Structure and Potential Application of Surfactant-Free Microemulsion Consisting of Heptanol, Ethanol and Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Diagram Construction
2.3. Electrical Conductivity Measurements
2.4. Determination of Surface Tension
2.5. DLS Measurements
2.6. FT-IR Measurements
2.7. Synthesis of Solid Silica Nanoparticles (SSNs)
2.8. The Preparation of Nanoparticle Samples for TEM Imaging
3. Results and Discussion
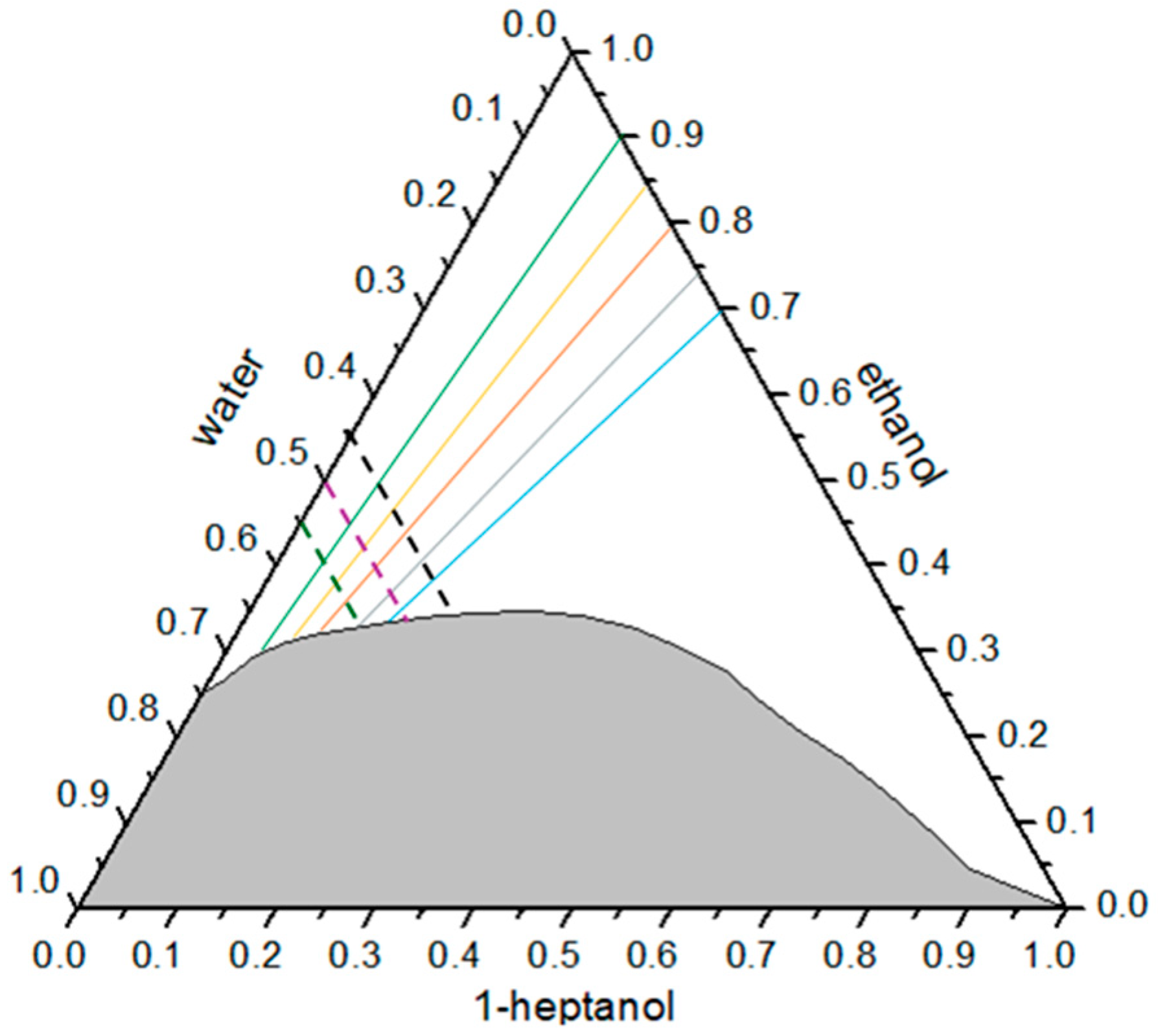
3.1. Phase Behavior of the Heptanol/Ethanol/Water Ternary System
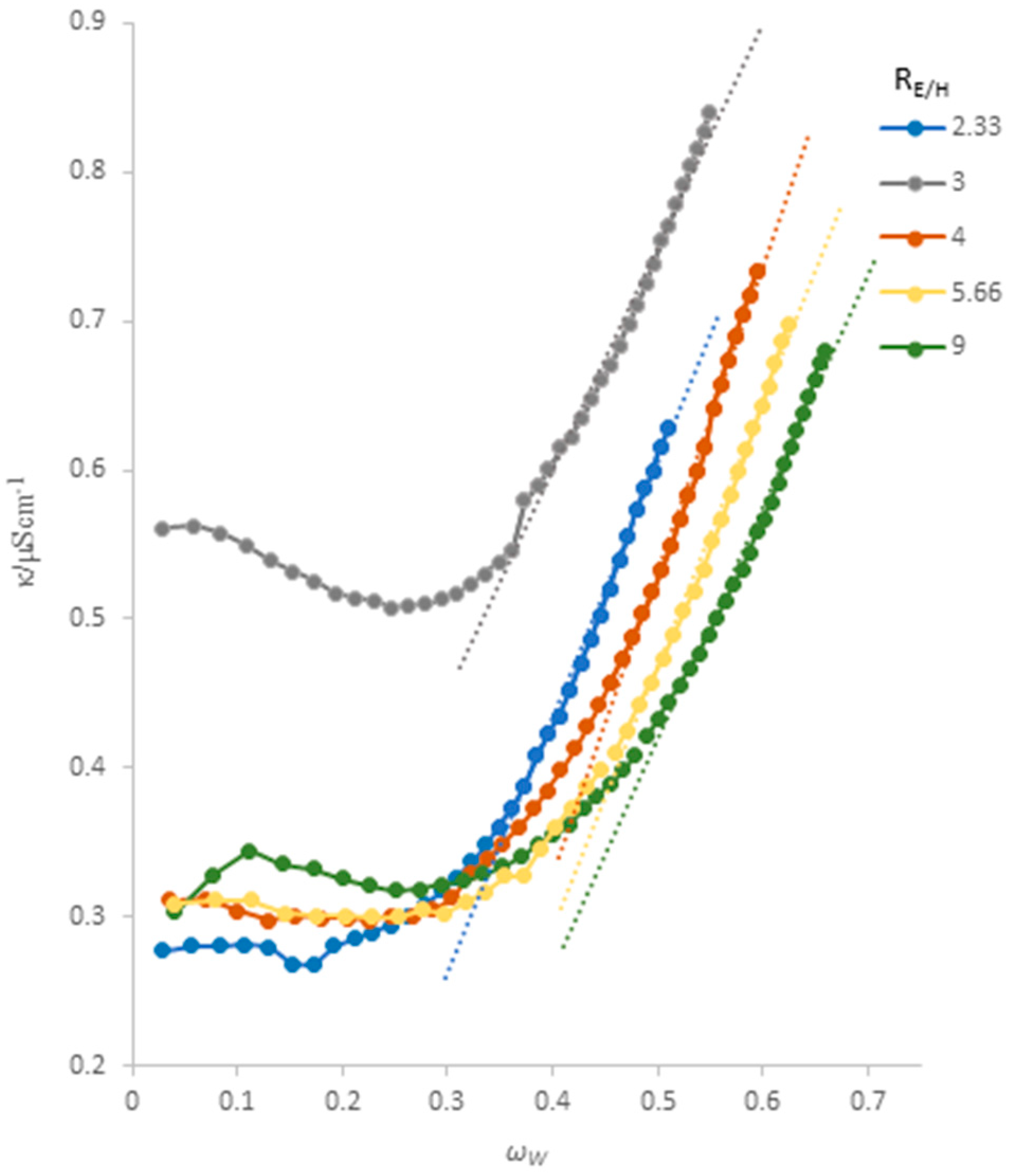
3.2. Electrical Conductivity Analysis
3.3. Surface Tension
3.4. DLS
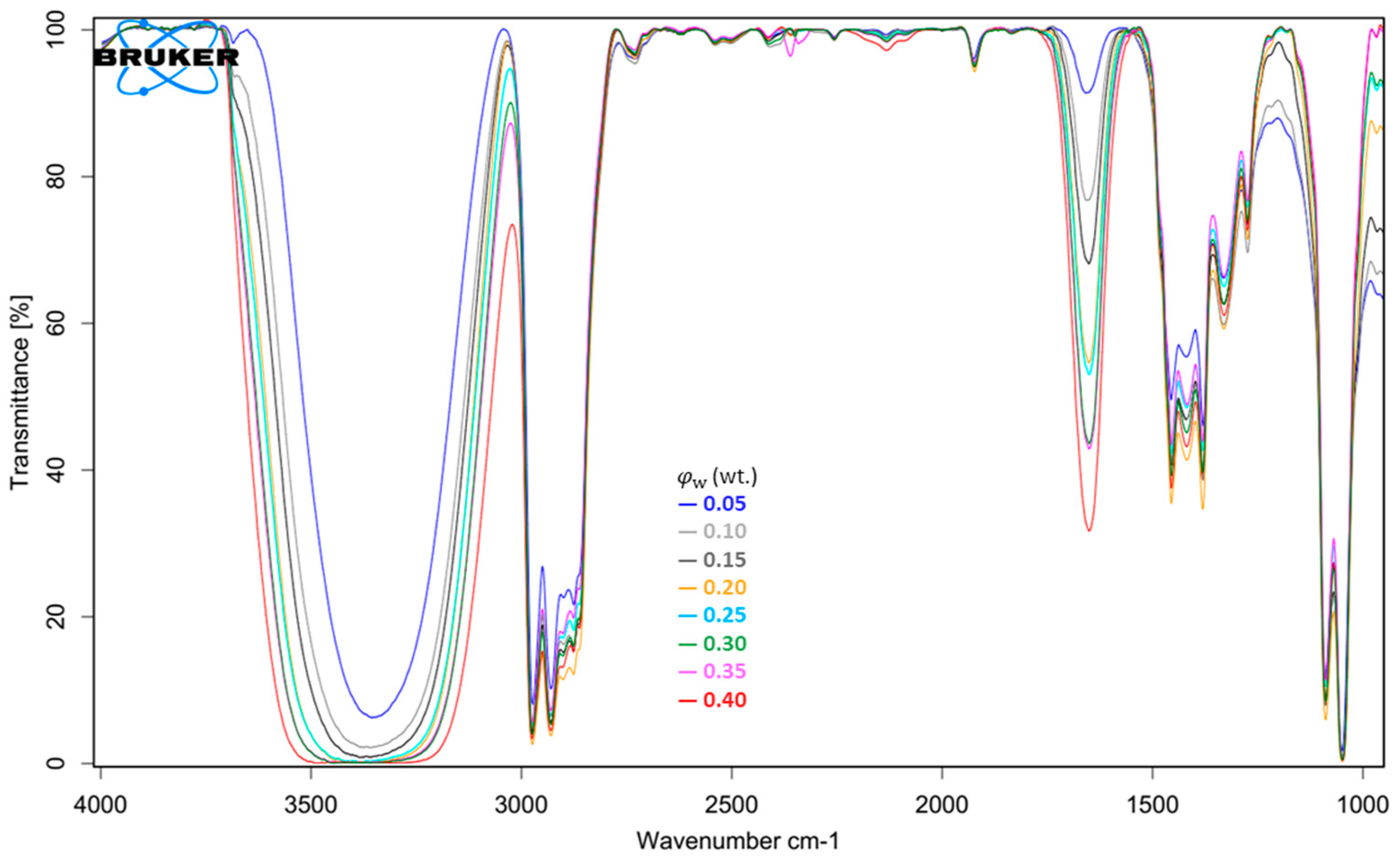
3.5. FT-IR Spectrum
3.6. Synthesized SSNs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, L.M. Microemulsions versus Micelles. J. Colloid Interface Sci. 1975, 52, 182–188. [Google Scholar] [CrossRef]
- Paul, B.K.; Moulik, S.P. Microemulsions: An Overview. J. Dispers. Sci. Technol. 1997, 18, 301–367. [Google Scholar] [CrossRef]
- Masahiko, A. Macro- and Microemulsions. J. Jpn. Oil Chem. Soc. 1998, 47, 819–843. [Google Scholar]
- Solans, C.; García-Celma, M.J. Surfactants for Microemulsions. Curr. Opin. Colloid Interface Sci. 1997, 2, 464–471. [Google Scholar] [CrossRef]
- Han, Y.; Pan, N.; Li, D.; Liu, S.; Sun, B.; Chai, J.; Li, D. Formation Mechanism of Surfactant-Free Microemulsion and a Judgment on Whether It Can Be Formed in One Ternary System. Chem. Eng. J. 2022, 437, 135385. [Google Scholar] [CrossRef]
