Ethanolic Cashew Leaf Extract Encapsulated in Tripolyphosphate–Chitosan Complexes: Characterization, Antimicrobial, and Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Ethanolic Cashew Leaf Extract (ECL-E) and the Encapsulation Process
2.2.1. Preparation of ECL-E
2.2.2. Dechlorophyllization of ECL-E
2.2.3. TPP–Chitosan (TPP-CS) Encapsulation Process
2.3. Analyses
2.3.1. Encapsulation Efficiency (EE)
2.3.2. Particle Size (PS), Polydispersity Index (PDI) and Zeta Potential Measurement
2.3.3. Color
2.3.4. In Vitro Releasing Efficiency (RE)
2.3.5. Antioxidant Activities
ABTS Radical Scavenging Activity
DPPH Radical Scavenging Activity
Ferric Reducing Ability Power
Metal Chelating Activity
Oxygen Radical Absorbance Capacity
2.3.6. Antimicrobial Activity
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.3.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.8. Scanning Electron Microscopic (SEM) Images of ECL-E-Ns
2.4. Light Stability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of ECL-E-Ns Prepared Using CS/ECL-E at Various Ratios by TPP-CS Complexation Methods
3.1.1. Encapsulation Efficiency (EE)
3.1.2. Particle Size (PS), Zeta Potential (ZP), and Polydispersity Index (PDI) of ECL-E-Ns
3.1.3. Color
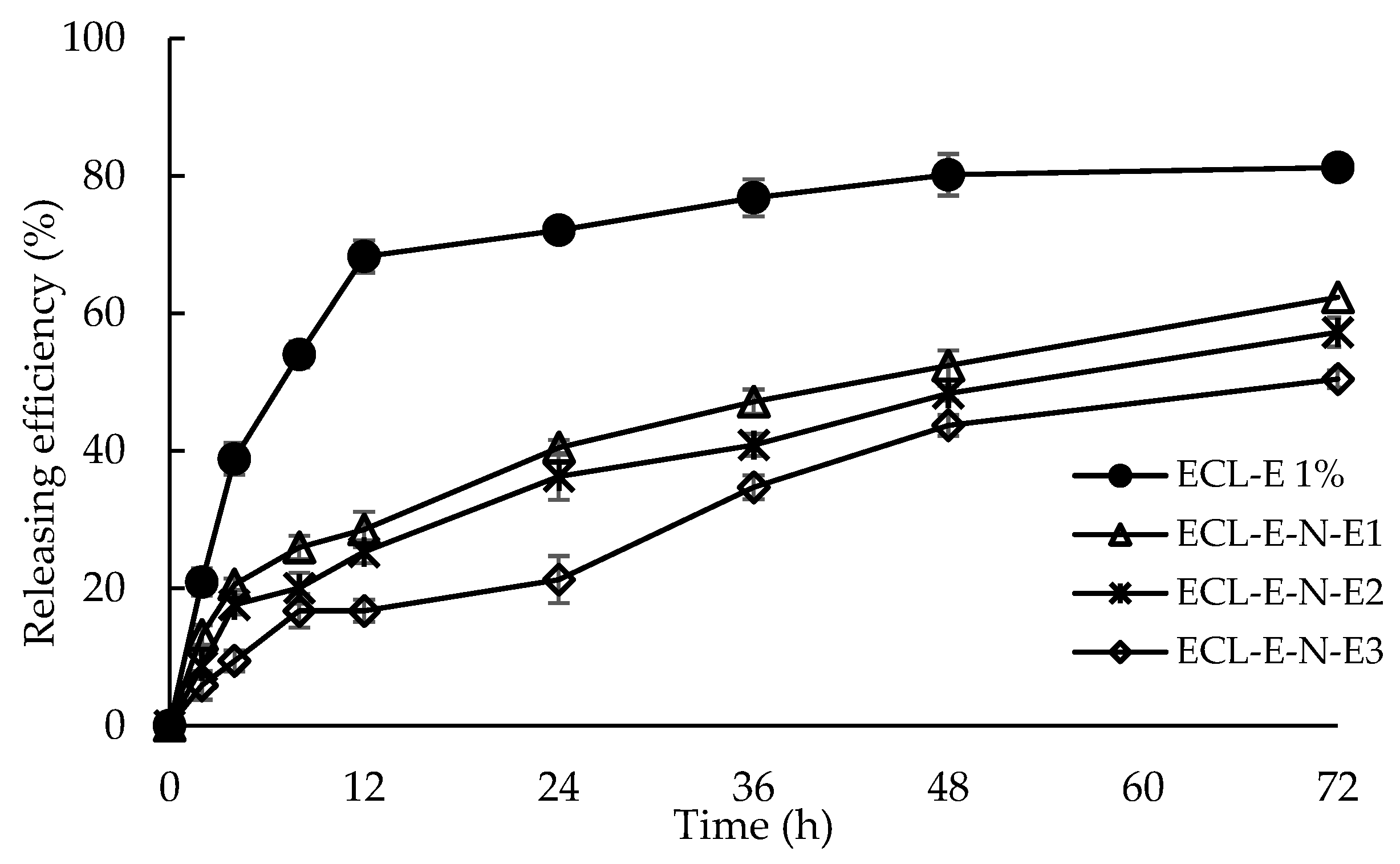
3.1.4. In Vitro Releasing Efficiency
3.1.5. Antioxidant Activities of TPP-CS Nanoparticles Prepared Using Different CS/ECL-E Ratios
3.1.6. Antimicrobial Activity
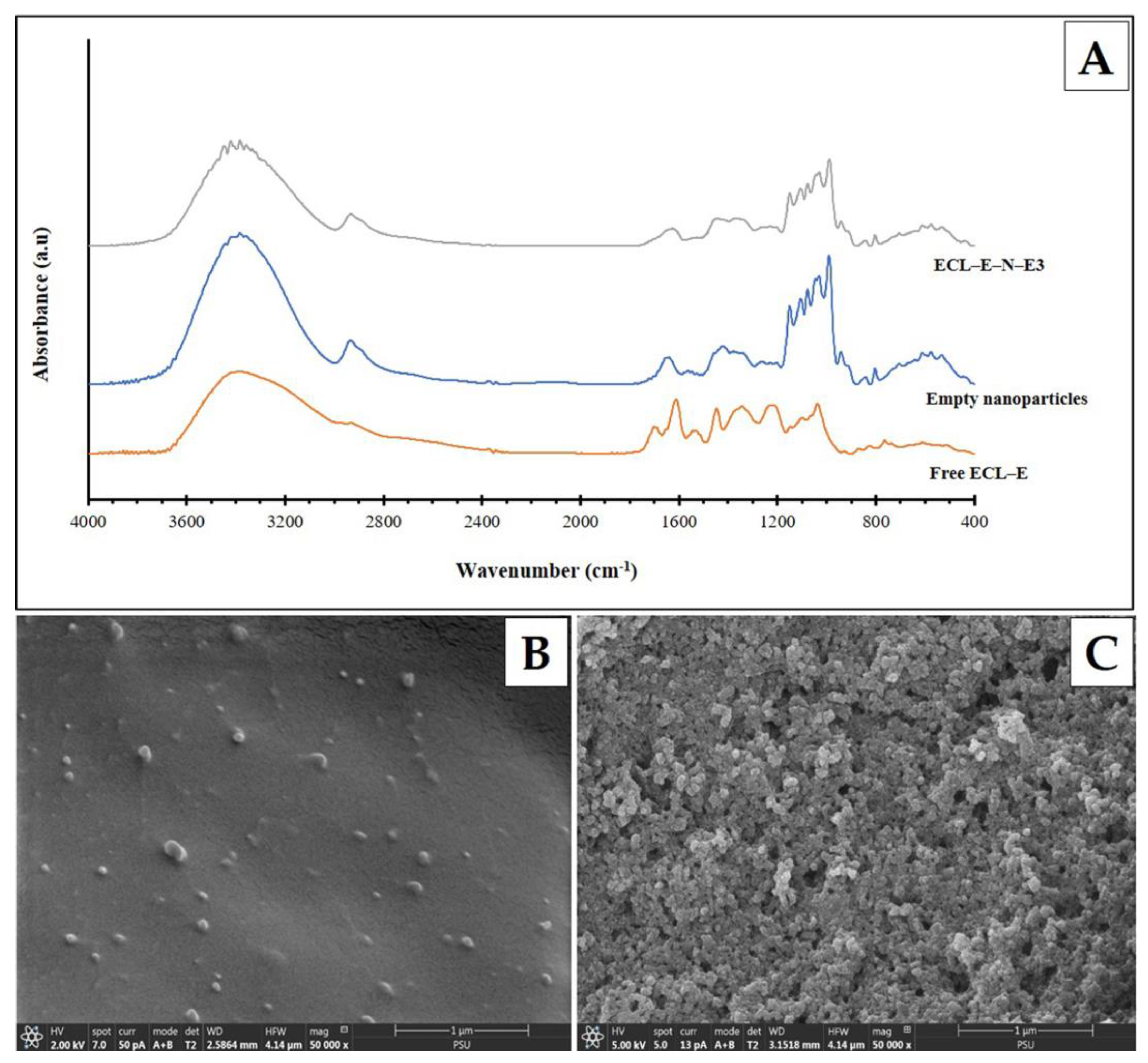
3.1.7. Fourier Transform Infrared Spectroscopic Spectra
3.1.8. Scanning Electron Microscopic Images
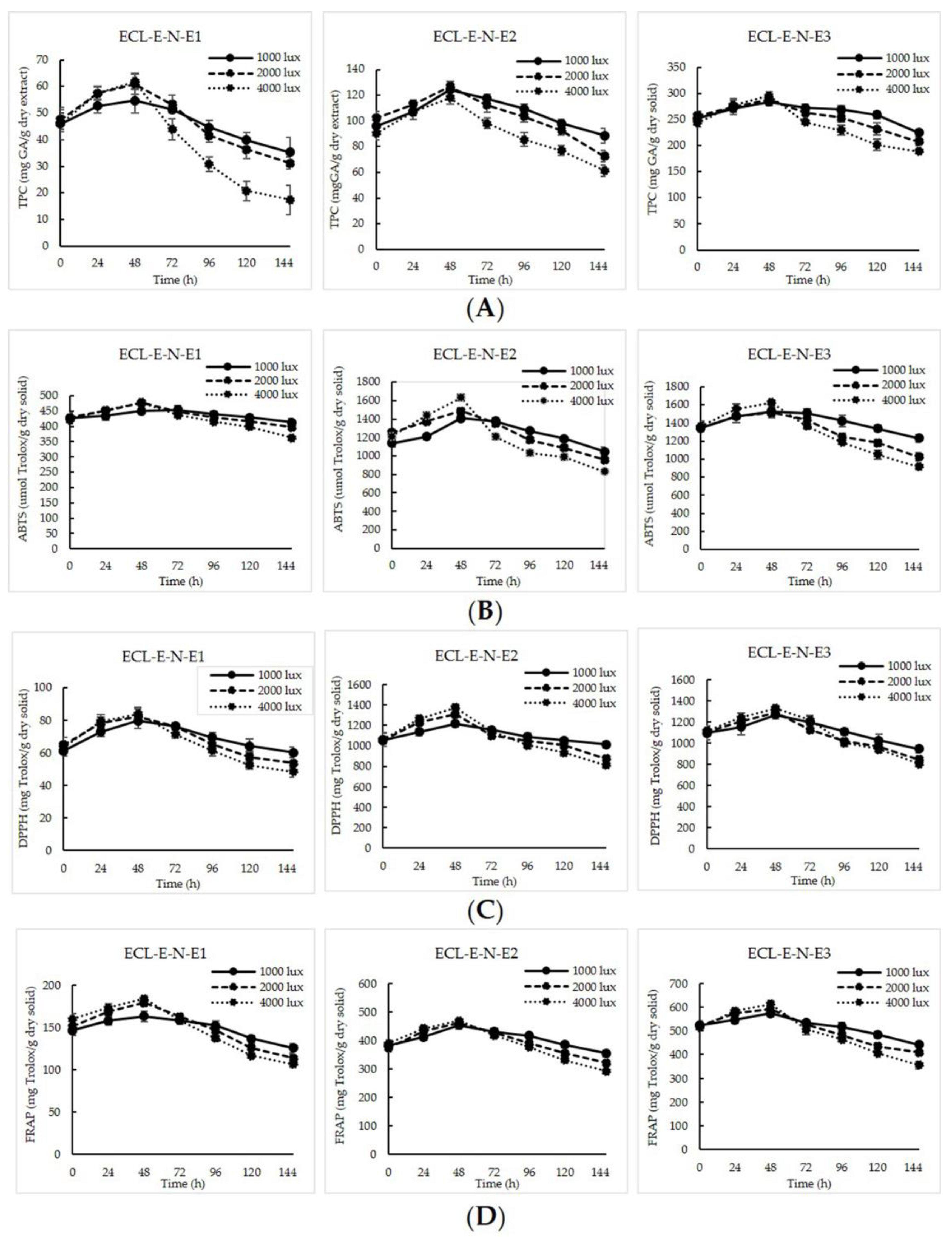
3.2. Light Stability of TPP-CS Nanoparticles Loaded with ECL-E as Affected by Light at Various Intensities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly) Phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Sinlapapanya, P.; Sumpavapol, P.; Nirmal, N.; Zhang, B.; Hong, H.; Benjakul, S. Ethanolic Cashew Leaf Extract: Antimicrobial Activity, Mode of Action, and Retardation of Spoilage Bacteria in Refrigerated Nile Tilapia Slices. Foods 2022, 11, 3461. [Google Scholar] [CrossRef]
