Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review
Abstract
1. Introduction
- (1)
- The design of novel packaging material with a controlled release mechanism.
- (2)
- For better experimental results in mathematical modeling and in vitro release testing, it is important to predict the release rate and the profile of the nanocarriers or polymers used as encapsulation materials.
- (3)
- In active packaging systems, mathematical modeling helps optimize the release process.
- (4)
- The release rate is affected by the physical attributes of packaging material such as morphology (shape, size, and composition), porosity, thickness, etc.
2. Materials and Methods
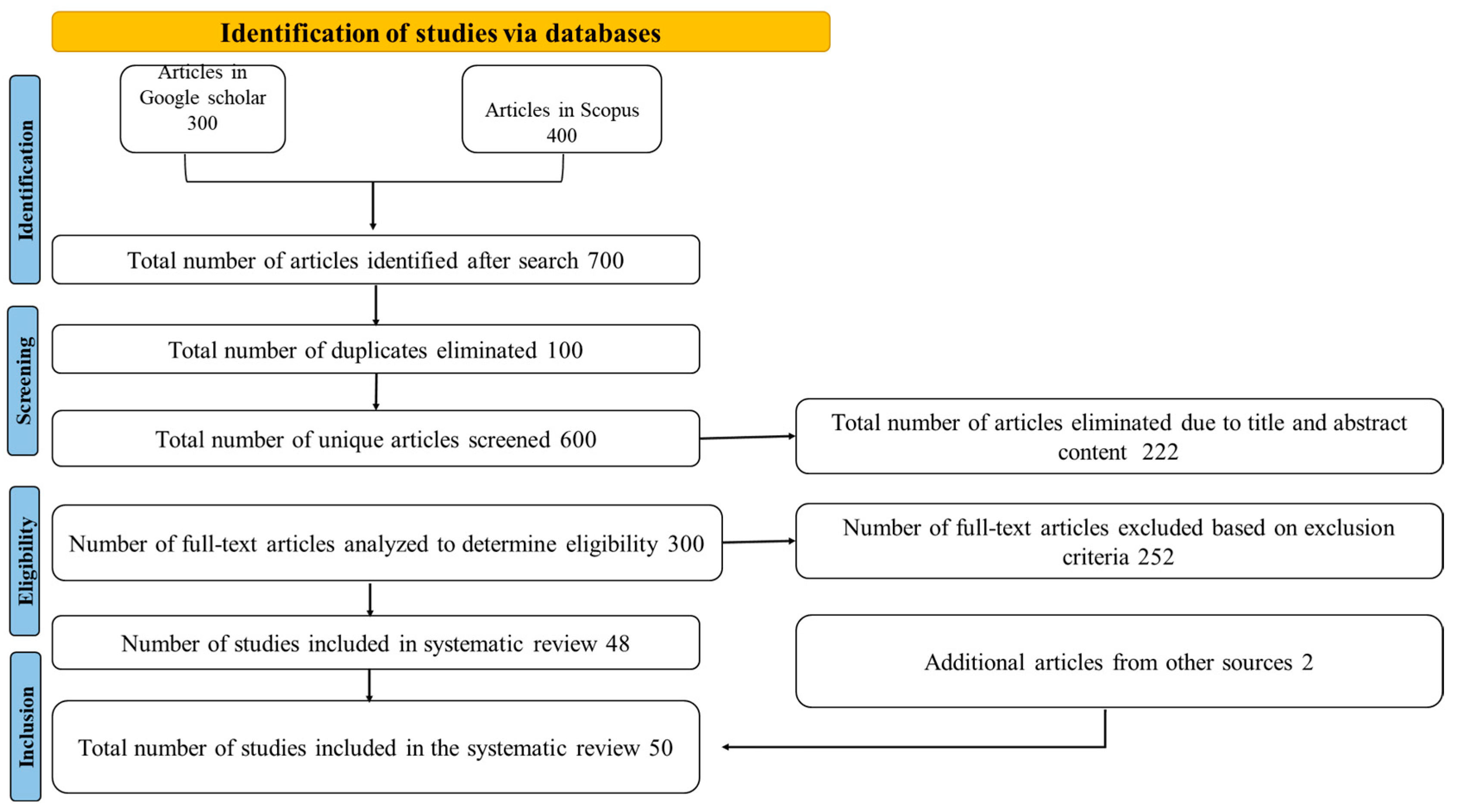
2.1. Study Evaluation
2.2. Inclusion and Exclusion Criteria
3. Results and Discussion
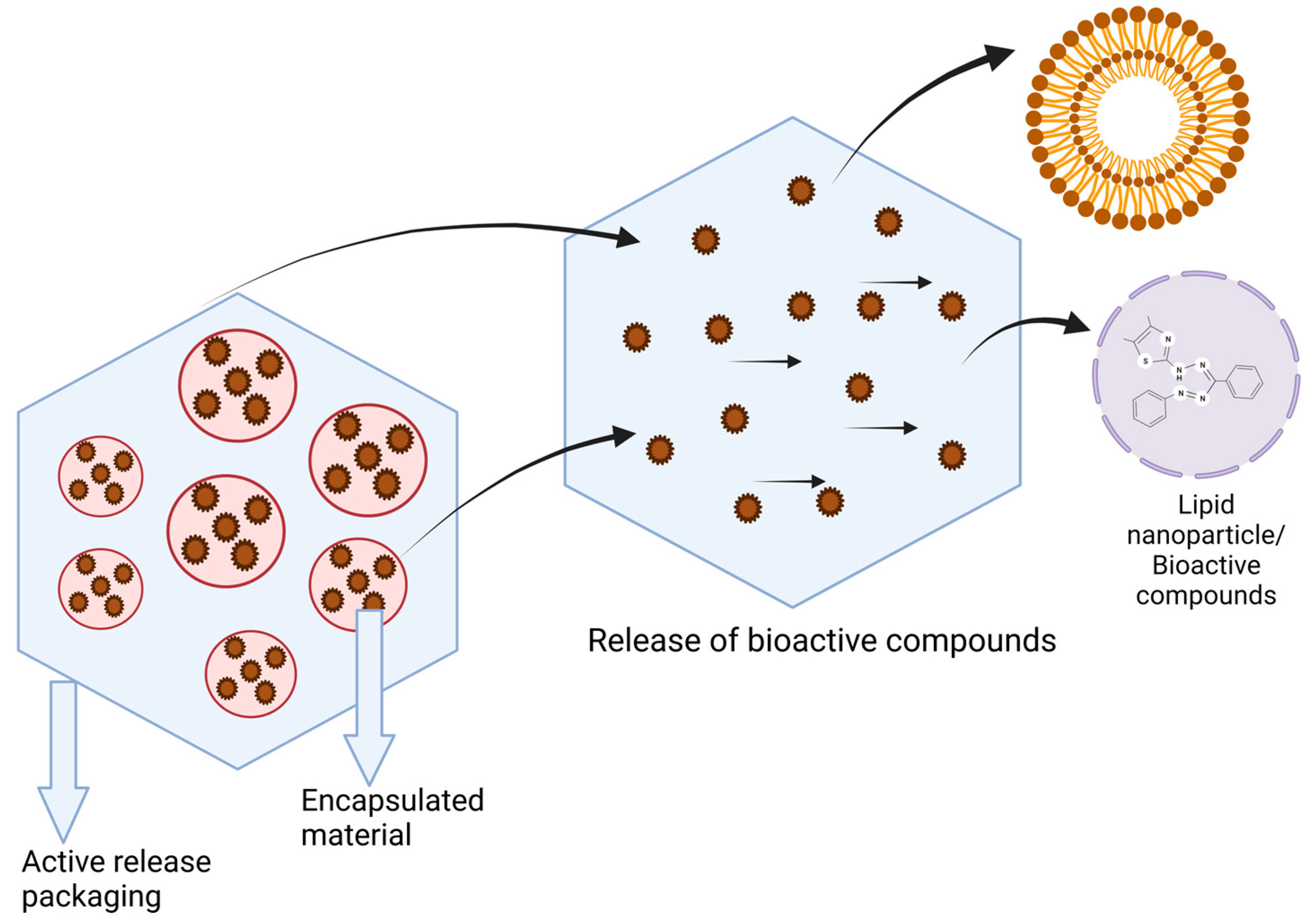
4. Encapsulation of Bioactives in Food Coating/Packaging Material
- (1)
- Oil-in-water emulsions for lipophilic compounds;
- (2)
- Water in oil emulsions for hydrophilic compounds;
- (3)
- Multiple emulsions such as oil-in-water-in-oil (O/W/O) or water-in-oil-in-water (W/O/W).