- Olkowska, E.; Polkowska, Z.; Namieśnik, J. Analytics of Surfactants in the Environment: Problems and Challenges. Chem. Rev. 2011, 111, 5667–5700. [Google Scholar] [CrossRef]
- Zhang, X.; Song, M.; Chai, J.; Cui, X.; Wang, J. Preparation, Characterization and Application of a Surfactant-Free Microemulsion Containing 1-Octen-3-Ol, Ethanol, and Water. J. Mol. Liq. 2020, 300, 112278. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Zhu, B.; Zhou, Y.; Liu, X.; Yang, C. Temperature-Switchable Surfactant-Free Microemulsion. Langmuir 2020, 36, 7356–7364. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Wang, L.; Dai, S.; Wang, B.; Wu, Y. Oil Removal from Solid Surface by Using Surfactant-Free Microemulsion Regulated by CO2: A Sustainable Approach for Treating Oily Waste. J. Clean. Prod. 2022, 345, 130990. [Google Scholar] [CrossRef]
- Hankel, R.F.; Rojas, P.E.; Cano-Sarabia, M.; Sala, S.; Veciana, J.; Braeuer, A.; Ventosa, N. Surfactant-Free CO2-Based Microemulsion-like Systems. Chem. Commun. 2014, 50, 8215–8218. [Google Scholar] [CrossRef]
- Hou, W.; Xu, J. Surfactant-Free Microemulsions. Curr. Opin. Colloid Interface Sci. 2016, 25, 67–74. [Google Scholar] [CrossRef]
- Gudelj, M.; Šurina, P.; Jurko, L.; Prkić, A.; Bošković, P. The Additive Influence of Propane-1,2-Diol on SDS Micellar Structure and Properties. Molecules 2021, 26, 3773. [Google Scholar] [CrossRef]
- Krickl, S.; Jurko, L.; Wolos, K.; Touraud, D.; Kunz, W. Surfactant-Free Microemulsions with Cleavable Constituents. J. Mol. Liq. 2018, 271, 112–117. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Z.; Xu, L.; Lou, N.; Li, M.; Liu, L. Controllable Click Synthesis of Poly(Ionic Liquid)s by Surfactant-Free Ionic Liquid Microemulsions for Selective Dyes Reduction. React. Funct. Polym. 2020, 147, 104464. [Google Scholar] [CrossRef]
