Abstract
Regenerated fibers can be prepared from a cooled solution of renneted casein micelles in a wet spinning process. For better handling and stability of the fiber, plasticizers, network modifiers or cross-linkers are used in the production process. For that reason, fibers with different glycerol and calcium content are prepared in this study and subsequently treated with the enzyme transglutaminase before being characterized after air drying. In addition to the swelling behavior in NaOH, H2O, simulated milk ultrafiltrate buffer as well as HCl, the mechanical properties of the fibers are investigated, taking into account their microscopic fine structure. Transglutaminase-treated fibers show sigmoidal absorption curves for all solvents and reach higher equilibrium swelling percentages than untreated fibers. When the calcium content in the coagulation bath is increased from 50 mM to 100 mM, more stabilizing calcium bridges lead to a denser fiber structure that swells more slowly in all solvents considered. With increasing glycerol content, the flexibility of the fibers increases, as indicated by the decrease in elastic moduli, and a fine structure in the sub-µm range becomes visible. The fibers also demonstrate lower elastic moduli when post-treated with transglutaminase. Besides the higher casein content due to the transglutaminase treatment, this could also contribute to the higher equilibrium swelling percentages compared to the untreated fibers.
1. Introduction
Protein fibers are a promising material for new innovative foods, tissue engineering, or the controlled release of drugs [1]. Since the proteins are obtained from natural raw materials, they represent an environmentally friendly alternative to petroleum-based fibers. Spinning processes in the food sector can be used to reproduce the bundle structure of flesh foods in order to produce meat analogs. Visser and coworkers used various blends of rennet casein or sodium- or calcium-caseinate and other additives to produce milk protein-based fibers in a dry spinning process. Depending on their composition, different mechanical properties with elasticities in the range of 50–182 kN m−2 and different swelling behavior in water were reported [2]. In contrast to fibers produced for the food sector, fibers for the textile or medical sector are manufactured in a wet spinning process. In literature, numerous studies deal with the use of casein as a raw material for such fibers. A basic common feature in their production is that the proteins are initially dissolved in an alkaline solution and subsequently spun in an acidic coagulation bath [1,3,4,5,6,7].
In this study, we report on fibers fabricated by our recently developed wet spinning process of rennet-treated casein micelles [8]. However, little is known about the stability of these fibers. In contrast, there is extensive literature on the casein micelles that build them up and their intra- and intermicellar stability. Caseins, as the main protein component in milk, are biocompatible, biodegradable, and bioresorbable and have been used for nano- and microencapsulation of drugs, probiotic cells, nutraceuticals and nutrients [9]. There are a total of four different caseins in cow’s milk (αS1-, αS2-, β- und κ-casein), which are intrinsically disordered proteins with an open, solvent-exposed structure [10]. In milk, caseins are organized in so-called casein micelles (CMs), which are highly hydrated (3.7 g of water per g of dry weight) spherical aggregates with diameters between 50 and 600 nm [11]. In addition, colloidal calcium phosphate exists as nanoclusters, which are randomly distributed in the protein matrix of CMs [12]. Recent structural models of CMs propose a sponge-like structure [13,14,15], with water both tightly bound to the casein surfaces or trapped in water channels [16]. Caseins were simplified as block copolymers to explain their adsorption behavior and micellar assembly [17]. In this modeling approach, the caseins consist of alternating clusters of phosphoseryl-residues interacting with colloidal calcium phosphate and hydrophobic blocks through which caseins are interconnected. Since κ-casein is the only casein lacking phosphoseryl-residues, it prevents further micellar growth and hence forms a surface layer of the CMs. If the surface layer of κ-casein is enzymatically cleaved, e.g., by chymosin, steric repulsion between neighboring CMs can no longer be ensured. With the loss of colloidal stability, aggregation of the so-formed para-casein micelles (p-CMs) starts. The p-CMs first associate to form µm-sized clusters, which then combine to form a coherent gel in the second step [18,19,20].
