Abstract
Fruits are a significant source of natural nutrition for human health. However, the perishable nature and short shelf life of fruits lead to spoilage, nutrition safety challenges, and other substantial postharvest losses. Edible coatings have emerged as a novel approach in order to enhance the shelf life of perishable fruits by forming a protective barrier against adverse environmental conditions and microbial infections. Sodium alginate is recognized as an excellent polysaccharide (derived from algae, seaweed, etc.) in the food industry for edible fruit coatings because of its non-allergic, biodegradable, non-toxic (safe for human health), inexpensive, and efficient gel/film-forming properties. However, the hydrophilicity of the polysaccharides is a significant concern to prevent the growth of mold and yeast. In recent years, various plant extracts (containing multiple bioactive compounds, including polyphenolic acids) and nanoparticles have been applied in sodium alginate-based edible films and fruit coatings to enhance antimicrobial activity. This review study summarized recent advancements in fabricating plant extracts incorporating sodium alginate-based films and coatings to enhance fruit shelf life. In addition, approaches to preparing edible films and the basic mechanism behind the role of coating materials in enhancing fruit shelf life are discussed. Moreover, the limitations associated with sodium alginate-based fruit coatings and films have been highlighted.
1. Introduction
In a balanced diet, fruits are essential components that facilitate regeneration and cellular detoxification and are also useful for healing several diseases in the human body [1]. Fresh fruits enriched with health-promoting compounds and good appearance are essential features for their marketability and attractiveness to consumers [1,2]. However, the perishable nature of fruits is a significant concern for their short shelf-life after harvest [3]. After the harvesting process, the synthesis of aromatic compounds, generation of reactive oxygen species, ethylene production, and increased respiration rate can degrade the quality of fruits [4]. Apart from the issue of biochemical changes, the contamination of pathogenic microorganisms in postharvest fruits is another serious concern for human health [5].
Traditional approaches to maintaining the quality and extending the shelf-life of fruits are commonly used in waxing, cold storage, modified atmosphere packaging, and chemical preservatives [6]. Waxing is a standard and prevailing method involving applying natural or synthetic waxes to fruits to reduce moisture loss and slow decay. Cold storage is another conventional method of storing fruits at low temperatures to inhibit microbial growth and slow ripening processes. Modified atmosphere packaging alters the gas composition around fruits to create an environment that retards ripening and reduces spoilage. Chemical preservatives have also controlled microbial activity and prolonged fruit freshness. However, these traditional approaches have notable limitations and drawbacks, raising concerns regarding consumer health and environmental impact. This stimulates the exploration and development of modern techniques like edible biodegradable coatings for fruit packaging [7].
Biocompatible materials-based edible coatings/films have emerged as a green environmental approach for increasing shelf-life and protecting fruits from quality deterioration [3,8,9,10,11]. Edible films and coatings are typically made from natural biopolymeric edible materials (base matrix) based on polysaccharides (such as starch, chitosan, alginate, pectin, etc.), proteins (such as casein, whey protein, gelatine, or collagen, etc.), and lipids (such as mineral oil, vegetable oil, waxes, natural resins, etc.) or in their combination in optimized proportions [12,13].
Among these biopolymeric materials, alginate is one of the materials that is widely used [14]. Alginate is a polysaccharide derived from seaweeds, which makes it safe for consumption. It is renewable and biocompatible, and the film-forming ability and gelation capacity make it an appropriate material for formulating edible coating [14]. Cations like calcium, sodium, or magnesium bound to alginic acid form an alginate matrix. The biotechnological applications of alginate are based on the alginate molecule and its variations depending on the covalent bonds with cations, such as calcium, sodium, or magnesium. Modifications in the configuration and proportion or ratio of its linear chains of β-D-mannuronic acid and α-L-guluronic acid give rise to its different and unique physicochemical characteristics [15,16,17]. Because of these structural distinctions, sodium alginate has specific functional qualities that make it an appropriate and edible coating material [13,14]. Due to its unique composition and characteristics, many studies have been conducted on sodium alginate, primarily derived from brown seaweeds like Laminaria hyperborea and Macrocystis pyrifera [18]. The first stage in the sequential extraction of alginate, which is subsequently purified, is the extraction of alginate-rich seaweed biomass. Sodium alginate has a high G-block content and possesses anionic properties from carboxylic groups attached to guluronic acid residues [19]. Due to its structure, alginic acid can form strong hydrogen bonds with the membranes of microorganisms, which prevents microbial growth and multiplication [19]. Since sodium alginate is hydrophilic, it dissolves readily in water as an edible coating on fruits and vegetables [16,20]. That acts as a barrier against physical and microbiological harm because of their dense gel formation ability [16]. The development of a gel-like viscosity serves as a protective layer against microbiological and physical damage [16]. Its viscoelastic qualities and capacity to form gel allow for even coating on irregular surfaces, which is necessary for efficient preservation [17].
Additionally, the properties of these base matrices of edible coatings can be further improved by incorporating natural phytochemicals to enhance antimicrobial activity, further extending the shelf-life of fruits [11,21]. The phytochemicals, or bioactive substances, present in natural additives like plant extracts, such as clove, contain eugenol [22]; guava leaves contain tannins, phlorotannins, saponins, terpenoids, alkaloids, polyphenols, quercetin, lycopene, and vitamin C [23]; purple onion peel contains anthocyanidin [24], etc., are highly efficient in enhancing the fruit shelf-life. They include significant antioxidant and antibacterial properties that help to maintain the nutritional value, inhibit microbial growth, and delay ripening, thus enhancing fruit shelf-life.
The application involves dipping, spraying, and brushing fruits with a thin layer of the edible coating, creating a barrier that maintains the fruit’s moisture content and reduces respiration rates [9,10]. These have tremendous advantages, like controlling respiration (the controlled exchange of gases), inhibiting the action of reactive oxygen species (ROS), and retaining moisture [10]. Therefore, this study summarizes the most commonly used sources of sodium alginate and recent strategies for fabricating alginate-based edible fruit coatings to improve shelf-life. In addition, recent research trends about plant extract-based edible coatings and their importance in the combination of alginate-based coating base matrices are also discussed.
