Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cell Lines
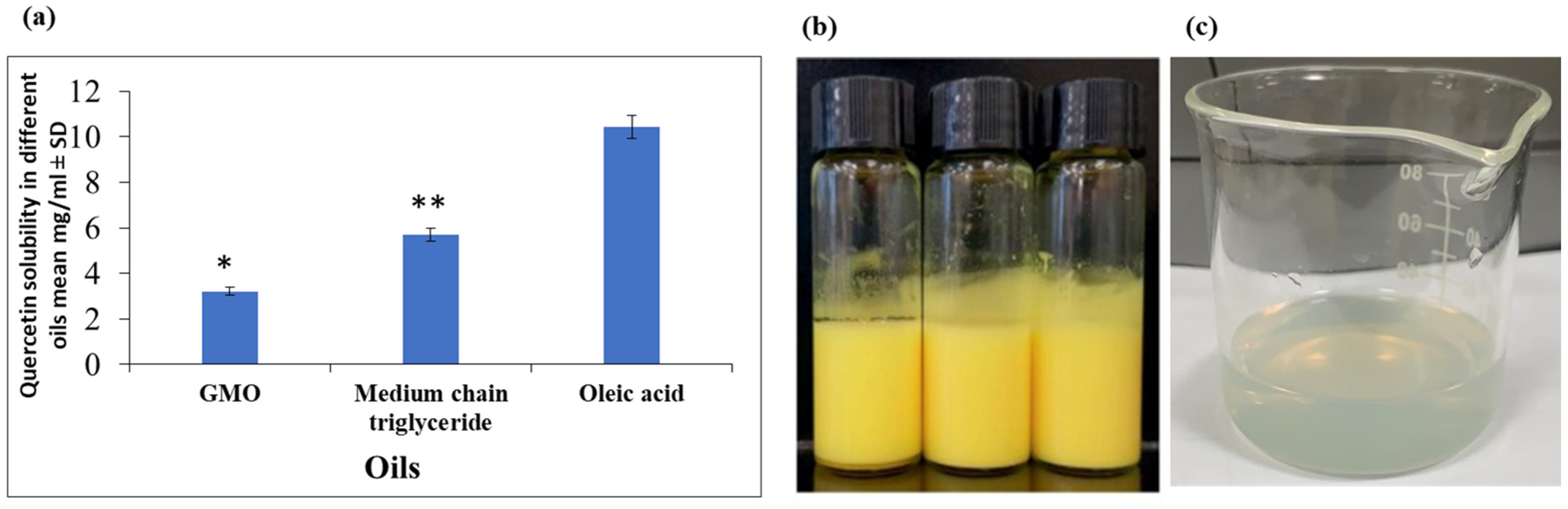
2.2. Selection of the Oil Phase
2.3. Preparation of Quercetin-Loaded NEs (QR-NEs)
2.4. Evaluation of the Prepared QR-NE
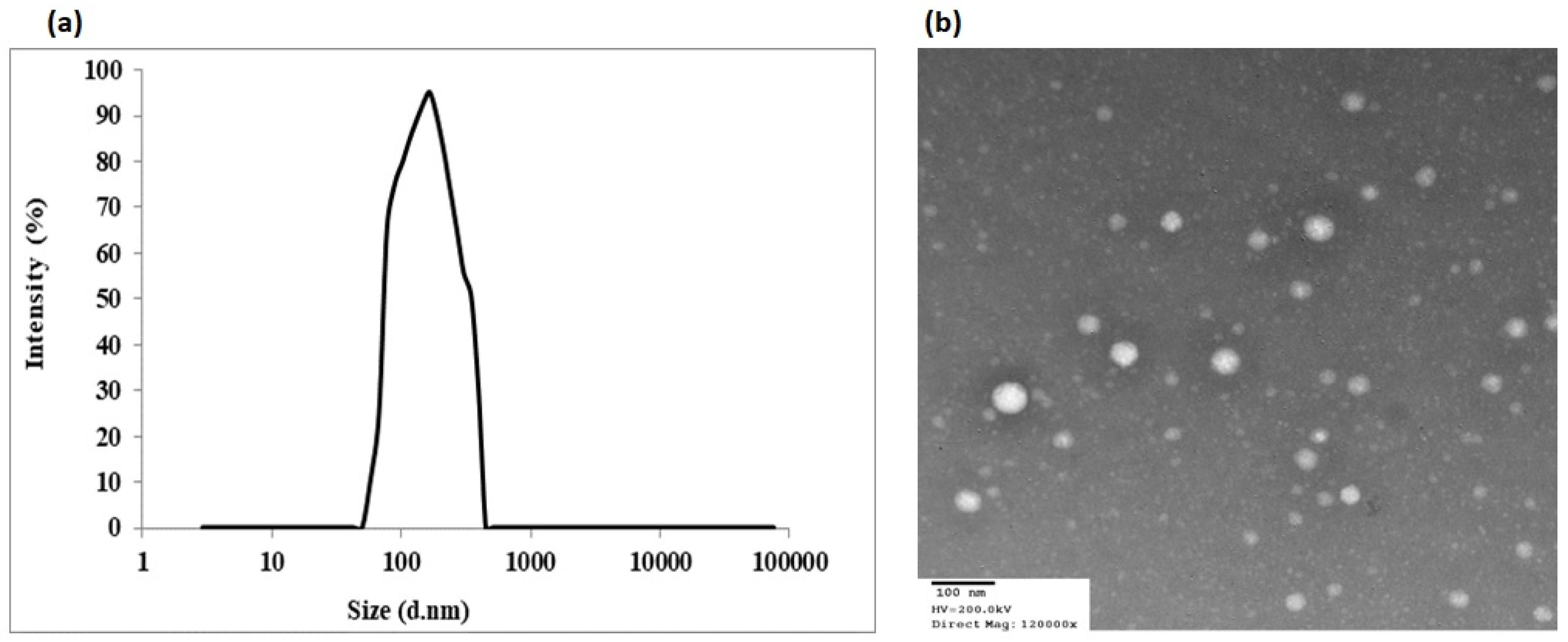
2.4.1. Nanoemulsion Particle Morphology
2.4.2. Determination of Entrapment Efficiency