CMs change their composition and structure depending on the milieu and process conditions, which affect intermicellar stability. After cooling, β-casein leaves the CMs [21] and the amount of calcium and phosphate increases slightly in the surrounding medium due to a better solubility of the calcium phosphate [22]. As a function of pH, the ionization of caseins changes and so too does the protein–protein and protein–mineral interactions within the CMs [23]. During acidification, a dissociation of caseins and dissolution of calcium and phosphate occurs. At a pH close to 4.5, the isoelectric point is reached, at which time, the electrostatic repulsion between the CMs is lowest, resulting in aggregation and gelation. When the pH is further lowered, positive charging of the polypeptide chains occurs, leading to electrostatic repulsion. In contrast, a stronger negative charging takes place the more the pH value shifts into the alkaline range. In addition, the demineralization of the micelle and dissociation of caseins into the serum takes place [23]. The disintegration of CMs also results from the weakening of protein–protein contacts, which is a consequence of the improvement in solvent quality for caseins at a higher pH [24]. For microparticles formed from intact CMs, it has been shown that the swelling rate increases strongly with pH. At pH 14, for example, the microparticles swell within seconds and irreversibly disintegrate [25]. In contrast, swelling of rennet casein fibers at pH 14 is slower, and disintegration is not complete but leaves µm-sized particles [8]. In addition to proton binding, caseins bind divalent cations preferentially to phosphoseryl and carboxyl groups [23]. Using surface-sensitive small-angle X-ray scattering, it was shown that CMs are more densely packed within the film structure at 100 mM of CaCl2 compared to films without additional CaCl2 [26,27]. High-pressure studies also showed that intramicellar stability reaches maximum values after the addition of 60–110 mM of CaCl2 [28]. The colloidal stability already mentioned above prevents aggregation, which can be triggered, e.g., by temperature and concentration increase, ethanol, acid or rennet. This forms the basis for the conversion of CMs into other dairy products, for example, for the production of yogurt or cheese or the production of casein microparticles or fibers [8,25,29]. Treatment with the enzyme transglutaminase (TGase) increases the colloidal stability of CMs and higher aggregated structures by cross-linking caseins [29,30,31,32].
Swelling experiments can be used to analyze hydrogels with regard to their stability, microbial safety, functional properties, texture and cost of production [32]. It was shown on disc-shaped acrylamide gels that with increasing cross-link concentration, the rate of swelling and the cooperative diffusion coefficient for the swelling process decreased [33]. For fluid transport into a polymer in the glassy state, non-fickian diffusion is normally observed, with a moving boundary front forming between swollen and still glassy regions [34]. The slow diffusion of the solvent into the glassy matrix of the dried hydrogels is a consequence of the limited rate at which changes in the polymer structure occur in response to the stresses imposed on the material [34,35]. Due to the cross-linking, the equilibrium swelling percentage generally decreases, as shown, e.g., for acrylamide hydrogels [36].
Regenerated fibers from rennet-treated casein micelles could be used in the future for applications that benefit from the various properties of spun micellar casein, such as good water-binding capacity, high calcium affinity or encapsulation possibilities for bioactive compounds. The aim of this study is to explore the fundamentals of a number of strategies to improve fiber properties, which are important for their handling and later application. For this purpose, we investigate the influence of glycerol and calcium ions during production and subsequent TGases treatment, respectively, on the stability of this novel casein fiber. For the discussion, we use SEM images of the fiber fine structure as well as swelling kinetics in different solvents and mechanical tests.
2. Materials and Methods
2.1. Sample Preparation
We isolated the casein micelles by ultracentrifugation of fresh whole milk, obtained from a local dairy farm (Soerser Milchkännchen, Aachen, Germany). After skimming at 3000 xRCF for 20 min, the milk was centrifuged at 70,000 xRCF for 60 min in an Optima XPN-80 (Beckman Coulter GmbH, Krefeld, Germany) ultracentrifuge at 25 °C. The remaining casein pellet was resuspended under agitation in simulated milk ultrafiltrate (SMUF) which was prepared according to Dumpler [37] at 37 °C for 2 h. To prevent microbial growth, 0.05% sodium azide (99%, Carl Roth, Karlsruhe, Germany) was added. The CM concentration was measured gravimetrically and adjusted to 5 wt%. The solution was then incubated with 50 IMCU L−1 rennet enzyme (CHY-MAX M, Chr. Hansen A/S, Hoersholm, Denmark) at 5 °C for 24 h, which yielded the non-aggregated para-CM solution used for the spinning process. For the glycerol fibers, an 8 wt% CMs solution was diluted with glycerol (99.5%, S. Lange, Berlin, Germany) and SMUF prior to rennet addition. The CMs concentration was again adjusted to 5 wt% with glycerol concentrations of 1 wt% and 10 wt%, respectively.
