Innovative Preparation of Salted Duck Egg White Lysozyme Functional Film and Its Application in Fresh Storage of Small Nectarines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of SDEWL-CMCS Composite Films
2.3. Properties of SDEWL-CMCS Composite Films
2.3.1. Measurement of Thickness
2.3.2. Water Solubility (WS)
2.3.3. Water Vapor Transmittance Rate (WVTR)
2.3.4. Oil Resistance Performance
2.3.5. Mechanical Properties
2.3.6. Antibacterial Properties
2.4. Characterization of SDEWL-CMCS Composite Films
2.4.1. UV Spectral Analysis
2.4.2. FTIR Spectrometer
2.4.3. XRD Analysis
2.4.4. SEM Analysis
2.5. Application of SDEWL-CMCS Composite Films
2.5.1. Soluble Solid Content (SSC)
2.5.2. Weight Loss Rate (WLR)
2.5.3. Color
2.5.4. Ascorbic Acid Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Basic Properties of SDEWL-CMCS Composite Films
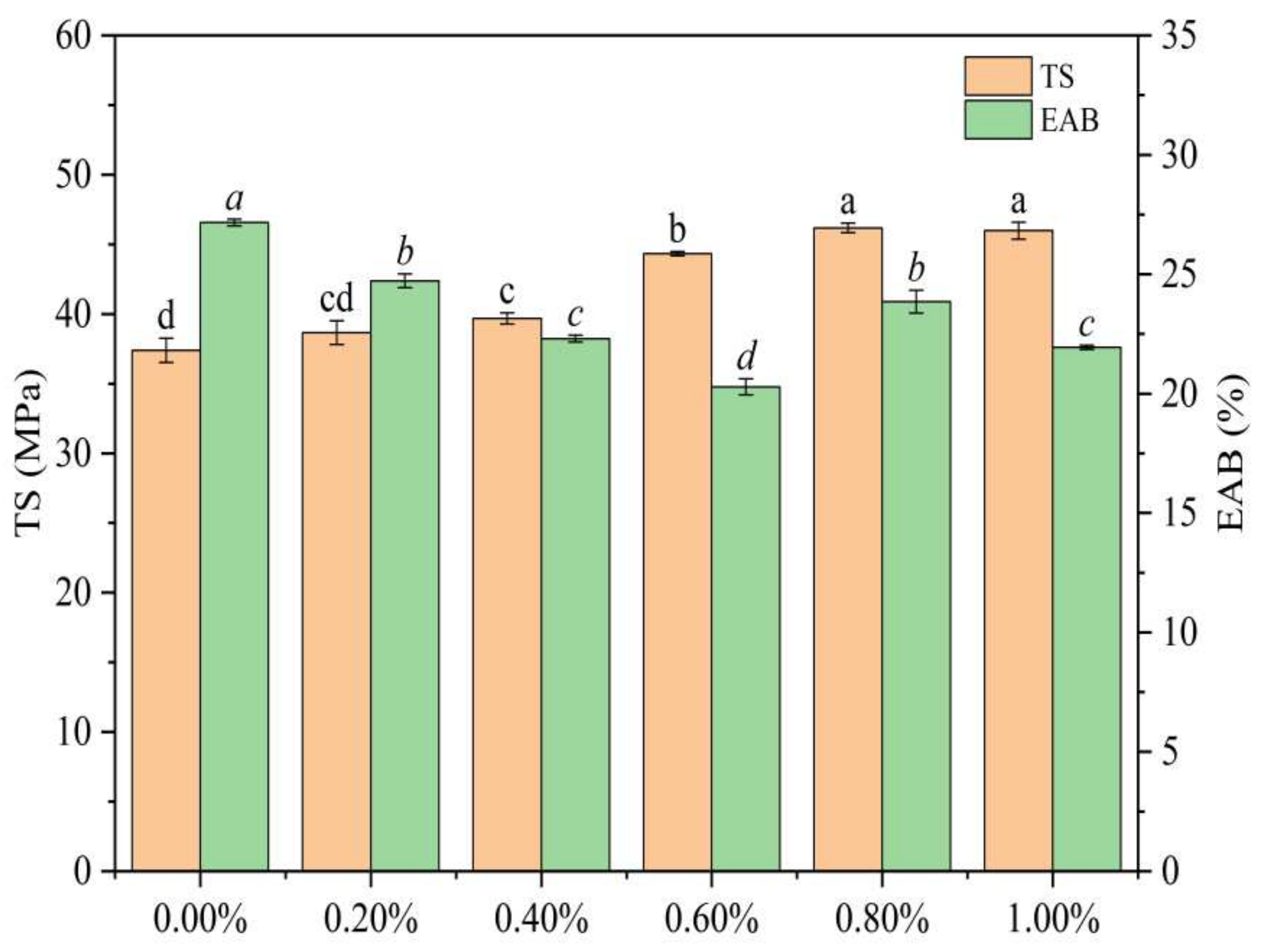
3.2. Analysis of Mechanical Properties of SDEWL-CMCS Composite Films
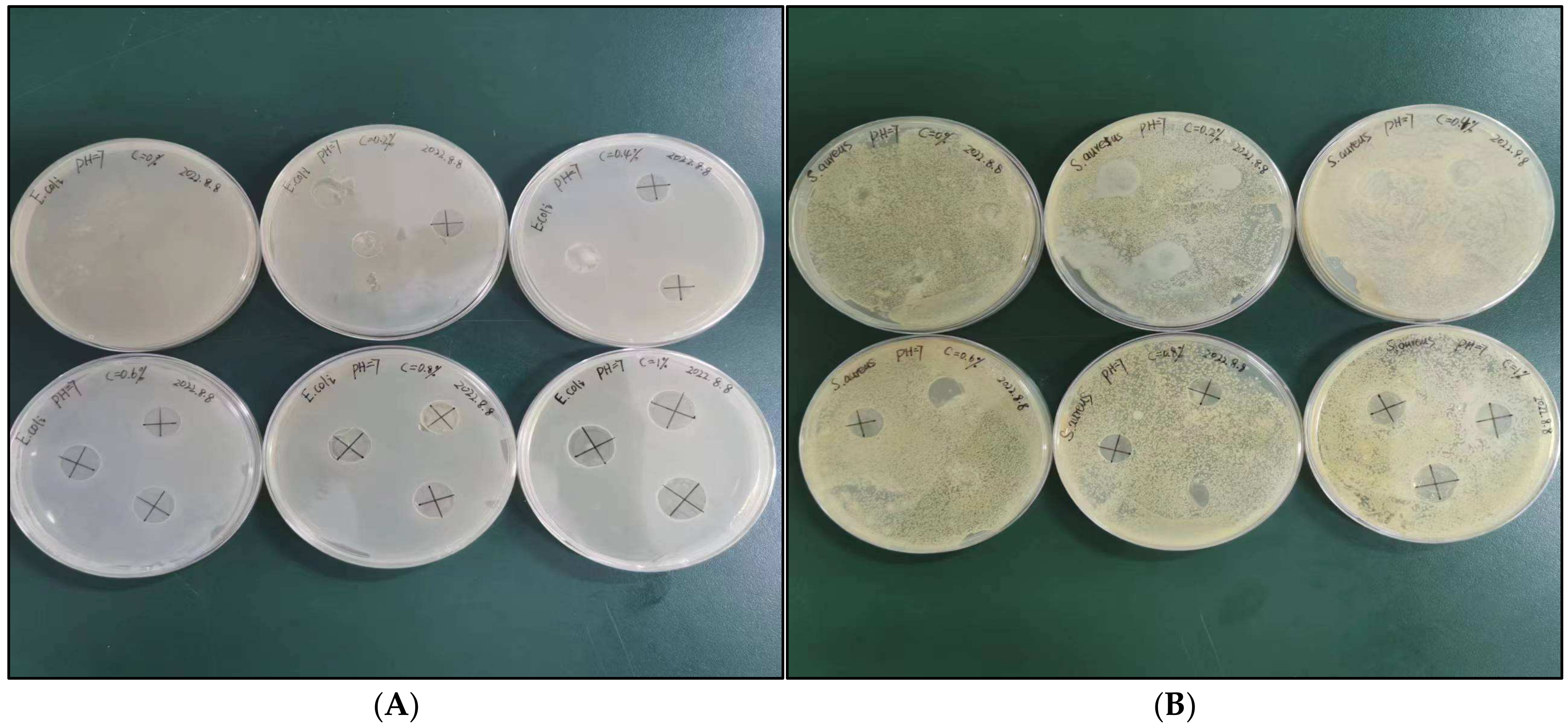
3.3. Analysis of Antibacterial Properties of SDEWL-CMCS Composite Films
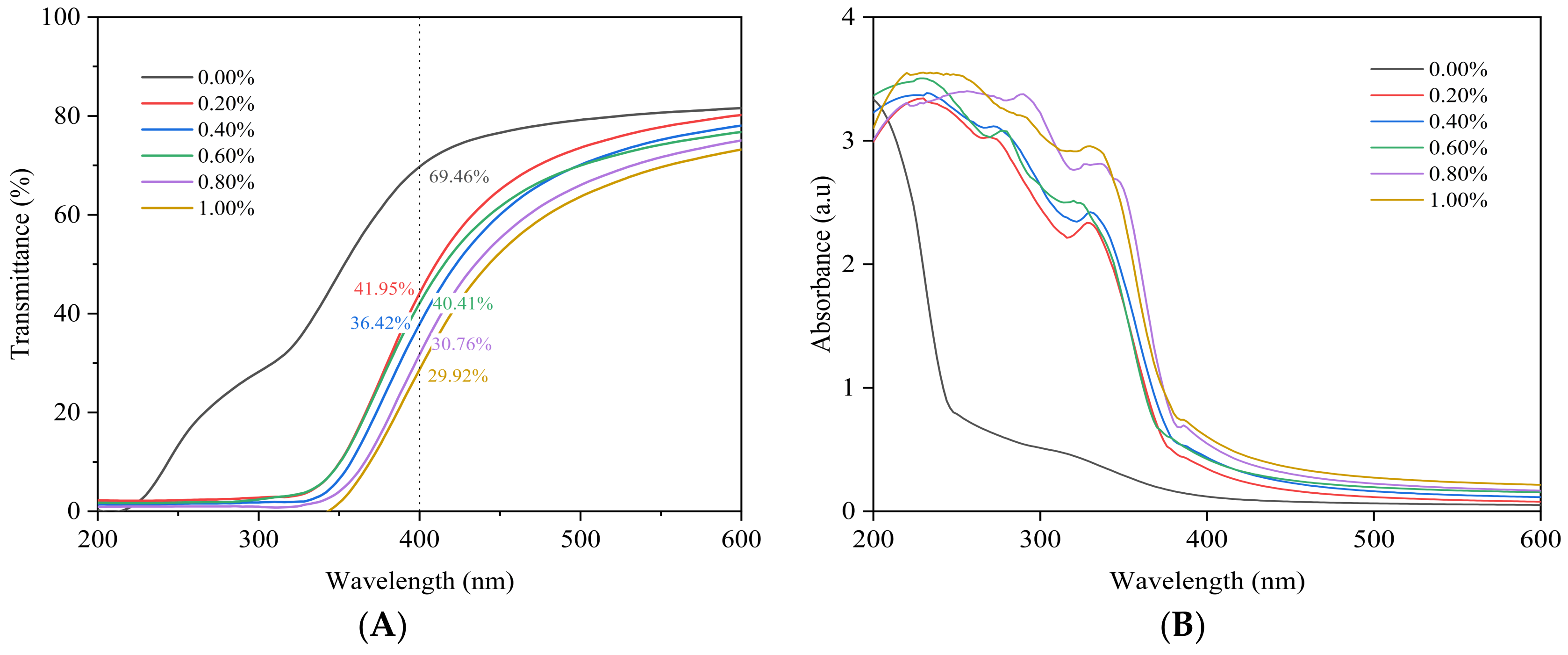
3.4. UV Spectral Analysis of SDEWL-CMCS Composite Films
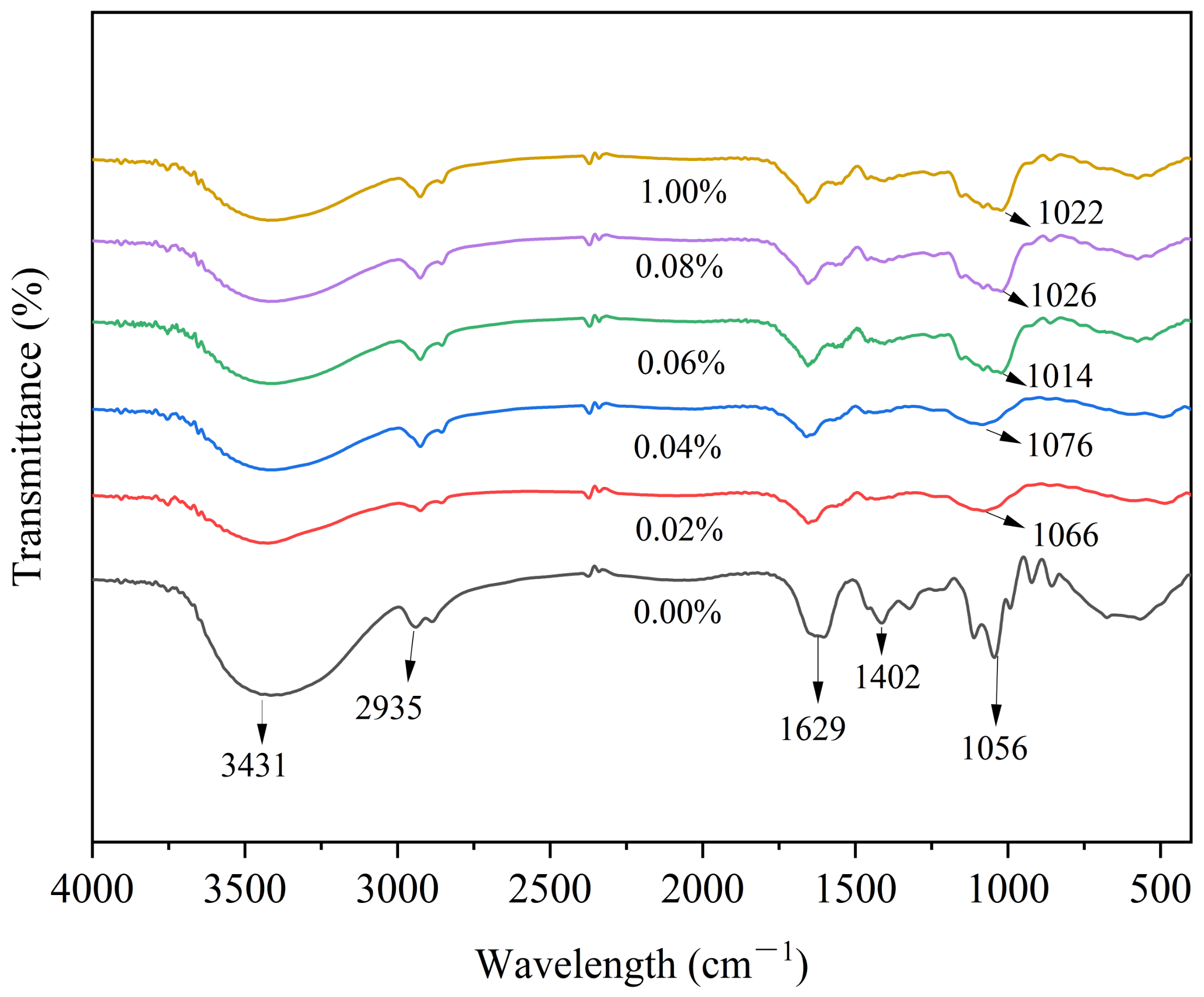
3.5. FTIR Analysis of SDEWL-CMCS Composite Films
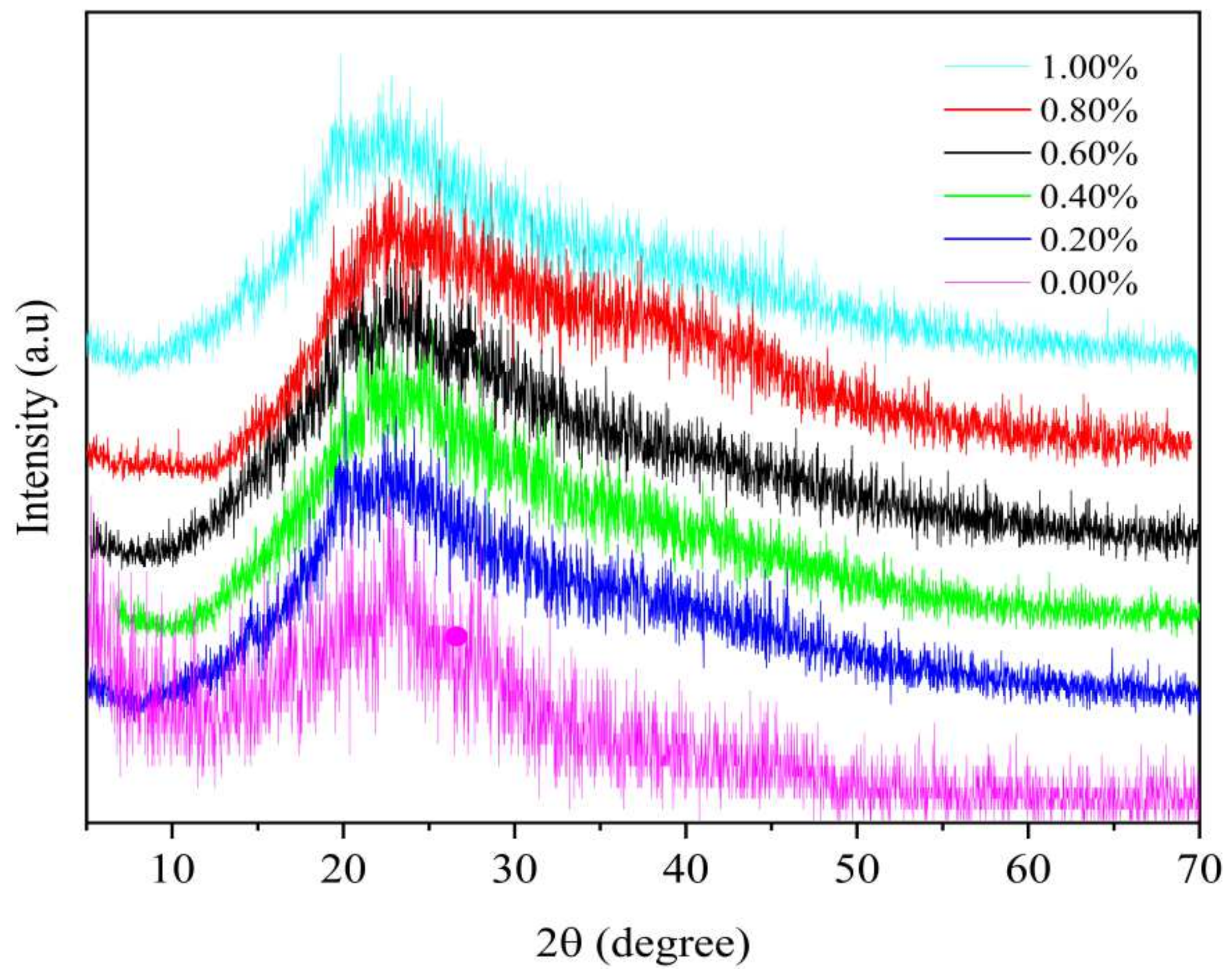
3.6. XRD Analysis of SDEWL-CMCS Composite Films
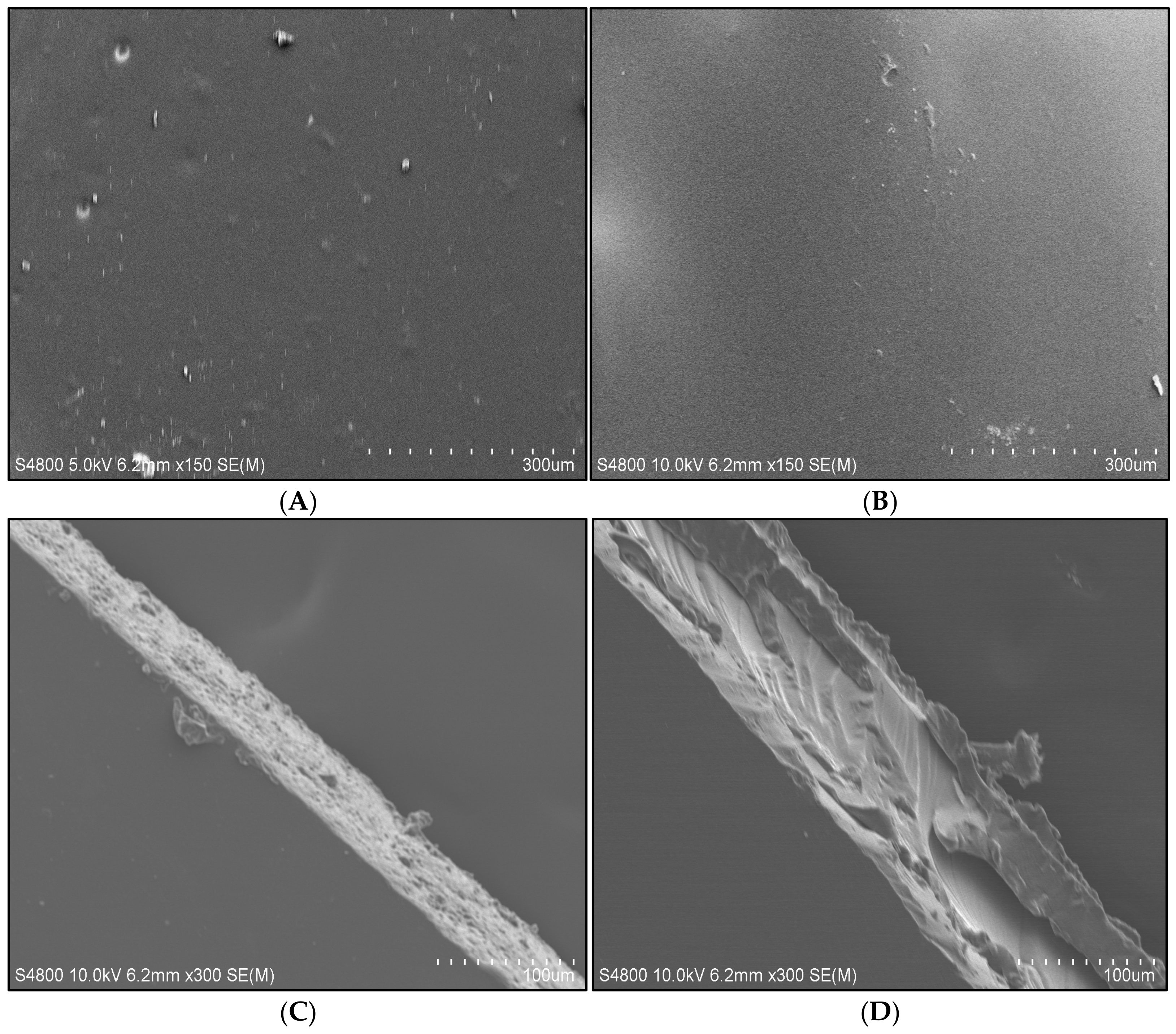
3.7. SEM Analysis of SDEWL-CMCS Composite Films
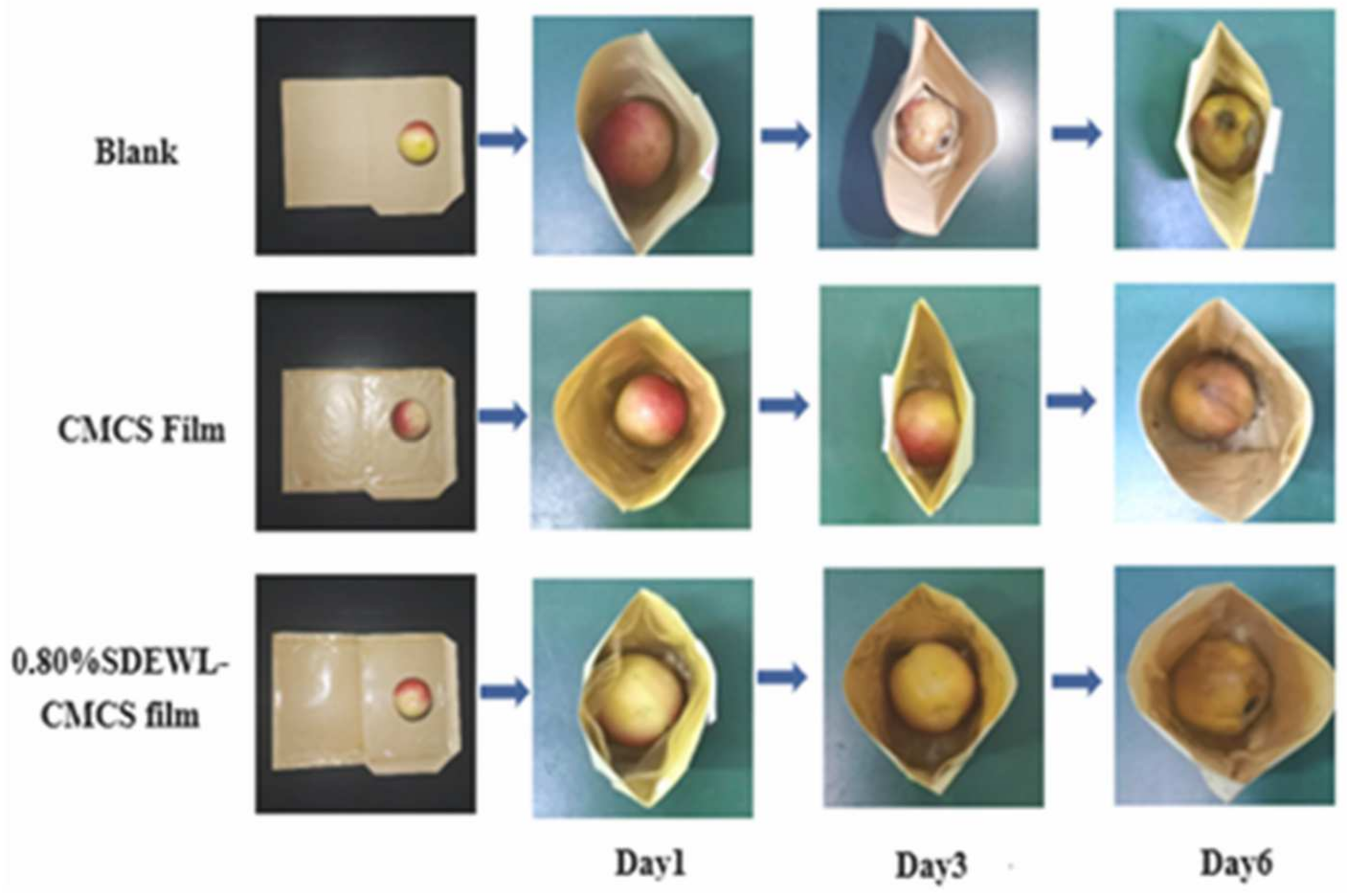
3.8. Effects of SDEWL-CMCS Composite Films on the Preservation of Small Nectarines
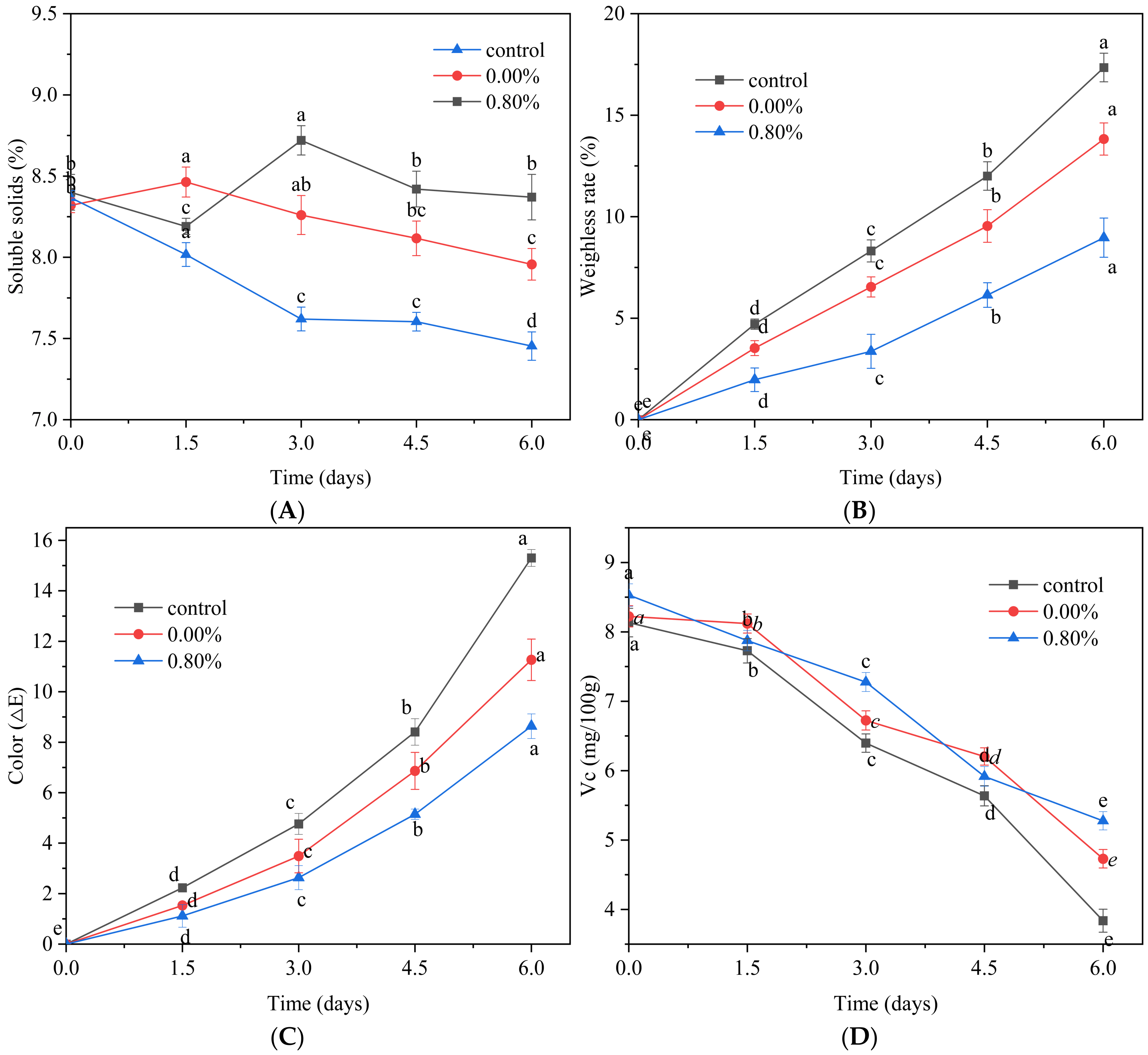
3.8.1. Influence of SDEWL-CMCS Composite Membranes on the SSC of Small Nectarines
3.8.2. Effects of SDEWL-CMCS Composite Film on the WLR of Small Nectarines
3.8.3. Effects of SDEWL-CMCS Composite Films on the Color of Small Nectarines
3.8.4. Effects of SDEWL-CMCS Composite Films on the Ascorbic Acid Content of Small Nectarines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silue, Y.; Fawole, O.A. Global Research Network Analysis of Edible Coatings and Films for Preserving Perishable Fruit Crops: Current Status and Future Directions. Foods 2024, 13, 2321. [Google Scholar] [CrossRef]