2. Sources and Extraction of Sodium Alginate
These natural sources offer renewable and sustainable alternatives for edible coating formulations and provide benefits such as moisture retention, preservation of sensory qualities in coated food products, and being environmentally friendly substitutes for synthetic polymers [9,19]. Alginate is a biocompatible, biodegradable, and anionic biopolymer with an excellent ability to prepare edible films to preserve food quality and enhance fruit shelf-life [25,26]. Alginates, crucial components (30–60%) of brown algae cell walls, mainly consist of salt combinations of alginic acid with calcium, sodium, potassium, and magnesium [26,27,28]. Brown seaweeds, particularly those in the Phaeophyceae family like Laminaria and Fucoides, contain high concentrations of alginate, with species such as Macrocystis pyrifera, Ascophyllum nodosum, and Laminaria hyperborea being primary sources globally [27,29,30]. While brown seaweeds are the primary sources of alginates due to their abundance and high alginate content, certain bacteria like Azotobacter vinelandii also produce alginate-like polymers, offering alternative sources for extraction [27,30]. As the demand for environmentally friendly food packaging rises, further exploration and refinement of extraction methods from these natural sources promise increased scalability and efficiency in sodium alginate production for edible coating applications [9,13,19]. The extraction process of alginate from brown seaweeds involves several intricate steps to isolate this natural polysaccharide, which has potential applications in edible coatings and various industries [Figure 1]. Initially, the primary sources of brown seaweed are harvested predominantly from cold water regions due to their higher alginic acid content [27]. The seaweeds undergo preparatory steps upon harvesting, including washing, drying, and milling into fine particles. The extraction of alginate relies on converting insoluble alginic acid salts into soluble alginate salts, such as sodium or potassium, through ion exchange reactions [30], wherein the seaweed is treated with a strong alkali following pre-treatment with hydrochloric acid. The alginate isolation process involves several methods to separate alginate from other soluble substances in the crude extract solution, including alcohol precipitation for sodium alginate and calcium chloride solution precipitation for calcium alginate. Mild acid treatments remove undesirable compounds and modify cell wall alginate into alginic acid, maximizing extraction efficacy. After extraction, the alginic acid is recovered in a soluble sodium form through neutralization with sodium carbonate or sodium hydroxide. Insoluble residue is removed through filtration, flotation, or centrifugation, leaving behind soluble alginate [29]. This soluble alginate is then precipitated by conversion into alginic acid or calcium/sodium alginate, with its counter ion adjusted through neutralization with appropriate hydroxides or chlorites. The meticulous extraction process, tailored according to the source and structure of constituents, ensures the efficient extraction of this valuable polysaccharide from brown seaweeds [31].
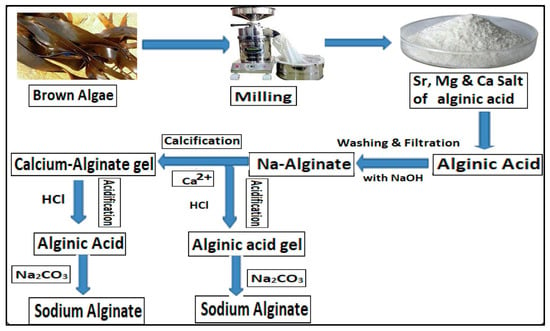
Figure 1.
Schematic representation of sodium alginate extraction from brown algae. Reproduced with permission from Ref. [26]. Copyright 2024, Elsevier.
Sodium alginate, obtained through this extraction process, holds immense potential for various applications, particularly in developing edible coatings for fruits, vegetables, and other food products [9,26]. These coatings offer numerous advantages, including shelf-life extension, improved appearance, and maintained nutritional value [13]. Moreover, sodium alginate-based coatings are environmentally friendly and safe for consumption, aligning with the growing demand for sustainable food packaging solutions [32].
2.1. Modification Approaches
When improving the qualities or enabling specific applications, modification techniques for sodium alginate entail changing its chemical structure. These methods in many industrial and biological domains usually seek to enhance properties like solubility, viscosity, stability, or usefulness. Enzymatic, physical (physical blending, physical cross-linking), and chemical modifications (hydroxyl modification, carboxyl modifications, etc.) are frequently used in modification processes [33,34]. Chemical modification involves reacting with substances such as bases, acids, or cross-linking agents to add functional groups to the alginate molecule [33,35]. This may alter some elements, such as the kinetics of drug release or gelation behavior. Physical modification techniques include irradiation, sonication, and mechanical therapy. These procedures can change sodium alginate’s molecular weight, particle size, and rheological properties [36]. These alterations usually enhance the material’s ability to interact with other materials or function better in specific applications, such as food preservation [33,37].
2.2. Properties of Sodium Alginate
Sodium alginate is a substance extracted from seaweed and widely used in the pharmaceutical and cosmetics industries, food preservation, and preparation of edible films because of their various unique properties (Figure 2) [29]. These are a few of its essential attributes, such as its safety and non-toxic properties, proving its biocompatibility. It forms a transparent film with good mechanical strength and flexibility. It possesses barrier properties against gases and moisture, which help minimize oxidative reactions, maintain the freshness and quality of fruit, and prolong their shelf life during postharvest storage.
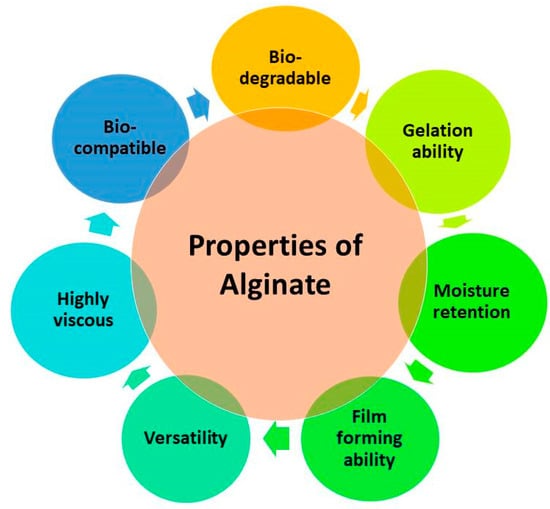
Figure 2.
