Abstract
Metal-based nanofillers are used as disperssants to enhance thermal conductivity for a minimal fuel requirement to extract maximum energy. To achieve this, metal-based nanofillers must be suspended uniformly into jet fuel so that desired propulsive characteristics can be achieved. However, the dispersion of the metal-based nanofillers into the jet fuel is a critical challenge due to the density and viscosity that are independent parameters with a scattered relation. Hence, in the current work, we intended to investigate the propulsive characteristics of the JP-10 (Exo-Tetra Hydro Dicyclopentadiene) jet fuel dispersed with boron particles (BP) at various concentrations. The challenge involved in the current work was to make dispersion stable for a longer period due to the absence of functional groups entailed to BPs. Alongside JP-10, is a single-component, high-density hydrocarbon that can that can exhibit thixotropic characteristic in nature and hence combining with BP makes it difficult; hence, there is a need for oligomerization or the addition of surfactants that are derived from oligomers. Hence, in the current work, the BPs were dispersed in jet fuel by the ultrasound probe with various surfactants, namely Hydroxyl Terminated Polybutadiene (HTPB), Triton X-100, Span 80, Oleic acid, and Sodium dodecyl sulfate (SDS), followed by an investigation of their stability. The experimental studies reported that the stability of the boron was longest, for 54 h, with 0.5 wt.% boron and 0.3 wt.% HTPB at a micron size of the boron particles (325 ± 25 nm). The uniform dispersion of the particles was achieved by the effect of the ultrasound probe. From the thermal analysis, a total weight loss of 25% was observed within a short range of temperatures, i.e., 50 to 200 °C.
1. Introduction
The need for high-energy-density fuels received significant attention in the scientific community to reduce the specific fuel consumption in aircraft engines alongside producing high thrust and regression rates. As a result, various fuels have been developed ever since the aviation industry explored a commercial horizon [1]. Due to the increase in the commercialization of the airline industry, the carbon footprint has drastically increased globally, reporting aviation as a primary vertical. Hence to reduce these footprints, the thrust for eco-friendly fuels with the potential to produce a high-energy density has foreseen scientific interest [2,3]. Though there is an ample amount of research that has been dedicated to the development of new fuels, it is desired that their compliance for use in a real-time application is long-awaited. In the meantime, amongst the available fuels, JP-10 is the most widely used fuel in the aviation industry. Though this fuel has excellent characteristics, it also possesses decent lacunae making it desirable for sustainable fuel [4,5].
Being polycyclic, JP-10 has a provision to generate aromatics in flames through additives to enhance the combustion kinetic characteristics. JP-10 has a fuel density of 0.94 g/cm3 and a volumetric heating value of 39.6 MJ/L with a single-component hydrocarbon making it advantageous to enhance the volumetric heating and energy density [6]. Alongside this, the other advantage is to introduce a thixotropic nature, thus making it more amenable to better regression and enhanced thermal conductivity [7]. From the literature, it was identified that by altering the molecular structure through artificial synthesis, the maximum density is 1 g/cc and the volumetric heating value is 42 MJ/L [8,9]. To surmount these challenges metal-based nano has been used as a dispersant in jet fuel to increase the regression rate and combustion characteristics of the fuel. Few metal-based nanoparticles, such as Zr, Mg, Si, Ti, Al, TiO2, Co3O2, Ni, Fe2O4, CNTs, CuO, MnO, CoCl2, Al-Mg, CuSO4, CeO, CuCl2, GNPs, and FeCl3 [10], are used as additives in petrol and diesel fuels and have displayed significant enhancement of the energy density of the fuel. However, very few works can be cited from the literature for JP-10 fuel. Few to name additives, such as aluminum [11], boron [12,13,14], and titanium-aluminum boron (Ti–Al–B) [15], are dispersed as particles at different scales to study the enhancement of combustion characteristics. However, these works have not reported on the stability of the particle dispersion in the JP-10 fuel, though there is very limited work wherein they have used sorbitane oleate, trioctylphosphine oxide [16] as a surfactant to stabilize the particles; however, it is highly uneconomical.
