Abstract
When we are asleep, we lose the ability to promptly respond to external stimuli, and yet we spend many hours every day in this inherently risky behavioral state. This simple fact strongly suggests that sleep must serve essential functions that rely on the brain going offline, on a daily basis, and for long periods of time. If these functions did not require partial sensory disconnection, it would be difficult to explain why they are not performed during waking. Paradoxically, despite its central role in defining sleep and what sleep does, sensory disconnection during sleep remains a mystery. We have a limited understanding of how it is implemented along the sensory pathways; we do not know whether the same mechanisms apply to all sensory modalities, nor do we know to what extent these mechanisms are shared between non-rapid eye movement (NREM) sleep and REM sleep. The main goal of this contribution is to review some knowns and unknowns about sensory disconnection during sleep as a first step to fill this gap.
Keywords:
arousal threshold; NREM sleep; REM sleep; auditory system; visual system; olfactory system; pain; OFF periods 1. Introduction
Partial sensorimotor disconnection is a defining feature of sleep, the one that distinguishes sleep from any other behavioral state, including quiet waking, anesthesia, and coma. By sensorimotor disconnection, we mean that, relative to waking, the ability to respond to a stimulus is reduced during sleep, i.e., the “arousal threshold” is increased. The arousal threshold is usually assessed in humans by measuring the ability to produce a behavioral response, such as pushing a button or providing a verbal response, often in association with the analysis of the global brain response as detected by the scalp EEG. Orienting responses or other complex behaviors are rarely used in animals, where the arousal threshold is measured by the ability of the stimulus to cause a shift in behavioral state, an “awakening”, as detected by the scalp EEG. Awakening from NREM sleep is usually defined as a clear switch in the EEG from the high-amplitude, low-frequency pattern to the low- amplitude and high- frequency pattern, while awakening from REM sleep requires an increase in muscle tone. Sensory disconnection during sleep is partial, i.e., reversible, which means that contrary to anesthesia and coma, we still always wake up from sleep if the stimulus is strong enough.
Despite the obvious risks associated with the reduced ability to respond to a potential threat, we sleep for many hours every day, as do all other animals carefully studied so far [1]. This simple fact is one of the strongest indicators that sleep fulfills some essential functions that require the brain to be offline. If the same functions were independent of sensory disconnection, evolution would have found a way to carry them out more safely during waking, when we can promptly react to a stimulus [2]. Thus, sensory disconnection not only defines sleep but also puts specific constraints on any idea about its putative functions. Paradoxically, however, the neural mechanisms responsible for sensory disconnection during sleep are still unclear, and most of the available evidence is restricted to the auditory system. Two main approaches have been used to understand these mechanisms: evoked potentials (evoked “responses”) and evoked unit activity. These measures of neuronal responsiveness are taken in specific brain regions, i.e., they are local, and they are usually compared in three conditions: subjects are awake when stimulated, they are asleep and do not wake up in response to the stimulus, or they do wake up. Evoked responses are usually collected using scalp EEG (or magnetoencephalography, or functional MRI) recordings or with invasive, deep intracortical local field potential (LFP) recordings. Evoked responses reflect mainly the synaptic input, not the firing output, which is why the analysis of unit activity is also critical to studying sensory disconnection.
Here, we review this fragmented literature, point to many seemingly inconsistent findings across sensory modalities, and discuss the evidence linking the reduced activity of specific arousal systems to sensory disconnection during sleep. We will point to identified sites of impaired sensory transmission (“gates”) and, when possible, discuss the underlying candidate mechanisms.
2. Sensory Disconnection in Sleep across Different Sensory Modalities
2.1. Auditory System
Partial sensory disconnection during sleep is best characterized in the auditory system. Relative to waking, in both humans [3,4] and rats [5,6], the acoustic arousal threshold is increased to a similar extent in REM sleep and non-rapid eye movement (NREM) stage 2, and highest in the deepest stage of NREM sleep, called stage 3 or slow-wave sleep. The arousal threshold depends on the amount of slow-wave activity (SWA): a higher SWA leads to a higher arousal threshold [5,6]. The arousal threshold is also higher in phasic REM sleep compared to tonic REM sleep [7,8].
Cortical auditory evoked potentials look similar in wake and REM sleep, but the amplitude of the late components is larger in NREM sleep, likely reflecting the bistability (ON/OFF firing) of cortical neurons during this sleep phase [9,10]. One EEG/fMRI study found that sounds elicit BOLD responses in the thalamus and primary auditory cortex when delivered in waking as well as when given during NREM sleep without causing an awakening [11]. In sleep, the responses are smaller and more variable when sounds are given during spindles, and larger and extend to broader cortical areas if the sound elicits a K complex, a large slow wave that reflects the presence of bistability in the thalamocortical system [11]. In general, the studies that measured the firing evoked by a sound found little difference between sleep and wake in subcortical regions and primary cortical areas. For instance, in guinea pigs, the majority of neurons in the primary auditory cortex (A1) show similar evoked firing activity in waking and NREM sleep, and the remaining neurons are split evenly between increased or decreased firing in sleep relative to wake [12]. In marmosets, the analysis of evoked unit firing in the A1 and higher order auditory cortex (mostly supragranular layers) showed that some neurons fire more in sleep than in waking; others do the opposite, with no obvious depressive effect of sleep [13]. In the same study, the response in NREM sleep was a poor predictor of the response in REM sleep, with a third of all units showing opposite responses in the two phases [13], suggesting that the mechanisms of auditory disconnection may differ between NREM sleep and REM sleep. A follow-up study in the supragranular layers of marmoset A1 found reduced unit firing in response to weak stimuli during NREM sleep [14]. However, louder sounds evoked similar unit responses in wake and sleep despite being unable to cause behavioral arousal, leaving the question of why the acoustic arousal threshold is increased in sleep unresolved [14]. Several studies that recorded from rat A1 also concluded that the firing evoked by different sounds does not fundamentally differ in wake, NREM sleep, or REM sleep [15,16,17]. On the other hand, it was recently found that most neurons in the perirhinal cortex, a higher order area, respond less to sounds in REM sleep, and even less so in NREM sleep, relative to waking [17]. A crucial insight provided by this study was that the reduced response in NREM sleep does not depend on the cortical area per se (high order vs. A1) but on the latency of the response, which likely reflects the position of the responding neurons within layers: early (<20 ms) responding neurons, which accounted for most of the recorded units in A1, were unaffected, while the few A1 neurons that were late-responding (>40 ms) showed a reduced response in NREM sleep. Consistently, most recorded neurons in the perirhinal cortex were late-responding and responded less in NREM sleep, while the few early-responding neurons were unaffected. Interestingly, a late response did not predict a reduced response in REM sleep, pointing again to different mechanisms for disconnection in the two sleep phases [17]. A recent study in epileptic patients implanted with depth electrodes in the lateral temporal lobe found that sounds evoked a moderately decreased spiking response in NREM sleep relative to wake, especially outside A1. A moderate decrease in evoked firing was also present in REM sleep [18]. Relative to waking, however, in both sleep phases, the most significant change was the attenuation of “alpha/beta desynchronization”, i.e., the large decrease in power in the alpha and beta frequencies triggered by the sound during waking was strongly attenuated in sleep [18]. Another recent study found that whether they cause an awakening from sleep or not, sounds evoke the same strong calcium response in the mouse primary auditory thalamus (ventral medial geniculate nucleus, vMGN) that projects to A1 [19], consistent with previous evidence that sounds reach the input layer of A1 equally well in waking and NREM sleep. Moreover, optogenetic excitation of vMGN neurons did not cause an awakening from sleep, and their optogenetic inhibition did not change the probability of sound-induced awakenings. By contrast, in the “high-order” posterior intralaminar thalamic nucleus, sounds or blue light applied during NREM sleep induced a large calcium response only when they caused an awakening. Furthermore, optogenetic inhibition of this nucleus, or of its dense projections to the temporal association cortex, strongly reduced the probability of waking up from sounds or blue light [19]. While evoked responses were not recorded during REM sleep, the optogenetic stimulation of the posterior intralaminar nucleus woke up the mouse from NREM sleep but not from REM sleep [19], again suggesting that sensory disconnection in REM sleep may be mediated differently than in NREM sleep.
