Abstract
Here, we prepared hydrophobic cryogel particles with monolithic supermacropores based on poly-trimethylolpropane trimethacrylate (pTrim) by combining the inverse Leidenfrost effect and cryo-polymerization technique. The hydrophobic cryogel particles prepared by adopting this method demonstrated the separation of the stabilized O/W emulsion with surfactant. The prepared cryogel particles were characterized in terms of macroscopic shape and porous structure. It was found that the cryogel particles had a narrow size distribution and a monolithic supermacroporous structure. The hydrophobicity of the cryogel particles was confirmed by placing aqueous and organic droplets on the particles. Where the organic droplet was immediately adsorbed into the particles, the aqueous droplet remained on the surface of the particle due to repelling force. In addition, after it adsorbed the organic droplet the particle was observed, and the organic solvent was diffused into the entire particle. It was indicated that monolithic pores were distributed from the surface to the interior. Regarding the application of the hydrophobic cryogel particles, we demonstrated the separation of a stabilized oil-in-water emulsion, resulting in the successful removal of the organic solvent from the emulsion.
1. Introduction
Water pollution caused by oil spill accidents and the leakage of industrial wastewater causes serious environmental problems [1]. Wasted oil disperses as an emulsion in a heterogenous system; therefore, the removal of oil is difficult compared to that of immiscible oil, which spontaneously separates from aqueous solution [2]. Especially, oil-in-water emulsion including surfactants can be regarded as a semi-equilibrium state with high stability in the dispersed state, and it is difficult to remove its oil fraction from the system [3]. Thus, the oil–water separation process is important for treating oily wastewater containing surfactants and other substances. As the major oil–water separation process, the gravity-driven separation, centrifugal precipitation, coagulation, and chemical demulsification are well known [4,5,6,7]. However, these separation techniques can be restricted because of low-cost performance with complex treatment and high energy consumption [8]. The membrane separation method uses hydrophobic materials to adsorb oil and are relatively inexpensive, making them a useful method for oil-removing strategies compared to the conventional treatment [9].
Despite the fact that they were discovered more than 30 years ago, cryogels have only recently gained widespread attention due to their extraordinary properties. Cryogels are extremely tough gels that can withstand significant deformation, such as elongation and torsion, and can be squeezed almost entirely without crack propagation [10,11,12]. In recent years, many cryogenically structured polymeric materials have been of significant scientific and applied interest in various fields, such as separation, waste-water treatment, biotechnology, and tissue engineering [13,14,15,16,17]. Ihlenburg et al. demonstrated that a sulfobetaine-based cryogel adsorbs methyl orange preferentially from mixed dye solutions. Methyl orange (MO)/methyl blue (MB) mixtures can be separated by the selective adsorption of the MO to the cryogels, while the MB remains in solution [18]. Cryogel is a porous material obtained by the polymerization of the polymer precursor, a solution containing monomers, a cross-linker, and polymerization initiators, under a frozen temperature (cryo-polymerization) [19,20,21]. In the process of cryo-polymerization, the phase separation occurs between the unfrozen region (concentrated monomers) and the frozen one (ice crystals), both inhabited by the polymer precursor [22]. Polymerization can occur only in the unfrozen regions of the system, where frozen solvent crystals act as porogens [10]. After the polymerization, the porous structure in the range of the µ-meter scale can be obtained by melting and drying porogen (ice crystals), which is referred to as a “supermacroporous structure” [23]. The nature of the solvent, the type (monomer or polymer precursors), the temperature of the cryogenic process, the rate of freezing/thawing dynamics, and some other factors influence the properties of polymeric cryogels [24]. Therefore, it is necessary to consider the polymer composition, such as hydrophobic monomers and hydrophilic monomers, and the preparation method according to the purpose of application. Consequently, the hydrophobic cryogel, which prefers oil to water, is useful for the adsorption and separation of organic material from aqueous solutions [25].