It can be inferred from the literature that there is not a substantial amount of work that has contributed toward the development of metal particle blended fuels. This was attributed to various complexities, such as stability through direct dispersion (fuel-particles) and constrain on the particle concentration, size, shape, and surfactant concentration. To overcome these challenges to some extent, we have introduced boron particles into JP-10 with a suitable surfactant, in the current case, namely HTPB, which is both eco-friendly and economical followed by good propulsive characteristics. In the literature, there has been substantial work implicating the use of HTPB because of its high energy level, excellent processability, and better mechanical properties. Moreover, there is an identified lacuna in achieving the stability of the boron particles for a longer time. In the process of achieving long-term stability, the role of the surfactant, mechanical agitation force during stirring, weight concentration of nanoparticles, and physical morphology of the nanoparticles play a crucial role. In the aspect of stability, several studies report that with the average range of nanoparticle sizes of 15–50 nm, boron shows significant stability of two days [17]. The intermolecular interactions of boron particles in the suspension are agglomerate and can occur with a similar mechanism of Ostwald ripening. The attractive forces of nanoparticles dominating the repulsive forces amongst nanoscale boron particles results in larger-diameter particles. This phenomenon induces instability in the suspension. The presence of randomness and meaningful kinetic energy in the nanoparticles during the Ostwald ripening develops unwanted collisions and agglomeration. Fateme Sadat Shariatmadar et al. [18] reported nano- and micro-size boron particles to prepare a fuel mixture and achieved stability by using sorbitane oleate, which was 57 h for nanoparticles, whereas for micro-sized particles, it was 6.5 h. In the economical aspect, the cost of the surfactant is higher; thus, the stability was achieved at a higher cost. Surfactant addition in the suspension of nanoparticles increases the overall density of the solution. With an increasing density of the solution, the nanoparticles will find the additional resistance through the solution layers to become closer and agglomerate. Thus, the role of the surfactant is crucial in achieving stability of the heterogeneous system of the solid and liquid. The concentration of the surfactant/gel was a major concern, as the higher surfactant increases the stability in the suspension for a longer time [19]. However, the added surfactant requires its initial energy during the burning of the composite fuel, and it increases the initial cost of the process. Though the use of ultrasound irradiation develops the cavitation effect in the suspension, the bubble collision phenomenon adds a higher amount of energy to the suspension. The molecules of boron nanoparticles will remain in the suspended form due to the absorption of kinetic energy that develops during the collision of a bubble. The mass transfer rate of nanoparticles will be enhanced due to the shock waves developed in cavitation. Thus, the nanoparticles are in the suspension for a longer period. Hence, to understand the feasibility, we have dispersed boron particles at various compositions along with surfactants to ensure stability behavior [20]. The higher composition of the surfactant affects the performance of the fuel. The optimum composition of surfactant with required stability was achieved by formulating the concentration of boron. Few works using HTPB as fuel in solid rocket motors can be inferred here [21,22,23,24]. Due to its derived chemical oligomeric nature, it is cross-linked with various other additives to enhance the energy density of the fuel [25,26,27]. However, to realize the potential for HTPB, the authors have reported various other surfactants for the pros and cons of the use of HTPB. Alongside this, the article also elucidates the correlation between the physical and chemical properties of the fuel, surfactants, and additives through characterizations, such as SEM, DSC, and XRD. From the characterizations, the fluid–particle interaction and stability of the dispersion were discussed.
2. Materials and Methods
2.1. Materials
The average boron particles size was about 325 ± 25 nm, and the base fuel (JP-10) Exo-Tetra Hydro Dicyclopentadiene (Exo-THTCPD), which is a non-polar solvent, was provided by Gas Turbine Research Establishment, Bangalore, India, and was used in the current work. The surfactants used for the stabilization of boron particles, Sodium dodecyl sulfate (SDS) (an ionic surfactant), Triton X-100, span 80, Oleic acid as a non-ionic surfactant, and HTPB, were procured from Neo Pharma Industry, at commercial grade. The physical and chemical properties of the surfactants and JP-10 fuel (Exo-THTCPD) are reflected in Table 1. The chemicals were used without further purification.

Table 1.
Various surfactants with different concentrations were loaded to observe the stable dispersion.
2.2. Methodology
The as-obtained chemicals and materials were treated in phases, with the as-received boron particles having their surfaces functionalized and the fuel slurry being made with the use of an ultrasonic probe. Initially, the as-received boron particles were surface functionalized because of the poor polar properties, which do not allow for stable dispersion in the JP-10. The boron particles were stirred in the ethanol to functionalize the boron surface and dried at 50 °C under a vacuum for improving the adsorption. In parallel, the surfactant HTPB was used to crosslink the JP-10 fuel. Trion X-100, Span 80, Oleic acid, and SDS were used to make it thixotropic at slightly above room temperature to enhance the density of the fuel. Now, the functionalized boron particles are dispersed into a thixotropic solution for ultrasonication. Because of its high energy intensity and ability to disperse particles with scattered morphology, high-frequency ultrasonication probes were utilized in this study as a particle-irradiation method maintained at low temperatures by an ice bath for 20 min. The fuel slurry was thus ready (as shown in Figure 1) to be studied for stability behavior.
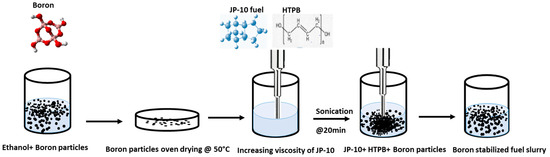
Figure 1.
Illustration of fuel slurry preparation methodology.
Detailed experimental studies were conducted to examine the surfactant behavior by varying the concentration with a different surfactant to obtain the stability of the fuel slurry. The concentration loading of the surfactant varied (0.3, 0.5, 1, 1.5, 2 wt.%) at a constant concentration of boron particles (0.5 wt.%). Different surfactants, such as HTPB, Triton X-100, Span 80, Oleic acid, and SDS surfactants, with JP-10 gasoline as a base fuel were used to monitor the stability of the boron particles.