Together, these studies show that sounds reach the input layer of A1 equally well in waking and NREM sleep, but their transmission to supragranular and infragranular layers, as well as the feedforward transmission to higher order cortices, are compromised. During NREM sleep, this effect likely depends on the occurrence of OFF periods, which impair corticocortical communication [20], a hypothesis that is consistent with the fact that higher SWA leads to a higher auditory arousal threshold [5,6]. It is also in line with an early report that a motor response (thumb press) is unlikely to occur if the tone is delivered during a K complex [21,22], and with more recent findings that spontaneous and evoked K complexes are associated with an increase in power in the low frequencies, although region-specific effects are present [23,24]. Other mechanisms likely exist, however, because during stage 2 of NREM sleep or REM sleep, when slow waves are rare or spatially restricted [25], subjects still respond only 50% of the time [3]. One proposed mechanism is the impairment of the feedback from higher to lower cortical areas, consistent with the reduced alpha/beta desynchronization caused by sounds in sleeping humans [18]. Of note, although many recent studies point to cortical “gates” located after the input layer, or gates in the high-order thalamus, one early study found that during sleep, neurons in the midbrain reticular formation and deep layers of the superior colliculus respond less to sounds and other stimuli [26]. Intriguingly, all units recorded in REM sleep showed a reduced response independent of latency, while during NREM sleep, the affected neurons were those with a late response [26], akin to what is seen in the cortex.
In summary, a prominent mechanism for partial auditory disconnection in NREM sleep is in place after the input layer of A1, but other mechanisms in the high-order thalamus are also involved, and a brainstem gate may exist for REM sleep.
2.2. Visual System
Most mammals, including humans, close their eyes while asleep, but sleeping invertebrates keep their eyes open, as do sleeping humans in certain pathological conditions (nocturnal lagophthalmos) or during experiments designed to test whether images presented during sleep are incorporated into dreams (they rarely are [27]). In head-fixed mice, sleep often occurs with the eyes open. In these experimental conditions, the pupils are tonically constricted in REM sleep, but their size can fluctuate during NREM sleep from small to as large as in wake [28], presumably allowing visual stimuli to enter the brain. In short, retinal deafferentation is unlikely to fully and consistently account for the reduced response to visual stimuli during sleep.
Seminal studies in cats pointed to less effective synaptic transmission in the primary visual thalamus, the lateral geniculate nucleus of the thalamus (LGN), during NREM sleep. LGN responses evoked by direct prethalamic (optic tract) stimulation are depressed in NREM sleep [29], and reduced thalamic transmission was found both postsynaptically in LGN neurons and presynaptically in the fibers of the optic tract [30]. Another study, using quasi-intracellular recordings of LGN neurons, measured the ratio between the number of spikes (output) and the number of excitatory postsynaptic potentials (input) in response to visual stimuli: the transfer (output/input) ratio was close to 1 in waking, decreased in drowsiness, and was even smaller in NREM sleep or light anesthesia [31]. In this preparation, pupils were dilated, and the activity of the optic fibers remained constant across levels of alertness, pointing to a postsynaptic mechanism in LGN neurons [31]. Unit recordings in cats also found that, relative to waking, during NREM sleep, LGN neurons respond to light flashes less strongly and in brief “clusters” [32]. Thus, there is converging evidence for impaired transmission of visual inputs from the LGN to the visual cortex during NREM sleep.
Light flashes, like acoustic stimuli, evoke larger late cortical responses in NREM sleep than in wake in humans [10] and rats [33]. Unit recordings performed in the cat’s visual cortex strongly suggest that even when the visual input reaches layer 4, its propagation beyond the thalamorecipient layer may be impaired. Specifically, it was found that in response to light, most neurons show an early (20–40 msec) and a late (80–100 msec) increase in firing during waking, while in NREM sleep only the early response is present [34]. Other neurons only showed a late response in waking and did not respond at all in NREM sleep [34]. Thus, during NREM sleep, a cortical mechanism may also restrict the processing of visual stimuli in the visual cortex, specifically for late responses, akin to the finding in the auditory cortex [17]. This mechanism may depend on bistable (ON/OFF) firing favored by the low noradrenergic tone during NREM sleep [20]. Moreover, like for the auditory system, the high-order thalamus may gate stimuli because, in mice, silencing the posterior intralaminar thalamic nucleus decreases the probability of waking up from NREM sleep after exposure to blue light [19].
During REM sleep, LGN neurons fire at wake-like levels with isolated bursts [35], and their synaptic responsiveness to electrical stimulation is similar or higher than in wake [30]. Increased excitability, however, does not necessarily promote the thalamocortical transmission of visual stimuli, and some early experiments suggested that it could even lead to occlusion [36,37]. In cat primary visual cortex, firing rates are higher in REM sleep than in NREM sleep [38]; however, laminar recordings in mice found that the tonic, wake-like activity is restricted to deep layers, while neurons in superficial and middle layers are bistable and slow waves are present, which could impair cortico-cortical transmission [25].
In summary, both thalamic and cortical disconnection mechanisms are likely to operate during NREM sleep. In REM sleep, suggested mechanisms include subcortical occlusion and cortical bistability in and above the input layer of the primary visual cortex.