The monolithic porous structure has a large specific area and high porosity; therefore, it is applicable in chromatographic separations [26]. Since the monolithic porous structure forms a hierarchical porous structure with an interconnected network of the polymer wall, it supplies efficient diffusive mass transfer. The properties of monolithic porous structure are classified into several parameters. For the application and optimization of monolithic porous materials as separation substances, the characterization of their properties is required. The parameters include (i) the size distribution of the particle diameter, (ii) the standard deviation of the particle diameter, (iii) the coefficient of variation of particle, (iv) the bulk density, and (v) the size distribution of the pore size. Furthermore, the macroscopic shape of the cryogel’s (a) cylinder, (b) sheet, (c) disk, and (d) particle is an essential factor in the design of separation devices such as the particle-packed column or monolithic column chromatography. The various shapes of the cryogel can be conventionally prepared by performing cryo-polymerization in a mold vessel. Cryogel particles can be prepared by the polymerization method using emulsion as a mold; cryo-polymerization occurs at a phase containing polymer precursor [27,28]. Due to the acceleration of cryogel research, various preparation methodologies and polymer components have been developed [29,30]. The preparation of the feed system, freezing, incubating the gelation system in a frozen state, and thawing the frozen sample are all required stages in fabricating the cryogel [11]. As a different preparation method, we recently developed a combination technique, i.e., the inverse Leidenfrost effect and the cryo-polymerization technique (the iLF cryo-method) [31,32].
In this study, we first adopted the iLF cryo-method to create hydrophobic monolithic supermacroporous cryogel particles. The cryogel particles revealed the narrow size distribution of the diameter, and numerous pores with monolithic supermacroporous structures. Furthermore, the properties such as hydrophobicity and adsorption preference were characterized by using dyed solvents. Unlike the aqueous droplet, the organic droplet was immediately adsorbed into the particles. The cross section of the cryogel particle after the adsorption of the organic solvent showed that the entire particle was stained with dye, indicating that the oil diffused thoroughly into the interior of the particle. For the separation of the oil-in-water emulsions stabilized with surfactants, the cryogel particles successfully removed emulsions from the system.
2. Materials and Methods
2.1. Materials
Trimethylolpropane trimethacrylate (Trim), benzoyl peroxide (BPO), etlyl-4-dimethylaminobenzoate (EDMAB), and nile red were purchased from Tokyo Chemical Industry Ltd. Acetic acid, toluene, n-hexane, N,N’-methylenebisacrylamide (MBA), liquid paraffine, sudan (IV), Coomassie brilliant blue G-250 (CBB), and polyoxyethylene sorbitan monooleate (Tween 80) were purchased from Wako Pure Chemical Industry Ltd., (Osaka, Japan). Ultra-pure water (conductive 18.2 MΩ cm) was purchased from a Merck Millipore. All materials were used as received without further treatment.
2.2. Preparation of Hydrophobic Cryogel Particles
The hydrophobic cryogel particles were prepared as described in previous works [31,32]. Briefly, the cryogel particles were prepared via a 2-step process: (1) the preparation of frozen droplets by utilizing the iLF effect and (2) cryo-polymerization under a frozen temperature. Trim used as monomer (Figure 1) and MBA used as cross-linker were dissolved in acetic acid. Then, BPO and EDMAB were added as initiators and the polymer precursor was obtained. Subsequently, the obtained polymer precursor was dropped onto liquid nitrogen and frozen droplets were prepared. These droplets were transferred to liquid paraffine at −15 °C and polymerized overnight. After cryo-polymerization, hydrophobic cryogel particles were washed with n-hexane and lyophilized.