2.3. Characterization
The crystallinity characteristics were evaluated for boron particles and modified boron particles, such as X-ray diffraction (XRD) patterns to evaluate whether the sample is crystalline or amorphous. The XRD sample was calibrated from the Panalytical Netherlands model X’Pert Powder XRD using a 2.2 kW Cu anode with a maximum-rated output of 60 kV and 55 mA. Fourier Transform Infrared (FTIR) Spectroscopy of various samples was calibrated to analyze the chemical shifts and interface behavior between the surfactant, additive, and fuel. FTIR has performed for both the pre- and post-slurry preparation of the samples to realize the chemical kinetic behavior. This will emphasize the chemical decomposition of the fuel for smooth and stable burning. FESEM was evaluated using the ZEISS SIGMA VP-FESEM to perform high-resolution microstructure, and Scanning Electron Microscopy (SEM) was performed to investigate the elemental composition of the constituents and the surface morphology, such as particle size and shape. The SEM calibrations were obtained from the Tescan Vega-3 Lmu model. The thermal characteristics, such as glass transition, crystallinity, and melting temperatures, were evaluated using Dynamic Thermal Analysis (DTA) and thermogravimetric analysis (TGA) under an argon atmosphere using the Netzsch model regular STA 2500.
3. Results and Discussion
The dispersion of the boron nanoparticles was achieved with ultrasound assistance. The cavitation phenomenon refers to the cavities that are produced due to the high-frequency ultrasound irradiation in a low-volatile solution, such as JP-10. Thus, the generated bubble during the cavitation had a decent impact on the physical destruction of boron particles. The sound transmission across JP-10 develops the cavities due to compression and rarefaction cycle exposure. These cavities are generated, grown, and collapse during each cycle of sound transmission. At the collapse of the cavity, the kinetic energy is released in teams of impact and temperature. The impact in terms of shock waves is produced in the solution, which induces a greater extent of dispersion of boron nanoparticles under initial conditions. The cavitation extent increases and the boron nanoparticles absorbed more and more energy. This higher energy state of boron nanoparticles develops the repulsive force amongst boron particles, which keeps them in a suspended form for a longer period. The results observed from the following analytical processes confirm the stability of the boron nanoparticle in the JP-10 solution.
3.1. Morphological Studies
The as-received samples were characterized for their morphology to verify the size, shape, and distribution of the particles in a given sample powder. It was identified that the average particle size was observed to be in the range of 325 ± 25 nm and the shape of the particles was completely scattered. At the same time, it can be pursued that no surface hydroxyl groups were also reported from the SEM micrograph indicating pure amorphous boron powder, as shown in Figure 2. In the current work, we aimed to disperse the boron particles into a jet fuel, which is a single-component high-density hydrocarbon that cannot host boron particles or any other foreign particles without hydroxyl groups that can enable a polymer chain. Hence, there is a need to add a structure-redirecting agent, which can serve the purpose of both creating a polymer network and has the ability to host the foreign particles; in the current case they were boron particles. In a more precise way, it should create a crosslink between the boron surface hydroxyl group and polymer chain, which is a combination of fuel and surfactant. However, since boron particles are unstable when used as a dispersant, adsorption must be entailed, which can be achieved by properly mixing the boron particles in ethanol solution [28].
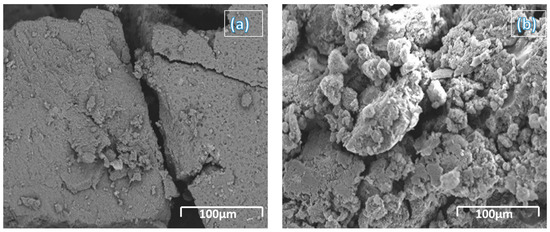
Figure 2.
SEM image of (a) boron particles and (b) surface-modified boron particles by HTPB.
This adsorption opens the structure to form a polymer network with the polymer chain formed with HTPB and JP-10 solution. At the same time, after the mixture, we identified that SEM micrographs indicated a decent uniformity in the particle size and shape, which is attributed to the ultrasound envelope. The ultrasound envelope includes time and frequency, wherein a larger time with a lower frequency is considered to prepare the HTPB-JP-10 slurry, whereas boron is gently mixed with ethanol for a longer time. This will allow for the formation of a long polymer chain of fuel with enhanced energy density. A similar procedure is adopted with the other surfactants to develop the slurry. The SEM micrographs are limited to only HTPB because of the better stability, low-cost, eco-friendly, and easily available characteristics, though other surfactants have displayed better stability periods, but not HTPB.
3.2. Effect of Ultrasound Irradiation on the Suspension of Boron Nanoparticles and JP-10
The X-ray diffraction studies indicated the presence of a boron trioxide phase for an as-received sample, and from the literature, it is inferred that the boron trioxide exists only in an amorphous form that is displayed in Figure 3a below. The boron trioxide is in a hexagonal crystal structure with a maximum peak at 2θ = 26.002 in the {1 0 1} plane. The shift in the peak after the oligomerizing, in XRD results, was 0.333 nm, which is nearly equal to the hexagonal boron powder, as shown in Figure 3b. A Scherrer analysis suggested that crystalline domains have a size of 24.34 nm. Since the given size for the hexagonal boron trioxide matches with the d-spacing, hence considering the boron powder as amorphous, it is agreeable.
