1. Introduction
Symmetric antennary oligoglycines have recently attracted research interest due to their specific performance in aqueous environments and because of their high potential for various applications, such as water purification from bacterial endotoxins [
1], antiviral activity [
2,
3], and ultra-regular coatings at solid substrates [
2,
4]. These compounds are synthetic products of a well-focused molecular design and are characterized by the presence of oligoglycine tails (antennae) of equal lengths attached to a common central portion: either to a C-atom or to both ends of a hydrocarbon chain. The antennary oligoglycines have a distinctive mode of self-organization in aqueous media and form nanostructures named
tectomers [
2]. The supramolecular entities appear as a result of specific couplings between structural portions of the molecules and the fine balance among several interaction tendencies. These include (1) the possibility for the onset of Polyglycine II (PGII) motifs; (2) electrostatic interactions; and (3) hydrophobic interactions. Polyglycine II arrangements are known to initiate crystalline structures of bolaamphiphiles, polyglycines, etc. [
5,
6,
7,
8,
9], and have been detected in aqueous solutions of antennary oligoglycines as well [
1,
2,
10,
11,
12]. Because of the existence of a common central portion at which the antennae are attached and due to the potential for the onset of intra- and intermolecular PGII-portions, highly coordinated intra- and intermolecular H-bonding networks of considerable stability can be formed, related to conspicuous preferences of additional ‘click-clack’ tendencies [
1,
2]. Depending on the number of the antennae (two-antennary, three-antennary, four-antennary) and on the type of the central connection portion (carbon atom, hydrocarbon chain), these compounds may be hydrophilic (four-antennary oligoglycines) or amphiphilic (two- and three-antennary oligoglycines). In addition, the presence of positive electric charges at the terminal amine groups of the antennae (NH
3+) in an aqueous environment presupposes the onset of electrostatic interactions with negatively charged species, e.g., bacterial lipopolysaccharides, mica substrates, etc. [
1,
2,
4].
So far, the hydrophilic four-antennary [
1] and the amphiphilic two-antennary [
11] oligoglycines have been systematically studied, with the accent on monitoring of their self-assembly via control of the solution conditions: pH, ionic strength, temperature, etc. Concerning the available information about the performance of three-antennary oligoglycines in aqueous media, only some data from single-test experiments have been shortly mentioned in an early overview of the general types of these oligopeptides [
2]. The test outcomes were related to the formation of highly ordered coatings on a solid support. The oligoglycine molecules initiated the onset of flat mono- and bilayers on a (charged) mica surface surrounded by a water environment, with layer thicknesses of 2.5 nm and/or 5 nm, as determined by the AFM techniques. The suggested adsorption mechanism was based on a provisional formation of initial bulk vesicular tectomers that collapse in the vicinity of the solid plate. However, definitive proof of the presence of such vesicles in the aqueous bulk has not been reported. In this case, the oligoglycine adsorption is governed exclusively by electrostatic attractions between the positive electric charges at the terminal amine groups (NH
3+) of the antennae and the negatively charged mica plate. The stacking between the molecules inside the layer is supposed to be due to the onset of PGII motifs, while the appearance of a second layer is allegedly related to hydrophobic interactions among the CH
3—groups attached to the core C-atom of the oligoglycines. So, to the best of our knowledge, a detailed investigation of the structure–property relationships for three-antennary oligoglycines in aqueous solution formulations is missing, and a dedicated examination regarding their possible assembly in aqueous solutions and at fluid interfaces has not been reported.
The specific molecular structure of the three-antennary oligoglycines and their properties stand somewhat in-between those of the four-antennary and the two-antennary compounds. These compounds possess three hydrophilic antennae of equal lengths but have a short hydrophobic portion (-CH
3), which still defines these substances as amphiphilic. It has been known that, for the onset of PGII motifs, the presence of at least several glycine residues per antenna (minimum of four) is required, and these were the cases of two- and four-antennary oligoglycines [
1,
2,
3,
4,
11]. Our previous results on the two-antennary species disclose the fact that the length of the hydrophilic antennae has an impact on several structure-property relationships: surface tension, stabilization of microscopic foam films, etc. [
11,
12]. These effects are related to a possible shift of the hydrophilic/hydrophobic balance of the oligoglycine molecule due to changing the glycine-tail lengths. So, in the present study we investigate two types of three-antennary specimens, containing either five (CH
3C(-CH
2-NH-Gly
5)
3, T3Gly
5) or seven (CH
3C(-CH
2-NH-Gly
7)
3, T3Gly
7) glycine units in their antennae, so as to ensure that these compounds are capable of developing highly ordered H-bonding portions (e.g., PGII).