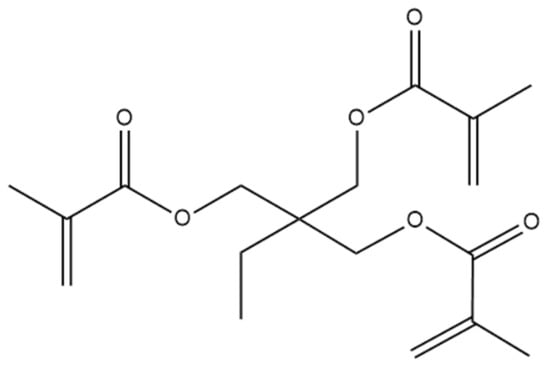
Figure 1.
Chemical structure of trimethylolpropane trimethacrylate (Trim).
2.3. Observation of Macroscopic Shape
The overall cryogel particles in the bottle of glass were observed as photographic images by a charge-coupled device (CCD, iPhone 12 MJNJ3J/A) camera. In order to evaluate the size distribution of particles, the diameter of particles was measured from optical microscope images. The mean diameter of the particles (Dv) and standard deviation (σ) were calculated from the optical microscope images by using image J software. Furthermore, the coefficient of variation (Cv) was calculated according to Equation (1).
Here, the tapped bulk density (ρbulk) was calculated according to Equation (2).
The Wparticles and Vcylinder were represented as the weight of the particles and the volume of the particles in the graduated cylinder, respectively. The scale of the graduated cylinder with particles was read after tapping 100 times [33].
2.4. Characterization of Porous Properties
The surface of the porous structure of the cryogen particle was observed by scanning electronic microscope (Hitachi, SU3500, Tokyo, Japan, SEM), operated at 20 keV. As pretreatment, gold was coated on cryogel for 30 s by spattering. The pore diameters were measured according to the size distribution that was analyzed using image J software.
2.5. Testing Hydrophobicity of Cryogel Particles
The hydrophobicity of cryogel particles was tested by using aqueous and organic droplets. The water was dyed with CBB as a blue-colored aqueous solution, and the toluene was dyed with Nile red as a red-colored organic solution. Then, the droplets placed on the cryogel particles were observed. Subsequently, the adsorption behavior of an organic solvent by cryogel particles was investigated. The red-dyed organic solution was separated from the water collecting at the top, and the cryogel particles were added to the water. A CCD camera was used to record the adsorption behavior over time three and ten seconds after the particle was added. Furthermore, the cryogel particle was cut in half after being adsorbed with the dyed organic solution, and the cross-section was observed.
2.6. Separation of Stabilized Oil-in-Water Emulsion
The oil-in-water emulsion stabilized with a surfactant was prepared by mixing 990 µL of n-hexane, 100 mg of Tween 80, and 99 mL of ultra-pure water under ultrasonication at room temperature (around 25–30 °C) and atmospheric pressure. After preparation, the sample was stored for over 24 h at room temperature and atmospheric pressure. Then, the stabilization of O/W emulsion (cloudy) was visually observed, and the light scattering was confirmed via laser at dark place (Tyndall phenomenon). The characterization of the emulsion after separation was recorded by photograph and using the optical microscope image. To investigate the separation of stabilized oil-in-water emulsion by cryogel particles, the organic phase of the emulsion was dyed with Sudan IV during the preparation of oil-in-water emulsion [34]. Then, the emulsion was separated by mixing the emulsion and cryogel particles in the glass bottle and kept for 3 h at room temperature. After that, the particles were removed from the solution by filter and measured the UV-Vis spectra by spectrophotometer (Shimadzu UV-1800, Kyoto, Japan).