- Mirhoseini, B.S.; Salabat, A. A Novel Surfactant-Free Microemulsion System for the Synthesis of Poly (Methyl Methacrylate)/Ag Nanocomposite. J. Mol. Liq. 2021, 342, 117555. [Google Scholar] [CrossRef]
- Drapeau, J.; Verdier, M.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Effective Insect Repellent Formulation in Both Surfactantless and Classical Microemulsions with a Long-Lasting Protection for Human Beings. Chem. Biodivers. 2009, 6, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Zoumpanioti, M.; Karali, M.; Xenakis, A.; Stamatis, H. Lipase Biocatalytic Processes in Surfactant Free Microemulsion-like Ternary Systems and Related Organogels. Enzyme Microb. Technol. 2006, 39, 531–539. [Google Scholar] [CrossRef]
- Han, Y.; Liu, S.; Du, Y.; Li, D.; Pan, N.; Chai, J.; Li, D. A New Application of Surfactant-Free Microemulsion: Solubilization and Transport of Drugs and Its Transdermal Release Properties. J. Taiwan Inst. Chem. Eng. 2022, 138, 104473. [Google Scholar] [CrossRef]
- Song, L.; Jia, H.; Zhang, F.; Jia, H.; Wang, Y.; Xie, Q.; Fan, F.; Wang, Q.; Wen, S. Sustainable Utilization of Surfactant-Free Microemulsion Regulated by CO2 for Treating Oily Wastes: A Interpretation of the Response Mechanism. Langmuir 2024, 40, 960–967. [Google Scholar] [CrossRef]