- Sinlapapanya, P.; Sumpavapol, P.; Buatong, J.; Benjakul, S. Ethanolic Cashew Leaf Extract: Antioxidant Potential and Impact on Quality Changes of Dried Fermented Catfish During Storage. Future Foods 2024, 9, 100296. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available Technologies on Improving The Stability of Polyphenols in Food Processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, T.; Ho, C.T.; Huang, Q.; Wu, Q.; Zhang, M. Improving The Stability and Bioavailability of Tea Polyphenols by Encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Gulzar, S.; Raju, N.; Prodpran, T.; Benjakul, S. Chitosan-Tripolyphosphate Nanoparticles Improves Oxidative Stability of Encapsulated Shrimp Oil Throughout The Extended Storage. Eur. J. Lipid Sci. Technol. 2022, 124, 2100178. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of Chitosan-Based Carrier as an Encapsulating Agent in Food Industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid Nano Scale Cargos for The Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Rostami, M.; Yousefi, M.; Khezerlou, A.; Mohammadi, M.A.; Jafari, S.M. Application of Different Biopolymers for Nanoencapsulation of Antioxidants via Electrohydrodynamic Processes. Food Hydrocoll. 2019, 97, 105170. [Google Scholar] [CrossRef]
- Jacob, P.L.; Brugnoli, B.; Del Giudice, A.; Phan, H.; Chauhan, V.M.; Beckett, L.; Gillis, R.B.; Moloney, C.; Cavanagh, R.J.; Krumins, E.; et al. Poly (diglycerol adipate) Variants as Enhanced Nanocarrier Replacements in Drug Delivery Applications. J. Colloid Interface Sci. 2023, 641, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-Based Blends for Biomedical Applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kishor, R. Chapter 1-Chitin and Chitosan: Origin, Properties, and Applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, B.; Mariano, A.; Simonis, B.; Bombelli, C.; Sennato, S.; Piozzi, A.; Taresco, V.; Chauhan, V.M.; Howdle, S.M.; d’Abusco, A.S.; et al. Self-assembled Chitosan-sodium Caseinate Drug Delivery Nanosystems: Synthesis, Characterization, Stability Studies, in vitro Cytotoxicity and in vivo Biocompatibility against 143 B Cells. Carbohydr. Polym. Technol. Appl. 2023, 6, 100373. [Google Scholar] [CrossRef]
- Shafiei, M.; Jafarizadeh-Malmiri, H.; Rezaei, M. Biological Activities of Chitosan and Prepared Chitosan-Tripolyphosphate Nanoparticles using Ionic Gelation Method Against Various Pathogenic Bacteria and Fungi Strains. Biologia 2019, 74, 1561–1568. [Google Scholar] [CrossRef]