The goal of the present investigation is to examine the interfacial behavior and the bulk self-assembly of these two types of three-antennary oligopeptides. The aim is to clarify the effect of the antennae lengths and of the oligoglycine concentrations on the bulk solution properties of aqueous solutions at ambient conditions (room temperature, ‘native’ pH), on the adsorption-layer formation at the water–air interface, and on the performance of microscopic foam films. The obtained results are discussed in view of the effects of the hydrophilic/hydrophobic balance on the possible formation of supramolecular species and, consequently, on the prospects for further fine-tuning of the structure–property relationships in aqueous solution formulations.
2. Materials and Methods
The two types of synthetic three-antennary oligoglycines, T3Gly5 and T3Gly7, used in the present study, were purchased from PlasmaChem (Berlin, Germany) in salt forms: CH3C(-CH2-NH-Gly5)3*3TFA and CH3C(-CH2-NH-Gly7)3*3HCl; here, TFA denotes trifluoroacetic acid (CF3COOH), and Gly stands for a glycine residue. The substances were dissolved in doubly distilled (2D) water, and the temperature during all the experiments was maintained at 20.0 ± 0.1 °C. Since no specific pH regulation was performed, the so-called ‘native’ pH values were obtained. They were found to be within the pH range of 5.2 (1 × 10−4 mol/L) to 4.3 (5 × 10−4 mol/L) for the T3Gly5 samples and of 5.3 (1 × 10−4 mol/L) to 4.2 (1 × 10−3 mol/L) for the T3Gly7 cases, showing a gradual decrease in solution pH with the rise in the concentrations.
Due to the specific structure of the compounds (presence of hydrophobic -CH3 groups and PGII motifs, options for charging the terminal NH2 group to NH3+), a combined experimental protocol was applied. The aim was to clarify how the changes in the antennae lengths and the concentrations affect the complex interplay of the bulk and interfacial performance of the three-antennary oligoglycines in aqueous media.
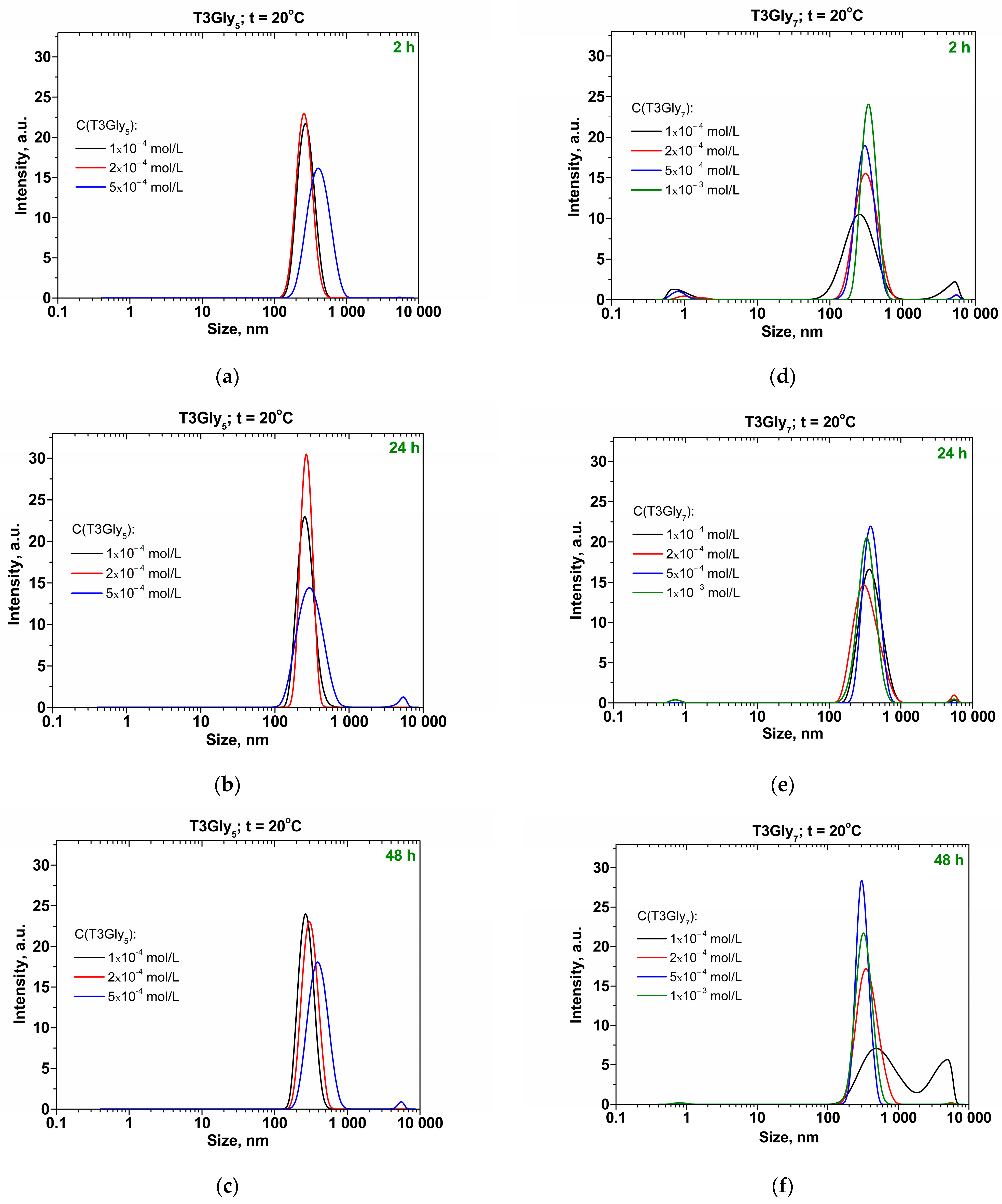
The investigation of the bulk solution properties was performed by dynamic light scattering [
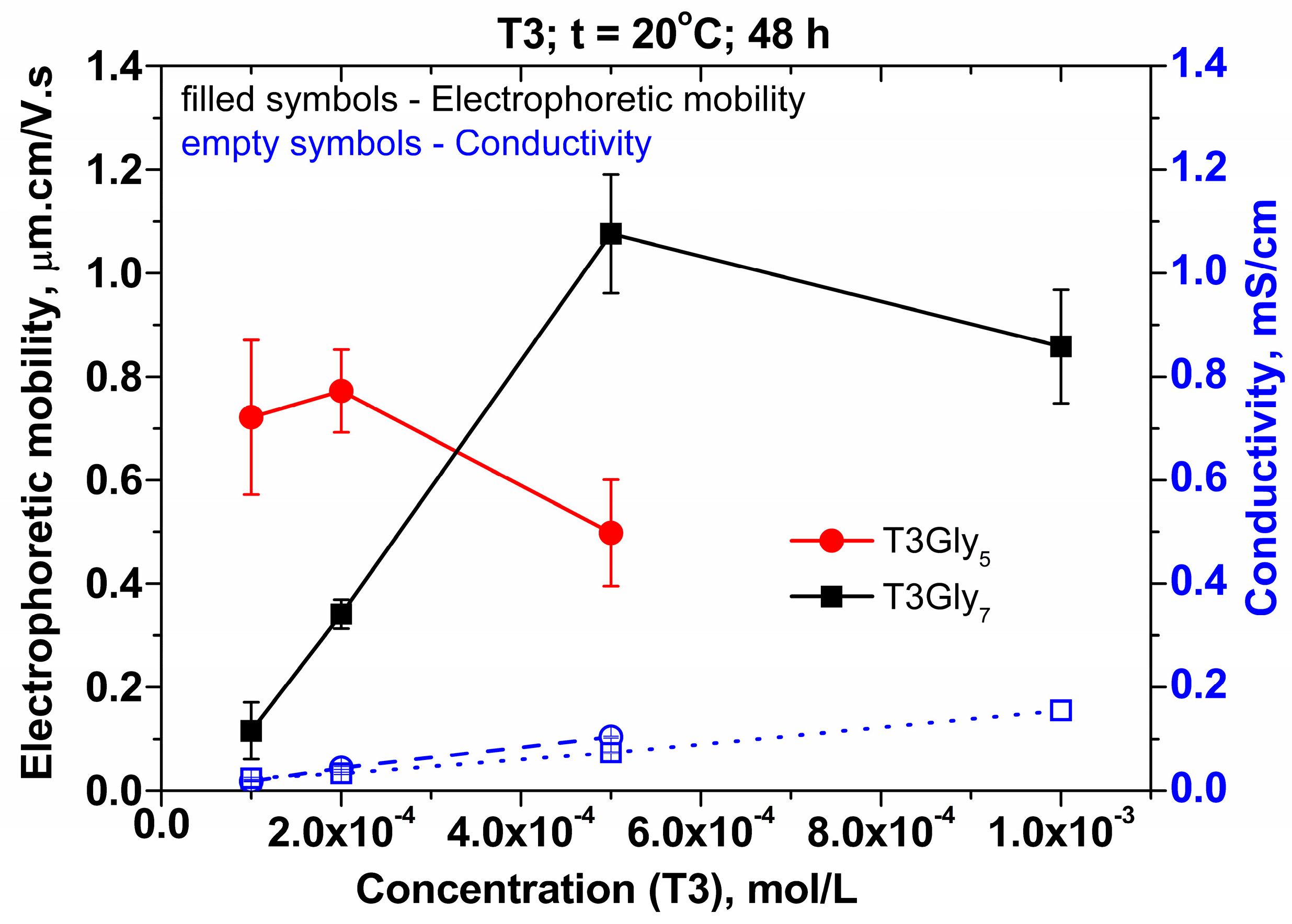