2.2. Wet Spinning Process
The fibers are fabricated by spinning the cold p-CMs solution through a two-substance nozzle into the heated coagulation bath, which contains either 100 mM of CaCl2 (99%, VWR, Darmstadt, Germany) or 50 mM of CaCl2 and 50 mM of MgCl2 (99%, Carl Roth, Karlsruhe, Germany). The spinning solution is drawn up to a 10-mL syringe placed on a syringe pump (Fusion 4000, Chemyx Inc., Stafford, TX, USA) and cooled with ice. The coagulation bath is heated to 60 °C and 50 mL of the coagulation solution is drawn up to the second syringe placed on the syringe pump. To ensure a bubble-free flow in the spinning nozzle, excess air is released through a ventilation valve. The fibers are spun at constant flow velocities of vp-CM = 6.6 cm/s for the para-CM solution and vcoag = 3.9 cm/s for the surrounding coagulation solution. When leaving the aligned flow inside the nozzle, the fiber is released into the coagulation bath and guided along the bath with tweezers. Directly after spinning, the fibers were harvested from the coagulation bath, rinsed with deionized water, and transferred to further post-treatment. Transglutaminase post-treatment was carried out by placing the fibers in a SMUF solution containing 1 wt% transglutaminase (Activia WM, Ajinomoto Foods Europe SAS, Mesnil-Saint-Nicaise, France) at 30 °C for 24 h. For comparison, reference fibers were stored under the same condition without transglutaminase. Afterward, the fibers were rinsed with deionized water and air-dried on a drying rack. We achieved a stabilization of the fibers against degradation in 1 M NaOH (97%, Merck, Germany) by post-treatment with 1 wt% TGase. Treatment with temperature deactivated TGase, however, did not lead to an increased stability in NaOH (see Supplementary Material Figures S1–S3).
2.3. SEM Images
Scanning electron microscopy images were taken with a TM3030 plus tabletop microscope (Hitachi High Tech Corporation, Krefeld, Germany). The dried fibers, prepared with 1% or 10% glycerol, were fixed with conductive tape on sample holders. The acceleration voltage was set to 15 kV and the backscattering electron (BSE) mode was used.
2.4. Swelling Experiments
The solvent uptake of the dried fibers was examined by recording the increase in fiber diameter after solvent addition. The dried fibers were fixed in a Petri dish, placed under a microscope (Leica DMI-LED, Leica Microsystems GmbH, Wetzlar, Germany) and an image of the dry fiber was taken as a reference. After the addition of 3 mL solvent, videos of the swelling process were recorded (Basler microscopy-ACE, 2.3 MP CMOS, Basler AG, Ahrensburg, Germany) at 2 fps for 10 min. We investigated the swelling in four different aqueous solvents. In the neutral pH regime, we used deionized water and SMUF buffer. For the acidic pH, 1 M HCl (>99%, Carl Roth, DE) was used and 1 M NaOH was used as a strongly alkaline solvent.
To calculate the fiber diameter, individual frames from the recorded video were evaluated at specific times. The diameters of the dry fibers were in a range between 50 and 80 µm. After binarization of the image, the fiber diameter was measured at 5 points along the fiber axis and the mean value was calculated. The diameters were then normalized to the initial diameter according to
and the increase in fiber width was plotted against time. Each swelling test was repeated at least three times.
2.5. Mechanical Properties
To characterize the mechanical properties of the fibers, stress–strain curves were recorded on a tensile testing machine (AGS-X, Shimadzu Deutschland GmbH, Duisburg, Germany). For this purpose, dried fiber pieces about 2 cm long were glued into a two-part template, resulting in an effective test length of 1 cm. Prior to the tensile tests, the fiber diameter was determined microscopically. The tensile test was carried out with a preload of 0.02 N and a test speed of 50 µm/s. The Young’s modulus was calculated by a linear fit between 0.3% and 1% strain (see Supplementary Note Figure S4).
2.6. Statistical Analysis
Two-way analysis of variance (ANOVA) was performed using the statistical computing software R (R 4.1.2, R Foundation for Statistical Computing, Vienna, Austria), allowing to analyze the effect of the glycerol content and TGase post-treatment on Young’s modulus. ANOVA was performed via type II sums of squares for unbalanced data sets and the values were considered statistically relevant for p < 0.05.