- Gutiérrez-Ruíz, S.C.; Cortes, H.; González-Torres, M.; Almarhoon, Z.M.; Gürer, E.S.; Sharifi-Rad, J.; Leyva-Gómez, G. Optimize The Parameters for The Synthesis By The Ionic Gelation Technique, Purification, and Freeze-Drying of Chitosan-Sodium Tripolyphosphate Nanoparticles for Biomedical Purposes. J. Biol. Eng. 2024, 18, 12. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of Food Bioactives and Nutraceuticals By Various Chitosan-Based Nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Di Santo, M.C.; D’Antoni, C.L.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-Tripolyphosphate Nanoparticles Designed to Encapsulate Polyphenolic Compounds for Biomedical and Pharmaceutical Applications—A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative Study of Encapsulated Peppermint and Green Tea Essential Oils in Chitosan Nanoparticles: Encapsulation, Thermal Stability, In-vitro Release, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Tagrida, M.; Prodpran, T.; Zhang, B.; Aluko, R.E.; Benjakul, S. Liposomes Loaded with Betel Leaf (Piper betle L.) Ethanolic Extract Prepared by Thin Film Hydration and Ethanol Injection Methods: Characteristics and Antioxidant Activities. J. Food Biochem. 2021, 45, e14012. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K.; Amnuaikit, T. Liposomal Encapsulated Ethanolic Coconut Husk Extract: AntiOxidant and Antibacterial Properties. J. Food Sci. 2019, 84, 3664–3673. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Kittiphattanabawon, P.; Sumpavapol, P.; Maqsood, S. Antioxidant Activities of Lead (Leucaena leucocephala) Seed as Affected By Extraction Solvent, Prior Dechlorophyllisation and Drying Methods. J. Food Sci. Technol. 2014, 51, 3026–3037. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant Activities and Selected Characteristics of Gelatin Hydrolysates from Seabass (Lates calcarifer) Skin as Affected by Production Processes. J. Food Sci. Technol. 2016, 53, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.K.N.; Hadisurya, M.; Cornwall, R.G. Antimicrobial Investigation and Structure Activity Analysis of Natural Eugenol Derivatives Against Several Oral Bacteria. J. Pharm. Microbiol. 2019, 5, 1–4. [Google Scholar]