2.4.3. Determination of pH
2.5. Studying the Physical Stability of QR-NE
2.5.1. Centrifugation Method
2.5.2. Agitation Test
2.5.3. Heating Cooling Cycle
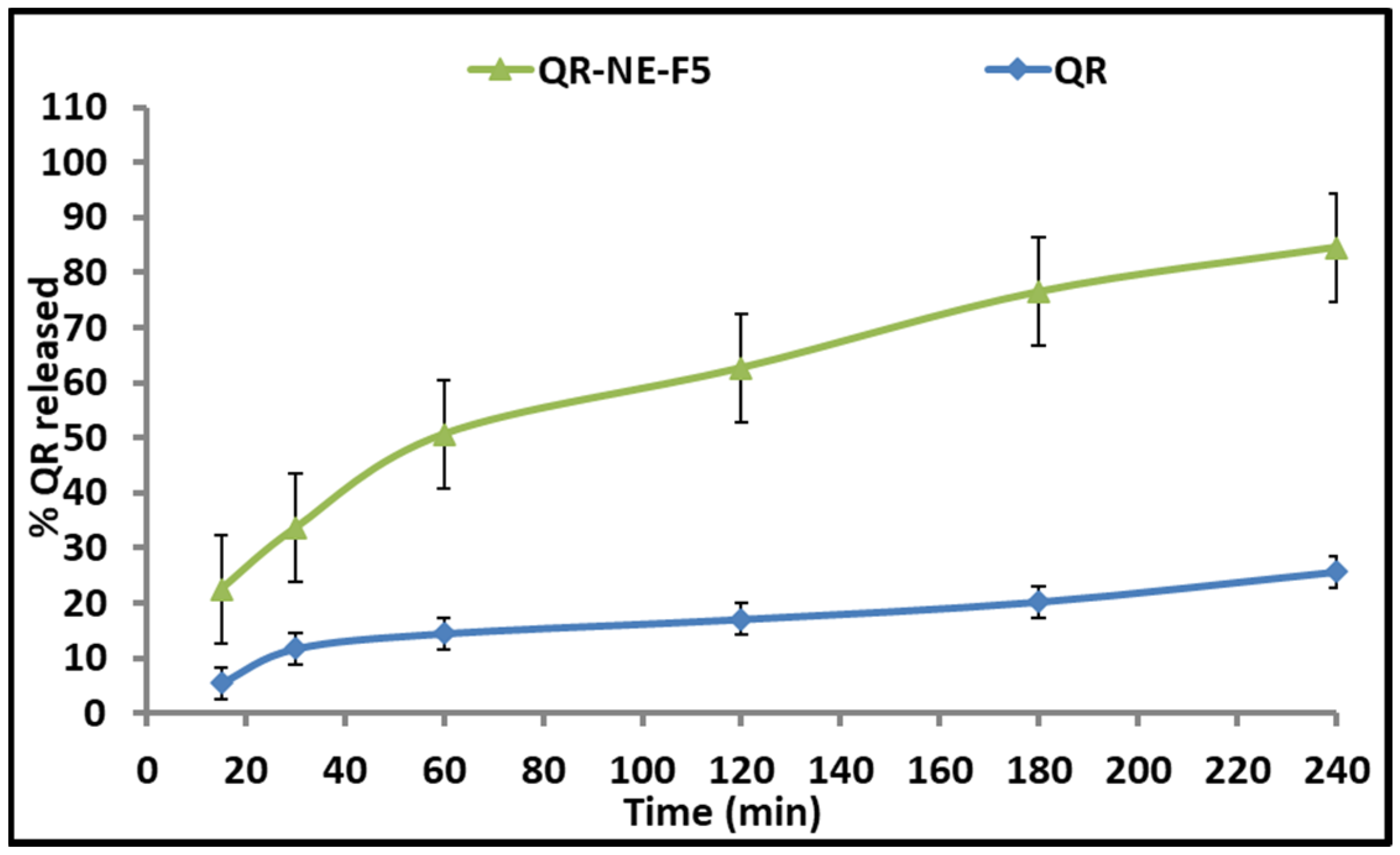
2.6. In Vitro Drug Release Study of QR from QR-NEs
2.7. Transmission Electron Microscopy (TEM)
2.8. Evaluation of the Anticancer Activity against HT-29 and HCT-116 Cells
