The Effect of Nonionic Surfactants on the Kinetics of Methane Hydrate Formation in Multiphase System
Abstract
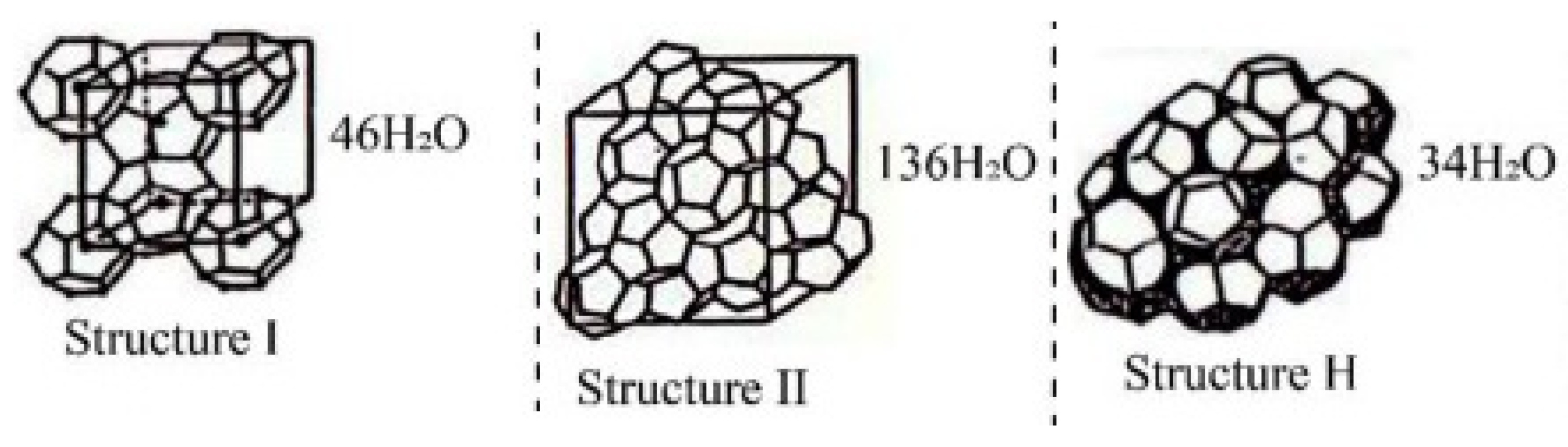
:1. Introduction
2. Materials and Methods
2.1. Material
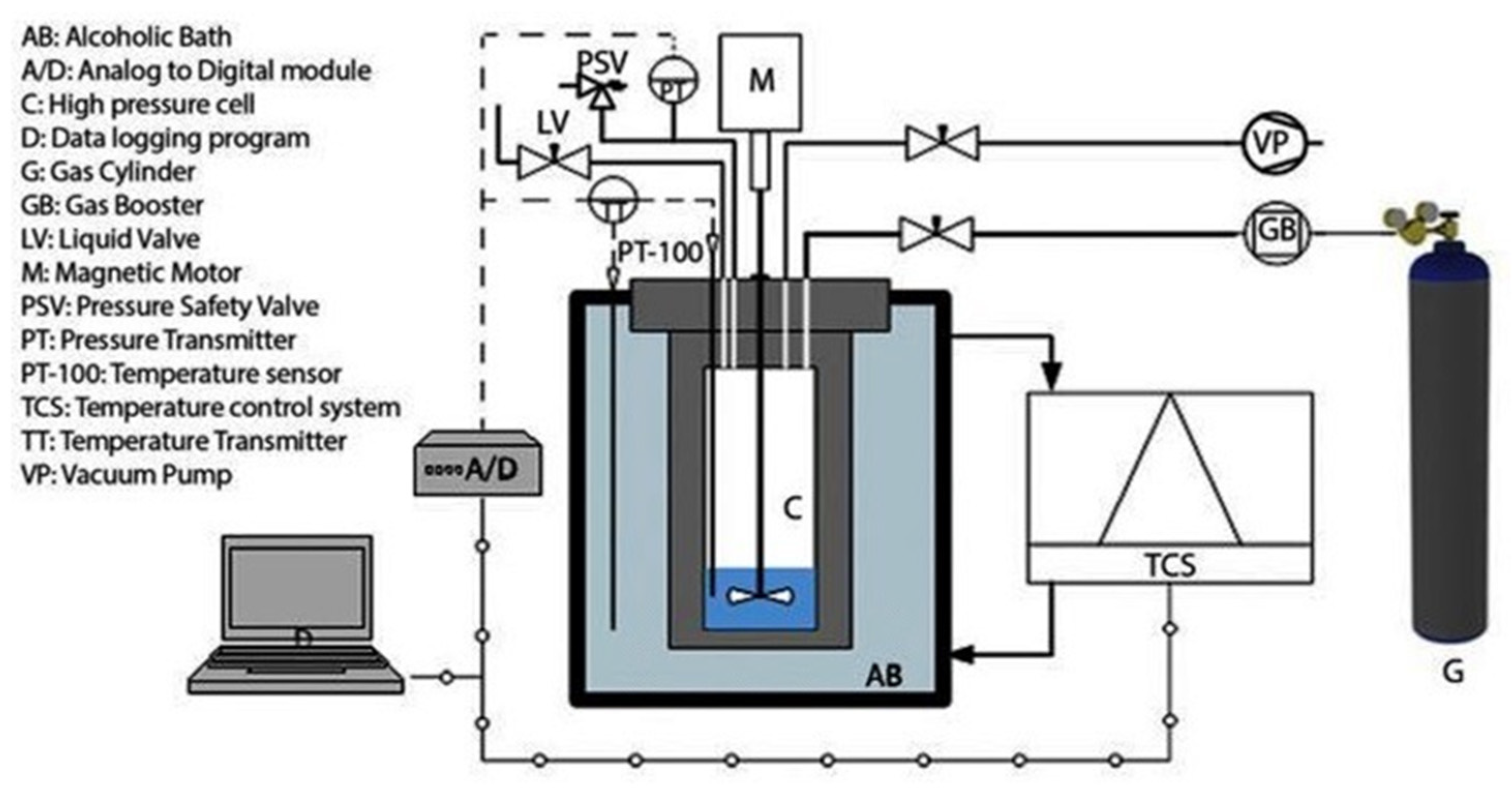
2.2. Experimental Apparatus and Procedure
2.2.1. Methane Hydrate Phase Equilibrium Measurement
2.2.2. Kinetics of Methane Hydrate Formation
2.3. Calculation of the Kinetic Parameters
2.3.1. Induction Time
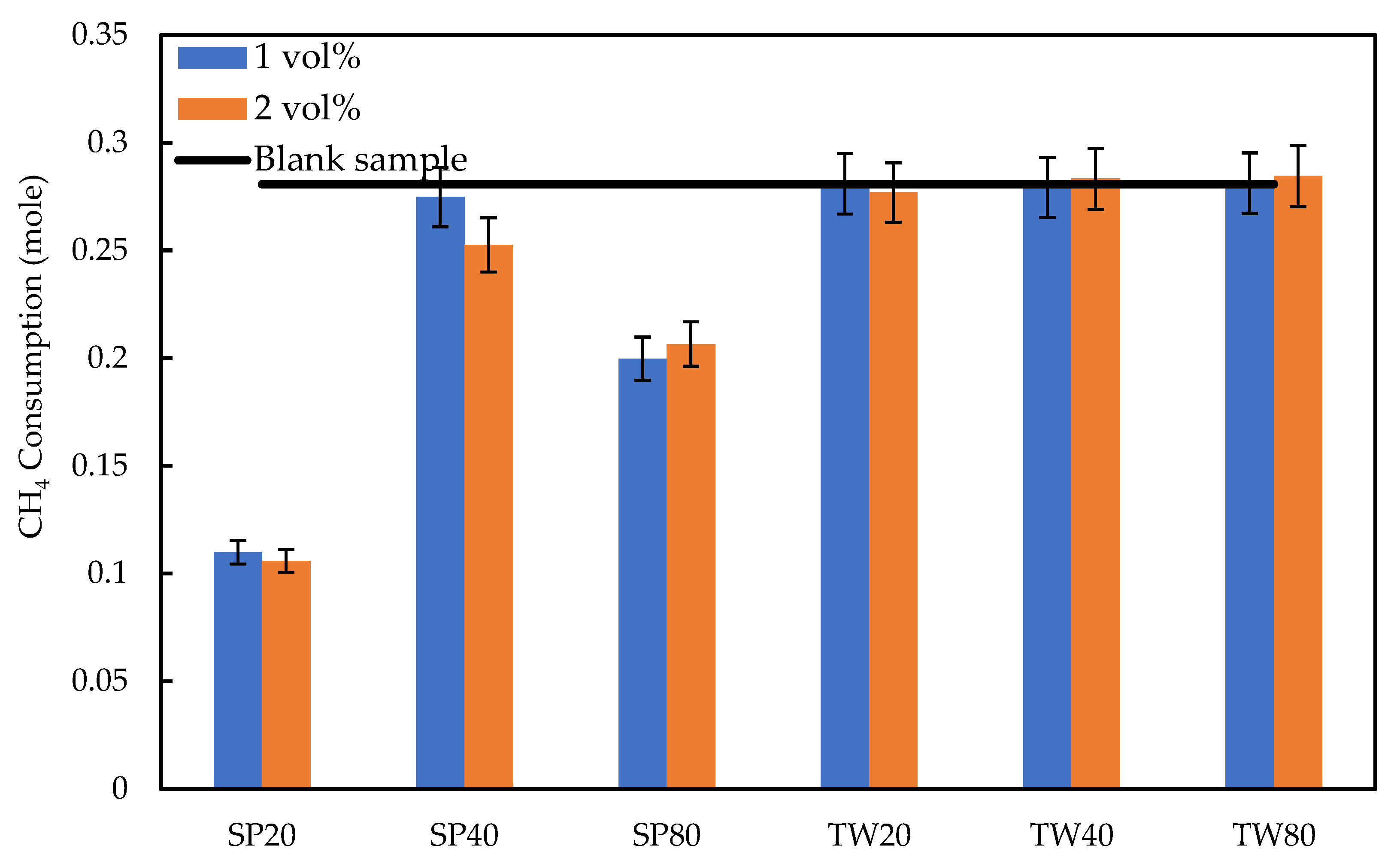
2.3.2. Amount of CH4 Consumption and Gas Uptake
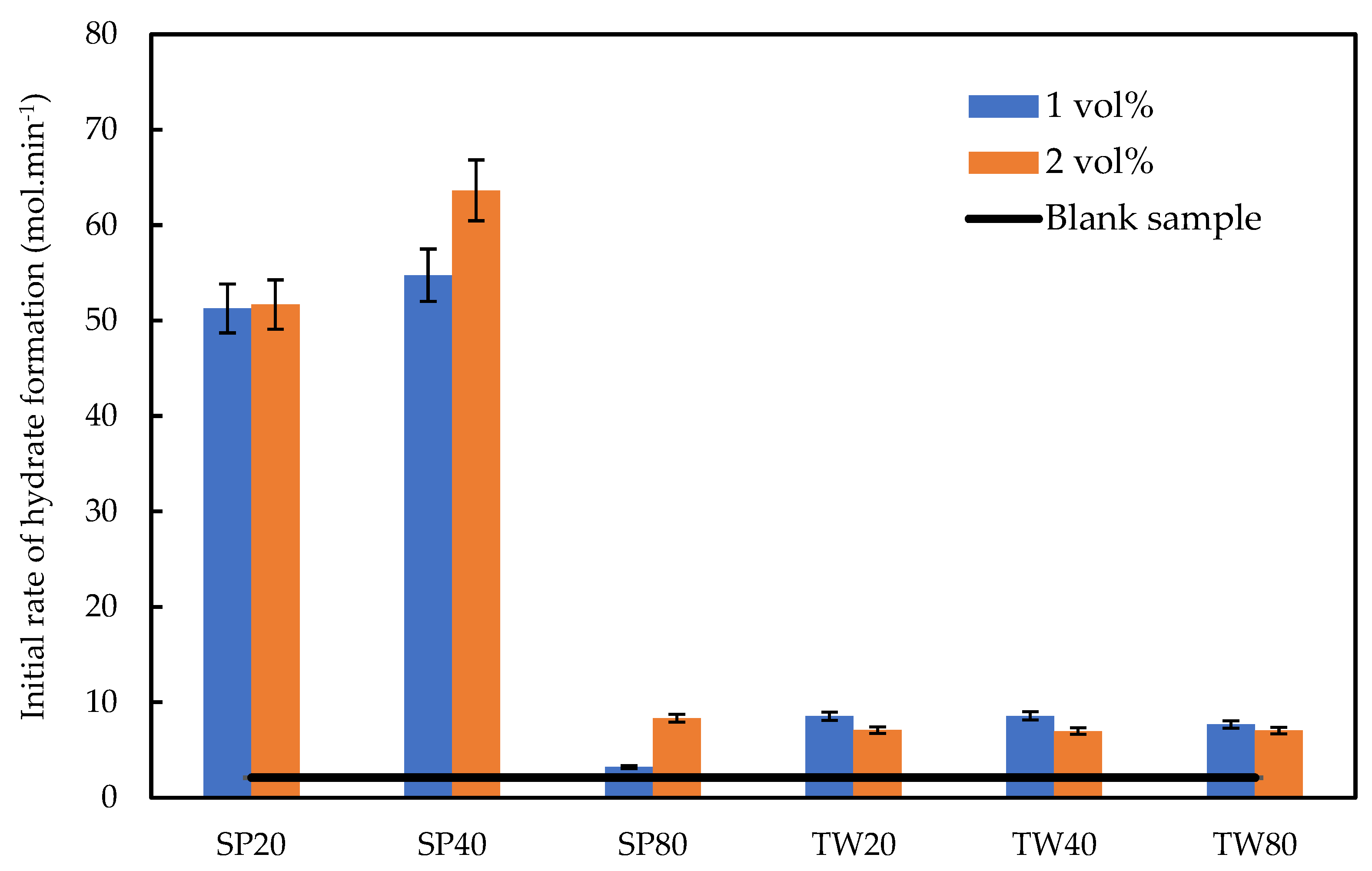
2.3.3. Initial Rate of the CH4 Consumption
2.3.4. Water to Hydrate Conversion
2.4. Kinetics Models Theories
Classical Nucleation Theory (CNT) for Induction Time Prediction
2.5. Prediction of Rate of Hydrate formation
3. Results
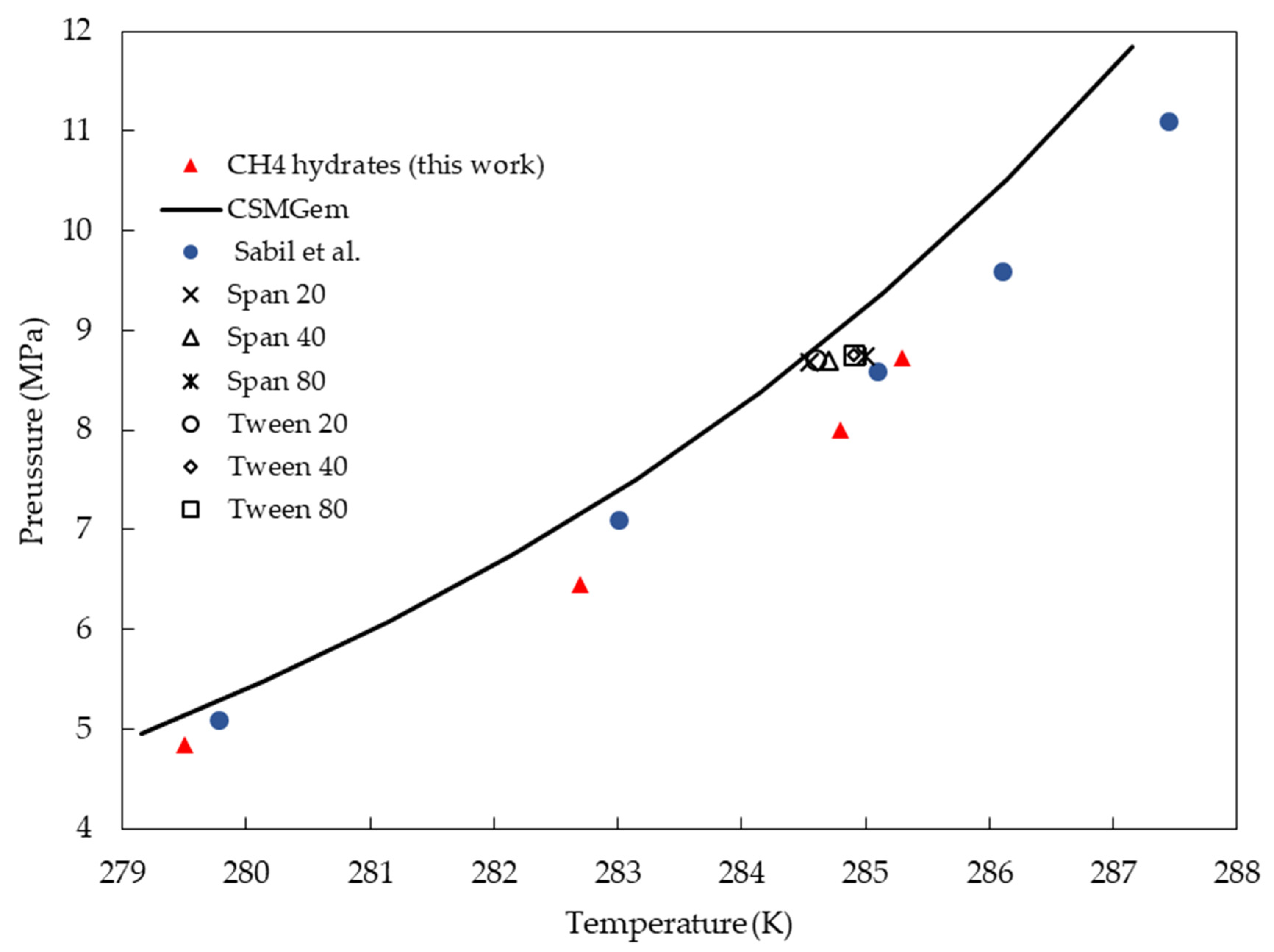
3.1. Hydrate Phase Equilibrium
3.2. Kinetics of the Methane Hydrate Formation
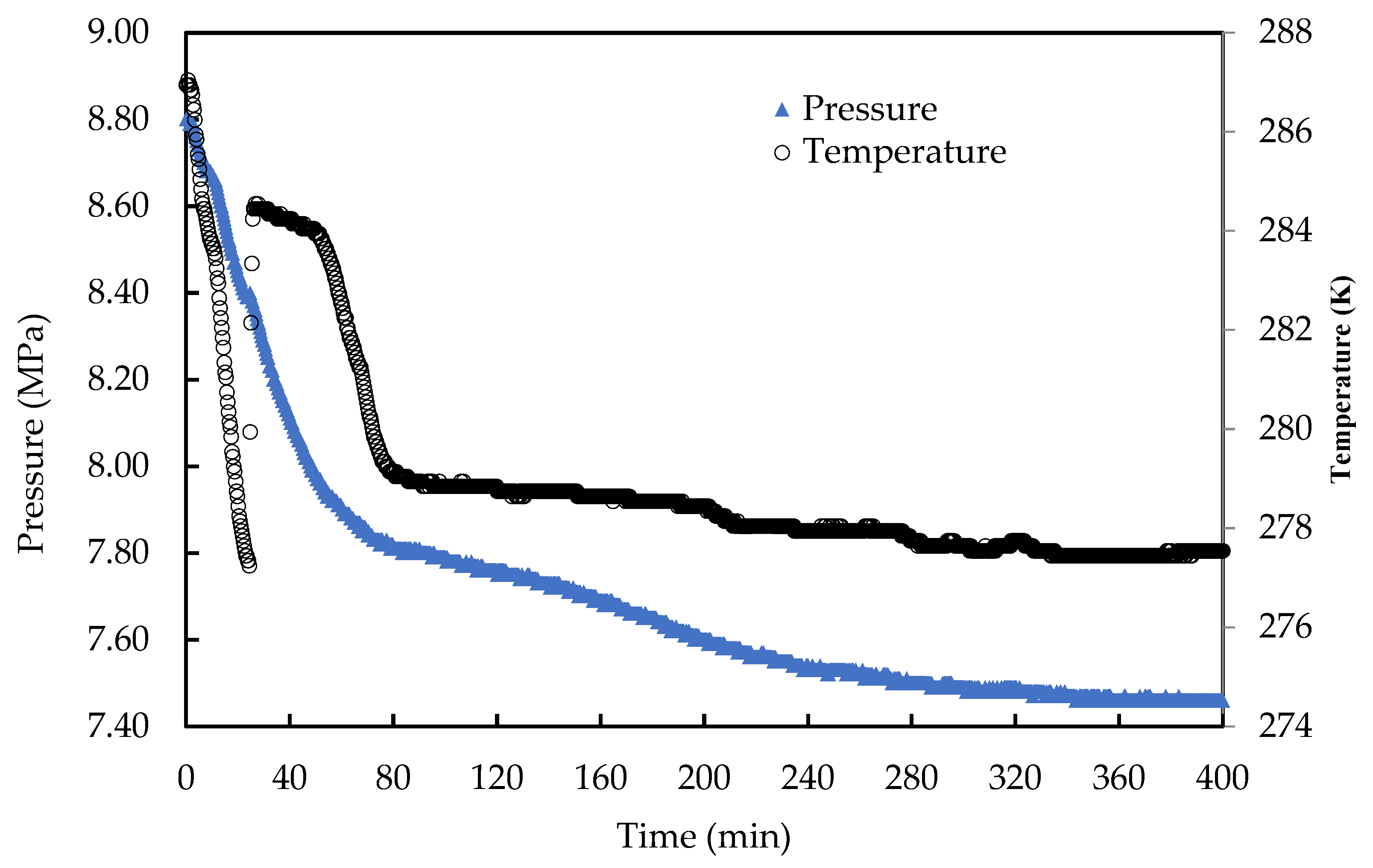
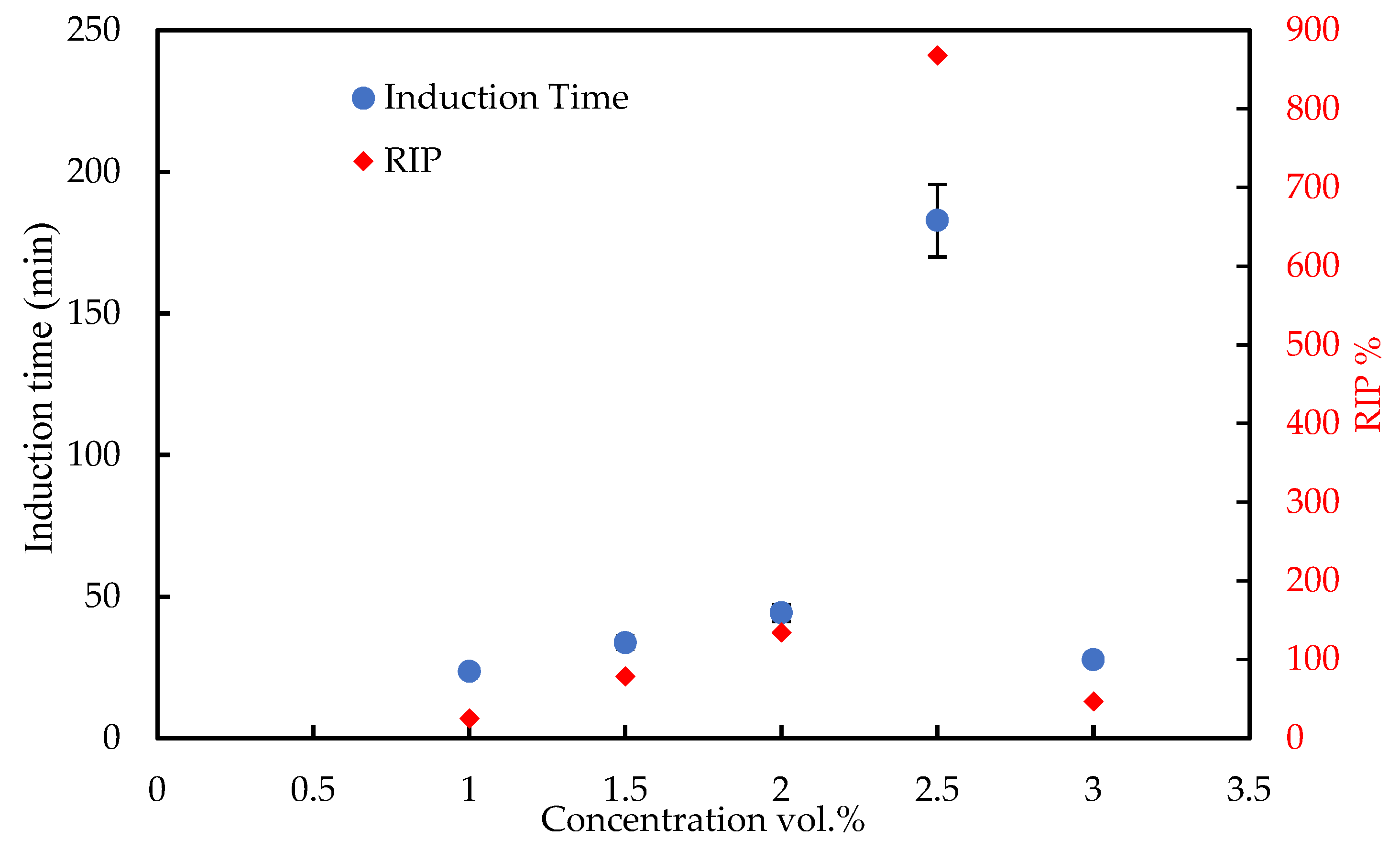
3.2.1. Induction Time Measurement
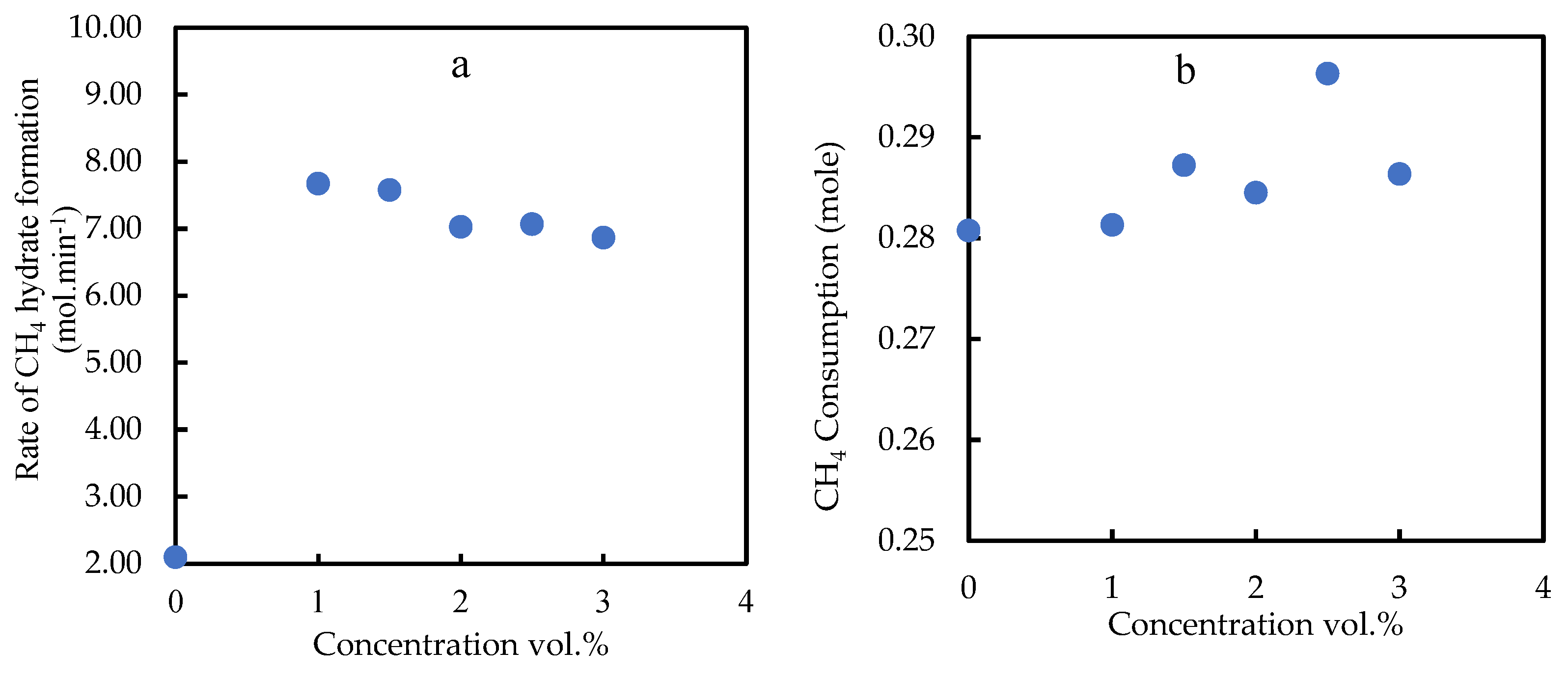
3.2.2. Initial Rate of the Gas Hydrate Consumption
3.2.3. Degree of Gas Consumption and Water to Hydrate Conversion
3.2.4. Further Evaluation of Tween-80 as KHI
3.3. Comparison of Tween 80 with PVP