- Jafarı-sales, A.; Jafari, B.; Khaneshpour, H.; Pashazadeh, M. Antibacterial Effect of Methanolic Extract of Rosa Damascena on Standard Bacteria Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa In Vitro. Int. J. Nat. Life Sci. 2020, 4, 40–46. [Google Scholar]
- Kadam, D.; Lele, S.S. Cross-Linking Effect of Polyphenolic Extracts of Lepidium sativum Seedcake on Physicochemical Properties of Chitosan Films. Int. J. Biol. Macromol. 2018, 114, 1240–1247. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of The Mechanical Properties of Chitosan. J. Biomater. Sci. Polym. Ed. 2020, 31, 350–375. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of The Phenolic Compounds of The Blackberry (Rubus fruticosus). LWT-Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Kaushalya, K.G.D.; Gunathilake, K.D.P.P. Encapsulation of Phlorotannins From Edible Brown Seaweed in Chitosan: Effect of Fortification on Bioactivity and Stability in Functional Foods. Food Chem. 2022, 377, 132012. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Dave, R.H. Preparation of Chitosan-TPP Nanoparticles: The Influence of Chitosan Polymeric Properties and Formulation Variables. Int. J. Appl. Pharm. 2018, 10, 60–65. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and In Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Danaei, M.R.M.M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on The Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Weng, J.; Wu, X.; Wang, W.; Yang, Q.; Guo, F.; Wu, D.; Song, Y.; Chen, F.; Yang, G. Characteristics, Cryoprotection Evaluation and In Vitro Release of BSA-Loaded Chitosan Nanoparticles. Mar. Drugs 2020, 18, 315. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Dos Santos, A.M.; Boni, F.I.; Dos Santos, K.C.; Robusti, L.M.G.; de Souza, M.P.; Ferreira, N.N.; Carvalho, S.G.; Cardoso, V.M.O.; Chorilli, M.; et al. Design of Chitosan-Lased Particle Systems: A Review of The Physicochemical Foundations for Tailored Properties. Carbohydr. Polym. 2020, 250, 116968. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.T.D.I.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Prakash, S.; Mishra, R.; Malviya, R.; Sharma, P.K. Measurement Techniques and Pharmaceutical Applications of Zeta Potential: A Review. J. Chronother. Drug Deliv. 2014, 5, 33–40. [Google Scholar]