- Liu, W.; Pan, N.; Han, Y.; Li, D.; Chai, J. Solubilization, Stability and Antioxidant Activity of Curcumin in a Novel Surfactant-Free Microemulsion System. LWT 2021, 147, 111583. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Dai, S.; Lu, H. Surfactant-Free Microemulsion Based on CO2-Induced Ionic Liquids. J. Phys. Chem. B 2019, 123, 9024–9030. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Wang, R.; Shi, Z.; Wu, C.; Li, D. Mesoporous La-Based Nanorods Synthesized from a Novel IL-SFME for Phosphate Removal in Aquatic Systems. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 624, 126689. [Google Scholar] [CrossRef]
- Marcus, J.; Touraud, D.; Prévost, S.; Diat, O.; Zemb, T.; Kunz, W. Influence of Additives on the Structure of Surfactant-Free Microemulsions. Phys. Chem. Chem. Phys. 2015, 17, 32528–32538. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jing, J.; Li, X.; Yue, W.; Qi, J.; Wang, N.; Lu, H. CO2-Responsive Hydrophobic Deep Eutectic Solvent Based on Surfactant-Free Microemulsion-Mediated Synthesis of BaF2 Nanoparticles. Langmuir 2023, 39, 1181–1189. [Google Scholar] [CrossRef]
- Li, M.; Yuan, J.; Yang, Q.; Liu, Z.; Meng, S.; Wang, X.; Peng, C.; Yin, T. Therapeutic Deep Eutectic Solvents Based on Natural Product Matrine and Caprylic Acid: Physical Properties, Cytotoxicity and Formation of Surfactant Free Microemulsion. J. Drug Deliv. Sci. Tehnol. 2023, 90, 105177. [Google Scholar] [CrossRef]