5. Mechanism Underlying the Release of Bioactives
5.1. Release Mechanisms from Different Packaging Materials
5.2. Controlled Release
- Types of bioactives;
- Dose of bioactives;
- Conditions for the release of media;
- Geometry and size of bioactives.
| Encapsulation System | Release Mechanisms | Factors Influencing the Releases | Key Findings | References |
|---|---|---|---|---|
| Cardamom-coated alginate-whey protein | First-order and Korsmeyer-Peppas models indicate Fickian diffusion | Release media temperature, pH, and shear force | An agent-based model for flavor release could be easily designed | [30] |
| Limonene-coated SPI fibrils and high methoxyl pectin | Rigter-Peppas model indicates non-Fickian diffusion | Size, uniformity, zeta potential, morphology, functional groups, modeling, and the release kinetics | Encapsulation method can be used for vegetarians since the material is plant-based | [36] |
| Curcumin coated liposome | Non-Fickian diffusion | Temperature and fluidity | Curcumin release was controlled by both diffusion and dissolution | [23] |
| Grape seed polyphenols coated liposomes | Diffusion-based release mechanism | Total phenolic content | The release rate of uncoated liposomes was higher than that of coated ones | [58] |
| Strawberry polyphenols coated chitosan | Diffusion-controlled non-Fickian | pH, particle size | pH 1.4–7.4 is perfect for the application of controlled release, either orally or externally on the skin | [29] |
| Curcumin-coated zein fibers | Fickian diffusion | morphology and size | Zein-CUR fibers were a promising material for antimicrobial applications to inhibit bacterial growth and propagation in food AP | [37] |
| Green tea polyphenols, coated choline, and cholesterol liposomes | Non-Fickian diffusion and erosion | pH, temperature, and release rate | Radical scavenging activity is observed | [34] |
| Curcumin-loaded pluronics, modifiedliposomes | Non-Fickian diffusion | pH, thermal stability, particle size, and PDI | Pluronics modification could improve absorption in the GIT tract | [59] |
| Quercetin coated microcapsules | Non-Fickian diffusion | Size and stability | Possess antioxidant functions | [60] |
| Curcumin-coated cress seed mucilage | Fickian diffusion | pH, morphology, release kinetics | Potential for the controlled release of hydrophobic food bioactives | [38] |
| Hesperetin-coated basil seed mucilage-PVA nanofibers | Fickian diffusion | EE and physical stability | Best carrier for encapsulation | [35] |
| Vitamin B12 coated chitosan microcapsules | Diffusion controlled mechanism | pH, temperature, release kinetics | Very stable microcapsule | [61] |
| Pantothenic acid-coated liposomes and hydrogel microcapsule | Diffusion controlled mechanism | pH, temperature, morphology, release kinetics | Production of a pantothenic acid capsules is possible | [22] |
| Coriander oil loaded chitosan/alginate/inulin microcapsules | Chitosan microcapsule-Fickian diffusion Other microcapsules, non-Fickian diffusion | pH, temperature, morphology, moisture, wettability, solubility, flowability, swelling, and release mechanisms | Chitosan, alginate, chitosan/alginate, and chitosan/inulin as wall materials, are resistant to pH and temperature variations | [62] |
| Thymol and carvacrol-coated maltodextrin and soy protein | Fickian diffusion | EE, release rate | The release rate is dependent on the encapsulating substance and concentrations | [32] |
| Green tea polyphenol-coated casein nanoparticles | Fickian diffusion | pH, temperature, morphology, release kinetics | Ideal for a sustained release system | [63] |
| Origanum vulgare and Thymus vulgaris oil-coated zein nanocapsules | Fickian diffusion | - | The nanoprecipitation method was effective for slow release without bursting | [64] |
| Beta-carotene coated citric acid and banana starch nanoparticles | Fickian diffusion | - | Nanoparticles with cross-linkage showed sustained release | [65] |
6. Empirical Release Models
- (a)
- There should be a balance between the simplicity of models and computational efforts for better prediction results and an understanding of the control mechanisms.
- (b)
- There must be comparative studies between theoretical and experimental data. In the first case, optimization of model parameters is performed to obtain the minimum difference between theoretical and experimental data. Thus, despite the model possibly not being efficient, it would frequently result in a good fit between experiment and theory. In this instance, not just one step in the process but the entire release profile should be described. In the second case, which defines the applicability of the concept as designed. Multiple sets of experimental data would be used to identify system-specific characteristics, and then the impact of various conditions on release kinetics would be assessed [15,69,70].
- (c)
- None of the mathematical models can be utilized for all types of systems.
- (d)
- Finally, despite strong correlations between diverse experimental and theoretical findings, some experimental evidence does not always coincide with model results [55].