3. Results and Discussion
After the treatment of CMs with the enzyme chymosin in the cold, the resulting p-CMs can be spun to fibers by extrusion into a warm coagulation bath [8]. The fiber quality can be improved by a spinning setup with an integrated two-substance nozzle, as shown in Figure 1. Afterward, the fibers are harvested from the coagulation bath and either stored in a buffer or in a TGase solution. TGase post-treatment significantly affected water binding and stability against degradation under alkaline conditions, as shown in Figures S1–S3. The fibers are then dried in air for further characterization and thereby transition to a brittle state. To improve the mechanical properties and handling of the fibers, we added 1% and 10% glycerol to the initial solution with CMs obtained from fresh milk. The fibers thus prepared with glycerol showed less brittleness in the dried state and were easier to handle. We investigated this effect in more detail in mechanical tensile tests. In the swollen state, on the other hand, fibers containing glycerol break very easily, which already made fiber harvesting in the coagulation bath difficult. For this reason, we used glycerol-free fibers to characterize the swelling properties and varied the calcium content instead, by using buffers with 50 mM and 100 mM of CaCl2 in the two-substance nozzle and in the coagulation bath during production.
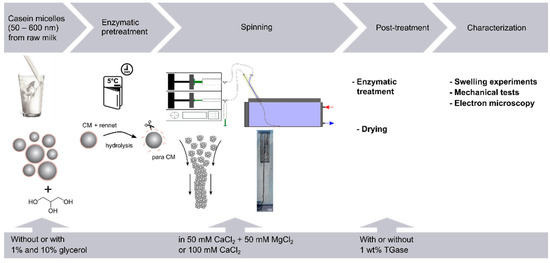
Figure 1.
Overview of the reaction mechanism and process of fiber preparation as well as the experimental procedure for preparation and characterization of the fiber.
3.1. Effects of TGase Post-Treatment on the Swelling of p-CM Fibers in Different Solvents
Figure 2 shows the typical swelling behavior in different solvents of dried TGase-treated p-CMs fibers prepared with 50 mM and 100 mM of CaCl2. Equilibrium values of reference fibers without additional TGase treatment are also plotted as dotted symbols for comparison. Electrostatic contacts are significantly involved in the stability of CMs and higher aggregated states, which strongly depend on the pH. Therefore, we varied the type of solvent and thus the pH during swelling. As Figure 2 shows, all fibers remain intact during swelling within the observation period of 500 s. For the fiber in NaOH in particular, this is due to the TGase post-treatment, as an untreated sample dissolves within this time period [8] and therefore, no comparative values for equilibrium swelling percentages can be plotted. All TGase-treated fibers reach an equilibrium value of a maximum increase in thickness within 100 s, ranging from 80% to 200% depending on the solvent. In detail, the increase in thickness for swelling is the largest with (a) NaOH and takes on smaller and smaller values via (b) deionized water, (c) SMUF buffer to (d) HCl.
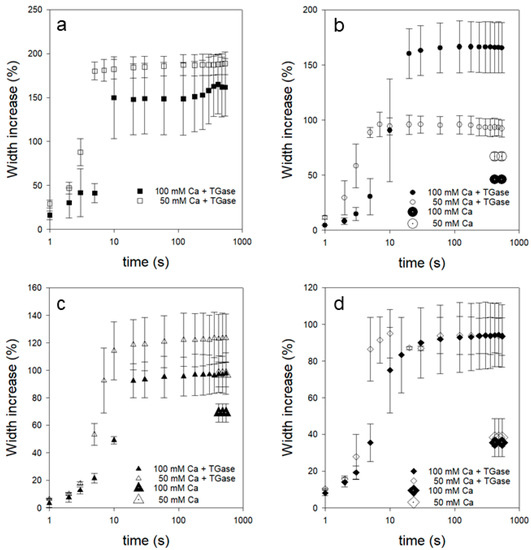
Figure 2.
Swelling curves of TGase-treated and dried p-CMs fibers prepared with 50 mM CaCl2 and 50 mM MgCl2 (open symbols) and 100 mM CaCl2 (closed symbols) in (a) NaOH, (b) distilled water, (c) SMUF, and (d) HCl. The reference values of maximum swelling for the samples without TGase treatment are shown as dotted symbols.