- Bilican, I. Preparation and Properties of Novel Mucilage Composite Films Reinforced with Polydimethylsiloxane. Macromol. Mater. Eng. 2023, 309, 2300317. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, R.; Zhou, C.; Gao, Z.; Gu, Y.; Chen, S.; Yang, Q.; Yan, B. Dynamically crosslinked chitosan/cellulose nanofiber-based films integrated with γ-cyclodextrin/curcumin inclusion complex as multifunctional packaging materials for perishable fruit. Food Hydrocoll. 2023, 144, 108996. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Zhou, L.; Jia, M.; Xiong, Y. Nanofillers in Novel Food Packaging Systems and Their Toxicity Issues. Foods 2024, 13, 2014. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, S.; Yousuf, B. Development and characterization of edible films based on flaxseed gum incorporated with Piper betle extract. Int. J. Biol. Macromol. 2023, 245, 125562. [Google Scholar] [CrossRef]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and characterization of chitosan-fungal extract films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Edible film of native jicama starch, agarwood Aetoxylon Bouya essential oil and calcium propionate: Processing, mechanical, thermal properties and structure. Int. J. Biol. Macromol. 2022, 209, 597–607. [Google Scholar] [CrossRef]
- Bizymis, A.-P.; Giannou, V.; Tzia, C. Contribution of Hydroxypropyl Methylcellulose to the Composite Edible Films and Coatings Properties. Food Bioprocess Technol. 2023, 16, 1488–1501. [Google Scholar] [CrossRef]
- Cui, H.; Cheng, Q.; Li, C.; Chen, X.; Lin, L. Improving packing performance of lily polysaccharide based edible films via combining with sodium alginate and cold plasma treatment. Int. J. Biol. Macromol. 2022, 206, 750–758. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, J.; Zhang, F.; Zhang, R.; Zhang, X.; Zhong, K.; Yan, B. Development of mussel-inspired chitosan-derived edible coating for fruit preservation. Carbohydr. Polym. 2023, 321, 121293. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Analysis of film-forming properties of chitosan with different molecular weights and its adhesion properties with different postharvest fruit surfaces. Food Chem. 2022, 395, 133605. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef]