7. Mechanistic Release Models
7.1. Mass Transport and Parameters
| First condition | Perfect sink condition | Cs = K Cb | Concentration on the surface of the release system (Cs) is constant as a function of bioactive concentration in the surrounding environment (Cb) with the partition coefficient between two concentrations (K) |
| Second condition | Perfect sink condition with limited mass transfer resistance at the surface of the system | In this system, surface concentration is defined using the convective mass transfer coefficient (h), and the bioactive concentration in the surrounding medium is constant | |
| Third condition | The volume of the surrounding medium is limited | Mass transfer resistance at the surface is either limited or not limited |
7.2. Systems of Mechanistic Models
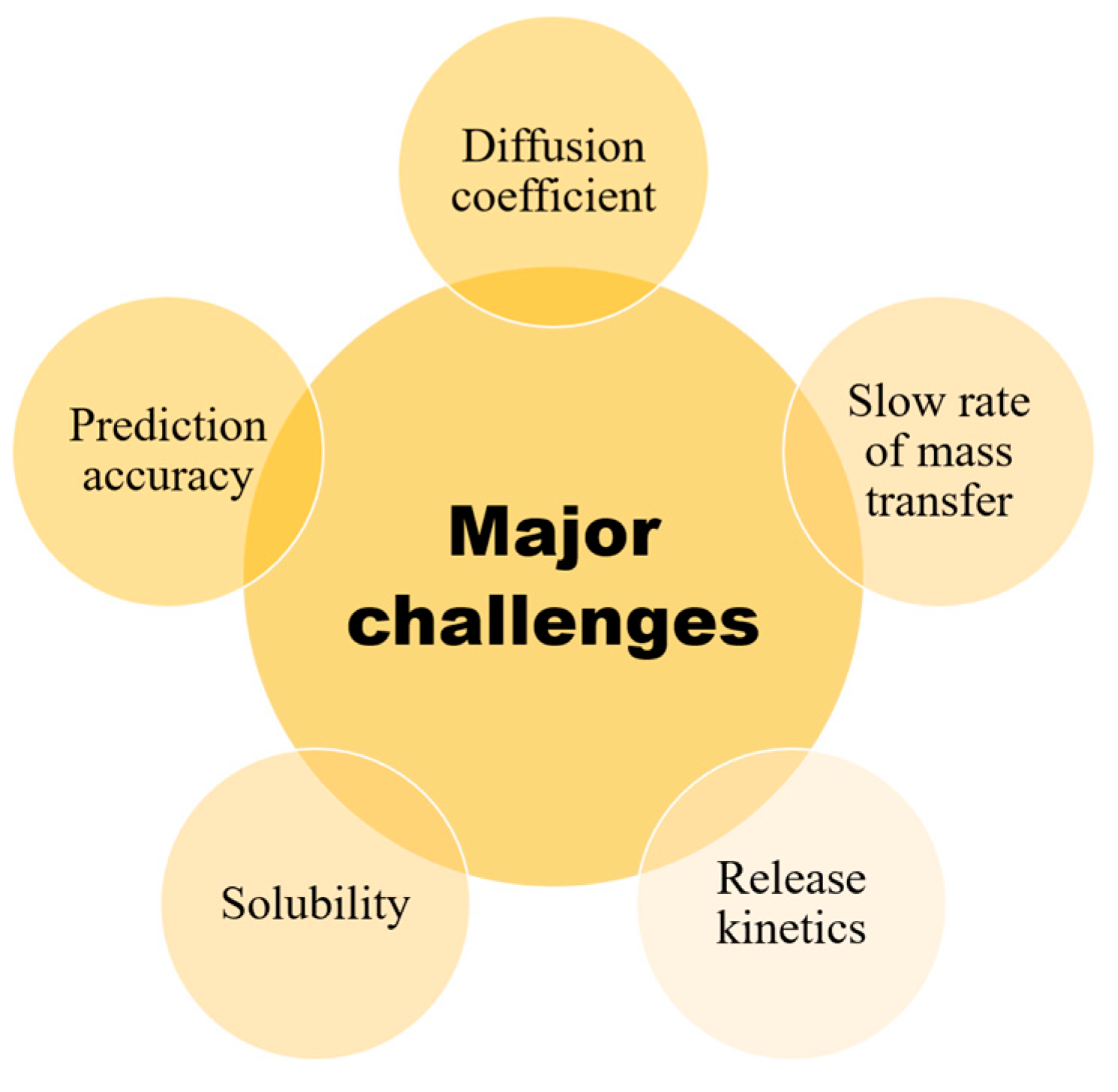
8. Challenges for the Application of Release Modeling in Food Packaging Systems
9. Conclusions and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Vin, M.; Herrera, E.; Palacin, H.; Javier, S.C.; Best, I.; Olivera-montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Kumar, K.V.P.; Suneetha, J.; Kumari, B.A. Active packaging systems in food packaging for enhanced shelf life. J. Pharmacogn. Phytochem. 2018, 7, 2044–2046. [Google Scholar]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Dekker, M.; Fogliano, V.; Heising, J. Development of a moisture-activated antimicrobial film containing ground mustard seeds and its application on meat in active packaging system. Food Packag. Shelf Life 2021, 30, 100753. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Nwakaudu, A.A.; Iheaturu, N.C. The Use of Natural Antioxidant Active Polymer Packaging Films for Food The Use of Natural Antioxidant Active Polymer Packaging Films for Food Preservation. Appl. Signals Rep. 2015, 2, 38–50. [Google Scholar]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Herculano, E.D.; de Paula, H.C.B.; de Figueiredo, E.A.T.; Dias, F.G.B.; Pereira, V.D.A. Physicochemical and antimicrobial properties of nanoencapsulated Eucalyptus staigeriana essential oil. Lwt 2015, 61, 484–491. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Pepping, L.; Dekker, M.; Fogliano, V.; Heising, J. Using particle size and fat content to control the release of Allyl isothiocyanate from ground mustard seeds for its application in antimicrobial packaging. Food Chem. 2020, 308, 125573. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- OECD. Regulatory Frameworks for Nanotechnology in Foods and Medical Products: Summary Results of a Survey Activity; OECD: Paris, France, 2013; Volume 4. [Google Scholar]
- Jafari, S.M.; Katouzian, I.; Rajabi, H.; Ganje, M. Bioavailability and release of bioactive components from nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128094365. [Google Scholar]
- Malekjani, N. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2020, 20, 3–47. [Google Scholar] [CrossRef]
- Urrútia, G.; Bonfill, X. PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Panichikkal, J.; Thomas, R.; John, J.C.; Radhakrishnan, E.K. Biogenic Gold Nanoparticle Supplementation to Plant Beneficial Pseudomonas monteilii was Found to Enhance its Plant Probiotic Effect. Curr. Microbiol. 2019, 76, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of plant probiotic performance of Pseudomonas sp. encapsulated in alginate supplemented with salicylic acid and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci. Technol. 2019, 91, 116–128. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Pandey, V.K.; Upadhyay, S.N.; Niranjan, K.; Mishra, P.K. Antimicrobial biodegradable chitosan-based composite Nano-layers for food packaging. Int. J. Biol. Macromol. 2020, 157, 212–219. [Google Scholar] [CrossRef]