2.9. Animals’ Treatment and Histological Analysis of Tissues after QR-NE Treatment
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Selection of Oils
3.2. Optimized QR-NE Formula Selection: Morphology and pH Determination
3.3. Studying the Physical Stability of QR-NEs
3.4. In Vitro Drug Release Study of QR from QR-NPs
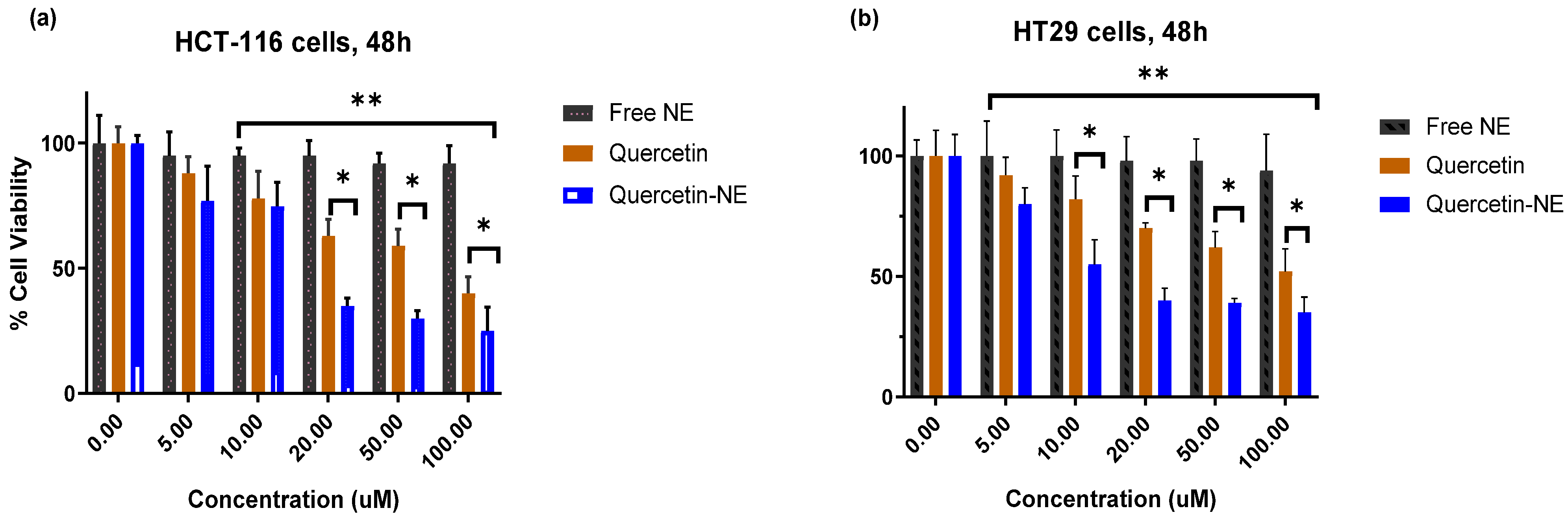