- Gómez, A.; López, L.; Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Effect of a Gelatin-Based Film Including Gelidium sp. Algal Flour on Antimicrobial Properties Against Spoilage Bacteria and Quality Enhancement of Refrigerated Trachurus trachurus. Foods 2025, 14, 1465. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Li, L.; Jiang, K.; Gao, J.; Zhong, K.; Pan, M.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Q.; Gao, Y.; Wan, S.; Meng, F.; Weng, W.; Zhang, Y. Characterization of silver nanoparticles loaded chitosan/polyvinyl alcohol antibacterial films for food packaging. Food Hydrocoll. 2022, 136, 108305. [Google Scholar] [CrossRef]
- Liu, W.; Kang, S.; Zhang, Q.; Chen, S.; Yang, Q.; Yan, B. Self-assembly fabrication of chitosan-tannic acid/MXene composite film with excellent antibacterial and antioxidant properties for fruit preservation. Food Chem. 2023, 410, 135405. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, C.; Zhou, C.; Pan, Q.; He, Z.; Wang, C.; Liu, Y.; Song, S.; Yu, L.; Qu, Y.; et al. Amphiphilic chitosan/carboxymethyl gellan gum composite films enriched with mustard essential oil for mango preservation. Carbohydr. Polym. 2022, 300, 120290. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of films produced using nanoemulsions stabilized by carboxymethyl chitosan-peptide conjugates and application in blueberry preservation. Int. J. Biol. Macromol. 2022, 202, 26–36. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Diao, Y.; Jing, Y. Free Radical Grafting of Epigallocatechin Gallate onto Carboxymethyl Chitosan: Preparation, Characterization, and Application on the Preservation of Grape Juice. Food Bioprocess Technol. 2020, 13, 807–817. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, S.; Xia, J.; Shao, P.; Sun, P.; Xiang, N. Enhanced Antibacterial Activity of Hen Egg-White Lysozyme against Staphylococcus aureus and Escherichia coli due to Protein Fibrillation. Biomacromolecules 2021, 22, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Du, T.; Guo, J.; Lv, W.; Adhikari, B.; Xu, J. Extraction