2.3. Somatosensory System and Pain Pathways
Pioneering experiments in cats measured the transmission of somatosensory stimuli in the caudal parts of the sensory pathway, below the thalamus and the cortex. These studies consistently found that, relative to waking, neuronal responses to tactile and proprioceptive stimuli are depressed mainly in REM sleep and much less so in NREM sleep. Specifically, synaptic transmission in the ascending medial lemniscal pathway is decreased in REM sleep in association with rapid eye movements, an effect that may be due to a postsynaptic mechanism in the cuneate nucleus as well as to a presynaptic mechanism involving fiber depolarization in the cuneate tract [39,40]. Relative to waking, during REM sleep, the spinoreticular, spinomesencephalic, and spinothalamic fibers also show reduced evoked response after electrical stimulation of the sciatic nerve [41], and lumbar spinoreticular neurons are spontaneously less active in REM sleep but show a similar firing in waking and NREM sleep [42]. Moving more rostrally in the sensory pathway, in the cat somatosensory relay nucleus of the thalamus, air puffs induce a smaller firing response, followed by a stronger decrease in firing during NREM sleep compared to waking [43]. In response to vibration, neurons in the primary sensory cortex of macaque monkeys show a small decrease in evoked firing during NREM sleep, and completely stop responding in REM sleep [44]. In summary, studies in animals have provided evidence for an early, pre-thalamic decrease in the transmission of somatosensory stimuli, specifically during REM sleep relative to waking, while a thalamic gate may be present during NREM sleep.
Consistent with the studies using somatosensory stimuli, experiments in animals that measured the transmission of painful stimuli at prethalamic levels found a depressed neuronal response mostly confined to REM sleep. Thus, after pain (tooth pulp) stimulation, the unit response of individual trigemino-thalamic tract neurons in the cat trigeminal sensory nuclear complex is similar in NREM sleep and waking but reduced in REM sleep [45], an effect that may depend on primary afferent depolarization [46]. More recent studies in rats focused on ON and OFF neurons in the medial medulla that are activated or inhibited by pain, respectively, and restricted the analysis to NREM sleep. ON neurons fire less during NREM sleep than in waking, while OFF neurons fire more [47], suggesting that the high spontaneous activity of OFF neurons during NREM sleep may contribute to the depression of the pain-evoked arousal response, thus helping to preserve sleep continuity [48]. However, during NREM sleep, ON neurons are still activated by heat pain, and OFF neurons are still inhibited (their behavior during REM sleep is unknown) [47]. In humans, sleep-dependent changes in the response to pain have been measured at the cortical level. The amplitude of the cortical potential evoked by a painful stimulus (laser-induced heat) correlates well with pain perception, that is, when subjects attend to the stimulus, the cortical response is larger and the perceived pain is stronger, compared to when they are distracted [49]. During NREM stage 2, this cortical evoked response is strongly depressed or abolished (no other sleep stages were studied) [10,49]. Another study found that the “activation” response to pain, defined as any change in EEG frequencies, is reduced in slow-wave sleep and REM sleep relative to NREM stage 2 [50]. In summary, pain perception is likely reduced in sleep via various mechanisms, and there is evidence for early, prethalamic gates in REM sleep but not NREM sleep.
2.4. Olfactory System
Humans are largely unresponsive to odors during sleep [51,52,53]. In response to strong unpleasant stimuli, arousal thresholds are higher for slow-wave sleep than for REM sleep and NREM stage 2 [52], consistent with the response to sounds. Mice also have a higher arousal threshold in NREM sleep than in REM sleep, at least in response to the scent of a predator [54]. The olfactory system is relatively simple: there is no first-order thalamic relay, and only two synapses divide the external world from the primary olfactory cortex, the first from the olfactory neurons in the olfactory epithelium to mitral/tufted cells in the olfactory bulb, and the second from the latter to the pyramidal neurons in the input layer of the primary olfactory cortex. One study found that the mitral cells are under stronger local inhibition from the granule cells during sleep than during waking [55]. The inhibition was strongest during NREM sleep, perhaps because acetylcholine, which inhibits granule cells, is low [55]. However, this explanation is unlikely to be valid for REM sleep, when mitral cells are more inhibited than in waking, but acetylcholine levels are presumably high. Under urethane anesthesia, mitral cells respond during both the slow-wave phase and the fast-wave phase, while the response of cortical pyramidal neurons is strongly depressed when slow waves are present [56]. To the extent that one assumes that slow waves are fundamentally similar in sleep and urethane anesthesia, these results suggest that the processing of olfactory stimuli is also gated at the cortical level, although direct evidence during physiological sleep is lacking. Like the hippocampus, the primary olfactory cortex shows sharp waves during NREM sleep that are generated by recurrent collaterals of cortical pyramidal neurons [57]. Whether these sharp waves play a role in olfactory disconnection during sleep is unknown.
3. Neuromodulators and Disconnection
Moruzzi and Magoun showed that the stimulation of the reticular activating system is sufficient to convert a synchronized EEG pattern with slow waves to an activated pattern with low-voltage fast activity [58]. Once viewed as a monolithic core, it is now clear that the original reticular activating system includes many ascending neuromodulatory systems that diffusely innervate both the cerebral cortex and thalamus and have overlapping and yet specific roles in regulating behavioral states [59]. In waking, neuromodulatory tone is generally high, while in NREM sleep, the activity of the noradrenergic [6,60,61], cholinergic [62,63,64,65], serotoninergic [66,67,68], histaminergic [69,70], orexinergic [71,72], and dopaminergic [73,74,75] neurons is reduced, leading to overall reduced levels of these neuromodulators throughout the brain. In contrast, during REM sleep, the activity in the noradrenergic [6,60,61], histaminergic [69,70], and serotoninergic [66,67,68] systems is markedly decreased relative to wake, but the activity of cholinergic [62,63,64,65] and dopaminergic midbrain neurons [73,74] is high. Thus, low levels of noradrenaline, serotonin, histamine, and/or orexin may play a key role in promoting sensory disconnection during sleep, while high levels of acetylcholine and dopamine may support conscious experience (dreaming) during REM sleep. The noradrenergic, serotoninergic, histaminergic, and orexinergic systems have widespread projections and reach their lowest levels of activity during REM sleep. Their role in sensory disconnection may therefore extend across most, if not all, sensory modalities and may be critical, especially in REM sleep. Below, we discuss whether the existing evidence supports these assumptions.