- Ota, A.; Istenič, K.; Skrt, M.; Šegatin, N.; Žnidaršič, N.; Kogej, K.; Ulrih, N.P. Encapsulation of pantothenic acid into liposomes and into alginate or alginate–pectin microparticles loaded with liposomes. J. Food Eng. 2018, 229, 21–31. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhu, L.; Gan, Q.; Le, X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, B.S.; Vasile, C. Encapsulation of natural bioactive compounds by electrospinning—Applications in food storage and safety. Polymers 2021, 13, 3771. [Google Scholar] [CrossRef] [PubMed]
- Arman Alim, A.A.; Mohammad Shirajuddin, S.S.; Anuar, F.H. A Review of Nonbiodegradable and Biodegradable Composites for Food Packaging Application. J. Chem. 2022, 2022, 7670819. [Google Scholar] [CrossRef]
- Nathalie, A.; Val, L.; Desloges, I.; Gontard, N.; Bras, J. Active bio-based food-packaging: Diffusion and release of active substances through and from cellulose nanofiber coating toward food-packaging design. Carbohydr. Polym. 2016, 149, 40–50. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Pulicharla, R.; Marques, C.; Das, R.K.; Rouissi, T.; Brar, S.K. Encapsulation and release studies of strawberry polyphenols in biodegradable chitosan nanoformulation. Int. J. Biol. Macromol. 2016, 88, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Dardmeh, N.; Pirsa, S.; Almasi, H. Identification of Cardamom Encapsulated Alginate-Whey Protein Concentrates Microcapsule Release Kinetics and Mechanism during Storage, Stew Process and Oral Consumption. J. Food Process. Eng. 2015, 40, e12314. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Guarda, A.; Valenzuela, X.; Rubilar, J.F. Modeling the release of antimicrobial agents (thymol and carvacrol) from two different encapsulation materials. Food Sci. Biotechnol. 2017, 26, 1763–1772. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; Sca, R.; Bilello, V.; Settanni, L.; Gaglio, R. Antibacterial biopolymeric foams: Structure–property relationship and carvacrol release kinetics. Eur. Polym. J. 2019, 121, 109298. [Google Scholar] [CrossRef]
- Theaj, R.; Upputuri, P.; Kalam, A.; Mandal, A. Sustained Release of Green Tea Polyphenols from Liposomal Nanoparticles; Release Kinetics and Mathematical Modelling. Iran. J. Biotechnol. 2017, 15, 277–283. [Google Scholar] [CrossRef]
- Kurd, F.; Fathi, M.; Shekarchizadeh, H. Nanoencapsulation of hesperetin using basil seed mucilage nanofibers: Characterization and release modeling. Food Biosci. 2019, 32, 100475. [Google Scholar] [CrossRef]
- Ansarifar, E.; Mohebbi, M.; Shahidi, F.; Koocheki, A.; Ramezanian, N. Novel Multilayer Microcapsules Based on Soy Protein Isolate Fibrils and High Methoxyl Pectin: Production, Characterization and Release Modeling. Int. J. Biol. Macromol. 2017, 97, 761–769. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S. Food Hydrocolloids Release kinetics and antibacterial activity of curcumin loaded zein fi bers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Kavousi, H.R.; Fathi, M.; Goli, S.A.H. Novel cress seed mucilage and sodium caseinate microparticles for encapsulation of curcumin: An approach for controlled release. Food Bioprod. Process. 2018, 110, 126–135. [Google Scholar] [CrossRef]
- Rezaeinia, H.; Ghorani, B.; Emadzadeh, B.; Tucker, N. Food Hydrocolloids Electrohydrodynamic atomization of Balangu (Lallemantia royleana) seed gum for the fast-release of Mentha longifolia L. essential oil: Characterization of nano-capsules and modeling the kinetics of release. Food Hydrocoll. 2019, 93, 374–385. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, L.; Xiong, X.; Qian, M.C.; Ji, H. Preparation and release mechanism of lavender oil microcapsules with different combinations of coating materials. Flavour Fragr. J. 2019, 35, 157–166. [Google Scholar] [CrossRef]