13] with a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK). The scattering angle was 173° (non-invasive backwards scattering). The recording of the size distribution in the aqueous solution bulk was carried out at the following incubation times of the aqueous samples: 2 h, 24 h, and 48 h. The electrophoretic mobility was recorded by the method of electrophoresis.
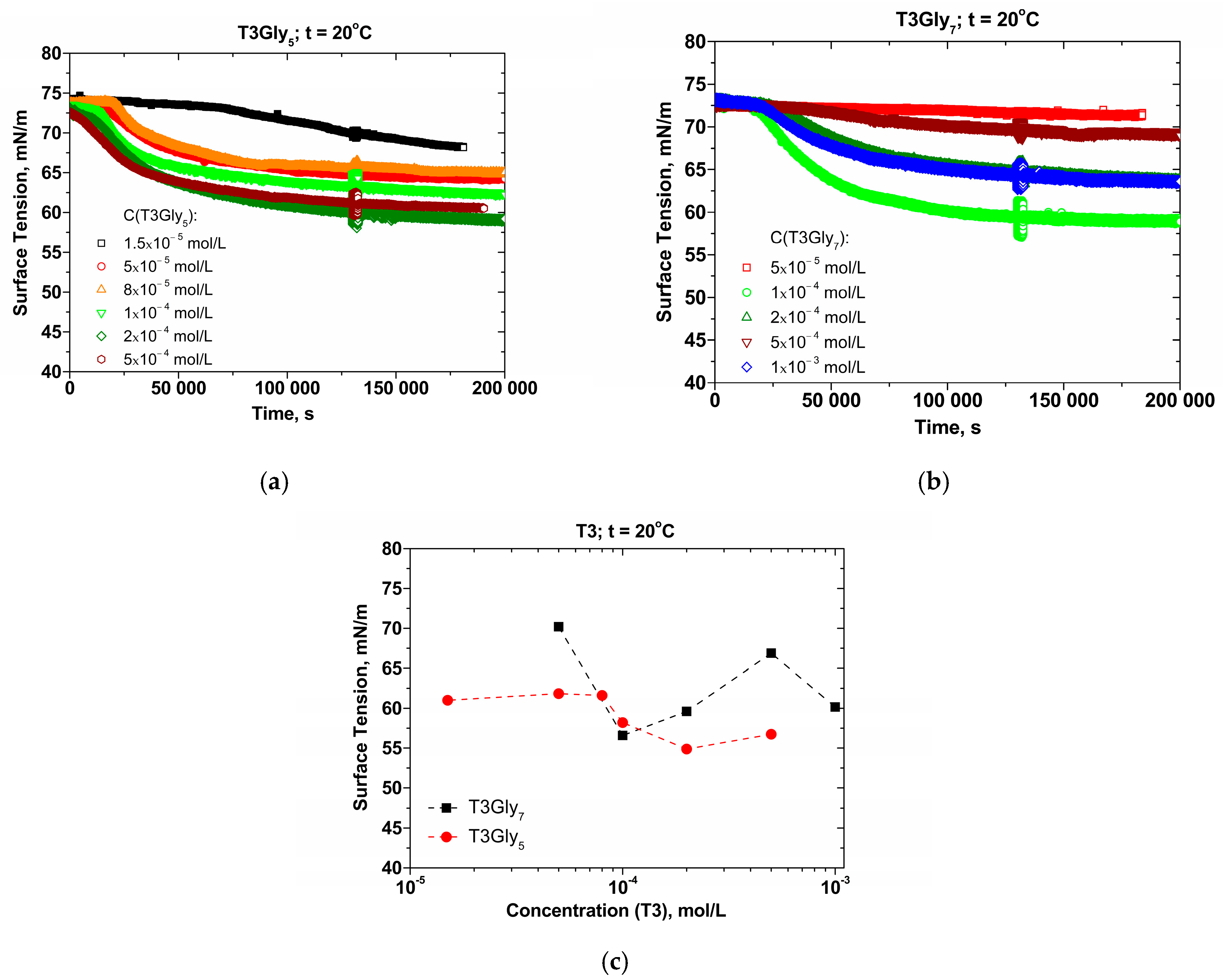
The adsorption layer properties at the air/solution interface were examined by the emerging-bubble option of the Profile Analysis Tensiometry (PAT-1, Sinterface, Berlin, Germany) [
14,
15]. The measurements were implemented in the course of more than 48 h, with the temperature kept at 20.0 ± 0.1 °C. The extended time duration was required because of the expected slow establishment of the surface tension equilibrium, as has already been found for other types of synthetic antennary oligoglycines [
1,
11,
12]. Surface dilational rheology was explored through oscillations within the low-frequency interval of 0.005–0.2 Hz; the oscillation amplitudes were in the range of 5–10% of the bubble’s surface area.
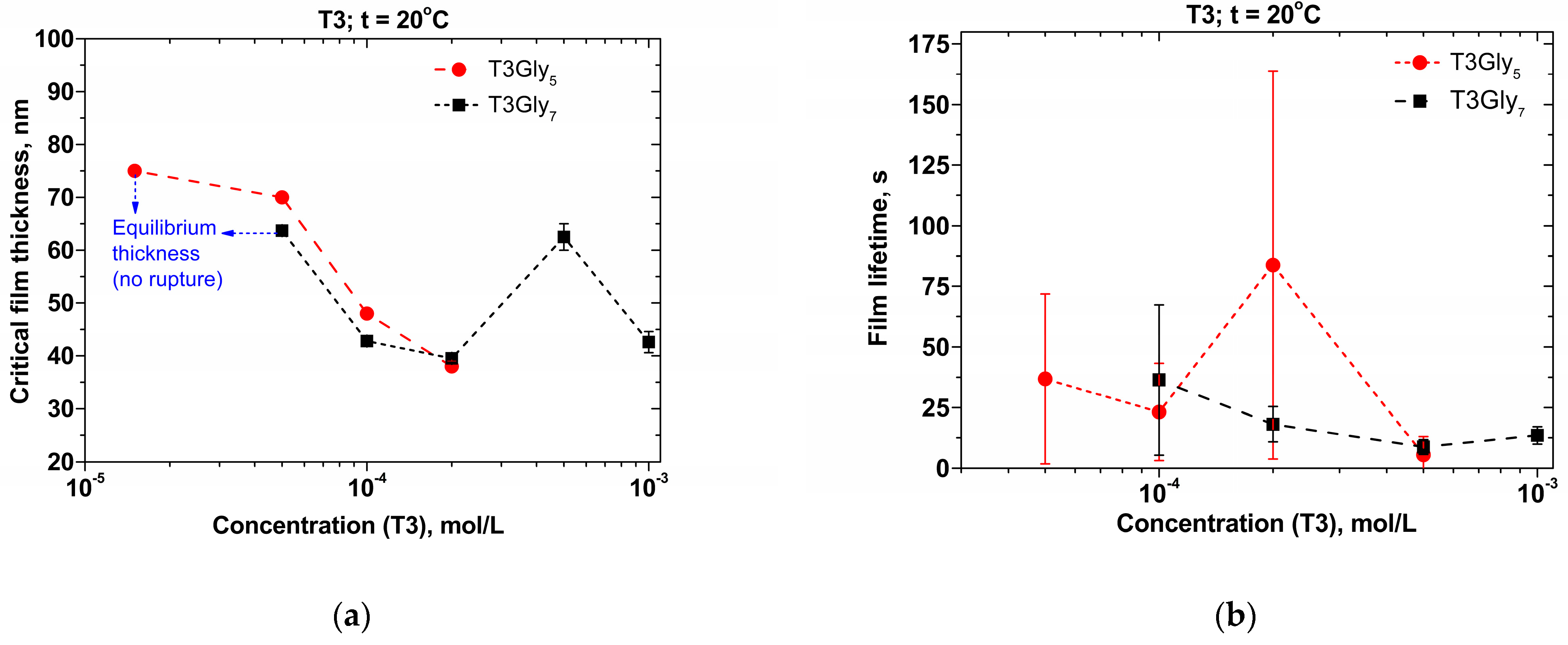
The drainage performance and the stability of microscopic foam films were studied using the original microinterferometric thin-liquid-film techniques (TLF) equipped with a Scheludko–Exerowa cell [