3. Results and Discussion
3.1. Macroscopic Shape of pTrim Cryogel Particles
The pTrim cryogel particles were prepared by combining the inverse Leidenfrost effect and cryo-polymerization technique (iLF cryo-method) [31,32]. By adding the water droplets containing polymer precursor into an extremely low temperature bath, the inverse Leidenfrost phenomenon was induced, and frozen droplets with a spherical shape were formed on the liquid nitrogen. The frozen droplet was then polymerized under a frozen temperature (−15 °C). After cryo-polymerization, the pTrim cryogel particles were obtained by thawing and lyophilization. Figure 2 shows the photo image of pTrim cryogel particles (Figure 2a) and the size distribution of the particles through the observation of an optical microscope (Figure 2b). As shown in Figure 2a, the pTrim cryogel particles had a spherical shape with a white color. Subsequently, after the diameter of more than 200 individual particles was observed from the optical microscopic images, the size distribution was then determined. Figure 2b shows the size distribution of pTrim cryogel particles. As a result, the pTrim cryogel particle, prepared by our iLF-cryo method, was distributed in the range of 700–2300 µm and has a high frequency of around 1600 µm. Herein, the basic properties of pTrim cryogel particles were summarized in Table 1; the value of the mean diameter, Dv, and its standard deviation, σ, were 1654 µm and 220, respectively. Furthermore, the variation coefficient, Cv, was low, at a value of 13 %. Hence, pTrim cryogel particles have a narrow size distribution, and the monodispersiblitiy of the particle was high in comparison with that of the conventional droplet [35]. The tapped bulk density, ρbulk, was calculated from the bulk-particle volume in a graduated cylinder and from the weight of the particle; the ρbulk value was 0.3 g/mL. Comprehensively, the macroscopic properties of pTrim cryogel particles adopted by the iLF cryo-method were characterized on the basis of the factors Dv, σ, Cv, and ρbulk.
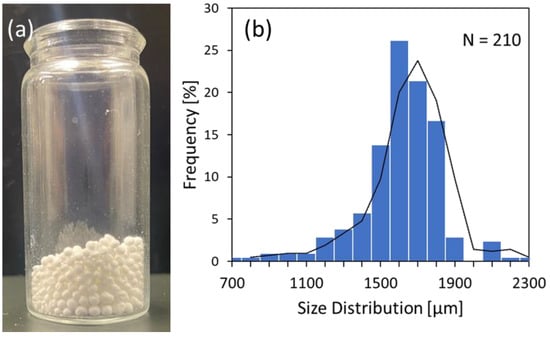
Figure 2.
Macroscopic shape of pTrim cryogel particles’ (a) appearance; and of (b) size distribution of particle diameter.

Table 1.
Particle properties of pTrim cryogel particles.
3.2. Characterization of Porous Properties
In general, cryogels have a monolithic porous structure with supermacropores in the range of the µ-meter scale. During the cryo-polymerization process, the crystals supplied from the solvent (i.e., acetic acid) were employed as porogens; the porous structure was therefore formed. As shown in Figure 3, the porous structure of the surface pTrim cryogel particle was observed via SEM analysis. The observations were performed for the surface section of the pTrim cryogel particle, as described in Figure 3a. From the overall image, the particles were found to have a rough surface (Figure 3b). Subsequently, the surface section of the particle was observed from the high-magnification SEM image to characterize its porous structure (Figure 3c). It was found that the pTrim cryogel particle had a monolithic supermacroporous structure on its surface. Interestingly, the polymer wall of the porous structure appeared to have a smooth surface. The fine morphology of the porous structure (i.e., surface roughness, undulation) is changed by employing different porogens [36]. Herein, the pore diameter was measured from 47 pore diameters by a high-magnification SEM image to characterize the porous property. As shown in Figure 3d, the size distribution of the porous diameter was evaluated. The porous diameters were distributed in the range of 1–12 µm, and the mean porous diameter dp was 4.7 µm. The results suggest that pTrim cryogel particles have a unique porous structure on their surface that is monolithic with a supermacroporous structure.