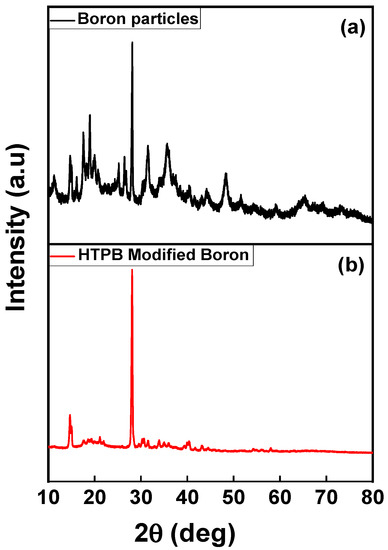
Figure 3.
XRD pattern. (a) Boron nanoparticles and (b) surface-modified with HTPB–boron slurry.
The XRD peaks suggested a density enhancement of boron from 2.34 to 2.56 g/cm3, which is attributed to the oligomerization. As a result, the cell volume was also decreased but with a very slight variation; this may be due to the accommodation of functional groups on the surface of the boron particles. This increment is possible since the boron trioxide in the FTIR has a confirmed peak range of 850–950 cm−1, which has symmetric stretching of B(3)-O, as shown in Figure 4. This symmetric stretching enables oligomerization when it is combined with ethanol thus creating Lorentz polarization to the boron phase that is reflected in the increase in the intensity of the peak without any shift for 2θ.
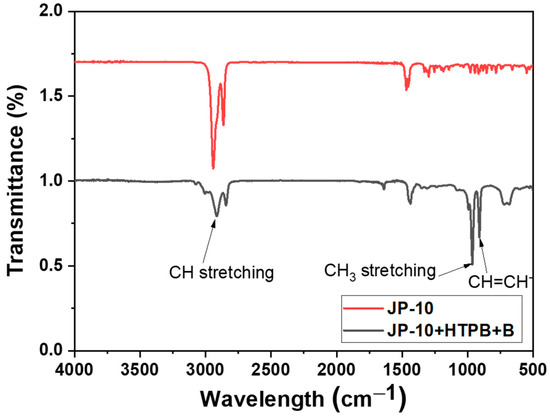
Figure 4.
FTIR pattern of received boron and boron-surface modified with HTPB suspension.
As shown in the FTIR and XRD peaks below, whereas the HTPB-boron particle slurry has weak C-H stretch at 2950 and 3000 cm−1, the CH3 methyl group is observed at 1478 cm−1, which is a stable hydrocarbon group that may be responsible for stabilizing the boron particles in the JP-10 fuel; 911 cm−1 represents the trans-CH=CH- isomers, which are highly stable for acyclic systems, i.e., open-chain compounds, such as JP-10 in the current work. The composition and the respective micrograph were studied to confirm the oligomerization and polymerization in the slurry; at the same time, the thixotropic nature was also evaluated for the jet fuel. It can be inferred that the boron is electronegative; hence, H+ ions have a lower tendency to join with O than boron. Therefore, H+ ions will join with boron trioxide particles because O atoms of the OH group will not share their free electron pairs with boron and will not be able to initiate covalent bonding. This behavior is exhibited by the other surfactants apart from HTPB, and hence, we have discoursed only HTPB, as greater stability was achieved in comparison. However, after the functionalization of boron with ethanol, carbon and silicon are electropositive and are readily allowed to form Si-O and C-O accumulating O in the boron trioxide system, which can be referred to in FESEM-EDS, which is the composition of the boron being observed to be lesser in comparison. It is also pursuable from the FESEM-EDS that carbon stretches and carbon-boron accumulation can be observed in the following Figure 5. However, this can more interestingly corroborated that a polymer network is well established and the boron surface is modified with the network of polymers, but this polymer chain indicates that a higher frequency with a longer time than the current processing time would not have created interfacial adhesion between boron particles. Hence, it is observable there is a partial local agglomeration that can be observed. The boron weight percentage for the as-received boron from FESEM-EDS was 51.94 and that modified from slurry was found to be 31.1.
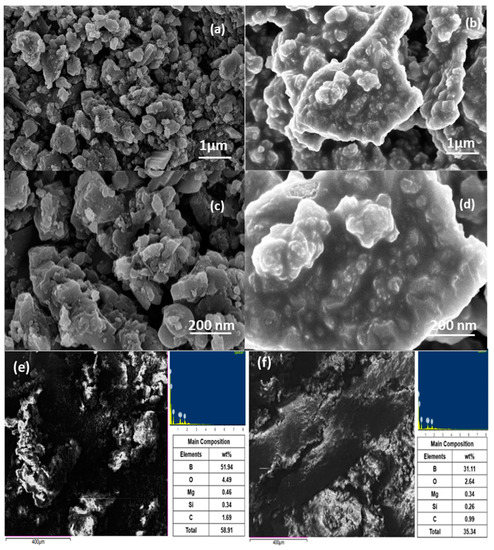
Figure 5.
Field emission scanning electron microscopy micrographs of (a,c) as-received boron particles and (b,d) modified HTPB-boron particles slurry, and (e) FESEM elemental composition of (a) as-received boron particles and (f) modified HTPB-boron slurry.