3.5. Anticancer Activity of QR-NE; Cytotoxicity Study against Colorectal Cancer Cells
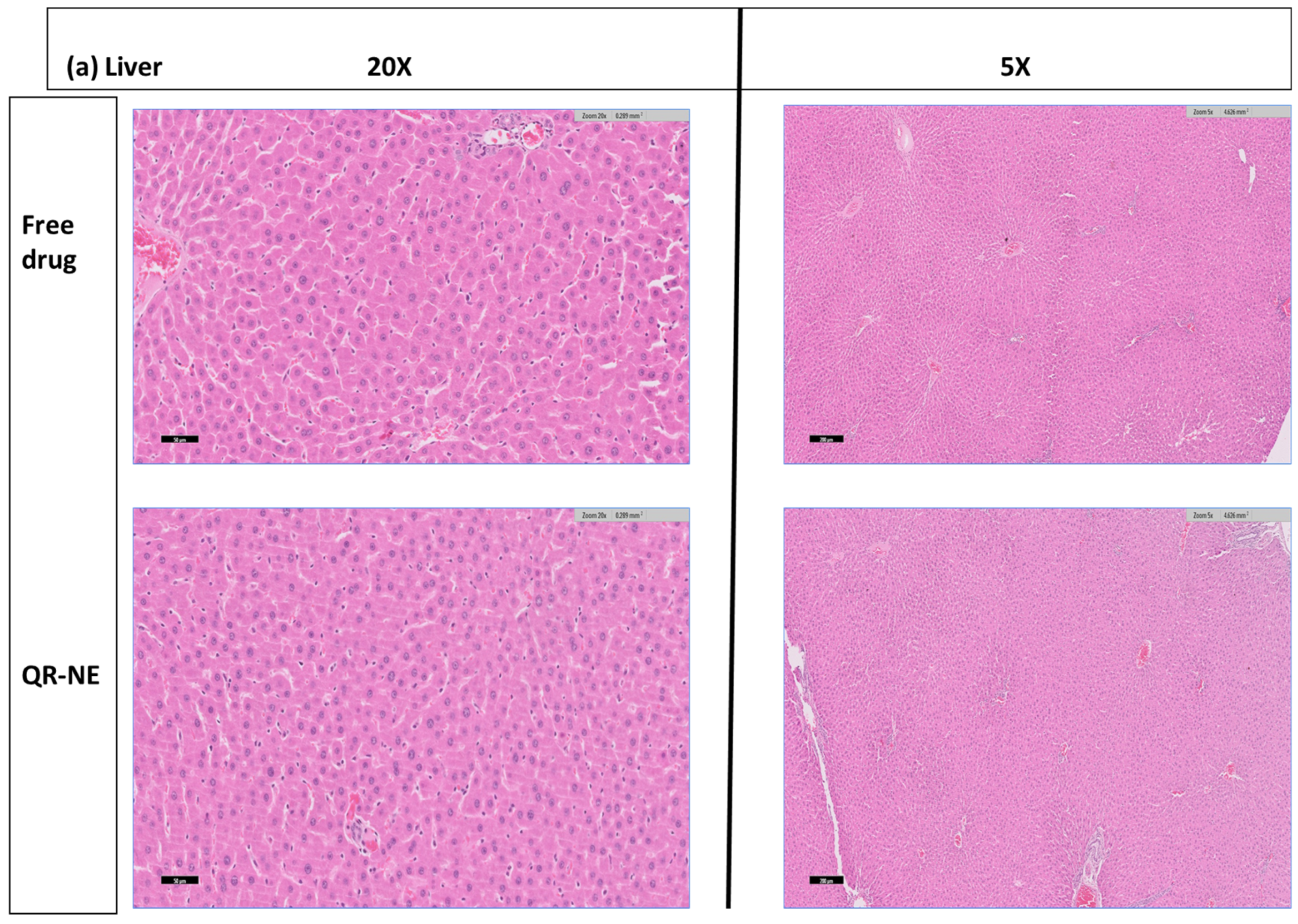
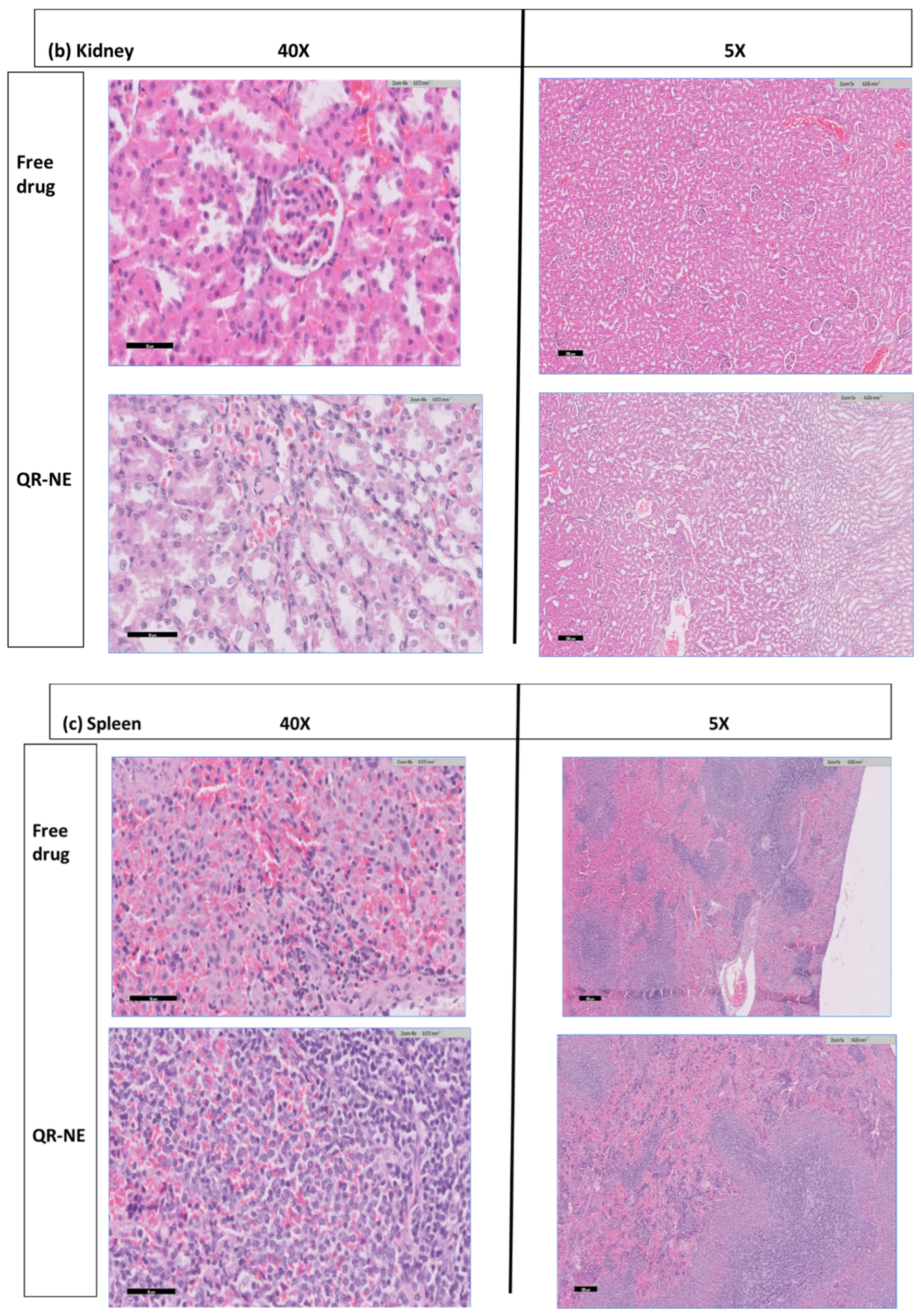
3.6. QR-NE Safety Evaluation on the Animal Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, S.A.; Flanders, W.D.; Ward, K.C.; Lin, C.C.; Jemal, A.; Goding Sauer, A.; Doubeni, C.A.; Goodman, M. Racial and ethnic disparities in interval colorectal cancer incidence: A population-based cohort study. Ann. Intern. Med. 2017, 166, 857–866. [Google Scholar] [CrossRef] [PubMed]
- US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report; US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute: Atlanta, GA, USA, 2017.