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant Concentration and pH Effects on The Zeta Potential Values of Alumina Nanofluids to Inspect Stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; Montalbán, M.G.; Peña-García, J.; Pérez-Sánchez, H.; Víllora, G.; Baños, F.G.D. Chitosan as Stabilizing Agent for Negatively Charged Nanoparticles. Carbohydr. Polym. 2017, 161, 63–70. [Google Scholar] [CrossRef]
- Ozturk, K.; Arslan, F.B.; Tavukcuoglu, E.; Esendagli, G.; Calis, S. Aggregation of Chitosan Nanoparticles in Cell Culture: Reasons and Resolutions. Int. J. Pharm. 2020, 578, 119119. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of Powder Particle Structure on The Oxidation Stability and Color of Encapsulated Crystalline and Emulsified Carotenoids in Carrot Concentrate Powders. J. Food Eng. 2019, 263, 398–408. [Google Scholar] [CrossRef]
- Zubko, E.; Shkuratov, Y.; Videen, G. Effect of Morphology on Light Scattering By Agglomerates. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 42–54. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Rahman, M.R.T.; Van Vuong, Q. Micro and Nano Encapsulation, Retention and Controlled Release of Flavor and Aroma Compounds: A Critical Review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Role of Powder Particle Size on The Encapsulation Efficiency of Oils During Spray Drying. Dry. Technol. 2007, 25, 1081–1089. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and Antioxidant Activity of Curcumin after Encapsulated by Nano and Pickerng Emulsion Based on Chitosan-Tripolyphosphate Nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Soleymanfallah, S.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Preparation, Physical Properties, and Evaluation of Antioxidant Capacity of Aqueous Grape Extract Loaded in Chitosan-TPP Nanoparticles. Food Sci. Nutr. 2022, 10, 3272–3281. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Li, Q.; Mi, Y.; Tan, W.; Guo, Z. Highly Efficient Free Radical-Scavenging Property of Phenolic-Functionalized Chitosan Derivatives: Chemical Modification and Activity Assessment. Int. J. Biol. Macromol. 2020, 164, 4279–4288. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, K.; Ma, Y.; Li, R.; Xing, R.; Yu, H.; Li, P. Preparation and Antioxidant Activity of Chitosan Dimers with Different Sequences. Mar. Drugs 2021, 19, 366. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of The Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. The Influence of Nanodelivery Systems on the Antioxidant Activity of Natural Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2022, 62, 3208–3231. [Google Scholar] [CrossRef]
- Li, Q.; Duan, M.; Liu, L.; Chen, X.; Fu, Y.; Li, J.; Zhao, T.; McClements, D.J. Impact of Polyphenol Interactions with Titanium dioxide Nanoparticles on Their Bioavailability and Antioxidant Activity. J. Agric. Food Chem. 2021, 69, 9661–9670. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Koilparambil, D.; Varghese, S.; Shaikmoideen, J.M. Chitosan Nanoparticles: A Novel Antimicrobial Agent. In Nanobiotechnology in Diagnosis, Drug Delivery, and Treatment; Rai, M., Razzaghi-Abyaneh, M., Ingle, A.P., Eds.; John Wiley and Sons: Chichester, UK, 2020; pp. 197–215. [Google Scholar]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled Release of Antioxidants from Active Food Packaging: A Review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of The Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and Morphological Properties of Size-Controlled Chitosan–Tripolyphosphate Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Nurhayati, M.; Dara, F.; Rizki, R.; Nasir, M.; Aziz, H.A.; Hendrawan, H.; Poh, N.E.; Kaewsaneha, C.; Opaprakasit, P. Physicochemical Properties of TPP-Crosslinked Chitosan Nanoparticles as Potential Antibacterial Agents. Fibers Polym. 2021, 22, 2954–2964. [Google Scholar] [CrossRef]
- Lubis, L.D.; Prananda, A.T.; Juwita, N.A.; Nasution, M.A.; Syahputra, R.A.; Sumaiyah, S.; Lubis, R.R.; Lubis, M.F.; Astyka, R.; Atiqah, J.F. Unveiling Antioxidant Capacity of Standardized Chitosan-Tripolyphosphate Microcapsules Containing PolyPhenol-Rich Extract of Portulaca oleraceae. Heliyon 2024, 10, e29541. [Google Scholar] [CrossRef]
- Luo, W.C.; Beringhs, A.O.R.