- Anjali; Pandey, S. Formation of Ethanolamine-Mediated Surfactant-Free Microemulsions Using Hydrophobic Deep Eutectic Solvents. Langmuir 2024, 40, 2254–2267. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Muller, L.; Degot, P.; Touraud, D.; Kunz, W. NADES-Based Surfactant-Free Microemulsions for Solubilization and Extraction of Curcumin from Curcuma Longa. Food Chem. 2021, 355, 129624. [Google Scholar] [CrossRef] [PubMed]
- Tarkas, H.; Rokade, A.; Upasani, D.; Pardhi, N.; Rokade, A.; Sali, J.; Patole, S.P.; Jadkar, S. Pioneering Method for the Synthesis of Lead Sulfide (PbS) Nanoparticles Using a Surfactant-Free Microemulsion Scheme. RSC Adv. 2024, 14, 4352–4361. [Google Scholar] [CrossRef]
- Tarkas, H.S.; Marathe, D.M.; Mahajan, M.S.; Muntaser, F.; Patil, M.B.; Tak, S.R.; Sali, J.V. Synthesis of Tin Monosulfide (SnS) Nanoparticles Using Surfactant Free Microemulsion (SFME) with the Single Microemulsion Scheme. Mater. Res. Express 2017, 4, 025018. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Zhang, X.; Wang, Q.; Song, M.; Chai, J. Preparation of Nano-TiO2 by a Surfactant-Free Microemulsion-Hydrothermal Method and Its Photocatalytic Activity. Langmuir 2019, 35, 9255–9263. [Google Scholar] [CrossRef]
- Sun, B.; Chai, J.; Chai, Z.; Zhang, X.; Cui, X.; Lu, J. A Surfactant-Free Microemulsion Consisting of Water, Ethanol, and Dichloromethane and Its Template Effect for Silica Synthesis. J. Colloid Interface Sci. 2018, 526, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Deng, Q.; Du, N.; Hou, W. Surfactant-Free Microemulsions of n-Butanol, Ethanol, and Water. J. Mol. Liq. 2023, 390, 122980. [Google Scholar] [CrossRef]