Schematic representation of some common properties of alginate.
Sodium alginate is hydrophilic because the presence of polar groups in large numbers efficiently produces a viscous colloidal solution in water [33,37]. Sodium alginate is insoluble in alcohol, having a content greater than 30% [38]. Due to several cationic or anionic groups in its structure, this polysaccharide exhibits a particular physical property through electrostatic contact [39]. The ability of aqueous solutions of alginate to form firm gels upon the addition of di- and trivalent metal ions, such as bivalent alkaline earth metals (Ca, Sr, and Ba), or trivalent Al and Fe+ ions, is a property that has been widely exploited for the sustained delivery of bioactive molecules. This results from intramolecular bonding and ionic contact between the cations and the carboxylic acid groups on the polymer backbone [40]. The most favored ions for forming microparticles are Ca2+ ones, and because Pb2+, Cu2+, and Cd2+ are poisonous, their use is restricted [41]. Because of its versatility, sodium alginate can be readily altered for use in various applications by blending it with other polymers, adding natural additives, or combining it with bioactive substances from natural resources, including plant extracts. This ability to adapt enables sodium alginate-based coatings to be customized to the unique requirements of various food products and processing conditions [37].
3. Plant Extract-Mediated Edible Coatings
In recent years, the uses of plant extracts have attracted wide attention in various research areas, including the synthesis of metallic nanoparticles [42,43,44], biomedical [45,46], antioxidant [47], antibacterial [48], cosmetics [49], sensing [50], edible films, and coating to enhance fruit shelf-life applications [4,51]. In the fabrication of food-grade edible coatings/films, there is an increasing demand for plant extracts for safe and natural additives (antibacterial, antioxidant, etc.) that do not harm human health and the environment. Plant extracts are considered a beneficial resource in formulating edible coatings, especially when preserving fruit’s postharvest quality and extending its shelf life. Incorporating plant-based substances and extracts into these coatings enhances their efficacy due to the phytochemicals in plant extracts, such as flavan-3-ols, catechin, gallic acid, quercetin, and kaempferol, which exhibit antimicrobial and antioxidant properties. For example, flavonoids inhibit microbial growth, while antioxidants reduce reactive oxygen species, delaying fruit decay and oxidation and enhancing fruit shelf life. Moreover, it is seen that plant extracts promote the activities of various antioxidant enzymes, contributing to detoxification processes and overall preservation of fruit quality and its nutritional value.
Notably, plant-based coatings extend shelf life and offer nutraceutical benefits, enhancing the nutritional value of treated fruits. Natural antioxidants and antimicrobials are mainly derived from plants through pure bioactive chemicals, fruit, root, leaf, seed extracts, essential oils, peptides, and so on (Figure 3) [52]. Various bioactive substances have significant antimicrobial action, like phenolics, terpenes, aliphatic alcohols, aldehydes, acids, and flavonoids [53]. For instance, extracts from sources like pomegranate peel, garlic, ginger, green tea, and karonda are being explored as potential alternatives to synthetic coatings [54,55,56]. These extracts, rich in bioactive compounds, mitigate postharvest losses and maintain fruit freshness.
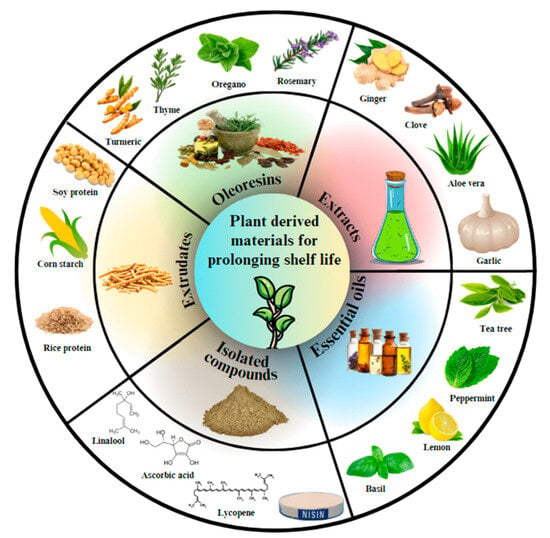
Figure 3.
Some commonly used plant-derived resources for the fabrication of edible coatings/films to enhance fruit shelf life. Reproduced with permission from Ref. [52]. Copyright 2024, RSC.
Recent studies have focused on developing edible coatings using plant extracts incorporated with diverse biopolymers (polysaccharides, proteins, lipids, etc.) [4,52]. For instance, cassava starch combined with cinnamon for guava preservation and rice starch blended with carrageenan for plum quality enhancement, peanut shell, pine nutshell, and jujube leaves increase the antioxidant properties of chitosan-based film efficiently [57,58,59]. The chitosan-based film enriched with dragon fruit extract and blended with ZnO nanoparticles has proven to be an effective way to enhance the films’ mechanical strength and thermal stability while reducing water vapor permeability [60]. Incorporation of 0.5% raspberry extract in soya protein isolates films for their enhanced applicability in food packaging as they noticed the improvement in tensile strength and percentage elongation at the break due to raspberry being a rich source of anthocyanin [61]. Pomegranate peel extract combined with mint extract improved the UV light protection of active films made of chitosan and polyvinyl alcohol and their tensile strength [62].