- Bhise, K.; Sau, S.; Alsaab, H.; Kashaw, S.K.; Tekade, R.K.; Iyer, A.K. Nanomedicine for cancer diagnosis and therapy: Advancement, success and structure–activity relationship. Ther. Deliv. 2017, 8, 1003–1018. [Google Scholar] [CrossRef]
- Hussain, Z.; Arooj, M.; Malik, A.; Hussain, F.; Safdar, H.; Khan, S.; Sohail, M.; Pandey, M.; Choudhury, H.; Ei Thu, H. Nanomedicines as emerging platform for simultaneous delivery of cancer therapeutics: New developments in overcoming drug resistance and optimizing anticancer efficacy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1015–1024. [Google Scholar] [CrossRef]
- Sau, S.; Alsaab, H.O.; Bhise, K.; Alzhrani, R.; Nabil, G.; Iyer, A.K. Multifunctional nanoparticles for cancer immunotherapy: A groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control. Release 2018, 274, 24–34. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Sivaram, A.J.; Mony, U.; Jayakumar, R. Combinatorial nanomedicines for colon cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 151–159. [Google Scholar] [CrossRef]
- Pellino, G.; Gallo, G.; Pallante, P.; Capasso, R.; De Stefano, A.; Maretto, I.; Malapelle, U.; Qiu, S.; Nikolaou, S.; Barina, A.; et al. Noninvasive biomarkers of colorectal cancer: Role in diagnosis and personalised treatment perspectives. Gastroenterol. Res. Pract. 2018, 2018, 2397863. [Google Scholar] [CrossRef]
- Bennie, L.A.; McCarthy, H.O.; Coulter, J.A. Enhanced nanoparticle delivery exploiting tumour-responsive formulations. Cancer Nanotechnol. 2018, 9, 10. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Liu, Z.; Hirvonen, J.; Santos, H.A. Bridging the knowledge of different worlds to understand the big picture of cancer nanomedicines. Adv. Healthc. Mater. 2018, 7, 1700432. [Google Scholar] [CrossRef] [PubMed]
- Subhadarshini, S.; Merchant, N.; Raju, G.S.R. Nanomaterials: Diagnosis and Therapeutic Properties. In Role of Tyrosine Kinases in Gastrointestinal Malignancies; Springer: Berlin/Heidelberg, Germany, 2018; pp. 235–241. [Google Scholar]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Gué, E.; Since, M.; Ropars, S.; Herbinet, R.; Le Pluart, L.; Malzert-Fréon, A. Evaluation of the versatile character of a nanoemulsion formulation. Int. J. Pharm. 2016, 498, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Gao, Z.; Huang, W.; Jin, M.; Wang, Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 2015, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Das, D.; Mishra, V.K.; Kashaw, V.; Kashaw, S.K. Nanoemulsion: A novel eon in cancer chemotherapy. Mini Rev. Med. Chem. 2017, 17, 1778–1792. [Google Scholar] [CrossRef]
- Park, J.B. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochem. Biophys. Res. Commun. 1999, 260, 568–574. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA A Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef]