3.1. Noradrenaline
The noradrenergic neurons of the locus coeruleus (LC) fire maximally during waking, less so during NREM sleep, and not at all in REM sleep [6,60,61]. LC neurons start firing before awakening from sleep and stop firing before the onset of EEG synchronization upon falling asleep [60,61]. There is both correlative and causal evidence linking high LC activity to arousal and connectedness, and low noradrenaline levels during sleep to partial sensory disconnection. Noradrenaline is involved in the orienting response, affects the sensory response properties of thalamic and cortical neurons, and modulates the signal-to-noise ratio in sensory systems [76]. Optogenetic activation of the LC quickly awakens mice [77] and rats [6], independent of whether the stimulation occurs in NREM sleep or REM sleep, showing that increased noradrenergic signaling is sufficient to switch to a connected state. Furthermore, sustained high-frequency optogenetic stimulation of the LC in awake mice decreases noradrenaline levels in the prefrontal cortex and triggers behavioral arrests, during which the animals become unresponsive to tail and toe pinches [77]. Relative to controls, mice unable to produce noradrenaline require louder sounds to wake up from recovery sleep following sleep deprivation [78]. More recent evidence linking low levels of noradrenaline with disconnection comes from experiments in mice using genetically encoded noradrenaline sensors [79]. These studies showed that noradrenaline levels continue to decline in REM sleep, while during NREM sleep they fluctuate up and down every 30–50 s in the thalamus [80] and prefrontal cortex [81]. These infraslow fluctuations co-occur with changes in spindle activity, which in turn co-occur with changes in arousability in response to noise [82]. Thus, within the same period of NREM sleep, there are fewer awakenings in response to acoustic stimuli when spindle activity is higher [82], which is also when noradrenaline levels are lower [80,81]. This latter finding is consistent with previous evidence that the firing of LC neurons is lowest just before spindle onset and resumes at its offset [60,83]. Finally, optogenetic experiments in rats found that the silencing of LC neurons reduces the likelihood of sound-evoked awakenings from NREM sleep from 25% to as low as 15%, while the probability of waking up from REM sleep remains at 50% [6]. A seminal study found that in response to auditory, somatosensory, and visual stimuli, LC neurons acutely increase their firing during wake but less so in NREM sleep, and not at all during REM sleep [84]. The same stimuli, however, evoked a strong field potential in the LC region in all behavioral states, suggesting that during REM sleep, LC neurons continue to receive excitatory postsynaptic potentials but do not fire because they are under strong direct inhibition [84]. Such strong local inhibition could be a key mechanism accounting for increased arousal threshold in REM sleep and would explain why, during this phase, optogenetic silencing of LC neurons has no effects [6]. However, while there is direct evidence that minimal optogenetic activation of LC neurons increases the probability of sound-evoked arousal from REM sleep [6], there is no direct evidence that arousability during this phase increases if LC neurons are relieved from inhibition. Also, in humans, tonic and phasic periods of REM sleep are associated with lower and higher arousal thresholds, respectively (reviewed in [85]), but whether subtle changes in LC activity can account for this difference is not known.
In summary, there is direct evidence that manipulating noradrenaline levels during NREM sleep can affect arousability to some extent, with higher auditory arousal thresholds being associated with lower LC activity and higher spindle activity. However, noradrenaline levels are lowest in REM sleep, when the probability of waking up from a sound is much higher than in NREM sleep (50% vs. 25%). Thus, the deep sensory disconnection of NREM sleep must rely on mechanisms that only partially depend on LC activity, while direct inhibition of LC neurons may be a key factor for decreased responsiveness during REM sleep. The extent to which depressed LC activity during sleep contributes to reduced responsiveness to light, pain, tactile, or olfactory stimuli is unknown.
3.2. Histamine
Histaminergic neurons fire in waking and are less active or silent in NREM sleep and REM sleep [69,70]. Histaminergic neurons resume firing only after awakening [61], and their loss or inhibition does not affect baseline waking levels (e.g., [86]), indicating that these cells are not required to initiate or maintain waking. On the other hand, at the transition from waking to sleep, histaminergic neurons stop firing before the onset of EEG synchronization [87], suggesting that their silence may promote disconnection. Strong positive emotions can trigger cataplectic episodes in narcoleptic dogs, like in humans. During these events, the animals can still track external objects with their eyes and appear awake [88], suggesting that, like human patients, they maintain awareness of the external world. Electrophysiological recordings in dogs showed that during cataplexy, the firing of noradrenergic LC cells ceases, as during REM sleep [68], while serotoninergic neurons maintain some degree of activity, as during NREM sleep [68], and histaminergic neurons are equally or more active than during quiet waking [69]. Other studies show that cholinergic activation of the basal forebrain triggers cataplexy in narcoleptic dogs [89]. Thus, the activity of histaminergic cells may be critical to maintaining the connection with the external world during cataplexy. On the other hand, there is no direct evidence linking the decrease in activity of histaminergic neurons during sleep with the increase in arousal threshold.
3.3. Serotonin
The firing pattern of serotoninergic neurons in the dorsal raphe nucleus, as well as in the raphe nuclei of the pons and medulla, is similar to that of LC neurons, highest in active wake, lower in quiet wake, further decreasing in NREM sleep, and lowest in REM sleep [66,67,68,90,91]. Consistent with this pattern, extracellular levels of serotonin are higher in wake than in sleep in the frontal cortex and all subcortical areas tested so far [92]. The activity of many caudal serotoninergic neurons and dorsal raphe neurons has been linked to tonic motor activity and repetitive behaviors such as chewing and grooming [91]. Cats with lesions of the dorsomedial pons display REM sleep without muscle atonia; in this state, the animals are unresponsive to bright light and mild tactile stimuli, as expected during sleep, but the activity of serotoninergic neurons in the dorsal raphe can approach wake-like levels [93]. Conversely, after carbachol injections in the same pontine area, cats can track visual stimuli but are unable to move, and serotoninergic neurons in the dorsal raphe are silent [94]. In short, serotoninergic activity appears to track motor activity and not behavioral state per se.
Despite sharing a similar pattern of firing during the sleep/waking cycle, noradrenaline and serotonin often have opposite effects on brain areas crucial for arousal and sensory processing. In freely moving rats, the injection of noradrenaline in the basal forebrain promotes waking, depresses SWA, and increases gamma activity in the EEG, while the injection of serotonin in the same area does not increase waking but increases SWA and decreases gamma activity [95]. Serotoninergic activity inhibits the processing of visual stimuli in the thalamus and cortex. Thus, both the spontaneous and synaptically evoked activity of lateral geniculate neurons is increased by the local application of noradrenaline and decreased by that of serotonin [96]. In the primary visual cortex, the response to moving visual stimuli is mostly enhanced by the local application of noradrenaline and suppressed by serotonin [97]. Furthermore, orienting to a novel stimulus is associated with a decrease in the firing of many serotoninergic neurons in the dorsal raphe [98]. Taken together, these results suggest that during waking, high serotonin levels may facilitate motor output, while sensory processing may be promoted when the animal is not moving and serotoninergic neurons are less active.