- Klemm, W.R. Biological Water and Its Role in the Effects of Alcohol. Alcohol 1998, 15, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Liu, X. Temperature-Induced Reversible-Phase Transition in a Surfactant-Free Microemulsion. Langmuir 2019, 35, 14358–14363. [Google Scholar] [CrossRef]
- Marcus, J.; Klossek, M.L.; Touraud, D.; Kunz, W. Nano-Droplet Formation in Fragrance Tinctures. Flavour Fragr. J. 2013, 28, 294–299. [Google Scholar] [CrossRef]
- Bošković, P.; Sokol, V.; Zemb, T.; Touraud, D.; Kunz, W. Weak Micelle-Like Aggregation in Ternary Liquid Mixtures as Revealed by Conductivity, Surface Tension, and Light Scattering. J. Phys. Chem. B 2015, 119, 9933–9939. [Google Scholar] [CrossRef]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-Extract-Mediated Synthesis of Metal Nanoparticles. J. Chem. 2021, 2021. [Google Scholar] [CrossRef]
- Hassan, P.A.; Rana, S.; Verma, G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Holthoff, H.; Egelhaaf, S.U.; Borkovec, M.; Schurtenberger, P.; Sticher, H. Coagulation Rate Measurements of Colloidal Particles by Simultaneous Static and Dynamic Light Scattering. Langmuir 1996, 12, 5541–5549. [Google Scholar] [CrossRef]
- Lagourette, B.; Peyrelasse, J.; Boned, C.; Clausse, M. Percolative Conduction in Microemulsion Type Systems. Nature 1979, 281, 60–62. [Google Scholar] [CrossRef]
- Bošković, P.; Sokol, V.; Touraud, D.; Prkić, A.; Giljanović, J. The Nanostructure Studies of Surfactant-Free-Microemulsions in Fragrance Tinctures. Acta Chim. Slov. 2016, 63, 138–143. [Google Scholar] [CrossRef][Green Version]
