M.A.; Morris, J.C.; Holtzman, D.M.; Ju, Y.S. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol. 2018, 75, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, O.; Delwiche, B.; Mairesse, O.; Peigneux, P. Impact of Sleep Fragmentation on Cognition and Fatigue. Int. J. Environ. Res. Public Health 2022, 19, 15485. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, K.A.; Punjabi, N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010, 137, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Laffan, A.; Caffo, B.; Swihart, B.J.; Punjabi, N.M. Utility of sleep stage transitions in assessing sleep continuity. Sleep 2010, 33, 1681–1686. [Google Scholar] [PubMed]
- Rolls, A.; Colas, D.; Adamantidis, A.; Carter, M.; Lanre-Amos, T.; Heller, H.C.; de Lecea, L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc. Natl. Acad. Sci. USA 2011, 108, 13305–13310. [Google Scholar] [CrossRef]
- Guida, J.L.; Alfini, A.J.; Gallicchio, L.; Spira, A.P.; Caporaso, N.E.; Green, P.A. Association of objectively measured sleep with frailty and 5-year mortality in community-dwelling older adults. Sleep 2021, 44, zsab003. [Google Scholar] [CrossRef]
- Solelhac, G.; Sánchez-de-la-Torre, M.; Blanchard, M.; Berger, M.; Hirotsu, C.; Imler, T.; Sánchez-de-la-Torre, A.; Haba-Rubio, J.; Marchi, N.A.; Bayon, V.; et al. Pulse Wave Amplitude Drops Index: A Biomarker of Cardiovascular Risk in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2023, 207, 1620–1632. [Google Scholar] [CrossRef]
- Windred, D.P.; Burns, A.C.; Lane, J.M.; Saxena, R.; Rutter, M.K.; Cain, S.W.; Phillips, A.J.K. Sleep regularity is a stronger predictor of mortality risk than sleep duration: A prospective cohort study. Sleep 2024, 47, zsad253. [Google Scholar] [CrossRef]
- Foster, R.G. Sleep, circadian rhythms and health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef]
- Burns, A.C.; Saxena, R.; Vetter, C.; Phillips, A.J.K.; Lane, J.M.; Cain, S.W. Time spent in outdoor light is associated with mood, sleep, and circadian rhythm-related outcomes: A cross-sectional and longitudinal study in over 400,000 UK Biobank participants. J. Affect. Disord. 2021, 295, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.; Riemersma, R.F.; Swaab, D.F. Functional plasticity of the circadian timing system in old age: Light exposure. Prog. Brain Res. 2002, 138, 205–231. [Google Scholar] [CrossRef] [PubMed]
- Dekker, K.; Benjamins, J.S.; Maksimovic, T.; Filardi, M.; Hofman, W.F.; van Straten, A.; Van Someren, E.J.W. Combined Internet-Based Cognitive-Behavioral and Chronobiological Intervention for Insomnia: A Randomized Controlled Trial. Psychother. Psychosom. 2020, 89, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Covassin, N.; Bock, J.M.; Mohamed, E.A.; Pappoppula, L.P.; Shafi, C.; Lopez-Jimenez, F.; Somers, V.K. Excessive Daytime Sleepiness and Cardiovascular Mortality in US Adults: A NHANES 2005–2008 Follow-Up Study. Nat. Sci. Sleep 2021, 13, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Wickham, S.R.; Amarasekara, N.A.; Bartonicek, A.; Conner, T.S. The Big Three Health Behaviors and Mental Health and Well-Being Among Young Adults: A Cross-Sectional Investigation of Sleep, Exercise, and Diet. Front. Psychol. 2020, 11, 579205. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Gower, J.; Lamperti, L.; Dreyse, J.; Jorquera, J. Chronic intermittent hypoxia in obstructive sleep apnea: A narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath 2020, 24, 751–760. [Google Scholar] [CrossRef]
- Högl, B.; Stefani, A.; Videnovic, A. Idiopathic REM sleep behaviour disorder and neurodegeneration—An update. Nat. Rev. Neurol. 2018, 14, 40–55. [Google Scholar] [CrossRef]
- Bernert, R.A.; Joiner, T.E. Sleep disturbances and suicide risk: A review of the literature. Neuropsychiatr. Dis. Treat. 2007, 3, 735–743. [Google Scholar] [CrossRef]