16,
17]. The cell was situated in a specially designed thermostatic unit of the instrumentation; the film experiments were performed following 2 h of thermal incubation at 20.0 ± 0.1 °C.
4. Discussion
The juxtaposition of the PAT and TLF results of the two types of oligoglycines, and in view of the obtained DLS data, allows the advance of the following hypotheses about the possible structure–property relationships in the investigated systems.
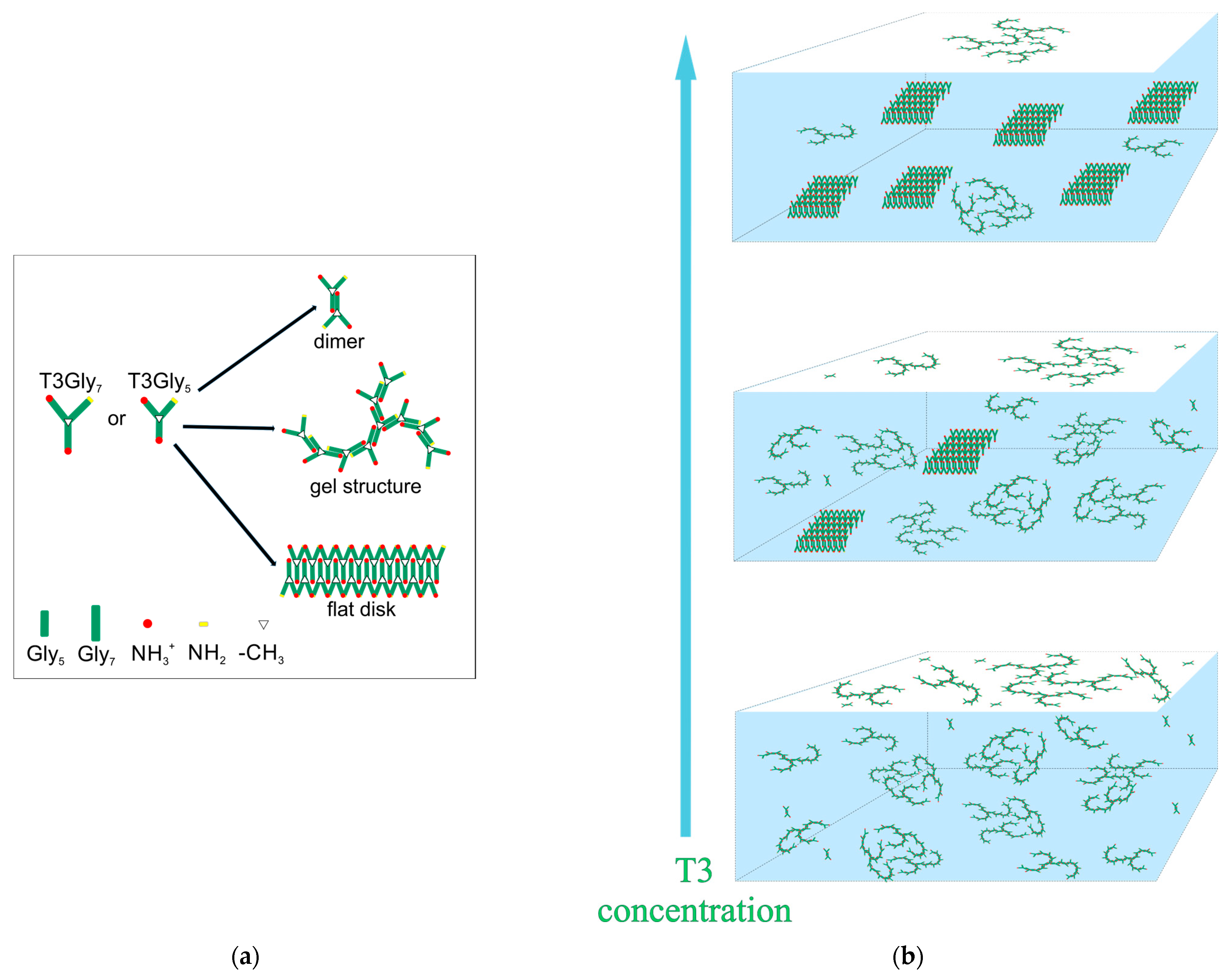
Three types of possible supramolecular items may appear in the bulk of aqueous solutions of the three-antennary oligoglycines T3Gly
5 and T3Gly
7:
dimers,
gel-like structures, and
disk-like tectomers (
Scheme 1). Most probably, these structural entities appear in this order upon an increase in the concentration of the oligopeptides. The role of the lengths of the antennae is also important. Shorter tails initiate gel-like structures at lower surfactant concentration as compared to the case of longer tails. This issue might be related to the weaker capacity to create PGII intra- and intermolecular motifs due to the relatively small oligoglycine antennae of the T3Gly