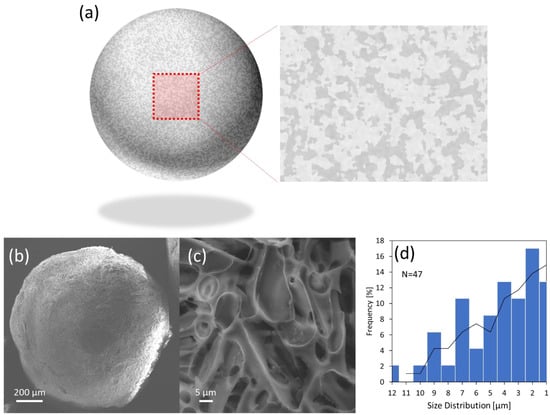
Figure 3.
Characterization porous structure of pTrim cryogel particle. (a) Schematic illustration of the morphological observation overall particle and a surface section of high magnification. (b) Overall particle SEM image and (c) surface section at high magnification. (d) Size distribution of pores diameter.
3.3. Hydrophobicity of pTrim Cryogel Particles
The hydrophobicity of polymer particles can be investigated by confirming the behavior of aqueous or organic droplets when they are contacting the polymer particles. When the aqueous droplet is placed on the hydrophilic polymer, the droplet penetrates the hydrophilic polymer, while the organic droplet is repelled. In the case of the hydrophobic polymer, where the aqueous droplet is repelled, and the organic droplet penetrates the hydrophobic polymer. As shown in Figure 4, the hydrophobicity of pTrim cryogel particles was investigated. Herein, the behavior of droplets, aqueous (dyed as blue), and organic (dyed as red) was placed on the pTrim cryogel particles and observed (Figure 4a). As the result, the aqueous droplet was repelled from the pTrim cryogel particles, whereas the organic droplet penetrated the particles. As stated previously, the cryogel particles presented here comprise pTrim and thus have a hydrophobic surface. Subsequently, the adsorption of organic solvent in the oil–water system by pTrim cryogel particles was examined in Figure 4b,c. Cyrogel particles were added to water containing the red-dyed organic solvent. As shown in Figure 4b, three seconds after the addition of the particles, the particles floated to the liquid surface, resulting in aggregate formation. Furthermore, the particles rapidly started to adsorb the dyed organic solvent. Eventually, the particles were found to adsorb all organic solvents in the beaker after only 10 s (Figure 4c). After the adsorption of organic solvent, pTrim cryogel particles were removed from the water and cut in half; the appearance of cross section was then then observed (Figure 4d). It was confirmed that the pTrim cryogel particle was colored red, including the interior side from the image of the cross section. Hence, the adsorbed organic solvent was diffused through the interior of the particle. It is expected that the porous structure of the pTrim cryogel particle has pores running from the exterior to the interior of the particle and monolithic structure. These results are supported by the evidence that the monolithic porous structure was formed in the pTrim cryogel particle (Figure 3). The pTrim cryogel particles were thus shown to have the potential to be applied as a separation material because of their permeability function with the expansive monolithic porous structure.
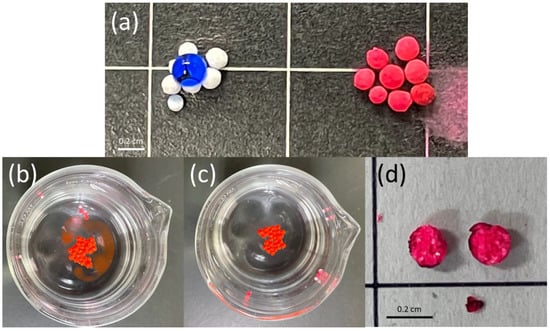
Figure 4.
Hydrophobicity of pTrim cryogel particles (a) left; aqueous droplet on the particles, right; organic droplet on the particles. The adsorption of organic solvent from water by pTrim cryogel particles (b) 3 s and (c) 10 s. (d) Appearance cross section of the particle after adsorption organic solvent.