and Characterization of Lysozyme from Salted Duck Egg White. Foods 2022, 11, 3567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, X.; Wang, D.; Fang, C.; Zhang, W.; Wang, C.; Huang, Z. Lysozyme-based composite membranes and their potential application for active packaging. Food Biosci. 2021, 43, 101078. [Google Scholar] [CrossRef]
- Pei, Y.; Li, Z.; McClements, D.J.; Li, B. Comparison of structural and physicochemical properties of lysozyme/carboxymethylcellulose complexes and microgels. Food Res. Int. 2019, 122, 273–282. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Freitas, J.A.M.; Mendonça, G.M.N.; Santos, L.B.; Alonso, J.D.; Mendes, J.F.; Barud, H.S.; Azeredo, H.M.C. Bacterial Cellulose/Tomato Puree Edible Films as Moisture Barrier Structures in Multicomponent Foods. Foods 2022, 11, 2336. [Google Scholar] [CrossRef]
- Vidmar, G.; Knez, F. Measuring and modelling of linear water vapour transmittance of steel sandwich panels with mineral wool. Bauphysik 2015, 37, 229–236. [Google Scholar] [CrossRef]
- Dhandapani, E.; Suganthi, S.; Vignesh, S.; Dhanalakshmi, M.; Sundar, J.K.; Raj, V. Fabrication and physicochemical assessment of L-Histidine cross-linked PVA/CMC bio-composite membranes for antibacterial and food-packaging applications. Mater. Technol. 2020, 37, 124–134. [Google Scholar] [CrossRef]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Ji, R.; Li, K.; Zhang, W. Preparation and Characterization of Pullulan/Carboxymethyl Cellulose/Nano-TiO2 Composite Films for Strawberry Preservation. Food Biophys. 2021, 16, 460–473. [Google Scholar] [CrossRef]
- Nikvarz, N.; Khayati, G.R.; Sharafi, S. Preparation of UV absorbent films using polylactic acid and grape syrup for food packaging application. Mater. Lett. 2020, 276, 128187. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, Y.; Li, Q. Preparation and Characterization of Corn Starch-Based Composite Films Containing Corncob Cellulose and Cassia Oil. Starch-Starke 2020, 72, 1900209. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wang, H.; He, H.; Bai, R. Preparation and characterization of Enteromorpha prolifera nanocellulose/polyvinyl alcohol composite films. Polym. Compos. 