- Bu, H.; He, X.; Zhang, Z.; Yin, Q.; Yu, H.; Li, Y. A TPGS-incorporating nanoemulsion of paclitaxel circumvents drug resistance in breast cancer. Int. J. Pharm. 2014, 471, 206–213. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin–pharmacodynamic and antioxidant studies. Drug Deliv. 2015, 22, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef] [PubMed]
- El-Enin, H.A.; Al-Shanbari, A.H. Nanostructured liquid crystalline formulation as a remarkable new drug delivery system of anti-epileptic drugs for treating children patients. Saudi Pharm. J. 2018, 26, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Riquelme, N.; Zúñiga, R.; Arancibia, C. Physical stability of nanoemulsions with emulsifier mixtures: Replacement of tween 80 with quillaja saponin. LWT 2019, 111, 760–766. [Google Scholar] [CrossRef]
- Ha, J.-W.; Yang, S.-M. Rheological responses of oil-in-oil emulsions in an electric field. J. Rheol. 2000, 44, 235–256. [Google Scholar] [CrossRef]
- Panapisal, V. Effects of surfactant mixture ratio and concentration on nanoemulsion physical stability. Thai J. Pharm. Sci. 2016, 40, 45–48. [Google Scholar]
- Pangeni, R.; Choi, S.W.; Jeon, O.-C.; Byun, Y.; Park, J.W. Multiple nanoemulsion system for an oral combinational delivery of oxaliplatin and 5-fluorouracil: Preparation and in vivo evaluation. Int. J. Nanomed. 2016, 11, 6379–6399. [Google Scholar] [CrossRef]
- Galaup, A.; Opolon, P.; Bouquet, C.; Li, H.; Opolon, D.; Bissery, M.-C.; Tursz, T.; Perricaudet, M.; Griscelli, F. Combined effects of docetaxel and angiostatin gene therapy in prostate tumor model. Mol. Ther. 2003, 7, 731–740. [Google Scholar] [CrossRef]
- Nesamony, J.; Kalra, A.; Majrad, M.S.; Boddu, S.H.S.; Jung, R.; Williams, F.E.; Schnapp, A.M.; Nauli, S.M.; Kalinoski, A.L. Development and characterization of nanostructured mists with potential for actively targeting poorly water-soluble compounds into the lungs. Pharm. Res. 2013, 30, 2625–2639. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Bei, D.; Marszalek, J.; Youan, B.-B.C. Formulation of dacarbazine-loaded cubosomes—part I: Influence of formulation variables. Aaps PharmScitech 2009, 10, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Mehmood, Z.; Baig, A.; Khalid, N. Formulation and characterization of food grade O/W nanoemulsions encapsulating quercetin and curcumin: Insights on enhancing solubility characteristics. Food Bioprod. Process. 2020, 123, 304–311. [Google Scholar] [CrossRef]
- Pillay, V.; Fassihi, R. In vitro release modulation from crosslinked pellets for site-specific drug delivery to the gastrointestinal tract: I. Comparison of pH-responsive drug release and associated kinetics. J. Control. Release 1999, 59, 229–242. [Google Scholar] [CrossRef]