Similarly, it was proposed that adding curry leaf and pomegranate peel extracts to gluten-based films would improve the shelf life of fruits and vegetables, credited to the antimicrobial potential of modified films [63]. Furthermore, it has been reported that extracts from the skin of plums, Italian red grapes, and other elderberry components are rich sources of phenolic compounds, including anthocyanins and flavonoids, which have potent antioxidant properties against various pathogens [64]. In addition to maintaining the physical characteristics, pectin films containing alcoholic extracts of some fruits, such as acerola, cashew apple, and strawberry, demonstrated increased antioxidant capacity. The films with acerola extract exhibited the highest level of antioxidant activity [65]. Likewise, several edible coatings were made by incorporating plant extract into polysaccharides, proteins, or lipids [54,56,66,67,68,69,70]. Plant-based edible coatings offer several advantages compared to synthetic coatings and packaging materials. They are biodegradable, safe for consumption, and contribute to reducing environmental pollution. While a few plant-based coatings have been commercialized, there’s immense scope for researchers to explore additional plant extracts for enhancing fruit preservation. This signifies the plant-enriched coatings and their potential to positively impact various physical, biochemical, antioxidant, and enzymatic parameters during the postharvest life of fruit crops. As we switch to safe and sustainable options, plant-based edible coatings emerge as a sustainable and effective solution in postharvest management.
4. Application of Sodium Alginate for Edible Coatings
4.1. Sodium Alginate-Based Edible Coatings Incorporated with Plant Extracts
Sodium alginate has proven to be a potential base material for the development of edible film because of its unique properties, as shown in Figure 2. However, as chemical preservatives, chemical coatings, and chemical pesticides are increasing daily, a safe, sustainable, biodegradable, strong antioxidant and antibacterial coating that is too edible is needed. For this reason, plant extracts have emerged as a remarkable substitute and are used extensively, as these extracts are abundant in bioactive substances like phytochemicals, antioxidants, and antimicrobials. These substances give the films advantages like increased mechanical strength, barrier qualities, antimicrobial and antioxidant activity, and controlled gaseous exchange. As we have already discussed, the importance of phytochemicals present in plant extract is that each component of the extract contributes a unique functionality to the sodium alginate matrix; for example, some contain green tea extract, which offers improved barrier qualities, and extracts of rosemary and oregano, which have strong antioxidant and antibacterial qualities.
Along with these plant extracts, adding plasticizers for better and improved flexibility and reduced brittleness is very important. Water, glycerol, sorbitol, acetylated monoglyceride, polyethylene glycol, sucrose, etc., have been used as plasticizers in developing edible coating. Adding hydrophilic plasticizers to the coating generally promotes water vapor permeability and influences the mechanical properties of the coating material [71]. The fruitful combination of other plant extracts. Some recent studies based on plant extract incorporated with sodium alginate matrix for preparing edible coatings/films to enhance fruit shelf life are summarized in Table 1. The details about coating materials, alginate, and coatings’ effect on selected fruits are tabulated.
4.2. Sodium Alginate-Based Edible Coatings Incorporated with Nanoparticles
In addition to using plant extract, researchers have shown significant interest in fabricating sodium alginate-based edible coatings with the incorporation of metallic nanoparticles [72,73]. Incorporating metallic nanoparticles in biopolymeric coatings/films improved the antifungal, antibacterial, and antioxidant properties. Several nanoparticles have been used in alginate-based edible fruit coatings to enhance fruit shelf life. For instance, Khan et al. [72] reported the fabrication of alginate-based (nano-engineered) edible coatings incorporated with titanium dioxide (TiO2) nanoparticles to extend the shelf life of peach fruits. In this study, initially, TiO2 nanoparticles (particle size, 5–20 nm) were synthesized via a green synthesis approach using mousami peel extract. The prepared TiO2 nanoparticles (0.5%) were added to sodium alginate solution (2% w/v) with glycerol (0.6%) and Tween 80 (1%) to prepare nano-emulsion coatings solution via homogenizer. Thus, the obtained coating nano-emulsion was applied to peach fruits as an active coating suspension to assess their effects on shelf life. After that, coated peach fruit were stored at room temperature and analyzed at intervals of 1, 3, 5, and 7 days. From the results of characterization analysis, it was observed that compared to uncoated, coated peach fruits retained antioxidant activity, reduced percent weight loss, and extended the shelf life of up to 7 days at ambient temperature. Based on experimental observation, authors conclude that the prepared nanoemulsion coating was effective and fruits may be stored at room temperature for a longer time.
Similarly, in another study, Tran et al. [74] reported the fabrication of selenium nanoparticles (Nano Se) incorporated into alginate-based edible coatings and examined their efficacy to extend the shelf life of strawberries at 24 ± 2 °C. In this study, viscous nanoformulations were synthesized using sodium alginate biopolymer (2.0 g) in distilled water (90 mL) and a specific amount (0.62–2.50 mM) of Nao Se (purchased from Merck, Rahway, NJ, USA) with constant stirring of the resultant solution. Strawberry fruits were dipped in the prepared nanoemulsion for about 5 min and then subjected to air drying. The coated and uncoated (control) strawberries were stored at 24 ± 2 °C room temperature for 5 days, and the biochemical and physiological changes were analyzed at definite interval days. The results of the characterization analysis confirmed that 26% less weight was observed in coated fruit samples compared to non-treated (control) samples. In addition, natural appearance was also maintained in coated samples of strawberries, which preserved the highest amount of vitamin C and B9 compared to uncoated references.

Table 1.
The combination of different plant extracts along with sodium alginate for edible fruit coatings to enhance shelf-life.
Table 1.