- Ni, P.; Hou, W.-G. A Novel Surfactant-Free Microemulsion System: Ethanol/Furaldehyde/H2O. Chin. J. Chem. 2008, 26, 1985–1990. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, J. Effect of Cosolvent Ethanol on Solubilization of Ionic Liquids in Supercritical CO2Microemulsions: Experiments and Simulations. J. Chem. Eng. Data 2021, 66, 347–359. [Google Scholar] [CrossRef]
- Magrode, N.; Poomanee, W.; Kiattisin, K.; Ampasavate, C. Microemulsions and Nanoemulsions for Topical Delivery of Tripeptide-3: From Design of Experiment to Anti-Sebum Efficacy on Facial Skin. Pharmaceutics 2024, 16, 554. [Google Scholar] [CrossRef] [PubMed]
- Klossek, M.L.; Touraud, D.; Zemb, T.; Kunz, W. Structure and Solubility in Surfactant-Free Microemulsions. ChemPhysChem 2012, 13, 4116–4119. [Google Scholar] [CrossRef]
- Zemb, T.N.; Klossek, M.; Lopian, T.; Marcus, J.; Schöettl, S.; Horinek, D.; Prevost, S.F.; Touraud, D.; Diat, O.; Marčelja, S.; et al. How to Explain Microemulsions Formed by Solvent Mixtures without Conventional Surfactants. Proc. Natl. Acad. Sci. USA 2016, 113, 4260–4265. [Google Scholar] [CrossRef]
- Xu, J.; Deng, H.; Song, J.; Zhao, J.; Zhang, L.; Hou, W. Synthesis of Hierarchical Flower-like Mg2Al-Cl Layered Double Hydroxide in a Surfactant-Free Reverse Microemulsion. J. Colloid Interface Sci. 2017, 505, 816–823. [Google Scholar] [CrossRef]
- Bourebrab, M.A.; Oben, D.T.; Durand, G.G.; Taylor, P.G.; Bruce, J.I.; Bassindale, A.R.; Taylor, A. Influence of the Initial Chemical Conditions on the Rational Design of Silica Particles. J. Sol-Gel Sci. Technol. 2018, 88, 430–441. [Google Scholar] [CrossRef]