2020, 42, 1712–1726. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Li, Z.; An, X.; Gao, Z.; Tian, S. Preparation and Performance Characterization of a Composite Film Based on Corn Starch, κ-Carrageenan, and Ethanol Extract of Onion Skin. Polymers 2022, 14, 2986. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Yue, T.; Cui, L. Preparation and Characterization of Chitosan–Nano-ZnO Composite Films for Preservation of Cherry Tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, W.; Yang, J.; Yang, Q. Preparation and characterization of chitosan/Nano-ZnO composite film with antimicrobial activity. Bioprocess Biosyst. Eng. 2021, 44, 1193–1199. [Google Scholar] [CrossRef]
- Niu, X.; Zhu, L.; Xi, L.; Guo, L.; Wang, H. An antimicrobial agent prepared by N-succinyl chitosan immobilized lysozyme and its application in strawberry preservation. Food Control. 2020, 108, 106829. [Google Scholar] [CrossRef]
- Sha, H.; Yuan, C.; Cui, B.; Zhao, M.; Wang, J. Pre-gelatinized cassava starch orally disintegrating films: Influence of β-Cyclodextrin. Food Hydrocoll. 2022, 123, 107196. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Q.; Zhang, Y.; Wang, Z.; Gao, M.; Li, S. Preparation and properties of antibacterial and antioxidant mango peel extract/polyvinyl alcohol composite films. J. Food Process. Preserv. 2021, 46, e16206. [Google Scholar] [CrossRef]
- Li, S.; Yi, J.; Yu, X.; Wang, Z.; Wang, L. Preparation and characterization of pullulan derivative/chitosan composite film for potential antimicrobial applications. Int. J. Biol. Macromol. 2020, 148, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xiao, N.; Zhang, X.; Luo, W.; Xiao, G.; Zhai, W.; Zhong, L.; Lan, B. Preparation and Characterization of Chinese Leek Extract Incorporated Cellulose Composite Films. Front. Bioeng. Biotechnol. 2021, 9, 731749. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, N.M.B.; Donnici, C.L.; de Mesquita, J.P.; Pereira, F.V. Preparation and characterization of hydrogels obtained from chitosan and carboxymethyl chitosan. J. Polym. Res. 2021, 28, 335. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, D.; Liu, X.; Ma, T.; He, J.; Dong, Q.; Din, Z.-U.; Zhou, J.; Chen, L.; Hu, Z.; et al. Improving the hydrophobicity and mechanical properties of starch nanofibrous films by electrospinning and cross-linking for food packaging applications. LWT 2022, 169, 114005. [Google Scholar] [CrossRef]