Serotoninergic neurons in the dorsal raphe continue to respond to acoustic stimuli during slow-wave sleep [99], contrary to LC neurons [84] and dopaminergic neurons of the substantia nigra [100], whose response is greatly attenuated compared to waking. Mice with genetic lesions of serotoninergic neurons wake up normally in response to air puffs and sounds but show a very delayed arousal response to hypercapnia [101,102]. Many serotoninergic neurons increase firing in response to hypercapnia and/or acidosis [101,103]; in the medulla, these neurons function as respiratory chemoreceptors that project to and excite respiratory neurons; in the midbrain, raphe neurons behave as “arousal chemoreceptors”, causing awakening from sleep via direct and indirect projections to the thalamus, cortex, hypothalamus, and basal forebrain [104]. In the thalamus, for instance, in vitro studies show that both noradrenaline and serotonin promote the transition from burst to tonic firing and do so by depolarizing the GABAergic neurons of the reticular nucleus via inhibition of leak potassium channels [105] as well as by promoting the hyperpolarization-activated cation current Ih in thalamocortical relay neurons [106,107].
To summarize, serotonin does not appear to be involved in the rapid (a few seconds) arousal response to classical stimuli such as sounds, but is required for arousal to CO2, which occurs over many seconds.
3.4. Orexin
Among the arousal systems, the orexin cells are the only ones indispensable for maintaining waking, as shown by the fact that their loss causes fragmentation of the sleep/waking cycle and narcolepsy [108,109]. Orexin neurons are most active during active waking, virtually silent in NREM sleep, and only transiently active in REM sleep; their activity is linked to arousal, exploration, response to sounds, and goal-directed behavior rather than to movement per se, and their occasional firing in REM sleep is not associated with phasic muscle activity [71,72,87]. Like LC neurons, orexin neurons resume firing before awakening [71,87], and their optogenetic stimulation leads to awakening from both NREM sleep and REM sleep [110]. However, awakening is slow, occurring around 25 s after stimulation and abolished by the silencing of LC neurons [111]. Thus, increased orexinergic activity helps switching to a connected state only when noradrenergic neurons are intact. On the other hand, orexin neurons are still firing, albeit at low levels, at the onset of EEG synchronization upon falling asleep [87]. Thus, the transition from waking to sleep is characterized by an early decline in noradrenergic and histaminergic activity, followed, one second or so later, by a decline in orexinergic activity [61,87]. Contrary to noradrenergic and cholinergic fibers, orexin axons do not have direct excitatory effects on sensory areas of the thalamus and cortex. In the thalamus, they avoid sensory relay nuclei but project to, and excite, midline and intralaminar nuclei [112]. In the cortex, orexin selectively excites layer 6b neurons in the cingulate, motor, somatosensory, and visual areas but has no effect on other layers [113].
In summary, orexin promotes consolidated periods of waking but does not seem to play a key role in waking initiation, and experiments testing directly the link between orexinergic activity and arousability from sleep are lacking. On the other hand, orexin’s selective targeting of high order thalamic nuclei and cortical layer 6b, which is involved in long-range intracortical communications and not in the classical thalamocortical loop [114], suggests that its relative inactivity during sleep, especially during REM sleep, may contribute to sensory disconnection.
4. Conclusions
The literature about sensory disconnection in sleep is limited, and many intriguing observations about the underlying mechanisms come from old studies in which few neurons were recorded. In several experiments, neuronal responses in waking were compared only to those in NREM sleep or REM sleep, and most studies focused on the auditory system. An emerging theme is that there must be multiple “gates” along the sensory pathways to ensure sensory disconnection during sleep, and the relative importance of these gates is likely to vary between NREM sleep and REM sleep (Figure 1). In NREM sleep, the early focus on specific thalamic nuclei and primary cortical areas yielded mostly negative findings, while the more recent focus on high-order, multisensory thalamic nuclei and cortico-cortical communication beyond the input layer is more promising. During REM sleep, however, the available evidence points to the importance of early (pre-thalamic) gates. Finally, there is strong supporting evidence that the low activity of the noradrenergic system during sleep is crucial for sensory disconnection. However, indirect evidence suggests that the histaminergic and orexin neurons may also play a role, calling for new experiments.
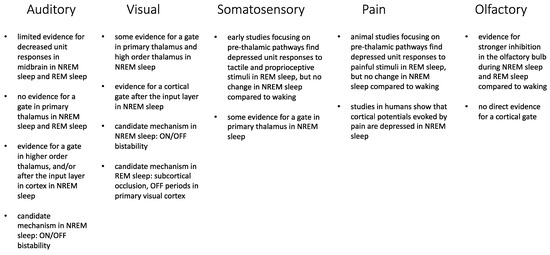
Figure 1.
Summary of the main sites of impaired sensory transmission (gates) during sleep. Some candidate mechanisms are also listed for the acoustic and visual systems, for which more data are available.
Author Contributions
C.C. and G.T. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Supported fully or in part by U.S. Department of Defense grant W911NF1910280 (CC, GT), NIH grant 1R01GM116916 (GT), and the Tiny Blue Dot Foundation (GT).
Conflicts of Interest
The authors declare no competing interests.
References
- Cirelli, C.; Tononi, G. Is sleep essential? PLoS Biol. 2008, 6, e216. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Hauri, P.; Zeitlin, M. Auditory awakening thresholds in REM and NREM sleep stages. Percept. Mot. Ski. 1966, 22, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Zepelin, H.; McDonald, C.S.; Zammit, G.K. Effects of age on auditory awakening thresholds. J. Gerontol. 1984, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Neckelmann, D.; Ursin, R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep 1993, 16, 467–477. [Google Scholar] [PubMed]
- Hayat, H.; Regev, N.; Matosevich, N.; Sales, A.; Paredes-Rodriguez, E.; Krom, A.J.; Bergman, L.; Li, Y.; Lavigne, M.; Kremer, E.J.; et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020, 6, eaaz4232. [Google Scholar] [CrossRef] [PubMed]
- Price, L.J.; Kremen, I. Variations in behavioral response threshold within the REM period of human sleep. Psychophysiology 1980, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ermis, U.; Krakow, K.; Voss, U. Arousal thresholds during human tonic and phasic REM sleep. J. Sleep Res. 2010, 19, 400–406. [Google Scholar] [CrossRef]