- Park, S.K.; Do Kim, K.; Kim, H.T. Preparation of Silica Nanoparticles: Determination of the Optimal Synthesis Conditions for Small and Uniform Particles. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 197, 7–17. [Google Scholar] [CrossRef]



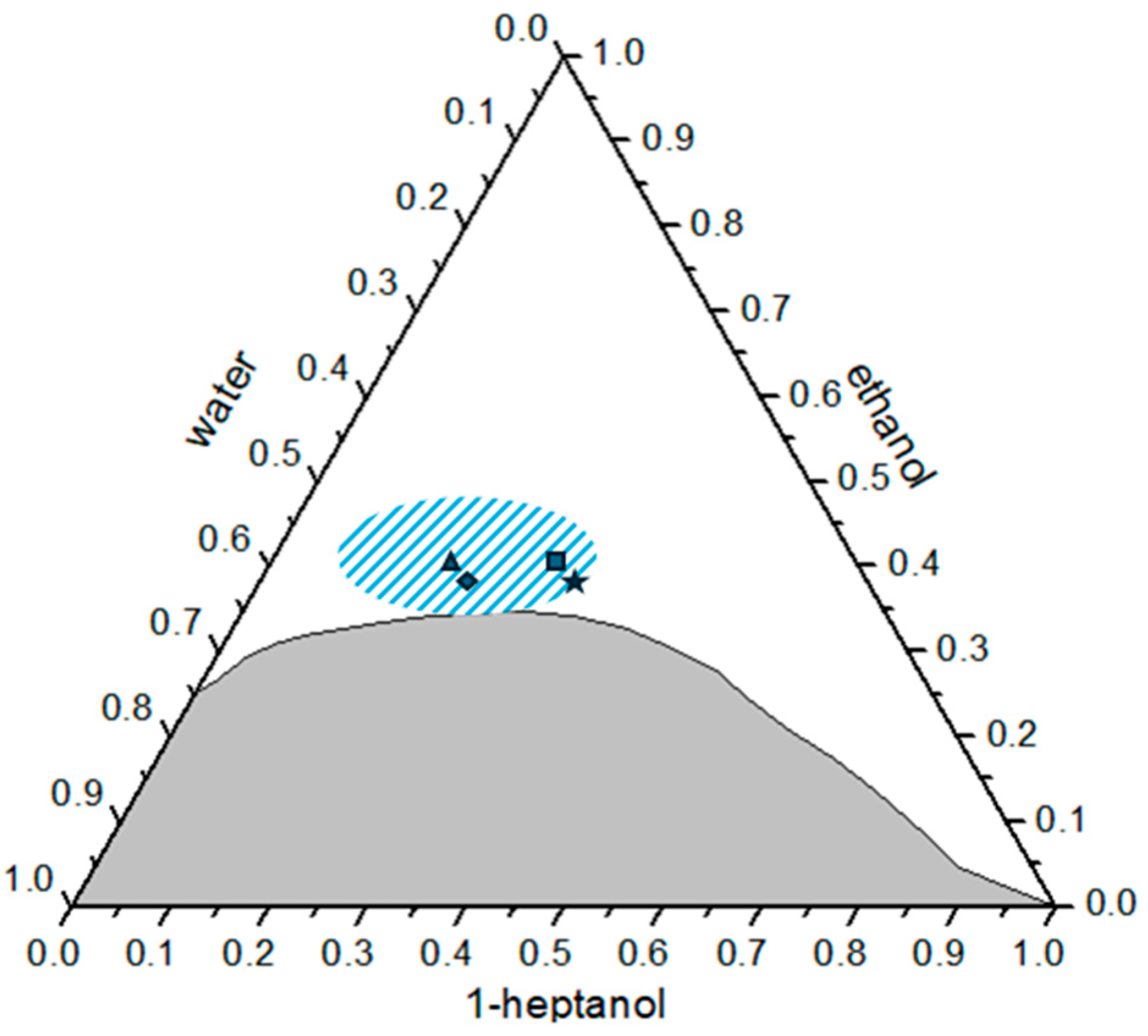

| Point | Hydrodynamic Radii/nm |
|---|---|
| AB 6 | 5.31 |
| AB 8 | 6.98 |
| AB 10 | 6.39 |
| B 8 | 7.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudelj, M.; Kranjac, M.; Jurko, L.; Tomšič, M.; Cerar, J.; Prkić, A.; Bošković, P. Structure and Potential Application of Surfactant-Free Microemulsion Consisting of Heptanol, Ethanol and Water. Colloids Interfaces 2024, 8, 53. https://doi.org/10.3390/colloids8050053
Gudelj M, Kranjac M, Jurko L, Tomšič M, Cerar J, Prkić A, Bošković P. Structure and Potential Application of Surfactant-Free Microemulsion Consisting of Heptanol, Ethanol and Water. Colloids and Interfaces. 2024; 8(5):53. https://doi.org/10.3390/colloids8050053
Chicago/Turabian StyleGudelj, Martina, Marina Kranjac, Lucija Jurko, Matija Tomšič, Janez Cerar, Ante Prkić, and Perica Bošković. 2024. "Structure and Potential Application of Surfactant-Free Microemulsion Consisting of Heptanol, Ethanol and Water" Colloids and Interfaces 8, no. 5: 53. https://doi.org/10.3390/colloids8050053
APA StyleGudelj, M., Kranjac, M., Jurko, L., Tomšič, M., Cerar, J., Prkić, A., & Bošković, P. (2024). Structure and Potential Application of Surfactant-Free Microemulsion Consisting of Heptanol, Ethanol and Water. Colloids and Interfaces, 8(5), 53. https://doi.org/10.3390/colloids8050053