- Eltayeb, M.; Stride, E.; Edirisinghe, M. Preparation, characterization and release kinetics of ethylcellulose nanoparticles encapsulating ethylvanillin as a model functional component. J. Funct. Foods 2015, 14, 726–735. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural polysaccharide nanomaterials: An overview of their immunological properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated nisin: An engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Heising, J.; Dekker, M. Multiresponse kinetic modelling of the formation, release, and degradation of allyl isothiocyanate from ground mustard seeds to improve active packaging. J. Food Eng. 2021, 292, 110370. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M. Ultrasound-assisted preparation of different nanocarriers loaded with food bioactive ingredients. Adv. Colloid Interface Sci. 2019, 270, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Padma Ishwarya, S. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Pothakamury, U.R.; Barbosa-Cánovas, G.V. Fundamental aspects of controlled release in foods. Trends Food Sci. Technol. 1995, 6, 397–406. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Advances in controlled release devices for food packaging applications. Trends Food Sci. Technol. 2010, 21, 591–598. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Han, J.H. Antimicrobial Packaging. Nonthermal Process. Technol. Food 2011, 26, 462–471. [Google Scholar] [CrossRef]
- Parmar, A.; Sharma, S. Engineering design and mechanistic mathematical models: Standpoint on cutting edge drug delivery. TrAC Trends Anal. Chem. 2018, 100, 15–35. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Liu, W.; Liu, C. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Vanden, N.L.; Paredes, A.J.; Rossi, Y.E.; Porporatto, C.; Allemandi, D.A.; Borsarelli, C.D.; Correa, S.G.; Montenegro, M.A. International Journal of Biological Macromolecules Controlled release and antioxidant activity of chitosan or its glucosamine water-soluble derivative microcapsules loaded with quercetin. Int. J. Biol. Macromol. 2018, 112, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Study of microencapsulation and controlled release of modified chitosan microparticles containing vitamin B12. Powder Technol. 2017, 318, 162–169. [Google Scholar] [CrossRef]
- Dima, C.; Pa, L.; Cantaragiu, A.; Alexe, P. The kinetics of the swelling process and the release mechanisms of Coriandrum sativum L. essential oil from chitosan/alginate/inulin microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef]
- Mandal, R.S.K.; Bhatt, S.; Jithin, M.V.; Lekshman, A.; Raguvaran, R.; Mondal, D.B. Evaluation of encapsulated catechin in chitosan-sodium tripolyphosphate nanoparticle. J. Pharmacogn. Phytochem. 2019, 8, 4153–4158. [Google Scholar]
- Gonçalves, C.; Paula, A.; Melo, Z.D.; Gustavo, W.; Heck, M.; Ramos, M.; Vinicius, M.; Brisola, D.O.; Cleber, F.; Luiz, P.; et al. Food Hydrocolloids Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Santoyo-aleman, D.; Sanchez, T.; Villa, C.C. Citric-acid modified banana starch nanoparticles as a novel vehicle for β-carotene delivery. J. Sci. Food Agric. 2019, 99, 6392–6399. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.T.; Lee, D. Controlled Release. Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Noureddine, S.; Moussawi, E.; Cladière, M.; Chébib, H.; Camel, V. Empirical models to predict the effect of sterilisation and storage on bisphenols migration from metallic can coatings into food simulants. Food Addit. Contam. Part A 2019, 36, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Mlalila, N.; Hilonga, A.; Kaale, L.; Swai, H. Release Kinetic Models of Vanillin and Physicomechanical Properties of Thermoplastic Starch and Chitosan Nanocomposite Films: Effects of Mixing Order. J. Packag. Technol. Res. 2020, 4, 13–22. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Carranza, E.J.M. Predictive modeling of mineral exploration targets. Handb. Explor. Environ. Geochem. 2009, 11, 3–21. [Google Scholar]