The combination of different plant extracts along with sodium alginate for edible fruit coatings to enhance shelf-life.
| S. No. | Coating Material | Composition | Effect of Coating/Key Findings of the Study | References |
|---|---|---|---|---|
| 1. | Sodium alginate | Caryophyllus aromaticus L. clove (flower buds) | Increased the shelf life of fresh-cut apples by up to 14 days with strong antioxidant and antimicrobial activity. | [22] |
| 2. | Sodium alginate | Flowers of the Cananga odorata tree | Effective for maintaining the quality and extending the shelf life of mandarins. | [75] |
| 3. | Sodium alginate | Pomegranates were rinsed in sodium hypochlorite solution, and then distilled water, and the fruit peel was manually segregated and oven-dried. Finely sieved powder was left overnight in 80% of 1000 mL ethanol solution at 25 °C. | It has a delayed respiration rate that lowers mass loss, pH, and sensory quality in ‘Punjab Beauty’ pear fruit, delaying fruit senescence throughout the storage. | [76] |
| 4. | Sodium alginate | Loquat leaf extract and alginate-based coating | Reduce postharvest decay with the preservation of the nutritional quality of Nanfeng tangerines. | [77] |
| 5. | Sodium alginate | Purple onion peel extract and butterfly pea flower extract. The extract was prepared using a solvent–ethanol 60% (v/v). | Films blended with the two extracts showed more excellent antioxidant, antimicrobial, and UV protection and functioned as a freshness sensor in foods compared to films containing single extracts. | [24] |
| 6. | Alginate and gellan | Sunflower oil, glycerol, and an antibrowning agent—N-acetylcysteine, cross-linking polymer—CaCl2 | Extended the shelf life approximately three times compared to uncoated apples, which deteriorate within 4 days of storage. Reduced ethylene production and preserving firmness and color during refrigerated storage of fresh-cut fuji apples. | [78] |
| 7. | Alginate-apple puree | Essential oils (Lemongrass, Oregano oil, Vanillin), glycerol, calcium chloride, N-acetylcysteine, | Coatings with calcium chloride and N-acetylcysteine helped maintain firmness and color. Lemongrass coatings induced severe texture softening. Oregano oil coatings exhibited intense antimicrobial activity against L. innocua, effectively inhibiting the growth of psychrophilic aerobic bacteria, yeasts, and molds. Vanillin coatings showed beneficial effects on the shelf life of fresh-cut ‘Fuji’ apples. Vanillin coatings (0.3% w/w) were the most effective in terms of sensory quality after 2 weeks of storage. | [79] |
| 8. | Alginate, gelatine | Glycerol, carboxymethylcellulose, sucroesters | 2% alginate and 5% gelatine coatings improved appearance, imparting an attractive, natural-looking sheen to the fruit, reduced weight loss, maintained fruit firmness, and preserved freshness. Extended the shelf life of “Bravo de Esmolfe” apples, even at room temperature. | [80] |
| 9. | Alginate | Prebiotics (oligofructose, inulin) | Prebiotic coatings containing oligofructose and inulin remained stable over a 14-day storage period. Coated apple wedges showed a stable browning index, firmness, and acidity compared to the control, with an increase in insoluble solids. | [81] |
| 10. | Alginate | Essential oils | Thyme, cinnamon, and oregano oils were the most potent essential oils against Listeria monocytogenes, Salmonella typhimurium, and Staphylococcus aureus, respectively, with inhibition zones ranging from 12.27 to 24.00 mm. Thyme oil (0.5 μL mL−1) was the most effective in inhibiting microorganisms and respiration, decreasing weight loss, maintaining firmness, and reducing browning reactions in fresh-cut apples. | [82] |
| 11. | Sodium Alginate | Glycerol, Lemongrass, sunflower oil | Coated samples with 0.3% and 0.5% (w/v) lemongrass showed significantly lower yeast and mold counts, as well as total plate counts (p < 0.05), compared to other samples, extending the shelf life and maintaining the quality of fresh-cut pineapple. | [83] |
| 12. | Sodium Alginate | Thymol, glycerol | Thymol improves sodium alginate film properties, offering antioxidant and antibacterial benefits for fresh-cut apple packaging, potentially substituting polyethylene cling wrap. | [84] |
| 13. | Sodium Alginate | Thyme and Oregano oils | Coatings with 1.0% thyme and oregano oils effectively maintained physicochemical properties and microbiological safety, enhancing papaya’s shelf life. | [85] |
| 14. | Sodium Alginate | Cellulose nanocrystals, ginger essential oil. | Enhanced mechanical strength, water vapor resistance, UV–visible light barrier capacity, and antioxidant properties, ultimately improving the postharvest quality of mango fruit. | [86] |
| 15. | Sodium Alginate | Whey protein isolate | Coating effectively slowed down water loss and retarded ripening and browning. It extended the shelf life of bananas by 6 days at 25 °C and exhibited excellent mechanical properties, water resistance, UV shielding, and gas-selective transmission. | [87] |
| 16. | Sodium Alginate | Loquat leaf extract | Enhances antioxidant defense systems, reduces postharvest decay rate and weight loss, and delays senescence in Nanfeng tangerines. | [77] |
| 17. | Sodium Alginate | Pomegranate peel extract | Reduces the respiration rate and mass loss and maintains the firmness and internal quality of ‘Punjab Beauty’ pears during cold storage. | [76] |
| 18. | Sodium Alginate | Ethanol, glycerol, ylang–ylang oil/cellulose nanocrystals Pickering emulsion | Alginate-based coating containing 0.5% ylang–ylang oil Pickering emulsion (YYO PE) effectively reduces weight loss, maintains firmness, and inhibits the growth of Penicillium italicum and Penicillium digitatum fungi on mandarins, thereby extending their shelf life. | [75] |
| 19. | Sodium Alginate | Cashew nutshell liquid | Non-toxic film with increased antimicrobial and antioxidant activity and better thermal properties | [88] |
5. Conclusions and Future Outlook