4. Characteristics, Thermal Behavior, and Stability of the Boron -JP-10 Suspension
4.1. Physical Properties of Boron-JP-10 Suspension
Physical properties of the boron and functionalized boron from slurry were characterized from the field emission scanning electron microscopy. The FESEM micrographs of the as-received boron are displayed in Figure 5. Below, it showed an irregular shape, size, and distribution of the particles over a sample space. The average size of the boron particles was 325 ± 25 nm, and the shape was highly scattered with sharp polygon shapes. This shape is attributed to the synthesis methodology adopted, i.e., either melt-quench or cryogenic milling [29]. Further, the as-received boron particles were oligomerized to make them suitable for stabilization in the JP-10 jet fuel. During the oligomerization process, as mentioned earlier, similar to the boron particles, the boron-carbon and carbon-oxygen stretch has been identified and the accumulation of these stretches has been agglomerated on the non-participating boron particles. The FESEM micrographs of the oligomerized boron particles display a boundary between the boron particle and adsorbed layer. This layer has extended O groups, which are ready for polymerization, and hence, when these particles are dispersed in the HTPB-JP-10 slurry, interfaces are formed between the particles also increasing the interfacial distances between the particles, as shown in Figure 5b,d. This interface network was achieved through the oligomerization of particles along with ultrasonication irradiation, by which we can restrict the participation of the local agglomeration network.
It can be observed from the FESEM micrographs that the boron restructures as a particle to agglomerates, agglomeration to network modifiers, and network modifiers to thin film-like structures. To realize the boron to be stable for a longer time in the fluid, it is desired that the process must be monitored for shapes between the phase of the network-modifier to the thin films. The other reason that this process may disappear or may be too fast to realize is due to the composition of the boron and surfactant constituent in the jet fuel. Hence, a proper ultrasonication time, the suitable composition of constituents, and the morphological characteristics play a crucial role in attaining long-term stability. However, we suggest for future research that ball milling of the as-received specimens may enhance the uniformity in shape, size, and distribution of the boron particles, and literature in this context is well established [30,31]. We have ball-milled the as-received cryo-milled boron particles and found that a proper distribution is achieved, which can be future work in support of this as an extension.
4.2. Thermal Characteristics of the Boron -JP-10 Suspension with Different Compositions
Thermal properties were evaluated from dynamical thermal analysis (DTA) and thermogravimetric analysis (TGA). The results reflected for weight loss and heat flow as a function of temperature were analyzed, and the following interpretations were made. The TGA trend for HTPB surfactant concentrations of 0.3, 0.5, 1.0, 1.5, and 2 wt.% at a constant boron particle concentration of 0.5 wt.% in the jet fuel JP-10 are reported in Figure 6. The trend of the TGA was similar for all concentrations of various surfactants irrespective of the sonication time; as a result, only one sonication time has been discoursed here for 20 min. It must also be conferred that only the TGA of the HTPB is considered because of the similar trend observed with other surfactants as well, but at a lower rate of stabilization. Within the presented calibration of the TGA for HTPB at various concentrations, though the trend is similar, the magnitude of the weight loss as a function of the temperature is varying, which can be seen in the following Figure 6 for the same. TGA was performed at air temperature up to a maximum temperature of 600 °C at 10 °C/min. The endothermic trench down at 150 °C may be due to the volatilization of boron trioxide with the JP-10, and hence, a sharp slope is observed. We could observe a total weight loss of 25% within a short range of temperatures i.e., 50 to 200 °C i.e., almost complete burnout of the JP-10 took place when adding boron particles as an additive. The other phase of the TGA exothermic curve is burnouts of residual boron. Meanwhile, for the boron after being modified at a particular weight composition of the HTPB, it was observed from the calibration that there is a delay in the peak, indicating the delay in the endothermic trench but with minimal weight loss. This implication suggests that the volumetric energy density of the slurry fuel is enhanced, and hence, the initial delay in the combustion begins until both fuel and additive attain a common temperature, which is 150 °C for 0.3 to 1.0 wt.% of HTPB, whereas for 1.5 and 2.0 wt.%, there is a drastic weight loss at the lowest temperatures. Hence, it is preferable to have a weight percent of 0.3–1.0% of HTPB in JP-10 for 0.5 wt.% of boron.
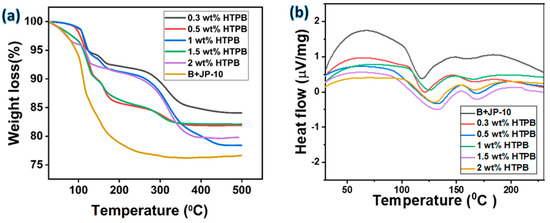
Figure 6.
Thermal behavior: (a) TGA and (b) DTA for boron particles with varying surfactant load fuel suspensions.