3.4. Separation of Stabilized Oil-in-Water Emulsion
Based on the characteristics of pTrim cryogel and its fundamental properties, it is possible to recover the oil-in-water emulsion from the aqueous solution. Typically, the oil-in-water emulsion can be formed by dispersing and stirring a small amount of oil in water. The formed oil-in-water emulsion is unstable in long-term and becomes a two-phase system which is thermodynamically stable. However, via the adsorption of a surfactant on the interface, the emulsion is stabilized and exists in a long-term heterogeneous system. It is difficult to remove the organic solvent from the stabilized emulsion because the emulsion does not spontaneously cause phase separation. Herein, the separation of stabilized oil-in-water emulsion with surfactant was performed by using pTrim cryogel particles in Figure 5. The emulsions were observed in a bulk-scale image and optical microscope images before the separation, and in a bulk-scale image and optical one after separation (Figure 5a–d). From bulk-scale images (Figure 5a,c), the solution was deeply cloudy in white before the addition of the cryogel particles, while the solution became transparent after that. The color change of these solutions indicates that the organic solvent was dispersed and became a heterogeneous system as a colloidal state (before the addition of cryogel particles), and then the colloid was removed (after the particle addition). As shown in Figure 5b,d, the states of solution were observed by using an optical microscope, the emulsions with several µ-meter scales were dispersed in the solution (before the particle addition), and then there was no colloidal emulsion (after the particle addition). It is possible that the slight turbidity of the solution after the particle addition could be caused by dispersed microemulsions that could not be observed with optical microscope. Subsequently, the separation property of pTrim cryogel particles was evaluated from UV-Vis spectra of the emulsion in which the organic phase was dyed with a probe Figure 5e. In the spectrum before the particle addition, the characteristic absorbance peak derived from the dye dissolved in the organic phase was detected. On the contrary, there was no absorbance peak in the spectra in the supernatant after the particle addition. The separation was induced simply by adding the cryogel particles with macroporous and hydrophobic nature through the interaction between the hydrophobicity of pTrim and the organic solvent as colloidal state dispersed in the solution. As a result, the separation of stabilized oil-in-water emulsion with surfactant has been demonstrated.
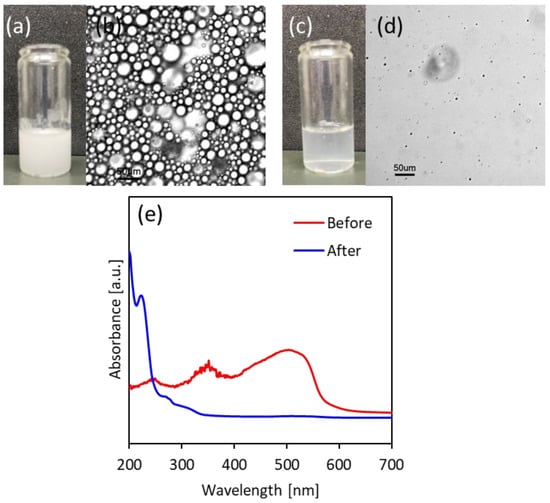
Figure 5.
Separation of stabilized emulsion by using pTrim cryogel particles. Before separation of (a) photograph of the emulsion in bulk scale and (b) optical microscope image, and after separation of (c) photograph of the emulsion in bulk scale and (d) optical microscope image. (e) UV-vis spectra of emulsion.
4. Conclusions
In summary, the pTrim-based cryogel particles were prepared by adopting our developed method, the iLF cryo-method. The optical microscope and SEM were used to characterize the particle properties (i) Dv, (ii) ρ, (iii) Cv, and (iv) σbulk, and (v) the size distribution of pore size. In addition, the cryogel particle was found to have a monolithic supermacroporous structure. Subsequently, the hydrophobicity of cryogel particles was tested by placing aqueous and organic droplets on the particles, where the aqueous droplet remained on the surface of the particle by a hydrophilic repulsive force and the organic droplet was immediately adsorbed into the particle due to the hydrophobic surface of the particles. Furthermore, it was found that the adsorbed organic solvent was diffused into the entire particle by observation cross-section of the cryogel particle. The separation of stabilized oil-in-water emulsion by using cryogel particles was performed. As a result, in the case study, the efficient removal of stabilized oil-in-water emulsion has been demonstrated. This cryogel particle material can be applied for the separation of oil phase in the emulsion state.