Alginate is observed as a remarkable and inexpensive material in active packaging due to its significant properties, like non-toxicity, biodegradability, and gelation capacity, derived from renewable natural sources, thus sustainable and potential to substitute plastic used for the packaging of fruits. The emergence of alginate as an edible coating material plays a vital role in the packaging industry by reducing plastic usage, thus reducing the generation of non-biodegradable waste. Due to its versatility in adjusting towards any additives (plasticizer and cross-linking agent) according to the formulation needed for the development of film/coating, alginate is an appropriate material. Incorporating plant extract into alginate containing various phytochemicals acts as an antioxidant and antimicrobial component, improving and increasing the efficiency of alginate-based coating, thus helping prolong the postharvest shelf life of fruits. Multiple studies have been conducted on incorporating plant extract and nanoparticles into alginate and coating materials. Based on the findings of the literature, it can be concluded that film enriched with plant extracts has the potential to increase tensile strength, reduce brittleness, improve flexibility, and control gas exchange and moisture retention. It helps maintain not only the physical aspects but also the nutritional value of fruit. Though researchers are working with alginate, there are some gaps. However, the focus should be on discovering new formulations and plant extracts that work best with alginate to increase the shelf life of fruits postharvest, thus reducing spoilage in natural environmental conditions at a commercial scale.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—review and editing, and supervision, A.K.S.; writing—original draft preparation and data analysis, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The author (A.K.S.) is thankful to the Council of Scientific & Industrial Research (CSIR), New Delhi, India, for the financial support of the research project grant (CSIR/22/0902/23/EMR–II).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors acknowledge the support from the Department of Chemistry and Research & Development Cell of Maharishi Markandeshwar (Deemed to be University), Mullana, Ambala, Haryana, India.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Tarancón, P.; Tárrega, A.; González, M.; Besada, C. External quality of mandarins: Influence of fruit appearance characteristics on consumer choice. Foods 2021, 10, 2188. [Google Scholar] [CrossRef] [PubMed]
- Dayeh, N.; Asefpour Vakilian, K.; Azadbakht, M. A fruit edible coating machine to protect the morphological, physiological, and biochemical properties of citrus fruits. Food Bioprod. Process. 2024, 148, 428–435. [Google Scholar] [CrossRef]
- Singh, A.K. Recent advancements in polysaccharides, proteins and lipids based edible coatings to enhance guava fruit shelf-life: A review. Int. J. Biol. Macromol. 2024, 262, 129826. [Google Scholar] [CrossRef]
- Shen, B.; Yan, Z.; Yang, T.; Zhu, L.; Wang, Y.; Jiang, L. Waste citrus pectin/garlic bionanohybrids for edible food preservation. J. Food Eng. 2024, 364, 111800. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, S.J.; Kim, T.I.; Chathuranga, K.; Lee, J.S.; Kim, S.; Kim, M.H.; Park, W.H. Chitosan-gallic acid conjugate edible coating film for perishable fruits. Food Chem. 2024, 463, 141322. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Patil, V.; Shams, R.; Dash, K.K. Techno-functional characteristics, and potential applications of edible coatings: A comprehensive review. J. Agric. Food Res. 2023, 14, 100886. [Google Scholar] [CrossRef]
- Summo, C.; De Angelis, D. The Importance of Edible Films and Coatings for Sustainable Food Development. Foods 2022, 11, 3221. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, A.M. Edible coatings and films for shelf-life extension of fruit and vegetables. Biomater. Adv. 2023, 154, 213632. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Such, A.; Wisła-Świder, A.; Węsierska, E.; Nowak, E.; Szatkowski, P.; Kopcińska, J.; Koronowicz, A. Edible chitosan-alginate based coatings enriched with turmeric and oregano additives: Formulation, antimicrobial and non-cytotoxic properties. Food Chem. 2023, 426, 136662. [Google Scholar] [CrossRef]
- Hamedi, H.; Kargozari, M.; Shotorbani, P.M.; Mogadam, N.B.; Fahimdanesh, M. A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: The antioxidant and antimicrobial activity, and application in food model. Food Hydrocoll. 2017, 72, 35–46. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, H.; Sun, S.; Yu, C. The interaction between sodium alginate and myofibrillar proteins: The rheological and emulsifying properties of their mixture. Int. J. Biol. Macromol. 2020, 161, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, R.; Benlloch, M.; Herrera, E.; García-Novelo, J.M. Effect of traditional and slow-release N fertilizers on growth of olive nursery plants and N losses by leaching. Sci. Hortic. 2004, 101, 39–49. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Borchard, W.; Kenning, A.; Kapp, A.; Mayer, C. Phase diagram of the system sodium alginate/water: A model for biofilms. Int. J. Biol. Macromol. 2005, 35, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, S.; Gontard, N.; Cuq, B. Technology and applications of edible protective films. Packag. Technol. Sci. 1995, 8, 339–346. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Singh, R.; Dar, A.H.; Dash, K.K. Effects of clove essential oil (Caryophyllus aromaticus L.) nanoemulsion incorporated edible coating on shelf-life of fresh cut apple pieces. J. Agric. Food Res. 2023, 14, 100791. [Google Scholar] [CrossRef]
- Dinh, T.A.; Le, Y.N.; Pham, N.Q.; Ton-That, P.; Van-Xuan, T.; Ho, T.G.T.; Nguyen, T.; Phuong, H.H.K. Fabrication of antimicrobial edible films from chitosan incorporated with guava leaf extract. Prog. Org. Coat. 2023, 183, 107772. [Google Scholar] [CrossRef]