- Hall, R.D.; Borbely, A.A. Acoustically evoked potentials in the rat during sleep and waking. Exp. Brain Res. 1970, 11, 93–110. [Google Scholar] [CrossRef]
- Kakigi, R.; Naka, D.; Okusa, T.; Wang, X.; Inui, K.; Qiu, Y.; Tran, T.D.; Miki, K.; Tamura, Y.; Nguyen, T.B.; et al. Sensory perception during sleep in humans: A magnetoencephalograhic study. Sleep Med. 2003, 4, 493–507. [Google Scholar] [CrossRef]
- Dang-Vu, T.T.; Bonjean, M.; Schabus, M.; Boly, M.; Darsaud, A.; Desseilles, M.; Degueldre, C.; Balteau, E.; Phillips, C.; Luxen, A.; et al. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc. Natl. Acad. Sci. USA 2011, 108, 15438–15443. [Google Scholar] [CrossRef] [PubMed]
- Velluti, R.A. Interactions between sleep and sensory physiology. J. Sleep Res. 1997, 6, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Issa, E.B.; Wang, X. Sensory responses during sleep in primate primary and secondary auditory cortex. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 14467–14480. [Google Scholar] [CrossRef] [PubMed]
- Issa, E.B.; Wang, X. Altered neural responses to sounds in primate primary auditory cortex during slow-wave sleep. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 2965–2973. [Google Scholar] [CrossRef]
- Nir, Y.; Vyazovskiy, V.V.; Cirelli, C.; Banks, M.I.; Tononi, G. Auditory responses and stimulus-specific adaptation in rat auditory cortex are preserved across NREM and REM sleep. Cereb. Cortex 2015, 25, 1362–1378. [Google Scholar] [CrossRef]
- Sela, Y.; Vyazovskiy, V.V.; Cirelli, C.; Tononi, G.; Nir, Y. Responses in Rat Core Auditory Cortex are Preserved during Sleep Spindle Oscillations. Sleep 2016, 39, 1069–1082. [Google Scholar] [CrossRef]
- Sela, Y.; Krom, A.J.; Bergman, L.; Regev, N.; Nir, Y. Sleep Differentially Affects Early and Late Neuronal Responses to Sounds in Auditory and Perirhinal Cortices. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 2895–2905. [Google Scholar] [CrossRef]
- Hayat, H.; Marmelshtein, A.; Krom, A.J.; Sela, Y.; Tankus, A.; Strauss, I.; Fahoum, F.; Fried, I.; Nir, Y. Reduced neural feedback signaling despite robust neuron and gamma auditory responses during human sleep. Nat. Neurosci. 2022, 25, 935–943. [Google Scholar] [CrossRef]
- Wang, Y.; You, L.; Tan, K.; Li, M.; Zou, J.; Zhao, Z.; Hu, W.; Li, T.; Xie, F.; Li, C.; et al. A common thalamic hub for general and defensive arousal control. Neuron 2023, 111, 3270–3287.e8. [Google Scholar] [CrossRef]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of cortical effective connectivity during sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef]
- Vetter, K.; Boker, W. Zur funktion des K-komplexes im Schlaf-Elektrencephalogramm. Nervenarzt 1962, 33, 390–394. [Google Scholar] [PubMed]
- Colrain, I.M. The K-complex: A 7-decade history. Sleep 2005, 28, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Forget, D.; Morin, C.M.; Bastien, C.H. The role of the spontaneous and evoked k-complex in good-sleeper controls and in individuals with insomnia. Sleep 2011, 34, 1251–1260. [Google Scholar] [PubMed]
- Latreille, V.; von Ellenrieder, N.; Peter-Derex, L.; Dubeau, F.; Gotman, J.; Frauscher, B. The human K-complex: Insights from combined scalp-intracranial EEG recordings. NeuroImage 2020, 213, 116748. [Google Scholar] [CrossRef]
- Funk, C.M.; Honjoh, S.; Rodriguez, A.V.; Cirelli, C.; Tononi, G. Local Slow Waves in Superficial Layers of Primary Cortical Areas during REM Sleep. Curr. Biol. 2016, 26, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R. Evoked and spontaneous activity in single units of medial brain stem during natural sleep and waking. J. Neurophysiol. 1961, 24, 451–468. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Foulkes, D. Effect of Visual Stimuli on Dream Content. Percept. Mot. Ski. 1965, 20 (Suppl. 3), 1149–1160. [Google Scholar] [CrossRef]
- Yuzgec, O.; Prsa, M.; Zimmermann, R.; Huber, D. Pupil Size Coupling to Cortical States Protects the Stability of Deep Sleep via Parasympathetic Modulation. Curr. Biol. 2018, 28, 392–400.e3. [Google Scholar] [CrossRef]
- Dagnino, N.; Favale, E.; Loeb, C.; Manfredi, M. Sensory Transmission in the Geniculostriate System of the Cat during Natural Sleep and Arousal. J. Neurophysiol. 1965, 28, 443–456. [Google Scholar] [CrossRef]
- Malcolm, L.J.; Bruce, I.S.; Burke, W. Excitability of the lateral geniculate nucleus in the alert, non-alert and sleeping cat. Exp. Brain Res. 1970, 10, 283–297. [Google Scholar] [CrossRef]
- Coenen, A.M.; Vendrik, A.J. Determination of the transfer ratio of cat’s geniculate neurons through quasi-intracellular recordings and the relation with the level of alertness. Exp. Brain Res. 1972, 14, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J. Physiol. 1960, 150, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Galambos, R.; Juhasz, G.; Kekesi, A.K.; Nyitrai, G.; Szilagyi, N. Natural sleep modifies the rat electroretinogram. Proc. Natl. Acad. Sci. USA 1994, 91, 5153–5157. [Google Scholar] [CrossRef] [PubMed]
- Evarts, E.V. Photically Evoked Responses in Visual Cortex Units during Sleep and Waking. J. Neurophysiol. 1963, 26, 229–248. [Google Scholar] [CrossRef]
- Sakakura, H. Spontaneous and evoked unitary activities of cat lateral geniculate neurons in sleep and wakefulness. Jpn. J. Physiol. 1968, 18, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Angel, A.; Strata, P. Relationship between cortical activity and the excitability of optic nerve terminals in the lateral geniculate body. Brain Res. 1967, 5, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Iwama, K.; Sakakura, H.; Kasamatsu, T. Presynaptic inhibition in the lateral geniculate body induced by stimulation of the cerebral cortex. Jap J. Physiol. 1965, 15, 310–322. [Google Scholar]
- Evarts, E.V. Activity of Neurons in Visual Cortex of Cat during Sleep with Low Voltage Fast Eeg Activity. J. Neurophysiol. 1962, 25, 812–816. [Google Scholar] [CrossRef]
- Carli, G.; Dietespi, K.; Pompeiano, O. Transmission of Sensory Information through Lemniscal Pathway during Sleep. Arch. Ital. De Biol. 1967, 105, 31–51. [Google Scholar]
- Carli, G.; Dietespi, K.; Pompeiano, O. Presynaptic and Postsynaptic Inhibition of Transmission O Somatic Afferent Volleys through Cuneate Nucleus during Sleep. Arch. Ital. De Biol. 1967, 105, 52–82. [Google Scholar]