- Somaratne, G.; Ye, A.; Nau, F.; Ferrua, M.J.; Dupont, D.; Paul Singh, R.; Singh, J. Role of biochemical and mechanical disintegration on β-carotene release from steamed and fried sweet potatoes during in vitro gastric digestion. Food Res. Int. 2020, 136, 109481. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, M.; Xu, C.; Yam, K.L. Critical review of controlled release packaging to improve food safety and quality. Crit. Rev. Food Sci. Nutr. 2019, 59, 2386–2399. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; de Sá Costa, B.; Siqueira, R.P.; Garcia-Rojas, E.E. Complex coacervates of β-lactoglobulin/sodium alginate for the microencapsulation of black pepper (Piper nigrum L.) essential oil: Simulated gastrointestinal conditions and modeling release kinetics. Int. J. Biol. Macromol. 2020, 160, 861–870. [Google Scholar] [CrossRef]
| Type of Bioactive Compounds | Encapsulation Materials | Encapsulation Method | Encapsulation Conditions/Parameters | Key Findings | References |
|---|---|---|---|---|---|
| Thymol and carvacrol (antimicrobials) | Maltodextrin and soy protein matrices | Microencapsulation | Microcapsules were prepared by O/W emulsions at different concentrations (10, 20% for MD and 2, 5% for SP) | Microencapsulation of AM agents (thymol and carvacrol) can be used in packaging materials | [32] |
| Carvacrol | Commercial biodegradable polymeric foams | Disc diffusion | Morphological analysis, mechanical tests, and measurements of CRV release kinetics in food samples | Carvacrol concentration promotes antibacterial activity in food items | [33] |
| Green tea polyphenols (GTP) | Casein nanoparticles | Spray drying | EE = 76.9% at 5 mg/mL GTP concentration | GTP nanoparticles are best for the prolonged release of bioactives | [34] |
| Hesperetin (HSP) | Basil seed mucilage (BSM)-polyvinyl alcohol (PVA) nanofibres | Electrospinning | EE and physicochemical properties were assessed | HSP and BSM have high EE and physical stability as packaging materials | [35] |
| Limonene | Microcapsule with methoxy pectin and soy protein isolate (SPI) fibrils | Layer adsorption | Size, uniformity, zeta potential, morphology, functional groups, modeling, and release kinetics were considered | Limonene microcapsules could be used as edible raw materials with vegetable-based protein sources when used as packaging materials | [36] |
| Cardamom | Whey protein concentrate (WPC) with alginate | Emulsification/internal gelation | Storage, stew processing, and simulated mouth situations | Can be used to design agent-based models for flavor release in food packaging | [30] |
| Curcumin | Liposomes coated with chitosan | Ethanol injection | The release rate is faster with temperature increase while chitosan decreases the release rate | Curcumin is protected from damage and leaks in food packaging | [23] |
| Curcumin (CUR) | Zein (zein-CUR) electrospun fibers | Electrospinning | EE is approximately 100%, and the encapsulated CUR still retained its antioxidant capacity | Zein-CUR fibers as antimicrobial applications to inhibit bacterial growth and propagation in food AP | [37] |
| Curcumin | Cress seed mucilage and sodium caseinate microparticles | Spray and freeze-drying methods | EE, load, and morphology, FTIR and release kinetics | These carriers had a high potential for encapsulation and controlled release of hydrophobic food bioactives | [38] |
| Hesperetin | Basil seed mucilage (BSM)/PVA nanofibers | Electrospinning | EE = 96, 93, and 89%, and loading capacity values of 10, 14, and 18% were obtained for 10, 15, and 20% (w/w) HSP-loaded nanofibers | Encapsulation of bioactives in the food industry | [35] |
| Pantothenic acid (B3) | Liposomes and alginate or alginate-pectin microparticles loaded with liposomes | Proliposome | EE = 0.75 was achieved, and for alginate microparticles, 0.60 | B3 release was mainly driven by a diffusion-controlled mechanism in food products | [22] |
| Mentha longifolia L. EOs | Balangu seed gum nano-capsules | Electrospinning | EOs emulsions with Balangu seed gum (0.25 and 0.5% w/w) and various PVA levels (0.5, 1, and 2%) combined with Tween-20 (0.06, 0.08, and 0.1%) were electrosprayed | Nano-capsules were a good choice for fast-flavor release systems | [39] |