- Rastriga, N.V.; Klimov, D.A.; Gasanova, D.A.; Levashov, P.A. Comparison of the individual and combined actions of charged amino acids and glycine on the lysis of Escherichia coli cells by human and chicken lysozyme. Process. Biochem. 2022, 125, 190–197. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Y.; Hu, J.; Bao, Z.; Wang, M. A novel c-type lysozyme from Litopenaeus vannamei exhibits potent antimicrobial activity. Fish Shellfish. Immunol. 2022, 131, 729–735. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Tang, H.; Zhang, J.; Zhang, L.; Yang, X.; Wang, F.; Chen, L. Effect of lysozyme and Chinese liquor on Staphylococcus aureus growth, microbiome, flavor profile, and the quality of dry fermented sausage. LWT 2021, 150, 112059. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Silveira, T.M.G.; Tapia-Blácido, D.R. Starch isolation from turmeric dye extraction residue and its application in active film production. Int. J. Biol. Macromol. 2022, 202, 508–519. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.-J.; Tu, Q.; Jian, Y.-Y.; Xie, D.-M.; Bai, T.; Li, S.-S.; Liu, Y.-T.; Li, C.; Wang, C.-X.; et al. Preparation and characterization of carboxymethyl chitosan/pullulan composite film incorporated with eugenol and its application in the preservation of chilled meat. Meat Sci. 2023, 198, 109085. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Zhu, D.; Jin, Y.; Jin, H.; Sheng, L. Preparation and characterization of edible carboxymethyl cellulose films containing natural antibacterial agents: Lysozyme. Food Chem. 2022, 385, 132708. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. Part B Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Dekina, S.; Romanovska, I.; Ovsepyan, A.; Tkach, V.; Muratov, E. Gelatin/carboxymethyl cellulose mucoadhesive films with lysozyme: Development and characterization. Carbohydr. Polym. 