The dynamical thermal analysis (DTA) calibration suggests that a 325 ± 25 nm particle size has reflected a similar trend elsewhere in the literature. For the particle size range received, it can be cited from the literature as well that the rate of oxidation increases and with corresponding heat release. Thus, the oxidation kinetics reveal that in a corroboration between both TGA and DTA, the mass loss during the combustion of boron occurs in two phases at high temperatures. With the evaporation of B2O2 and combination with the O to form B2O3, the sample starts losing the weight if the ratio of evaporation of B2O2 is more than the formation of B2O3. This can be realizable from the fact that the XRD peak suggests that the B2O3 phase exists and is amorphous. Hence, the formation of B2O3 will be faster than the evaporation of B2O2. In the current case, the mass loss is greater between 50 and 150 °C, i.e., it is indicative that higher mass loss and faster burning occur at a lower HTPB content, which is 0.3 to 0.5 wt.%, as observed in the DTA plot.
4.3. Stability Investigations of JP-10 Fuel Boron Particles through Visual Observation
The aforementioned characteristics are few to realize how the stability of dispersed boron influences the characteristics of combustion. It is also realized that physical, chemical, and thermal properties are crucial in achieving stability. Interestingly, the processing i.e., modifying the as-received boron, received very crucial attention in the current work. To understand the stability of the boron particles in the jet fuel JP-10, an asset of experiments discussed in Table 2 has been investigated for their sedimentation time after the slurry preparation. From the XRD calibration, it is realizable that amorphous B2O3 is present, and hence, the given boron particles are in an amorphous state in a hexogen crystal phase. This hexagonal crystal phase allows for the introduction of H2 molecules to pass through their rings. Hence, there is a possibility of modifying the surface of the boron. In post-surface modification of the boron particles, oligomerization plays a crucial role as all the boron particles must be properly adsorbed; otherwise, it may result in agglomeration, and to avoid this, it is important to sonicate for a longer time but to a threshold. The functionalized boron will now have to be ready to accept the polymer chain interactions with the jet fuel JP-10.

Table 2.
Influence of surfactant parameters at constant boron particles loaded to dispersed in JP-10 fuel.
Since jet fuel JP-10 is a single-compound, high-density carbon compound, it cannot form a chain with the oligomer compounds, and hence, there is a need for a hydroxyl compound; hence, the JP-10 carbon compound is accommodated by the O of the HTPB to create bonding. The H2 molecules left over will pass through the rings of the hexagonal boron due to functionalization with ethanol, thus enabling a proper chain network. Now, during the preparation of the slurry, which has modified boron with the HTPB-mixed JP-10 fuel, it was identified that the sonication time seems to be crucial. The SEM of the sonicated boron displayed that there is C-O stretch accumulation onto the boron surface and B-C that has shown the reduced weight of the boron sample in the composition, indicating that B-O is ready for oxidation during combustion with fuel. However, there was a typical behavior observed in the sample histology, the gradient stabilization and sedimentation observed as shown in Figure 7 that is displayed in A-D for various compositions of the HTPB-based jet fuel constant boron samples. The samples imply that this gradient distribution of sedimentation is due to insufficient sonication time as indicated in C. It is desired that the increase in the sonication time would have allowed for all chemical kinetics to be well established to make the complete fuel matrix into an ordered form. The faster-settled boron particles did not get enough time to participate to establish a network due to less sonication time as indicated in D; at the same time, only a few were able to settle in the top as indicated in B, due to their excellent morphological characteristics and proper adsorption of boron and finally, ultrasonication frequency that created a bulk phase, i.e., an interfaced agglomeration of unbiased boron particles at the interface along with the accumulation of HTPB on the interface, which is creating a polymer chain with local cross-linking. It was identified that 10 mL of JP-10 fuel with 0.3 wt.% of HTPB surfactant and 20 min of sonication time resulted in the best stability of 54 h with gradient variation, as shown in Table 2 and A from Figure 7.
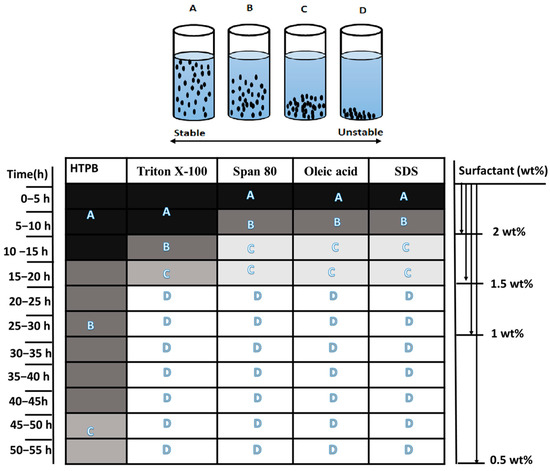
Figure 7.
Sedimentation study of various surfactants at 0.5 wt.% of boron particles.
4.4. Comparative Analysis of Boron-JP-10 Suspension
It can be realizable from Table 2 and Figure 8 that other surfactants though have reflected better properties in terms of the microstructure–property correlation; they have not shown the potential sedimentation time for the same. It can be observed from Table 2 that HTPB has displayed an excellent sedimentation time at room temperature for the same weight percent of the boron particles. It can also be inferred that other surfactants have poor chemical kinetics to form the network with the JP-10. Alongside, it can also be realized that very less or no work has been reported specifically with JP-10 fuel. However, there have been few reported works in the literature pertaining to the other surfactants with various fuels. As the surfactant concentration is increasing, the sedimentation time is decreasing; this is because an increase in the surfactant content will agglomerate and initiate the interface between the particles, thus allowing for the faster settling of the boron particles. An understanding in this context was explained afore in the stability studies.