Author Contributions
The manuscript was written through the contributions of all of the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was primarily supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aids for Scientific Research (A) (26249116, 21H04628), Grant-in-Aids for Scientific Research (B) (18H02005), Grant-in-Aids for Scientific Research (C) (20K05198), and JST SPRING (JPMJSP2138).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doshi, B.; Sillanpää, M.; Kalliola, S. A Review of Bio-Based Materials for Oil Spill Treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Wang, X.; Zhang, Z. Emulsification and Improved Oil Recovery with Viscosity Reducer during Steam Injection Process for Heavy Oil. J. Ind. Eng. Chem. 2018, 61, 348–355. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.S.J.; Lai, Z. Selective Separation of Oil and Water with Mesh Membranes by Capillarity. Adv. Colloid Interface Sci. 2016, 235, 46–55. [Google Scholar] [CrossRef]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J. Centrifugal Separation Efficiency in the Treatment of Waste Emulsified Oils. Chem. Eng. Res. Des. 2006, 84, 69–76. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Patel, S.U.; Chase, G.G. Layered Hydrophilic/Hydrophobic Fiber Media for Water-in-Oil Coalescence. Sep. Purif. Technol. 2012, 85, 157–164. [Google Scholar] [CrossRef]
- Abdulrazzaq Hadi, A.; Abdulkhabeer Ali, A. Chemical Demulsification Techniques in Oil Refineries: A Review. Mater. Today Proc. 2022, 53, 58–64. [Google Scholar] [CrossRef]
- Ammann, S.; Ammann, A.; Ravotti, R.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Effective Separation of Awater in Oil Emulsion from a Direct Contact Latent Heat Storage System. Energies 2018, 11, 2264. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Lozinsky, V.I. A Brief History of Polymeric Cryogels. Adv. Polym. Sci. 2014, 263, 1–48. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic Principles of Cryotropic Gelation, Polymeric Cryogels: Macroporous Gels with Remarkable Properties Book Series. Adv. Polym. Sci. 2014, 264, 49–101. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis and Structure-Property Relationships of Cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.; Montagnaro, F.; Inglezakis, V.J.; Balsamo, M. Experimental and Modeling Studies of Sr2+ and Cs+ Adsorption on Cryogels and Comparison to Commercial Adsorbents. Ind. Eng. Chem. Res. 2022, 61, 8204–8219. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Chen, M.; Wang, C.; Kuang, S.; Pan, Q.; Ren, D.; Chen, Z. Lotus-Root-like Supermacroporous Cryogels with Superphilicity for Rapid Separation of Oil-in-Water Emulsions. ACS Appl. Polym. Mater. 2019, 1, 2273–2281. [Google Scholar] [CrossRef]
- Juan, L.T.; Lin, S.H.; Wong, C.W.; Jeng, U.S.; Huang, C.F.; Hsu, S.H. Functionalized Cellulose Nanofibers as Crosslinkers to Produce Chitosan Self-Healing Hydrogel and Shape Memory Cryogel. ACS Appl. Mater. Interfaces 2022, 14, 36353–36365. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Sands, R.W.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G.; et al. Injectable Cryogel-Based Whole-Cell Cancer Vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef]
- Ihlenburg, R.B.J.; Lehnen, A.C.; Koetz, J.; Taubert, A. Sulfobetaine Cryogels for Preferential Adsorption of Methyl Orange from Mixed Dye Solutions. Polymers 2021, 13, 208. [Google Scholar] [CrossRef]