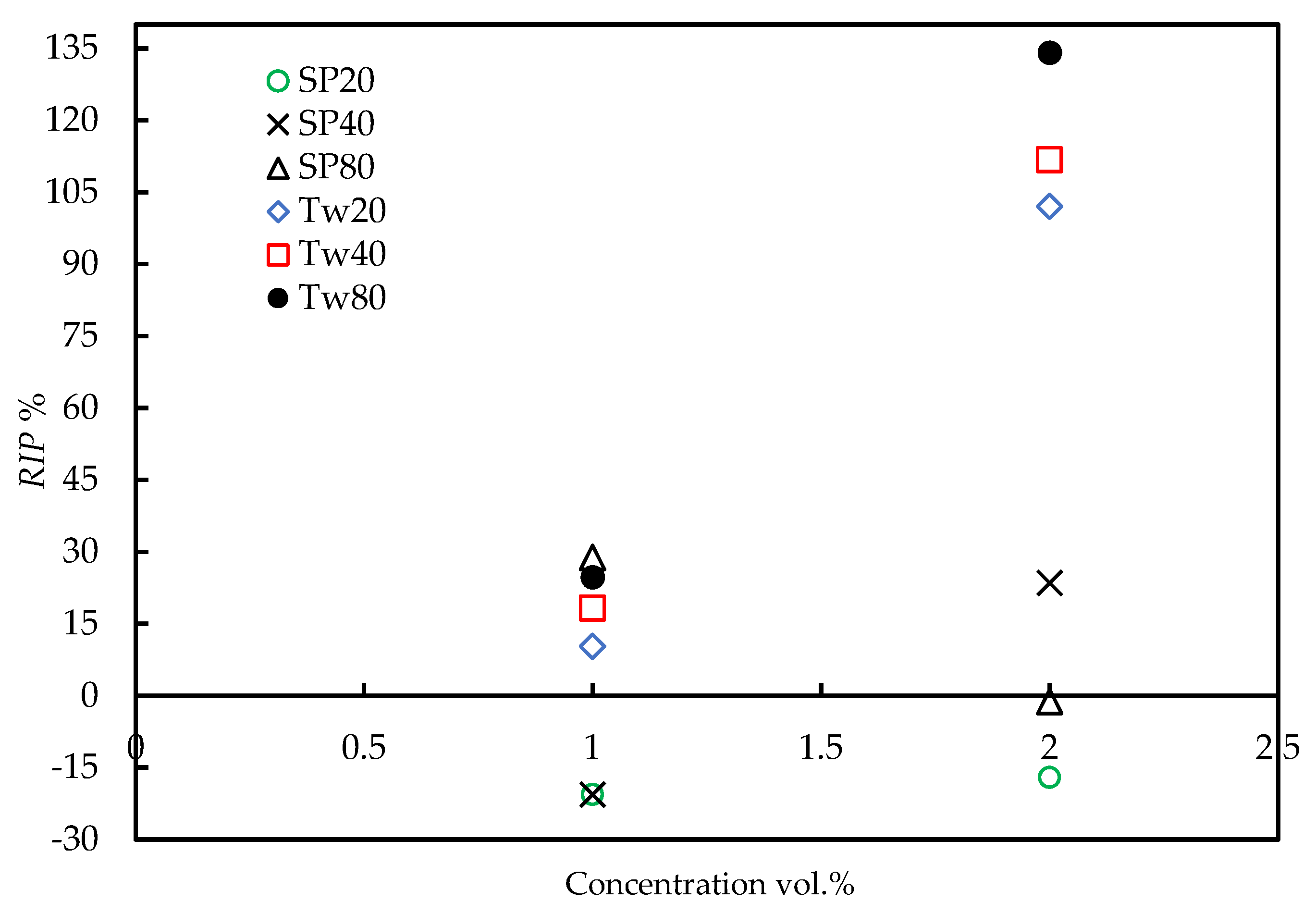
3.4. Induction Time Prediction Using CNT
3.5. Modeling the Rate of CH4 Hydrate Formation in the Presence of Tween-80
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-30749-3. [Google Scholar]
- Nashed, O.; Lal, B.; Shariff, A.M.; Sabil, K.M. Gas Hydrate Promoters; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Bavoh, C.B.; Nashed, O.; Rehman, A.N.; Othaman, N.A.A.B.; Lal, B.; Sabil, K.M. Ionic liquids as gas hydrate thermodynamic inhibitors. Ind. Eng. Chem. Res. 2021, 60, 15835–15873. [Google Scholar] [CrossRef]
- Mohamad Sabil, K.; Nashed, O.; Lal, B.; Foo, K.S. Formation Kinetics and Self-Preservation of CO2 Hydrates in the Presence of Carboxylated Multiwall Carbon Nanotubes. In Proceedings of the International Petroleum Technology Conference, Virtual, 16 March 2021. [Google Scholar]
- Takeya, S.; Mimachi, H.; Murayama, T. Methane storage in water frameworks: Self-preservation of methane hydrate pellets formed from NaCl solutions. Appl. Energy 2018, 230, 86–93. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Lal, B. Seawater and produced water treatment via gas hydrate: Review. J. Environ. Chem. Eng. 2021, 9, 105053. [Google Scholar] [CrossRef]