5 molecules as compared to the T3Gly
7 molecule.
At low oligoglycine concentrations, it is supposed that gel-like configurations and some dimer species are the prevailing self-assembled entities. The gel-like elements are formed due to the specific three-tailed structure of the single molecule, and because of the possibility for the onset of PGII motifs between single antennae, each of them is connected to two different molecules. Then, a third molecule could form another PGII motif on the tail portions of the two already click-clacked molecules, etc., initiating branched thread-like arrangements. Similar gelation phenomena are known to appear in water environments containing low-molecular-mass (LMM) hydrogelators due to the active role of noncovalent interactions that promote ‘anisotropic aggregation’ [
18]. Owing to the presence of the hydrophobic -CH
3 groups, these gel-like structures can also be adsorbed at the air/solution interface, forming an oligoglycine network that might initiate, e.g., the obtained surface dilational viscosity effects in the T3Gly
5 samples, as presented in
Figure 4b. In the bulk of the microscopic foam films and because of the confined space between the two air/solution interfaces, the presence of such gel-like elements may also cause the formation of loose bulk gel-like network elements that lead to an enhancement of the foam-film stability in the case of the lowest concentration samples, thus preventing TLF rupture (
Figure 5 and
Figure 6a).
At higher T3 concentrations, the onset of PGII options is boosted, and two-layered disc-like tectomers may appear as well. Insofar as the latter are hydrophilic, their onset affects the outcomes of surface-tension and surface-rheology measurements, and these nanostructures cannot stabilize the foam films during the drainage processes. Such phenomena may be related to the obtained data about all the films at a higher T3 content, which always rupture, usually in less than one minute (
Figure 6b). Moreover, the disc-like entities should be more easily formed in the T3Gly
7 samples and will be more stable than those in the T3Gly
5 solutions because of the higher capacity to form PGII motifs due to the longer oligoglycine antennae.