- DeSantis, A.S.; Dubowitz, T.; Ghosh-Dastidar, B.; Hunter, G.P.; Buman, M.; Buysse, D.J.; Hale, L.; Troxel, W.M. A preliminary study of a composite sleep health score: Associations with psychological distress, body mass index, and physical functioning in a low-income African American community. Sleep Health 2019, 5, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Martinez, A.J.; Buysse, D.J.; Harvey, A.G. A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep Health 2019, 5, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ulsa, M.C.; Xi, Z.; Li, P.; Gaba, A.; Wong, P.M.; Saxena, R.; Scheer, F.A.J.L.; Rutter, M.; Akeju, O.; Hu, K.; et al. Association of Poor Sleep Burden in Middle Age and Older Adults with Risk for Delirium During Hospitalization. J. Gerontol. Ser. A 2021, 77, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.E.; Chasens, E.R.; Bizhanova, Z.; Sereika, S.M.; Buysse, D.J.; Imes, C.C.; Kariuki, J.K.; Mendez, D.D.; Cajita, M.I.; Rathbun, S.L.; et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int. J. Obes. 2021, 45, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Furihata, R.; Hall, M.H.; Stone, K.L.; Ancoli-Israel, S.; Smagula, S.F.; Cauley, J.A.; Kaneita, Y.; Uchiyama, M.; Buysse, D.J.; Study of Osteoporotic Fractures Research, G. An Aggregate Measure of Sleep Health Is Associated with Prevalent and Incident Clinically Significant Depression Symptoms Among Community-Dwelling Older Women. Sleep 2017, 40, zsw075. [Google Scholar] [CrossRef]
- Duss, S.B.; Bernasconi, C.; Steck, A.; Brill, A.K.; Manconi, M.; Dekkers, M.; Schmidt, M.H.; Bassetti, C.L.A. Multiple sleep-wake disturbances after stroke predict an increased risk of cardio-cerebrovascular events or death: A prospective cohort study. Eur. J. Neurol. 2023, 30, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Dalmases, M.; Benitez, I.; Sapina-Beltran, E.; Garcia-Codina, O.; Medina-Bustos, A.; Escarrabill, J.; Salto, E.; Buysse, D.J.; Plana, R.E.; Sanchez-de-la-Torre, M.; et al. Impact of sleep health on self-perceived health status. Sci. Rep. 2019, 9, 7284. [Google Scholar] [CrossRef]
- Benitez, I.; Roure, N.; Pinilla, L.; Sapina-Beltran, E.; Buysse, D.J.; Barbe, F.; de Batlle, J. Validation of the Satisfaction, Alertness, Timing, Efficiency and Duration (SATED) Questionnaire for Sleep Health Measurement. Ann. Am. Thorac. Soc. 2020, 17, 338–343. [Google Scholar] [CrossRef]
- Janssen, H.; Venekamp, L.N.; Peeters, G.A.M.; Pijpers, A.; Pevernagie, D.A.A. Management of insomnia in sleep disordered breathing. Eur. Respir. Rev. 2019, 28, 190080. [Google Scholar] [CrossRef]
- Duarte, R.L.M.; Rabahi, M.F.; Magalhaes-da-Silveira, F.J.; de Oliveira, E.S.T.S.; Mello, F.C.Q.; Gozal, D. Simplifying the Screening of Obstructive Sleep Apnea with a 2-Item Model, No-Apnea: A Cross-Sectional Study. J. Clin. Sleep Med. 2018, 14, 1097–1107. [Google Scholar] [CrossRef]
- Klingman, K.; Jungquist, C.; Perlis, M. Introducing the Sleep Disorders Symptom Checklist-25: A Primary Care Friendly and Comprehensive Screener for Sleep Disorders. Sleep Med. Res. 2017, 8, 17–25. [Google Scholar] [CrossRef]
- Ferri, R.; Lanuzza, B.; Cosentino, F.I.; Iero, I.; Tripodi, M.; Spada, R.S.; Toscano, G.; Marelli, S.; Arico, D.; Bella, R.; et al. A single question for the rapid screening of restless legs syndrome in the neurological clinical practice. Eur. J. Neurol. 2007, 14, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorster, A.P.A.; van Someren, E.J.W.; Pack, A.I.; Huber, R.; Schmidt, M.H.; Bassetti, C.L.A. Sleep Health. Clin. Transl. Neurosci. 2024, 8, 8. https://doi.org/10.3390/ctn8010008
Vorster APA, van Someren EJW, Pack AI, Huber R, Schmidt MH, Bassetti CLA. Sleep Health. Clinical and Translational Neuroscience. 2024; 8(1):8. https://doi.org/10.3390/ctn8010008
Chicago/Turabian StyleVorster, Albrecht P. A., Eus J. W. van Someren, Allan I. Pack, Reto Huber, Markus H. Schmidt, and Claudio L. A. Bassetti. 2024. "Sleep Health" Clinical and Translational Neuroscience 8, no. 1: 8. https://doi.org/10.3390/ctn8010008
APA StyleVorster, A. P. A., van Someren, E. J. W., Pack, A. I., Huber, R., Schmidt, M. H., & Bassetti, C. L. A. (2024). Sleep Health. Clinical and Translational Neuroscience, 8(1), 8. https://doi.org/10.3390/ctn8010008