2016, 147, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Soubhagya, A.S.; Balagangadharan, K.; Selvamurugan, N.; Seeli, D.S.; Prabaharan, M. Preparation and characterization of chitosan/carboxymethyl pullulan/bioglass composite films for wound healing. J. Biomater. Appl. 2021, 36, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, W.; Niu, B.; Fang, X.; Chen, H.; Wang, Y.; Gao, H. Characterization of Zizania latifolia polysaccharide-corn starch composite films and their application in the postharvest preservation of strawberries. LWT 2022, 173, 114332. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.; Xue, C. Preparation and characterization of multifunctional films based on pectin and carboxymethyl chitosan: Forming microchambers for high-moisture fruit preservation. Food Packag. Shelf Life 2023, 37, 101073. [Google Scholar] [CrossRef]
- Tang, H.; Han, Z.; Zhao, C.; Jiang, Q.; Tang, Y.; Li, Y.; Cheng, Z. Preparation and characterization of Aloe vera polysaccharide-based packaging film and its application in blueberry preservation. Prog. Org. Coat. 2023, 177, 107445. [Google Scholar] [CrossRef]
- Ren, M.; Cai, Z.; Chen, L.; Wahia, H.; Zhang, L.; Wang, Y.; Yu, X.; Zhou, C. Preparation of zein/chitosan/eugenol/curcumin active films for blueberry preservation. Int. J. Biol. Macromol. 2022, 223, 1054–1066. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Rashid, A.; Qayum, A.; Chi, Z.; Ren, X.; Ma, H. Ultrasound-assisted development and characterization of novel polyphenol-loaded pullulan/trehalose composite films for fruit preservation. Ultrason. Sonochemistry 2022, 92, 106242. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Zhang, S.; Tang, J.; Shi, X.; Qin, S.; Pan, L.; Xiao, H. Enhancing the applicability of gelatin-carboxymethyl cellulose films by cold plasma modification for the preservation of fruits. LWT 2023, 178, 114612. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, J.; Xie, G.; Xiong, T.; Xu, H. Preparation and characterization of sodium alginate/gelatin/Ag nanocomposite antibacterial film and its application in the preservation of tangerine. Food Packag. Shelf Life 2022, 33, 100928. [Google Scholar] [CrossRef]