- Soja, P.J.; Oka, J.I.; Fragoso, M. Synaptic transmission through cat lumbar ascending sensory pathways is suppressed during active sleep. J. Neurophysiol. 1993, 70, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Soja, P.J.; Pang, W.; Taepavarapruk, N.; McErlane, S.A. Spontaneous spike activity of spinoreticular tract neurons during sleep and wakefulness. Sleep 2001, 24, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, M.; Formenti, A.; Mancia, M. Responses of VPL thalamic neurones to peripheral stimulation in wakefulness and sleep. Neurosci. Lett. 1989, 102, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gucer, G. The effect of sleep upon the transmission of afferent activity in the somatic afferent system. Exp. Brain Res. 1979, 34, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Cairns, B.E.; McErlane, S.A.; Fragoso, M.C.; Jia, W.G.; Soja, P.J. Spontaneous discharge and peripherally evoked orofacial responses of trigemino-thalamic tract neurons during wakefulness and sleep. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 8149–8159. [Google Scholar] [CrossRef] [PubMed]
- Cairns, B.E.; Fragoso, M.C.; Soja, P.J. Active-sleep-related suppression of feline trigeminal sensory neurons: Evidence implicating presynaptic inhibition via a process of primary afferent depolarization. J. Neurophysiol. 1996, 75, 1152–1162. [Google Scholar] [CrossRef]
- Leung, C.G.; Mason, P. Physiological properties of raphe magnus neurons during sleep and waking. J. Neurophysiol. 1999, 81, 584–595. [Google Scholar] [CrossRef]
- Foo, H.; Mason, P. Brainstem modulation of pain during sleep and waking. Sleep Med. Rev. 2003, 7, 145–154. [Google Scholar] [CrossRef]
- Beydoun, A.; Morrow, T.J.; Shen, J.F.; Casey, K.L. Variability of laser-evoked potentials: Attention, arousal and lateralized differences. Electroencephalogr. Clin. Neurophysiol. 1993, 88, 173–181. [Google Scholar] [CrossRef]
- Lavigne, G.; Zucconi, M.; Castronovo, C.; Manzini, C.; Marchettini, P.; Smirne, S. Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems. Pain 2000, 84, 283–290. [Google Scholar] [CrossRef]
- Badia, P.; Wesensten, N.; Lammers, W.; Culpepper, J.; Harsh, J. Responsiveness to olfactory stimuli presented in sleep. Physiol. Behav. 1990, 48, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Herz, R.S. Minimal olfactory perception during sleep: Why odor alarms will not work for humans. Sleep 2004, 27, 402–405. [Google Scholar] [PubMed]
- Stuck, B.A.; Stieber, K.; Frey, S.; Freiburg, C.; Hormann, K.; Maurer, J.T.; Hummel, T. Arousal responses to olfactory or trigeminal stimulation during sleep. Sleep 2007, 30, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Zhao, B.; Chen, S.; Ye, J.; Liu, J.; Liang, L.; Ding, H.; Schaefke, B.; Yang, Q.; Wang, L.; et al. The subthalamic corticotropin-releasing hormone neurons mediate adaptive REM-sleep responses to threat. Neuron 2022, 110, 1223–1239.e8. [Google Scholar] [CrossRef]
- Tsuno, Y.; Kashiwadani, H.; Mori, K. Behavioral state regulation of dendrodendritic synaptic inhibition in the olfactory bulb. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 9227–9238. [Google Scholar] [CrossRef]
- Murakami, M.; Kashiwadani, H.; Kirino, Y.; Mori, K. State-dependent sensory gating in olfactory cortex. Neuron 2005, 46, 285–296. [Google Scholar] [CrossRef]
- Yamaguchi, M. The role of sleep in the plasticity of the olfactory system. Neurosci. Res. 2017, 118, 21–29. [Google Scholar] [CrossRef]
- Moruzzi, G.; Magoun, H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 455–473. [Google Scholar] [CrossRef]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Bloom, F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. Off. J. Soc. Neurosci. 1981, 1, 876–886. [Google Scholar] [CrossRef]
- Takahashi, K.; Kayama, Y.; Lin, J.S.; Sakai, K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 2010, 169, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- el Mansari, M.; Sakai, K.; Jouvet, M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp. Brain Res. 1989, 76, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Boucetta, S.; Cisse, Y.; Mainville, L.; Morales, M.; Jones, B.E. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 4708–4727. [Google Scholar] [CrossRef]
- Lee, M.G.; Hassani, O.K.; Alonso, A.; Jones, B.E. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 4365–4369. [Google Scholar] [CrossRef]
- Xu, M.; Chung, S.; Zhang, S.; Zhong, P.; Ma, C.; Chang, W.C.; Weissbourd, B.; Sakai, N.; Luo, L.; Nishino, S.; et al. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 2015, 18, 1641–1647. [Google Scholar] [CrossRef]
- McGinty, D.J.; Harper, R.M. Dorsal raphe neurons: Depression of firing during sleep in cats. Brain Res. 1976, 101, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Trulson, M.E.; Jacobs, B.L. Raphe unit activity in freely moving cats: Correlation with level of behavioral arousal. Brain Res. 1979, 163, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; John, J.; Boehmer, L.N.; Yau, D.; Nguyen, G.B.; Siegel, J.M. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J. Physiol. 2004, 554, 202–215. [Google Scholar] [CrossRef]
- John, J.; Wu, M.F.; Boehmer, L.N.; Siegel, J.M. Cataplexy-active neurons in the hypothalamus: Implications for the role of histamine in sleep and waking behavior. Neuron 2004, 42, 619–634. [Google Scholar] [CrossRef]
- Takahashi, K.; Lin, J.S.; Sakai, K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10292–10298. [Google Scholar] [CrossRef]
- Lee, M.G.; Hassani, O.K.; Jones, B.E. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 6716–6720. [Google Scholar] [CrossRef]
- Mileykovskiy, B.Y.; Kiyashchenko, L.I.; Siegel, J.M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 2005, 46, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Dahan, L.; Astier, B.; Vautrelle, N.; Urbain, N.; Kocsis, B.; Chouvet, G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology 2007, 32, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Eban-Rothschild, A.; Rothschild, G.; Giardino, W.J.; Jones, J.R.; de Lecea, L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016, 19, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.R.; Treweek, J.B.; Robinson, J.E.; Xiao, C.; Bremner, L.R.; Greenbaum, A.; Gradinaru, V. Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron 2017, 94, 1205–1219.e8. [Google Scholar] [CrossRef] [PubMed]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; de Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Hunsley, M.S.; Palmiter, R.D. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharm. Biochem. Behav. 2004, 78, 765–773. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.; Lischinsky, J.E.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H.; et al. A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron 2019, 102, 745–761.e8. [Google Scholar] [CrossRef]
- Osorio-Forero, A.; Cardis, R.; Vantomme, G.; Guillaume-Gentil, A.; Katsioudi, G.; Devenoges, C.; Fernandez, L.M.J.; Luthi, A. Noradrenergic circuit control of non-REM sleep substates. Curr. Biol. 2021, 31, 5009–5023.e7. [Google Scholar] [CrossRef]