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of Formulation on The Quality and Stability of Freeze-Dried Nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Kaptso, G.K.; Kwaw, E.; Cheno, R.W.; Wu, M.; Osae, R.; Ma, S.; Farooq, M. Carrier Effects on The Chemical and Physical Properties of Freeze-Dried Encapsulated Mulberry Leaf Extract Powder. Acta Chim. Slov. 2018, 65, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Wais, U.; Jackson, A.W.; He, T.; Zhang, H. Nanoformulation and Encapsulation Approaches for Poorly Water-Soluble Drug Nanoparticles. Nanoscale 2016, 8, 1746–1769. [Google Scholar] [CrossRef] [PubMed]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. Effects of Simultaneous UV-C Radiation and Ultrasonic Energy Postharvest Treatment on Bioactive Compounds and Antioxidant Activity of Tomatoes During Storage. Food Chem. 2019, 270, 113–122. [Google Scholar] [CrossRef]
- Yue, W.; Yao, P.; Wei, Y.; Mo, H. Synergetic Effect of Ozone and Ultrasonic Radiation on Degradation of Chitosan. Polym. Degrad. Stab. 2008, 93, 1814–1821. [Google Scholar] [CrossRef]
- Bussiere, P.O.; Gardette, J.L.; Rapp, G.; Masson, C.; Therias, S. New Insights into The Mechanism of Photodegradation of Chitosan. Carbohydr. Polym. 2021, 259, 117715. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-Edge Advances in Tailoring Size, Shape, and Functionality of Nanoparticles and Nanostructures: A review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of Phenolic Compounds in Grape Stem Extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef]
| Treatment | Ratio | EE (%) | PS (nm) | ZP (mV) | PDI | Color | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| ECL-E | - | - | - | - | - | 59.34 ± 0.07 e | 1.33 ± 0.03 a | 10.16 ± 0.04 a |
| TPP-CS | CS | 43.01 ± 8.89 b | 47.30 ± 4.60 d | 43.96 ± 0.96 a | 0.56 ± 0.06 a | 63.02 ± 0.36 d | −0.54 ± 0.06 d | −0.65 ± 0.01 d |
| ECL-E-N-E1 | CS(1):ECL-E(1) | 47.62 ± 5.71 b | 314.60 ± 20.65 a | 44.24 ± 0.92 a | 0.30 ± 0.03 b | 64.71 ± 0.03 c | 0.73 ± 0.05 c | 10.07 ± 0.01 b |
| ECL-E-N-E2 | CS(1):ECL-E(2) | 86.72 ± 2.28 a | 273.60 ± 3.70 b | 41.13 ± 1.01 b | 0.20 ± 0.02 c | 66.91 ± 0.03 b | 1.26 ± 0.02 b | 9.73 ± 0.09 c |
| ECL-E-N-E3 | CS(1):ECL-E(3) | 89.47 ± 1.78 a | 256.05 ± 7.70 c | 40.37 ± 0.66 b | 0.22 ± 0.05 c | 69.55 ± 0.04 a | 1.22 ± 0.05 b | 10.10 ± 0.01 a |
| Treatment | TPC (mg GAE/g Dry Solid) | ABTS (μmol TE/g Dry Solid) | DPPH (mg TE/g dry Solid) | FRAP (mg TE/g Dry Solid) | MCA (μmol EE/g Dry Solid) | ORAC (μmol TE/g Dry Extract) |
|---|---|---|---|---|---|---|
| ECL-E | 345.81 ± 16.22 a | 1409.09 ± 24.26 a | 362.42 ± 22.24 c | 892.25 ± 36.87 a | 4.51 ± 0.14 c | 73.91 ± 8.67 b |