- Bharathi, A.; Nashed, O.; Lal, B.; Foo, K.S. Experimental and modeling studies on enhancing the thermodynamic hydrate inhibition performance of monoethylene glycol via synergistic green material. Sci. Rep. 2021, 11, 2396. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Foo, K.S.; Shariff, A.M.; Kassim, Z.; Othman, N.A.B.; Lal, B.; Ahmed, I.; Rahman, M.A.; Gomari, S.R. Kinetic behavior of quaternary ammonium hydroxides in mixed methane and carbon dioxide hydrates. Molecules 2021, 26, 275. [Google Scholar] [CrossRef]
- Tariq, M.; Rooney, D.; Othman, E.; Aparicio, S.; Atilhan, M.; Khraisheh, M. Gas hydrate inhibition: A review of the role of ionic liquids. Ind. Eng. Chem. Res. 2014, 53, 17855–17868. [Google Scholar] [CrossRef]
- Qasim, A.; Lal, B.; Shariff, A.M.; Ismail, M.C. Role of Ionic Liquid-Based Multipurpose Gas Hydrate and Corrosion Inhibitors in Gas Transmission Pipeline. In Nanotechnology-Based Industrial Applications of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2020; pp. 221–244. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and applications of gas hydrates. Ann. Rev. Chem. Biomolec. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Investigation of functionalized carbon nanotubes’ performance on carbon dioxide hydrate formation. Energy 2019, 174, 602–610. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Ganji, H.; Manteghian, M.; Sadaghiani zadeh, K.; Omidkhah, M.R.; Rahimi Mofrad, H. Effect of different surfactants on methane hydrate formation rate, stability and storage capacity. Fuel 2007, 86, 434–441. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Z.; Zhang, Z.; Ma, G.; Zhang, L.; Huang, Y. Natural gas hydrate formation dynamics in a diesel water-in-oil emulsion system. Pet. Sci. Technol. 2018, 36, 1649–1656. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, R.; Ma, R.; Guo, K.; Fan, S. Effect of surfactants and liquid hydrocarbons on gas hydrate formation rate and storage capacity. Int. J. Energy Res. 2003, 27, 747–756. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Sun, D.-l. Effect of surfactant Tween on induction time of gas hydrate formation. J. China Univ. Min. Technol. 2008, 18, 18–21. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Nashed, O.; Lal, B.; Mellon, N.B. Impacts of ammonium based ionic liquids alkyl chain on thermodynamic hydrate inhibition for carbon dioxide rich binary gas. J. Mol. Liq. 2018, 261, 283–290. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Ismail, L.; Japper-Jaafar, A.; Lal, B. Mean induction time and isothermal kinetic analysis of methane hydrate formation in water and imidazolium based ionic liquid solutions. J. Chem. Thermodyn. 2018, 117, 147–154. [Google Scholar] [CrossRef]