- Santos, L.G.; Martins, V.G. Multifunctional alginate films blended with polyphenol-rich extract from unconventional edible sources: Bioactive properties, UV-light protection, and food freshness monitoring. Int. J. Biol. Macromol. 2024, 262, 130001. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, L.; Wardana, A.A.; Tanaka, F.; Tanaka, F. The physicochemical, mechanical, and antifungal properties of sodium alginate film containing Japanese rice vinegar and peppermint (Mentha piperita) oil as bio-composite packaging. Int. J. Biol. Macromol. 2024, 281, 136511. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Singh, N.; Kumar, P. A review on sources, modification techniques, properties and potential applications of alginate-based modified polymers. Eur. Polym. J. 2024, 213, 113078. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview. In Alginates—Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of alginate extraction processes: Impact on alginate molecular structure and techno-functional properties. Trends Food Sci. Technol. 2023, 140, 104142. [Google Scholar] [CrossRef]
- Shilpa, A.; Agrawal, S.S.; Ray, A.R. Controlled delivery of drugs from alginate matrix. J. Macromol. Sci.-Polym. Rev. 2003, 43, 187–221. [Google Scholar] [CrossRef]
- Mancini, F.; Montanari, L.; Peressini, D.; Fantozzi, P. Influence of alginate concentration and molecular weight on functional properties of mayonnaise. LWT 2002, 35, 517–525. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Yerramathi, B.B.; Muniraj, B.A.; Kola, M.; Konidala, K.K.; Arthala, P.K.; Sharma, T.S.K. Alginate biopolymeric structures: Versatile carriers for bioactive compounds in functional foods and nutraceutical formulations: A review. Int. J. Biol. Macromol. 2023, 253, 127067. [Google Scholar] [CrossRef]
- Yan, P.; Lan, W.; Xie, J. Modification on sodium alginate for food preservation: A review. Trends Food Sci. Technol. 2024, 143, 104217. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Mousavi Khaneghah, A. Alginate and derivatives hydrogels in encapsulation of probiotic bacteria: An updated review. Food Biosci. 2023, 52, 102433. [Google Scholar] [CrossRef]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation, characterizations and properties of sodium alginate grafted acrylonitrile/polyethylene glycol electrospun nanofibers. Int. J. Biol. Macromol. 2019, 137, 420–425. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: A review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef] [PubMed]
- Metha, C.; Pawar, S.; Suvarna, V. Recent advancements in alginate-based films for active food packaging applications. Sustain. Food Technol. 2024, 2, 1246–1265. [Google Scholar] [CrossRef]
- Das, M.; Senapati, P. Furosemide-loaded alginate microspheres prepared by ionic cross-linking technique: Morphology and release characteristics. Indian J. Pharm. Sci. 2008, 70, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Kubo, W.; Attwood, D. Oral sustained delivery of theophylline using in-situ gelation of sodium alginate. J. Control. Release 2000, 67, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Katchalsky, A.; Cooper, R.E.; Upadhyay, J.; Wassermann, A. 1028. Counter-ion fixation in alginates. J. Chem. Soc. 1961, 5198–5204. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. A review on plant extract-based route for synthesis of cobalt nanoparticles: Photocatalytic, electrochemical sensing and antibacterial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100270. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhardwaj, K. Mechanistic understanding of green synthesized cerium nanoparticles for the photocatalytic degradation of dyes and antibiotics from aqueous media and antimicrobial efficacy: A review. Environ. Res. 2024, 246, 118001. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Singh, A.K. Bio-waste and natural resource mediated eco-friendly synthesis of zinc oxide nanoparticles and their photocatalytic application against dyes contaminated water. Chem. Eng. J. Adv. 2023, 16, 100536. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Bakhsh, E.M.; Chani, M.T.S. Development of plant extract impregnated bacterial cellulose as a green antimicrobial composite for potential biomedical applications. Ind. Crops Prod. 2022, 187, 115337. [Google Scholar] [CrossRef]
- Li, T.; Sun, W.; Qian, D.; Wang, P.; Liu, X.; He, C.; Chang, T.; Liao, G.; Zhang, J. Plant-derived biomass-based hydrogels for biomedical applications. Trends Biotechnol. 2024. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, N.; Tyagi, B.; Yadav, P.; Samanta, A.K.; Tyagi, A.K. Exploring bioactive compounds and antioxidant properties of twenty-six Indian medicinal plant extracts: A correlative analysis for potential therapeutic insights. Food Humanit. 2023, 1, 1670–1679. [Google Scholar] [CrossRef]
- Singh, A.K. Flower extract-mediated green synthesis of bimetallic Cu–Zn oxide nanoparticles and its antimicrobial efficacy in hydrocolloid films. Bioresour. Technol. Rep. 2022, 18, 101034. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Yayla, S.; Hurkul, M.M.; Cetinkaya, A.; Uzun, L.; Ozkan, S.A. Selective apigenin assay in plant extracts and herbal supplement with molecularly imprinted polymer-based electrochemical sensor. Talanta 2025, 281, 126895. [Google Scholar] [CrossRef]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food-packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- Sapna; Sharma, C.; Pathak, P.; Yadav, S.P.; Gautam, S. Potential of emerging “all-natural” edible coatings to prevent post-harvest losses of vegetables and fruits for sustainable agriculture. Prog. Org. Coat. 2024, 193, 108537. [Google Scholar] [CrossRef]
- Bodana, V.; Swer, T.L.; Kumar, N.; Singh, A.; Samtiya, M.; Sari, T.P.; Babar, O.A. Development and characterization of pomegranate peel extract-functionalized jackfruit seed starch-based edible films and coatings for prolonging the shelf life of white grapes. Int. J. Biol. Macromol. 2024, 254, 127234. [Google Scholar] [CrossRef] [PubMed]
- Assocle, M.S.; Okidi, L.; Ongeng, D. The effect of rosemary, ginger, or garlic on microbial shelf life and sensory acceptability of nutritionally enriched cassava-based pancake (Kabalagala). Appl. Food Res. 2024, 4, 100384. [Google Scholar] [CrossRef]