- Jadhav, C.; Kate, V.; Payghan, S.A. Investigation of effect of non-ionic surfactant on preparation of griseofulvin non-aqueous nanoemulsion. J. Nanostructure Chem. 2015, 5, 107–113. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.M.; Cheriyan, V.T.; Polin, L.A.; Vaishampayan, U.; Rishi, A.K.; Iyer, A.K. Tumor hypoxia directed multimodal nanotherapy for overcoming drug resistance in renal cell carcinoma and reprogramming macrophages. Biomaterials 2018, 183, 280–294. [Google Scholar] [CrossRef]
- Cheriyan, V.T.; Alsaab, H.O.; Sekhar, S.; Stieber, C.; Kesharwani, P.; Sau, S.; Muthu, M.; Polin, L.A.; Levi, E.; Iyer, A.K.; et al. A CARP-1 functional mimetic loaded vitamin E-TPGS micellar nano-formulation for inhibition of renal cell carcinoma. Oncotarget 2017, 8, 104928–104945. [Google Scholar] [CrossRef]
- Buyukozturk, F.; Benneyan, J.C.; Carrier, R.L. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J. Control. Release 2010, 142, 22–30. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; A Fahmy, U.; Alfaleh, M.A.; Asfour, H.Z. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in A549 lung cancer cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
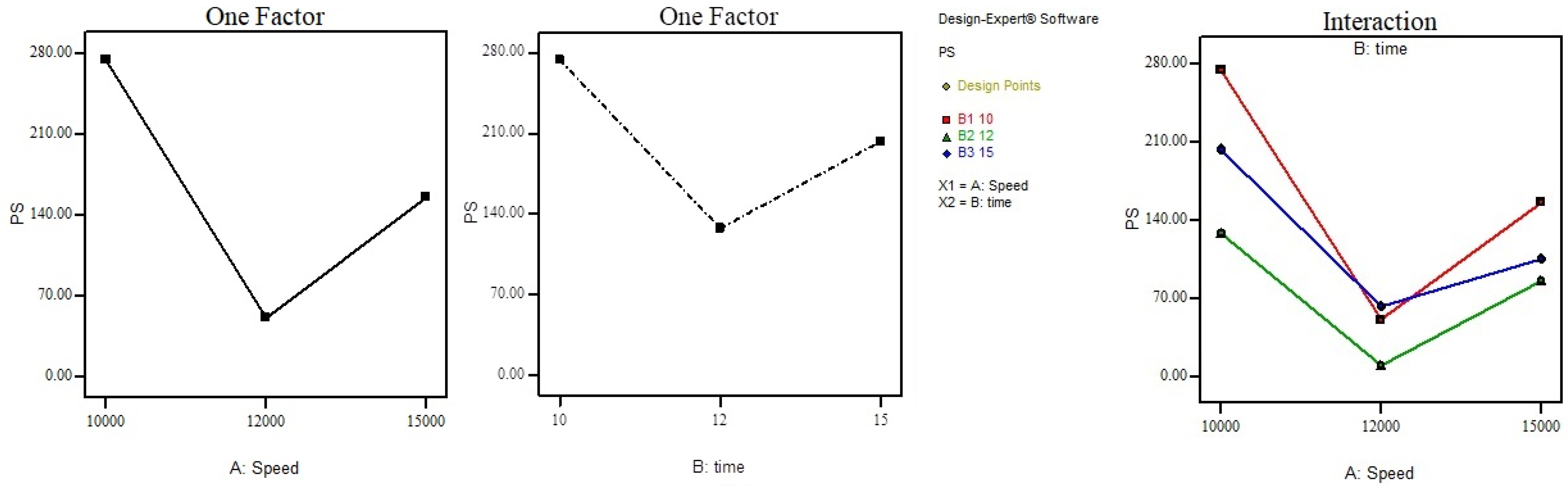

| PN | Speed (rpm) | Time (Min) | EE% | DL | PS (nm) |
|---|---|---|---|---|---|
| 1 | 10,000 | 10 | 87.3 ± 2.5 | 46.87% | 273.9 ± 2.22 |
| 2 | 10,000 | 12 | 91.2 ± 2.12 | 48.97% | 127.7 ± 1.14 |
| 3 | 10,000 | 15 | 93.2 ± 1.87 | 51.84% | 203.2 ± 1.98 |
| 4 | 12,000 | 10 | 83.5 ± 1.09 | 53.65% | 50.59 ± 0.56 |
| 5 | 12,000 | 12 | 85.5 ± 1.11 | 50.98% | 9.522 ± 0.11 |
| 6 | 12,000 | 15 | 88.9 ± 2.01 | 52.115 | 62.37 ± 0.87 |
| 7 | 15,000 | 10 | 79.1 ± 1.76 | 47.87% | 155.3 ± 1.53 |
| 8 | 15,000 | 12 | 78.8 ± 1.65 | 49.97% | 85.6 ± 0.16 |
| 9 | 15,000 | 15 | 77.1 ± 1.23 | 50.84% | 105.2 ± 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enin, H.A.A.; Alquthami, A.F.; Alwagdani, A.M.; Yousef, L.M.; Albuqami, M.S.; Alharthi, M.A.; Alsaab, H.O. Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids Interfaces 2022, 6, 49. https://doi.org/10.3390/colloids6030049
Enin HAA, Alquthami AF, Alwagdani AM, Yousef LM, Albuqami MS, Alharthi MA, Alsaab HO. Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids and Interfaces. 2022; 6(3):49. https://doi.org/10.3390/colloids6030049
Chicago/Turabian StyleEnin, Hadel A. Abo, Ahad Fahd Alquthami, Ahad Mohammed Alwagdani, Lujain Mahmoud Yousef, Majd Safar Albuqami, Miad Abdulaziz Alharthi, and Hashem O. Alsaab. 2022. "Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition" Colloids and Interfaces 6, no. 3: 49. https://doi.org/10.3390/colloids6030049
APA StyleEnin, H. A. A., Alquthami, A. F., Alwagdani, A. M., Yousef, L. M., Albuqami, M. S., Alharthi, M. A., & Alsaab, H. O. (2022). Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids and Interfaces, 6(3), 49. https://doi.org/10.3390/colloids6030049