- Englezos, P.; Dholabhai, P.D.; Kalogerakis, N. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Sear, R.P. Quantitative studies of crystal nucleation at constant supersaturation: Experimental data and models. CrystEngComm 2014, 16, 6506–6522. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Md Yuha, Y.B.; Tay, W.H.; Ofei, T.N.; Lal, B.; Mukhtar, H. Experimental and modelling of the impact of quaternary ammonium salts/ionic liquid on the rheological and hydrate inhibition properties of xanthan gum water-based muds for drilling gas hydrate-bearing rocks. J. Pet. Sci. Eng. 2019, 183, 106468. [Google Scholar] [CrossRef]
- Malegaonkar, M.B.; Dholabhai, P.D.; Bishnoi, P.R. Kinetics of carbon dioxide and methane hydrate formation. Can. J. Chem. Eng. 1997, 75, 1090–1099. [Google Scholar] [CrossRef]
- Nashed, O.; Lal, B.; Partoon, B.; Sabil, K.M.; Hamed, Y. Kinematic Study of Methane Hydrate Formation and Self-Preservation in the Presence of Functionalized Carbon Nanotubes. Energy Fuels 2019, 33, 7684–7695. [Google Scholar] [CrossRef]
- Karaaslan, U.; Parlaktuna, M. Surfactants as hydrate promoters? Energy Fuels 2000, 14, 1103–1107. [Google Scholar] [CrossRef]