- Kirsebom, H.; Rata, G.; Topgaard, D.; Mattiasson, B.; Galaev, I.Y. Mechanism of Cryopolymerization: Diffusion-Controlled Polymerization in a Nonfrozen Microphase. An NMR Study. Macromolecules 2009, 42, 5208–5214. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel Applications in Microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing Unveiled by Thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A. Cell Separation Using Cryogel-Based Affinity Chromatography. Nat. Protoc. 2010, 5, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the Basis of Natural and Synthetic Polymers: Preparation, Properties and Application. Usp. Khim. 2002, 71, 579–584. [Google Scholar] [CrossRef]
- Chen, X.; Sui, W.; Ren, D.; Ding, Y.; Zhu, X.; Chen, Z. Synthesis of Hydrophobic Polymeric Cryogels with Supermacroporous Structure. Macromol. Mater. Eng. 2016, 301, 659–664. [Google Scholar] [CrossRef]
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity Monolith Chromatography: A Review of Principles and Recent Analytical Applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Lu, D.P.; Lin, D.Q.; Yao, S.J. Preparation and Characterization of Supermacroporous Polyacrylamide Cryogel Beads for Biotechnological Application. J. Appl. Polym. Sci. 2013, 130, 3082–3089. [Google Scholar] [CrossRef]
- Yun, J.; Dafoe, J.T.; Peterson, E.; Xu, L.; Yao, S.J.; Daugulis, A.J. Rapid Freezing Cryo-Polymerization and Microchannel Liquid-Flow Focusing for Cryogel Beads: Adsorbent Preparation and Characterization of Supermacroporous Bead-Packed Bed. J. Chromatogr. A 2013, 1284, 148–154. [Google Scholar] [CrossRef]
- Hiramure, Y.; Suga, K.; Umakoshi, H.; Matsumoto, J.; Shiomori, K. Preparation and Characterization of Poly-N-Isopropylacrylamide Cryogels Containing Liposomes and Their Adsorption Properties of Tryptophan. Solvent Extr. Res. Dev. 2018, 25, 37–46. [Google Scholar] [CrossRef]
- Plieva, F.; Xiao, H.; Galaev, I.Y.; Bergenståhl, B.; Mattiasson, B. Macroporous Elastic Polyacrylamide Gels Prepared at Subzero Temperatures: Control of Porous Structure. J. Mater. Chem. 2006, 16, 4065–4073. [Google Scholar] [CrossRef]
- Takase, H.; Shiomori, K.; Okamoto, Y.; Watanabe, N.; Matsune, H.; Umakoshi, H. Micro Sponge Balls: Preparation and Characterization of Sponge-like Cryogel Particles of Poly (2-Hydroxyethyl Methacrylate) via the Inverse Leidenfrost Effect. ACS Appl. Polym. Mater. 2022, 4, 7081–7089. [Google Scholar] [CrossRef]
- Takase, H.; Watanabe, N.; Shiomori, K.; Okamoto, Y.; Matsune, H.; Umakoshi, H. Versatility of the Preparation Method for Macroporous Cryogel Particles Utilizing the Inverse Leidenfrost Effect. ACS Omega 2023, 8, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Jain, S.K.; Pandey, R.S. Controlling Release of Metformin HCl through Incorporation into Stomach Specific Floating Alginate Beads. Mol. Pharm. 2011, 8, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Huang, H.C.; Chen, L.T. Protonated Melamine Sponge for Effective Oil/Water Separation. Sci. Rep. 2015, 5, 14294. [Google Scholar] [CrossRef]
- Merakchi, A.; Bettayeb, S.; Drouiche, N.; Adour, L.; Lounici, H. Cross-Linking and Modification of Sodium Alginate Biopolymer for Dye Removal in Aqueous Solution. Polym. Bull. 2019, 76, 3535–3554. [Google Scholar] [CrossRef]
- Kirsebom, H.; Mattiasson, B. Cryostructuration as a Tool for Preparing Highly Porous Polymer Materials. Polym. Chem. 2011, 2, 1059–1062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).