| TPP-CS | 0.33 ± 0.07 e | 2.15 ± 0.06 e | 29.01 ± 0.57 e | 0.08 ± 0.02 e | 5.81 ± 0.41 b | 120.75 ± 2.26 a |
| ECL-E-N-E1 | 47.41 ± 0.74 d | 438.18 ± 0.83 d | 68.81 ± 0.24 d | 156.37 ± 0.35 d | 6.83 ± 0.38 a | 26.99 ± 1.44 d |
| ECL-E-N-E2 | 97.55 ± 0.35 c | 1302.86 ± 12.06 b | 1058.81 ± 1.61 b | 394.20 ± 0.62 c | 3.30 ± 0.31 d | 28.93 ± 1.42 d |
| ECL-E-N-E3 | 253.34 ± 0.20 b | 1240.80 ± 13.02 c | 1112.75 ± 13.89 a | 518.56 ± 0.38 b | 4.73 ± 0.32 c | 50.08 ± 1.31 c |
| Treatment | P. aeruginosa (mg/mL) | S. putrefaciens (mg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| ECL-E | 2.34 a | 9.38 a | 1.17 a | 4.69 a |
| TPP-CS | 37.50 d | 300.00 d | 18.75 d | 150.00 c |
| ECL-E-N-E1 | 18.75 c | 150.00 c | 9.38 c | 75.00 b |
| ECL-E-N-E2 | 9.38 b | 75.00 b | 9.38 c | 75.00 b |
| ECL-E-N-E3 | 9.38 b | 75.00 b | 4.69 b | 75.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinlapapanya, P.; Buatong, J.; Palamae, S.; Nazeer, R.A.; Zhang, B.; Prodpran, T.; Benjakul, S. Ethanolic Cashew Leaf Extract Encapsulated in Tripolyphosphate–Chitosan Complexes: Characterization, Antimicrobial, and Antioxidant Activities. Colloids Interfaces 2024, 8, 52. https://doi.org/10.3390/colloids8050052
Sinlapapanya P, Buatong J, Palamae S, Nazeer RA, Zhang B, Prodpran T, Benjakul S. Ethanolic Cashew Leaf Extract Encapsulated in Tripolyphosphate–Chitosan Complexes: Characterization, Antimicrobial, and Antioxidant Activities. Colloids and Interfaces. 2024; 8(5):52. https://doi.org/10.3390/colloids8050052
Chicago/Turabian StyleSinlapapanya, Pitima, Jirayu Buatong, Suriya Palamae, Rasool Abdul Nazeer, Bin Zhang, Thummanoon Prodpran, and Soottawat Benjakul. 2024. "Ethanolic Cashew Leaf Extract Encapsulated in Tripolyphosphate–Chitosan Complexes: Characterization, Antimicrobial, and Antioxidant Activities" Colloids and Interfaces 8, no. 5: 52. https://doi.org/10.3390/colloids8050052
APA StyleSinlapapanya, P., Buatong, J., Palamae, S., Nazeer, R. A., Zhang, B., Prodpran, T., & Benjakul, S. (2024). Ethanolic Cashew Leaf Extract Encapsulated in Tripolyphosphate–Chitosan Complexes: Characterization, Antimicrobial, and Antioxidant Activities. Colloids and Interfaces, 8(5), 52. https://doi.org/10.3390/colloids8050052