- Sabil, K.M.; Nashed, O.; Lal, B.; Ismail, L.; Japper-Jaafar, A. Experimental investigation on the dissociation conditions of methane hydrate in the presence of imidazolium-based ionic liquids. J. Chem. Thermodyn. 2015, 84, 7–13. [Google Scholar] [CrossRef]
- Partoon, B.; Sahith, S.J.K.; Lal, B.; Maulud, A.S. Bin Gas Hydrate Models. In Green Energy and Technology; Springer Verlag: Berlin/Heidelberg, Germany, 2020; pp. 67–85. [Google Scholar]
- Parreidt, T.S.; Schott, M.; Schmid, M.; Müller, K. Effect of Presence and Concentration of Plasticizers, Vegetable Oils, and Surfactants on the Properties of Sodium-Alginate-Based Edible Coatings. Int. J. Mol. Sci. 2018, 19, 742. [Google Scholar] [CrossRef]
- Qin, H.B.; Sun, Z.F.; Wang, X.Q.; Yang, J.L.; Sun, C.Y.; Liu, B.; Yang, L.Y.; Chen, G.J. Synthesis and Evaluation of Two New Kinetic Hydrate Inhibitors. Energy Fuels 2015, 29, 7135–7141. [Google Scholar] [CrossRef]
- Yin, Z.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
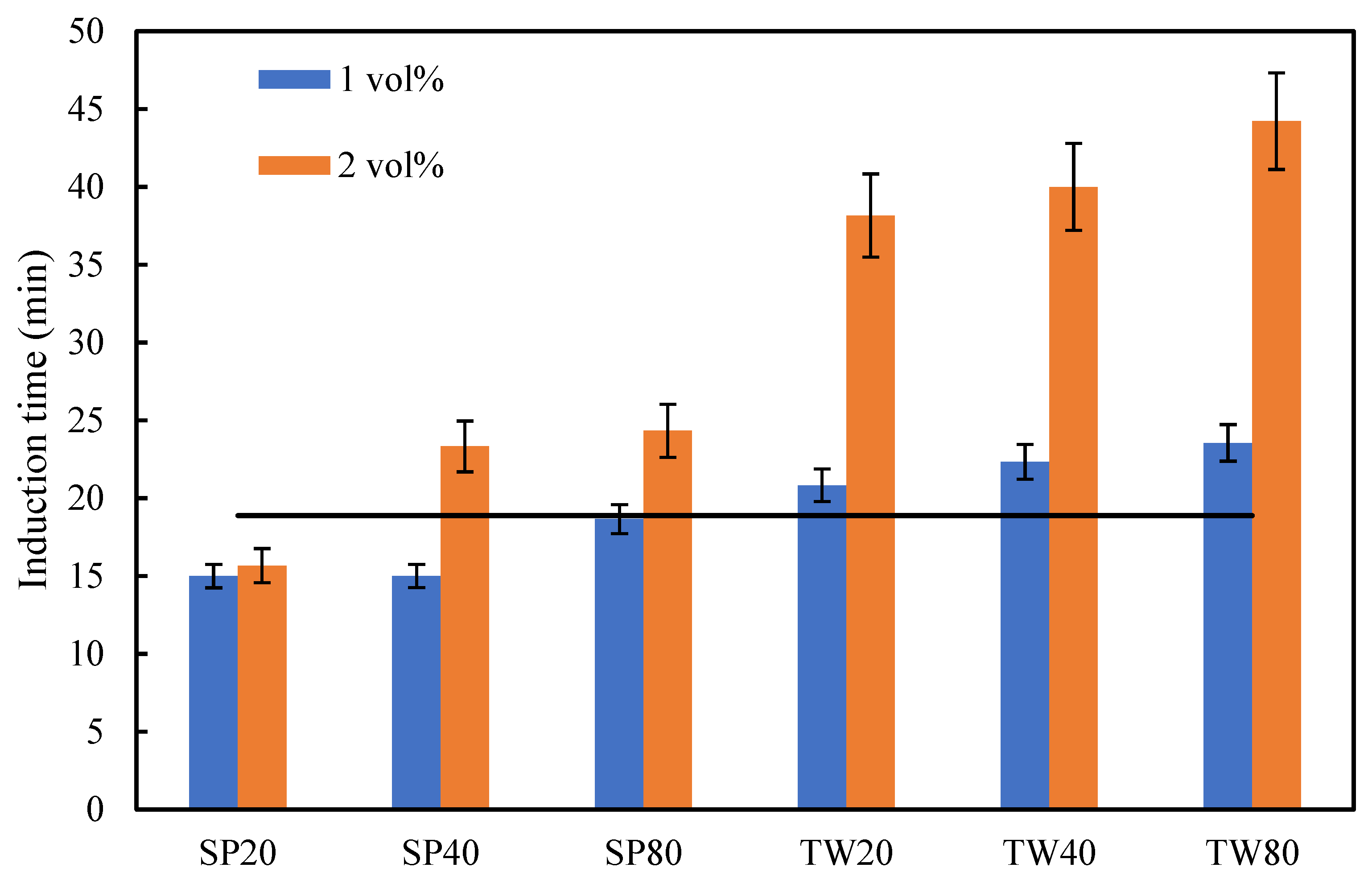
| Chemical | Abbreviation | HLB | Purity | Supplier |
|---|---|---|---|---|
| Sorbitan monolaurate | Span-20 | 8.6 | >95% | Sigma Aldrich |
| Sorbitan monopalmitate | Span-40 | 6.7 | >95% | |
| Sorbitane monooleate | Span-80 | 4.3 | >95% | |
| Polyoxyethylenesorbitan monolaurate | Tween-20 | 16.7 | 99% | |