Figure 8.
Snapshots of boron slurry fuel of 0.5 wt.% boron particles in JP-10 fuel after sonication, for 1 days, 2 days, and 3 days, using various surfactants/gel concentrations of 0.3 0.5, 1, 1.5 and 2 wt.%.
5. Conclusions
The stability of the boron in the current work appraises the complexities encountered during the modification and preparation of the propellant. The chemical kinetics suggest that the stability of the boron is longest for 54 h at 0.5 wt.% boron and 0.3 wt.% HTPB. The uniform dispersion of the particles can be achieved by the effect of the ultrasound probe and the surfactant that chelate the boron surface in JP-10 fuel. It is observed that surface-modification along with the surfactant occurs for the boron particles when they are subjected to high pressure near the cavitation. The particles near the cavitation ozone can enhance the dispersion rate to achieve good stability. This may be attributed to the particle shape and size, time for ultrasonication, and composition of the constituent. The thermal analysis also suggests that the lower concentration of HTPB with a constant boron wt.% displayed excellent heat flow and delayed time, i.e., energy density with stable combustion at low weight loss. At the same time, other surfactants have not shown a significant impact on the enhancement of energy density and lesser stability compared to HTPB.
Author Contributions
Conceptualization, supervision, and methodology by S.H.S. and S.S.; resources, validation, writing—original daft preparation by P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Gas Turbine and Research Establishment GTRE Bangalore, India, grant number GTRE/MMG/BMR1/1046/19 dated on 11/June/2019.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Gas Turbine and Research establishment GTRE Bangalore Team for continuous support, especially, the scientist at the combustion unit, G Sivaramakrishna, Dalton Maurya, and Sonal Lucy, for their valuable suggestions for conducting the experimental studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hileman, J.I.; Stratton, R.W.; Donohoo, P.E. Energy content and alternative jet fuel viability. J. Propuls. Power 2010, 26, 1184–1196. [Google Scholar] [CrossRef]
- Cabrera, E.; de Sousa, J.M.M. Use of Sustainable Fuels in Aviation—A Review. Energies 2022, 15, 2440. [Google Scholar] [CrossRef]
- Yusaf, T.; Fernandes, L.; Abu Talib, A.R.; Altarazi, Y.S.; Alrefae, W.; Kadirgama, K.; Ramasamy, D.; Jayasuriya, A.; Brown, G.; Mamat, R.; et al. Sustainable aviation—Hydrogen is the future. Sustainability 2022, 14, 548. [Google Scholar] [CrossRef]
- Fortin, T.J.; Bruno, T.J. Heat capacity measurements of conventional aviation fuels. Fuel Process. Technol. 2022, 235, 107341. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Zeng, Z.-M.; Zhang, H.-Z. An experimental and kinetic modeling study of JP-10 combustion. Fuel 2021, 312, 122900. [Google Scholar] [CrossRef]
- Shi, L.; Xu, P.; Wang, R.; Tang, W.; Ding, T.; Jiang, R.; Zhang, C. Experimental and kinetic study on JP-10/air autoignition and the effect of NO2 at high temperatures. Fuel 2023, 333, 126418. [Google Scholar] [CrossRef]
- Chen, A.; Guan, X.; Li, X.; Zhang, B.; Zhang, B.; Song, J. Preparation and Characterization of Metalized JP-10 Gel Propellants with Excellent Thixotropic Performance. Propellants Explos. Pyrotech. 2017, 42, 1007–1013. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wang, X.; Sheng, X.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of diesel or jet fuel range cycloalkanes with 2-methylfuran and cyclopentanone from lignocellulose. Energy Fuels 2014, 28, 5112–5118. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Zhang, X.; Han, P.; Xie, J.; Pan, L.; Zou, D.-R.; Liu, S.-H.; Zou, J.-J. Synthesis of high-density and low-freezing-point jet fuel using lignocellulose-derived isophorone and furanic aldehydes. Sustain. Energy Fuels 2018, 2, 1863–1869. [Google Scholar] [CrossRef]
- Chen, B.H.; Liu, J.Z.; Yao, F.; Li, H.P.; Zhou, J.H. Effect of oleic acid on the stability and rheology of nanoaluminium/JP-10 bi-phase system. Micro Nano Lett. 2017, 12, 675–679. [Google Scholar] [CrossRef]