In the investigated cases, aside from the onset of the described tectomeric structures presented in
Scheme 1a,b, no vesicular tectomers are expected to appear. If vesicular bulk entities appear, the small hydrophobic portion of the tectomers (-CH
3) should be situated predominantly inside the vesicle in a double or single oligoglycine layer and cannot cause the clearly outlined decrease in the surface tension values shown in
Figure 3a–c. If there is an eventual formation of vesicular species, and if these vesicles prevail in the size distribution of the bulk supramolecular entities, they will not be surface active and/or easily reorganized at the soft air/solution interface so as to substantiate the ob-tained experimental data.
The hypotheses about the described three types of supramolecular entities are supported by the synchrony of the obtained experimental data from the surface tension and surface rheology studies and by the TLF drainage performance against the oligoglycine concentration. The slightly changeable bulk size distributions of the tectomers registered in the DLS data (
Figure 1) may then be related to the specific concentration ranges, investigated in the present study. Here, only the number of the tectomers is increased upon higher oligoglycine content, but the incubation time (up to 48 h) is not sufficient for these entities to proceed further in the formation of larger species from the smaller tectomeric structures. The only exception is at the lowest C(T3Gly
7) = 1 × 10
−4 mol/L, and this might then be interpreted as a plausible combination of stronger PGII tendencies that initiate (extended) gel-like elements during the larger incubation time (48 h), in the case of longer oligoglycine antennae T3Gly
7, compared to the solutions of T3Gly
5 (
Figure 1c,f).
Test DLS experiments with samples of considerably higher oligoglycine concentration C(T3Gly
7) = 1 × 10
−2 mol/L, following 24 h incubation time, and at three different temperatures give additional support for this notion. As can be seen, at moderate temperature changes, the tectomeric aggregates are quite stable, and there is only a minor shift of the size distribution towards smaller entities at 40 °C (
Figure 7).
The proposed idea about the three types of tectomeric aggregates formed in aqueous solutions of the investigated three-antennary oligoglycines is well-evidenced by the present experimental data, and the obtained results constitute a good basis for further structure-properties investigations. The present outcomes will be further substantiated through additional systematic studies related to the impact of higher oligoglycine concentrations and changes in the aqueous environment conditions (e.g., pH regulation, addition of low-molecular-mass electrolytes, temperature variations, etc.). The prospective application of other types of research instrumentation is envisaged as well.
5. Conclusions
The bulk and interfacial structural peculiarities in aqueous solutions of two types of synthetic three-antennary oligoglycines—T3Gly5 and T3Gly7—are investigated. The accent is on the effects of moderate changes in the concentrations and of the lengths of the antennae. The key results are the following:
The bulk size distributions of the tectomeric entities are established within a 2 h incubation time of the samples. They remain of the same order of magnitude within the examined concentration ranges and do not change substantially upon an increase in the initial incubation times of up to 48 h.
Although the structure of the three-antennary oligoglycines does not imply that they are well-defined surface-active substances, a considerable surface tension decrease is obtained upon an increase in their concentrations. Generally, the values achieved for the compound with shorter tails (T3Gly5) are lower as compared to those with the longer antennae (T3Gly7). The attained surface dilational elasticities are also quite high.
All microscopic foam films for the investigated concentrations are rupturing, except for the lowest oligoglycine quantities where TLFs equilibrium thickness values are registered (non-rupturing films).
The results from the combined experimental procedures, based on the application of DLS, PAT, and TLF instrumentations, allow us to advance plausible hypotheses about the structural peculiarities of the investigated systems. Due to synchronized coupling of noncovalent interactions in the aqueous solutions of the three-antennary oligoglycines, specific bulk and interfacial tectomeric species are formed. The tectomers are predominantly of three types: dimers, gel-like entities, and disk-like tectomers.
To the best of our knowledge, systematic studies on the structure–property relationships in aqueous environments and at the air–solution interface for three-antennary oligoglycines are performed and reported here for the first time. Further investigations for the performance of these aqueous solution formulations and application of additional research methodologies (e.g., TEM analysis) are planned.
The experimental data reported here unlock important options for possible innovative applications. Being biocompatible and comparatively easy to be synthesized, the three-antennary oligoglycines have an extraordinary potential to be used for various biomedical, tissue-engineering, biocatalytic, and biosensing purposes.