| Polyoxyethylene sorbitan monopalmitate | Tween-40 | 15.6 | 99.95% | |
| Polyoxyethylenesorbitan monooleate | Tween-80 | 15.0 | ||
| Methane | CH4 | - | 99.95% | Linde Malaysia Sdn Bhd |
| Base oil | MG3 | NA | NA | Petronas Sdn Bhd |
| Sample | Concentration (vol%) | Water to Hydrate Conversion (mol%) |
|---|---|---|
| Blank | 0 | 0.606 |
| SP20 | 1 | 0.240 |
| 2 | 0.234 | |
| SP40 | 1 | 0.600 |
| 2 | 0.602 | |
| SP80 | 1 | 0.436 |
| 2 | 0.456 | |
| TW20 | 1 | 0.614 |
| 2 | 0.611 | |
| TW40 | 1 | 0.610 |
| 2 | 0.625 | |
| TW80 | 1 | 0.614 |
| 2 | 0.628 |
| Concentration vol.% | Tween-80 | PVP | ||||
|---|---|---|---|---|---|---|
| Induction Time (min) | r (mol·min−1) | Gas Consumption (mol) | Induction Time (min) | r (mol·min−1) | Gas Consumption (mol) | |
| 1.0 | 23.6 | 0.00767 | 0.2813 | 48.33 | 0.00192 | 0.0780 |
| 2.0 | 44.2 | 0.00703 | 0.2845 | 60.495 | 0.00078 | 0.1250 |
| 2.5 | 182.8 | 0.00707 | 0.2963 | 57.3 | 0.00193 | 0.1248 |
| Temperature | Induction Time (min) | APE% | |
|---|---|---|---|
| Experimental | Predicted | ||
| 274.15 | 32.17 | 34.00 | 5.70 |
| 275.15 | 124.00 | 127.33 | 2.69 |
| 276.15 | 164.83 | 168.50 | 2.22 |
| 277.15 | 182.83 | 189.00 | 3.37 |
| rpre (mol·min−1) | rexp (mol·min−1) | APE% |
|---|---|---|
| 0.00999 | 0.01000 | 1.04 |
| 0.00896 | 0.00907 | 1.19 |