| Lavender oil (LO) | Different mixtures of coating materials (GA, sodium caseinate [SC], gelatin [GE], CS, β cyclodextrin [β-CD], and PVA) | Spray drying | Encapsulating efficiency (EE), loading capacity, mean particle size, and morphology | Proper encapsulating coating materials can help in the controlled release of LO microcapsules | [40] |
| Ethylvanillin | Ethylcellulose | Electro- hydrodynamic process | Particle size varied between 45 and 85 nm, and polydispersity index (PDI) was between 16 and 34%, loading capacity 67 and 81%, and EE between 71 and 84% | Modified nanoparticles for the encapsulation and controlled release of specific bioactives to engineer the functional characteristics of food products | [41] |
| Model Type | Kinetics Equations | Variables |
|---|---|---|
| Zero-order Model | M is the mass of solute dissolved during the time t, dM/dt is the velocity of mass dissolved (mass/time), D is the diffusion coefficient of the solute in solution, S is the solute area exposed, l is the thickness of the diffusion layer, Cs is the solid solubility, and C is the solute concentration in the solution on time t | |
| First-order Model | C is the concentration in the drug molecule, and k is the first-order release constant | |
| Higuchi Model | Q = t | Q is the amount of drug released on time t by area unit, C is the initial amount of drug contained in dosage form, Cs is the solubility of bioactives in the matrix medium, and D is the diffusion coefficient in matrix medium |
| Korsmeyer-Peppas Model | F is the fraction of drug releases at time t, Mt is the amount of drug releases at time t, M is total amount of the drug in dosage form, Km is the kinetic constant, n is the diffusion or release exponent, and t is time in hours | |
| Hixson-Crowell Model | W0 is the initial amount of the drug in the system, Wi is the amount remaining in the system on time t, and KHC is the constant of incorporation, which relates surface and volume | |
| Weibull Model | The drug fraction accumulated (m) in the solution at time t, and the scale parameter (a) defines the timescale of the process. The localization parameter (Ti) represents the latency time of the release process, often being zero | |
| Hopfenberg Model | is the fraction of the drug dissolved, k0 is the erosion grade constant, C0 is the initial concentration of drug in the matrix, and a0 is the initial radius of the sphere or cylinder, or a half part of the thickness of the film | |
| Baker-Lonsdale Model | = | Mt is the amount of the drug released at the time t, and is the amount released at the infinite time |
| Mechanistic Models | Advantage | Disadvantage |
|---|---|---|
| Reservoir system | Widely used in lipophilic nutraceuticals | Not suitable for all types of bioactives |
| Matrix system | Used in antimicrobial food packaging systems | No concentration gradient of bioactives is observed in the food systems |
| Swelling based | Widely used for hydrogels | The Fickian model does not work for this phenomenon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Mehany, T.; Shyu, D.J.H.; Assadpour, E.; Malekjani, N.; Castro-Muñoz, R.; Jafari, S.M. Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review. Colloids Interfaces 2023, 7, 25. https://doi.org/10.3390/colloids7020025
Siddiqui SA, Singh S, Bahmid NA, Mehany T, Shyu DJH, Assadpour E, Malekjani N, Castro-Muñoz R, Jafari SM. Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review. Colloids and Interfaces. 2023; 7(2):25. https://doi.org/10.3390/colloids7020025
Chicago/Turabian StyleSiddiqui, Shahida Anusha, Shubhra Singh, Nur Alim Bahmid, Taha Mehany, Douglas J. H. Shyu, Elham Assadpour, Narjes Malekjani, Roberto Castro-Muñoz, and Seid Mahdi Jafari. 2023. "Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review" Colloids and Interfaces 7, no. 2: 25. https://doi.org/10.3390/colloids7020025
APA StyleSiddiqui, S. A., Singh, S., Bahmid, N. A., Mehany, T., Shyu, D. J. H., Assadpour, E., Malekjani, N., Castro-Muñoz, R., & Jafari, S. M. (2023). Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review. Colloids and Interfaces, 7(2), 25. https://doi.org/10.3390/colloids7020025