- Zaidi, M.; Akbar, A.; Ali, S.; Akram, H.; Ercisli, S.; Ilhan, G.; Sakar, E.; Marc, R.A.; Sonmez, D.A.; Ullah, R.; et al. Application of Plant-Based Edible Coatings and Extracts Influences the Postharvest Quality and Shelf Life Potential of “Surahi” Guava Fruits. ACS Omega 2023, 8, 19523–19531. [Google Scholar] [CrossRef] [PubMed]
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. ‘Pedro Sato’ treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina). Sci. Hortic. 2018, 237, 59–66. [Google Scholar] [CrossRef]
- Zhang, X.; Lian, H.; Shi, J.; Meng, W.; Peng, Y. Plant extracts such as pine nut shell, peanut shell and jujube leaf improved the antioxidant ability and gas permeability of chitosan films. Int. J. Biol. Macromol. 2020, 148, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Shahid-ul-Islam; Jaiswal, V.; Butola, B.S.; Majumdar, A. Production of PVA-chitosan films using green synthesized ZnO NPs enriched with dragon fruit extract envisaging food packaging applications. Int. J. Biol. Macromol. 2023, 252, 126457. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Marcone, M.; Barbut, S.; Lim, L.T. The Impact of Anthocyanin-Rich Red Raspberry Extract (ARRE) on the Properties of Edible Soy Protein Isolate (SPI) Films. J. Food Sci. 2012, 77, C497–C505. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Kumari, M.; Mahajan, H.; Joshi, R.; Gupta, M. Development and structural characterization of edible films for improving fruit quality. Food Packag. Shelf Life 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.C.; Hubinger, M.D.; Menegalli, F.C. Development of Active Films From Pectin and Fruit Extracts: Light Protection, Antioxidant Capacity, and Compounds Stability. J. Food Sci. 2015, 80, C2389–C2396. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, X.; Qin, Y.; Yan, J.; Li, J.; Yang, Q. A novel edible packaging film based on chitosan incorporated with persimmon peel extract for the postharvest preservation of banana. Food Qual. Saf. 2022, 6, fyac028. [Google Scholar] [CrossRef]
- Rather, S.A.; Hussain, P.R.; Suradkar, P. Evaluating the effects of chitosan incorporated with seabuckthorn leaf extract composite edible coatings on the shelf life of peach (Prunus persica L.) fruits. Food Humanit. 2024, 2, 100280. [Google Scholar] [CrossRef]
- do Nascimento, A.; Toneto, L.C.; Lepaus, B.M.; Valiati, B.S.; Faria-Silva, L.; de São José, J.F.B. Effect of Edible Coatings of Cassava Starch Incorporated with Clove and Cinnamon Essential Oils on the Shelf Life of Papaya. Membranes 2023, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Dey, A. Phycoremediation—An emerging technique for dye abatement: An overview. Process Saf. Environ. Prot. 2021, 147, 214–225. [Google Scholar] [CrossRef]
- Abera, B.; Duraisamy, R.; Birhanu, T. Study on the preparation and use of edible coating of fish scale chitosan and glycerol blended banana pseudostem starch for the preservation of apples, mangoes, and strawberries. J. Agric. Food Res. 2024, 15, 100916. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.A.; Zaidi, S.; Islam, R.U.; Naseem, S.; Junaid, P.M. Enhanced shelf-life of peach fruit in alginate based edible coating loaded with TiO2 nanoparticles. Prog. Org. Coat. 2023, 182, 107688. [Google Scholar] [CrossRef]
- Rout, S.S.; Pradhan, K.C. A review on antimicrobial nano-based edible packaging: Sustainable applications and emerging trends in food industry. Food Control 2024, 163, 110470. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, X.C.; Tran, T.N.M.; Nguyen, N.T.T.; Pham, B.N.; Vu, D. Nano selenium–alginate edible coating extends hydroponic strawberry shelf life and provides selenium fortification as a micro-nutrient. Food Biosci. 2023, 53, 102597. [Google Scholar] [CrossRef]
- Nkede, F.N.; Wardak, M.H.; Wardana, A.A.; Fanze, M.; Yan, X.; Jothi, J.S.; Thi Hang Phuong, N.; Tanaka, F.; Tanaka, F. Preparation and characterization of edible coating and film composed of sodium alginate/ylang-ylang oil/cellulose nanocrystals Pickering emulsion and its application to post-harvest control of mandarin (Citrus reticulata). Colloids Surfaces A Physicochem. Eng. Asp. 2024, 691, 133859. [Google Scholar] [CrossRef]
- Megha, M.; Gill, P.P.S.; Jawandha, S.K.; Kaur, P.; Sinha, A. Sodium alginate enriched with pomegranate peel extract maintains pear cv. Punjab Beauty cell membrane integrity and postharvest life. S. Afr. J. Bot. 2023, 158, 149–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Chen, C.; Gan, Z.; Chen, J.; Wan, C. Loquat leaf extract and alginate based green composite edible coating for preserving the postharvest quality of Nanfeng tangerines. Sustain. Chem. Pharm. 2022, 27, 100674. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Moldão-Martins, M.; Beirão-da-Costa, S.M.; Beirão-da-Costa, M.L. The effects of edible coatings on postharvest quality of the “Bravo de Esmolfe” apple. Eur. Food Res. Technol. 2003, 217, 325–328. [Google Scholar] [CrossRef]
- Rößle, C.; Brunton, N.; Gormley, R.T.; Wouters, R.; Butler, F. Alginate Coating as Carrier of Oligofructose and Inulin and to Maintain the Quality of Fresh-Cut Apples. J. Food Sci. 2011, 76, H19–H29. [Google Scholar] [CrossRef] [PubMed]
- Sarengaowa; Hu, W.; Jiang, A.; Xiu, Z.; Feng, K. Effect of thyme oil–alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, Y.; Jiang, H.; Chen, L.; Shen, C.; Zhang, W.; Cao, J.; Jiang, W. Improved sustained-release properties of ginger essential oil in a Pickering emulsion system incorporated in sodium alginate film and delayed postharvest senescence of mango fruits. Food Chem. 2024, 435, 137534. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, Y.; Deng, Q.; Sun, Y.; Li, Y.; Wang, Z.; Jiang, F. Multifunctional sodium alginate-based self-healing edible cross-linked coating for banana preservation. Food Hydrocoll. 2024, 151, 109753. [Google Scholar] [CrossRef]
- Vasconcelos, L.; de Souza, M.; de Oliveira, J.; Filho, E.S.; Silva, A.; Mazzetto, S.E.; Pereira, E.S.; Oliveira, R.L.; Bezerra, L. Elaboration and characterization of bioactive films obtained from the incorporation of cashew nut shell liquid into a matrix of sodium alginate. Antioxidants 2021, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).