| 0.00797 | 0.00760 | 4.93 |
| 0.00698 | 0.00707 | 1.29 |
| 0.00764 | 0.00767 | 0.38 |
| 0.00749 | 0.00758 | 1.22 |
| 0.00712 | 0.00703 | 1.30 |
| 0.00675 | 0.00687 | 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foo, K.S.; Nashed, O.; Lal, B.; Bavoh, C.B.; Shariff, A.M.; Tewari, R.D. The Effect of Nonionic Surfactants on the Kinetics of Methane Hydrate Formation in Multiphase System. Colloids Interfaces 2022, 6, 48. https://doi.org/10.3390/colloids6030048
Foo KS, Nashed O, Lal B, Bavoh CB, Shariff AM, Tewari RD. The Effect of Nonionic Surfactants on the Kinetics of Methane Hydrate Formation in Multiphase System. Colloids and Interfaces. 2022; 6(3):48. https://doi.org/10.3390/colloids6030048
Chicago/Turabian StyleFoo, Khor Siak, Omar Nashed, Bhajan Lal, Cornelius Borecho Bavoh, Azmi Mohd Shariff, and Raj Deo Tewari. 2022. "The Effect of Nonionic Surfactants on the Kinetics of Methane Hydrate Formation in Multiphase System" Colloids and Interfaces 6, no. 3: 48. https://doi.org/10.3390/colloids6030048
APA StyleFoo, K. S., Nashed, O., Lal, B., Bavoh, C. B., Shariff, A. M., & Tewari, R. D. (2022). The Effect of Nonionic Surfactants on the Kinetics of Methane Hydrate Formation in Multiphase System. Colloids and Interfaces, 6(3), 48. https://doi.org/10.3390/colloids6030048