- Gupta, S.K.; Prabhudeva, P.; Kumar, M.; Ojha, P.K.; Karmakar, S. Investigation on spray combustion characteristics of Boron-Loaded slurry fuel in a Swirl-Stabilized combustor. Fuel 2022, 323, 124316. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Zhang, J.; Zhu, Z.; Ren, X.; Yang, Y.; Lin, K.; Pang, A.; Shuai, Y. Encapsulated boron-based energetic spherical composites with improved reaction efficiency and combustion performance. Chem. Eng. J. 2022, 433, 134478. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, X.; Wang, X.; Dou, S.; Yang, Q.; Pan, L. Propulsive and combustion behavior of hydrocarbon fuels containing boron nanoparticles in a liquid rocket combustor. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2022, 236, 2580–2591. [Google Scholar] [CrossRef]
- Brotton, S.J.; Perera, S.D.; Misra, A.; Kleimeier, N.F.; Turner, A.M.; Kaiser, R.I.; Palenik, M.; Finn, M.T.; Epshteyn, A.; Sun, B.J.; et al. Combined spectroscopic and computational investigation on the oxidation of exo-tetrahydrodicyclopentadiene (jp-10; c10h16) doped with titanium–aluminum–boron reactive metal nanopowder. J. Phys. Chem. A 2021, 126, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Xiu, E.; Zhi, X.; Zhang, Y.; Li, C.; Zou, J.J.; Zhang, X.; Wang, L. Jet fuel containing ligand-protecting energetic nanoparticles: A case study of boron in JP-10. Chem. Eng. Sci. 2015, 129, 9–13. [Google Scholar]
- Ferrão, I.A.S.; Mendes, M.A.A.; Moita, A.S.O.H.; Silva, A.R.R. The Addition of Particles to an Alternative Jet Fuel. Fuels 2022, 3, 184–206. [Google Scholar] [CrossRef]
- Shariatmadar, F.S.; Pakdehi, S.G. Effect of various surfactants on the stability time of kerosene–boron nanofluids. Micro Nano Lett. 2016, 11, 498–502. [Google Scholar] [CrossRef]
- Xue, K.; Cao, J.; Pan, L.; Zhang, X.; Zou, J.-J. Review on design, preparation and performance characterization of gelled fuels for advanced propulsion. Front. Chem. Sci. Eng. 2022, 16, 819–837. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W. Ultrasonication an intensifying tool for preparation of stable nanofluids and study the time influence on distinct properties of graphene nanofluids—A systematic overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar] [CrossRef]
- Han, J.; Hu, S.; Liu, L.; He, P. High-Pressure Combustion Characteristics of Hydroxyl-Terminated Polybutadiene Propellants. J. Propuls. Power 2022, 39, 158–166. [Google Scholar] [CrossRef]
- Kawata, K.; Chung, H.L.; Itabashi, M. Mechanical characterization and high velocity ductility of HTPB propellant binder. In Composite Materials, 6th Japan US Conferences; CRC Press: Boca Raton, FL, USA, 2022; pp. 771–781. [Google Scholar]
- Amado, J.C.Q.; Ross, P.G.; Murakami, L.M.S.; Dutra, J.C.N. Properties of Hydroxyl-Terminal Polybutadiene (HTPB) and Its Use as a Liner and Binder for Composite Propellants: A Review of Recent Advances. Propellants Explos. Pyrotech. 2022, 47, e202100283. [Google Scholar]
- Xu, Y.; Li, D.; Zhou, S.; Shen, Z.; Li, Y. A Rate-Dependent Constitutive Model of HTPB Propellant Based on Parallel Rheological Framework. Propellants Explos. Pyrotech. 2022, 47, e202100334. [Google Scholar] [CrossRef]
- Thomas, J.C.; Petersen, E.L. HTPB Heat of Formation: Literature Survey, Group Additive Estimations, and Theoretical Effects. AIAA J. 2022, 60, 1269–1282. [Google Scholar] [CrossRef]
- Rang, S.; Jeong, J.; Bhosale, V.K.; Kwon, S. Reactivity of hypergolic hybrid solid fuel with industrial grade hydrogen peroxide. Fuel 2022, 330, 125543. [Google Scholar] [CrossRef]
- Meng, X.; Tian, H.; Zhu, H.; Wang, Z.; Yu, R.; Guo, Z.; Cai, G. Effects of aluminum and aluminum hydride additives on the performance of hybrid rocket motors based on 95% hydrogen peroxide. Aerosp. Sci. Technol. 2022, 130, 107914. [Google Scholar] [CrossRef]
- Smith, K.K.; Redeker, N.D.; Rios, J.C.; Mecklenburg, M.H.; Marcischak, J.C.; Guenthner, A.J.; Ghiassi, K.B. Surface modification and functionalization of boron nitride nanotubes via condensation with saturated and unsaturated alcohols for high performance polymer composites. ACS Appl. Nano Mater. 2019, 2, 4053–4060. [Google Scholar] [CrossRef]
- Sreedhara, S.S.; Joardar, J.; Ravula, V.; Tata, N.R. Preparation and characterization of nanoboron by cryo-milling. Adv. Powder Technol. 2020, 31, 3824–3832. [Google Scholar] [CrossRef]
- Yang, M.; Jin, H.; Sun, Z.; Gui, R. Experimental synthesis, functionalized modifications and potential applications of monoelemental zero-dimensional boron nanomaterials. J. Mater. Chem. A 2022, 10, 5111–5146. [Google Scholar] [CrossRef]
- Shariatmadar, F.S.; Pakdehi, S.G. Synthesis and Characterization of Aviation Turbine Kerosene Nanofuel Containing Boron Nanoparticles. Appl. Therm. Eng. 2017, 112, 1195–1204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).