| Z | Thickness (mm) | Water Solubility (%) | Water Vapor Transmittance Rate (10−10 g·m−1·s−1·Pa−1) | Oil Resistance (%) |
|---|---|---|---|---|
| 0.00% | 0.051 ± 0.003 b | 31.66 ± 0.37 a | 3.25 ± 0.08 a | 2.70 ± 0.02 a |
| 0.20% | 0.058 ± 0.006 ab | 27.90 ± 0.34 b | 2.71 ± 0.04 b | 2.37 ± 0.01 b |
| 0.40% | 0.056 ± 0.005 ab | 27.25 ± 0.28 b | 2.48 ± 0.03 c | 2.14 ± 0.06 c |
| 0.60% | 0.062 ± 0.005 a | 24.55 ± 0.14 c | 2.39 ± 0.02 c | 1.89 ± 0.03 d |
| 0.80% | 0.060 ± 0.004 a | 23.33 ± 0.25 d | 2.19 ± 0.02 d | 1.77 ± 0.02 e |
| 1.00% | 0.059 ± 0.004 a | 21.84 ± 0.19 e | 2.16 ± 0.03 d | 1.62 ± 0.03 f |
| Diameter of Inhibition Zones (mm) | ||||||
|---|---|---|---|---|---|---|
| 0.00% SDEWL-CMCS | 0.20% SDEWL-CMCS | 0.40% SDEWL-CMCS | 0.60% SDEWL-CMCS | 0.80% SDEWL-CMCS | 1.00% SDEWL-CMCS | |
| E. coli | 0.00 e | 10.37 ± 0.04 d | 10.95 ± 0.12 c | 11.15 ± 0.17 c | 13.34 ± 0.21 b | 15.06 ± 0.16 a |
| S. aureus | 0.00 d | 0.00 d | 0.00 d | 10.54 ± 0.02 c | 12.22 ± 0.36 b | 13.09 ± 0.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Li, W.; Guo, J.; Han, F.; Usman, M.; Wu, L. Innovative Preparation of Salted Duck Egg White Lysozyme Functional Film and Its Application in Fresh Storage of Small Nectarines. Colloids Interfaces 2025, 9, 74. https://doi.org/10.3390/colloids9060074
Yao X, Li W, Guo J, Han F, Usman M, Wu L. Innovative Preparation of Salted Duck Egg White Lysozyme Functional Film and Its Application in Fresh Storage of Small Nectarines. Colloids and Interfaces. 2025; 9(6):74. https://doi.org/10.3390/colloids9060074
Chicago/Turabian StyleYao, Xinjun, Wanrong Li, Jun Guo, Fangkai Han, Muhammad Usman, and Lipeng Wu. 2025. "Innovative Preparation of Salted Duck Egg White Lysozyme Functional Film and Its Application in Fresh Storage of Small Nectarines" Colloids and Interfaces 9, no. 6: 74. https://doi.org/10.3390/colloids9060074
APA StyleYao, X., Li, W., Guo, J., Han, F., Usman, M., & Wu, L. (2025). Innovative Preparation of Salted Duck Egg White Lysozyme Functional Film and Its Application in Fresh Storage of Small Nectarines. Colloids and Interfaces, 9(6), 74. https://doi.org/10.3390/colloids9060074