- Kjaerby, C.; Andersen, M.; Hauglund, N.; Untiet, V.; Dall, C.; Sigurdsson, B.; Ding, F.; Feng, J.; Li, Y.; Weikop, P.; et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat. Neurosci. 2022, 25, 1059–1070. [Google Scholar] [CrossRef]
- Lecci, S.; Fernandez, L.M.; Weber, F.D.; Cardis, R.; Chatton, J.Y.; Born, J.; Luthi, A. Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci. Adv. 2017, 3, e1602026. [Google Scholar] [CrossRef] [PubMed]
- Swift, K.M.; Gross, B.A.; Frazer, M.A.; Bauer, D.S.; Clark, K.J.D.; Vazey, E.M.; Aston-Jones, G.; Li, Y.; Pickering, A.E.; Sara, S.J.; et al. Abnormal Locus Coeruleus Sleep Activity Alters Sleep Signatures of Memory Consolidation and Impairs Place Cell Stability and Spatial Memory. Curr. Biol. 2018, 28, 3599–3609.e4. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Bloom, F.E. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. Off. J. Soc. Neurosci. 1981, 1, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Simor, P.; van der Wijk, G.; Nobili, L.; Peigneux, P. The microstructure of REM sleep: Why phasic and tonic? Sleep Med. Rev. 2020, 52, 101305. [Google Scholar] [CrossRef] [PubMed]
- Venner, A.; Mochizuki, T.; De Luca, R.; Anaclet, C.; Scammell, T.E.; Saper, C.B.; Arrigoni, E.; Fuller, P.M. Reassessing the Role of Histaminergic Tuberomammillary Neurons in Arousal Control. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 8929–8939. [Google Scholar] [CrossRef]
- Takahashi, K.; Lin, J.S.; Sakai, K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience 2008, 153, 860–870. [Google Scholar] [CrossRef]
- Nishino, S.; Mignot, E. Pharmacological aspects of human and canine narcolepsy. Prog. Neurobiol. 1997, 52, 27–78. [Google Scholar] [CrossRef]
- Nishino, S.; Tafti, M.; Reid, M.S.; Shelton, J.; Siegel, J.M.; Dement, W.C.; Mignot, E. Muscle atonia is triggered by cholinergic stimulation of the basal forebrain: Implication for the pathophysiology of canine narcolepsy. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 4806–4814. [Google Scholar] [CrossRef]
- Lydic, R.; McCarley, R.W.; Hobson, J.A. Serotonin neurons and sleep. I. Long term recordings of dorsal raphe discharge frequency and PGO waves. Arch. Ital. De Biol. 1987, 125, 317–343. [Google Scholar]
- Jacobs, B.L.; Fornal, C.A. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 1999, 21, 9S–15S. [Google Scholar] [CrossRef] [PubMed]
- Portas, C.M.; Bjorvatn, B.; Ursin, R. Serotonin and the sleep/wake cycle: Special emphasis on microdialysis studies. Prog. Neurobiol. 2000, 60, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Trulson, M.E.; Jacobs, B.L.; Morrison, A.R. Raphe unit activity during REM sleep in normal cats and in pontine lesioned cats displaying REM sleep without atonia. Brain Res. 1981, 226, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Steinfels, G.F.; Heym, J.; Strecker, R.E.; Jacobs, B.L. Raphe unit activity in freely moving cats is altered by manipulations of central but not peripheral motor systems. Brain Res. 1983, 279, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cape, E.G.; Jones, B.E. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 2653–2666. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Aghajanian, G.K. Norepinephrine and serotonin: Opposite effects on the activity of lateral geniculate neurons evoked by optic pathway stimulation. Exp. Neurol. 1980, 69, 678–694. [Google Scholar] [CrossRef]
- Waterhouse, B.D.; Azizi, S.A.; Burne, R.A.; Woodward, D.J. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 1990, 514, 276–292. [Google Scholar] [CrossRef]
- Fornal, C.A.; Metzler, C.W.; Marrosu, F.; Ribiero-do-Valle, L.E.; Jacobs, B.L. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral-buccal movements. Brain Res. 1996, 716, 123–133. [Google Scholar] [CrossRef]
- Heym, J.; Trulson, M.E.; Jacobs, B.L. Raphe unit activity in freely moving cats: Effects of phasic auditory and visual stimuli. Brain Res. 1982, 232, 29–39. [Google Scholar] [CrossRef]
- Steinfels, G.F.; Heym, J.; Strecker, R.E.; Jacobs, B.L. Response of dopaminergic neurons in cat to auditory stimuli presented across the sleep-waking cycle. Brain Res. 1983, 277, 150–154. [Google Scholar] [CrossRef]
- Buchanan, G.F.; Richerson, G.B. Central serotonin neurons are required for arousal to CO2. Proc. Natl. Acad. Sci. USA 2010, 107, 16354–16359. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; De Luca, R.; Khanday, M.A.; Bandaru, S.S.; Thomas, R.C.; Broadhurst, R.Y.; Venner, A.; Todd, W.D.; Fuller, P.M.; Arrigoni, E.; et al. Role of serotonergic dorsal raphe neurons in hypercapnia-induced arousals. Nat. Commun. 2020, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.E.; Hodges, M.R.; Wu, Y.; Wang, W.; Wylie, C.J.; Deneris, E.S.; Richerson, G.B. Medullary serotonin neurons and central CO2 chemoreception. Respir. Physiol. Neurobiol. 2009, 168, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Saper, C.B. Neural Circuitry Underlying Waking Up to Hypercapnia. Front. Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef]
- McCormick, D.A.; Wang, Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J. Physiol. 1991, 442, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; McCormick, D.A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 1989, 340, 715–718. [Google Scholar] [CrossRef]
- Lee, K.H.; McCormick, D.A. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron 1996, 17, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Faraco, J.; Li, R.; Kadotani, H.; Rogers, W.; Lin, X.; Qiu, X.; de Jong, P.J.; Nishino, S.; Mignot, E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999, 98, 365–376. [Google Scholar] [CrossRef]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.C.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef]
- Adamantidis, A.R.; Zhang, F.; Aravanis, A.M.; Deisseroth, K.; de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007, 450, 420–424. [Google Scholar] [CrossRef]
- Carter, M.E.; Brill, J.; Bonnavion, P.; Huguenard, J.R.; Huerta, R.; de Lecea, L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc. Natl. Acad. Sci. USA 2012, 109, E2635–E2644. [Google Scholar] [CrossRef] [PubMed]
- Bayer, L.; Eggermann, E.; Saint-Mleux, B.; Machard, D.; Jones, B.E.; Muhlethaler, M.; Serafin, M. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 7835–7839. [Google Scholar] [CrossRef] [PubMed]
- Bayer, L.; Serafin, M.; Eggermann, E.; Saint-Mleux, B.; Machard, D.; Jones, B.E.; Muhlethaler, M. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6760–6764. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, T.A.; Ledderose, J.; Toumazou, M.; Trimbuch, T.; Oram, T.; Rosenmund, C.; Eickholt, B.J.; Sachdev, R.N.S.; Larkum, M.E. Layer 6b Is Driven by Intracortical Long-Range Projection Neurons. Cell Rep. 2020, 30, 3492–3505.e5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).