It is particularly striking that fibers, post-treated with TGase, show larger equilibrium values of swelling, although, e.g., as studies on hydrogels show, the opposite would be expected [33]. However, acidified milk gels with TGase treatment were also shown to have better water-holding properties compared to the untreated sample [38]. This was due to a finer gel structure caused by smaller gel particle sizes. Therefore, we also used electron microscopy to investigate the morphology of the fibers. Figure 3 shows the dry fine structure of two identically prepared fibers that were post-treated after spinning either in SMUF only (a) or in SMUF mixed with TGase (b) at 30 °C for 24 h. For both fiber preparations, no major structural changes can be detected in the porous fiber structure (Figure 3(a1,b1)). It consists of single strands aligned in the fiber direction and embedded irregularly-shaped material. The higher resolution images in Figure 3(a2,b2) also reveal a few µm-sized gel particles, which we have already identified in a previous study as building blocks in rennet gel formation [20]. In the fibers treated with TGase, these gel particles appear finer and rather more irregularly oriented than in strands. However, based on the SEM images shown here, it is not yet possible to draw definitive conclusions about possible structural changes, as the measurements could be strongly influenced by the preparative artifacts that occur, for example, due to the drying of the fibers. However, we mention that this finding shows similarities to the acid gels treated with TGase and that such a finer gel network of smaller gel particles could explain the better swelling properties. However, it could also be that casein material leaves the fiber during post-treatment without TGase, without us being able to detect this so far. In this case, casein fibers held together only by physical contacts have less tendency to swell due to their lower casein volume fraction.
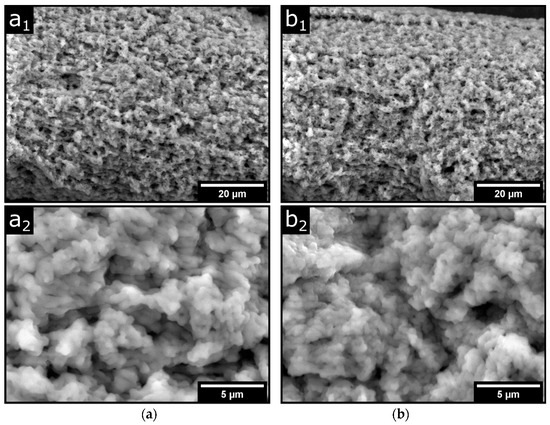
Figure 3.
Scanning electron micrographs of dried p-CMs fibers (a) without and (b) with TGase post-treatment. (a1,b1): ×2000 magnification, (a2,b2): ×8000 magnification.
Thus, casein fibers held together only by physical contacts have less tendency to swell due to their lower casein volume fraction. To interpret the different equilibrium values, the sometimes drastic structural changes that occur within the fiber during swelling must be taken into account. The dissociation of stabilizing salt bridges and negative and positive charging of the polypeptide chains occur in NaOH and HCl, respectively, due to large changes in pH, which finally increase the electrostatic repulsion. In NaOH, the solvent conditions for caseins improve due to the pH increase [24], resulting in stronger swelling and a more expanded network structure compared to other solvents. In contrast, the relatively low swelling in HCl can be explained by a collapsed gel structure with reduced solvent binding capacity. Moreso, for fibers held together only by physical contacts, the smallest equilibrium values of swelling are obtained in HCL with <40% (see dashed symbols in Figure 2d). However, the pH change within the fiber after the addition of SMUF and deionized water solvents is negligible. Nevertheless, mean equilibrium swelling values between 80–160% are obtained for TGase-treated fibers in both aqueous solvents. In these cases, the hydration of the protein strands and the associated softening of the fiber network, in particular, must be taken into account to explain the expansion of the fibers.
We studied the swelling behavior of fibers prepared at two different calcium concentrations since both the stability and the aggregation behavior of p-CMs is calcium dependent. Under standard conditions, p-CMs are made with a calcium content of 100 mM of CaCl2 in the buffer of the sheathing flow of the nozzle or coagulation bath. However, reproducible production of fibers with a homogeneous surface structure is also possible when the calcium content is reduced by half to 50 mM. In order to enable comparable ionic strengths in this case with regard to charge shielding, another 50 mM of MgCl2 was added to the 50 mM of CaCl2.
In general, it can be observed that fibers with 50 mM of calcium absorb more solvents than those produced with 100 mM of calcium. An exception is the swelling of TGase-treated fibers in water (see Figure 2b), about which we can only speculate for now. One reason could be that at 50 mM of calcium, a more ordered network structure is formed, which has a higher solvent binding capacity. In water, however, the TGase-treated fibers prepared with 100 mM of CaCl2 swell 50% more. Since this effect was not observed during swelling with the second aqueous solvent SMUF, the effect can only be caused by the difference in ionic strength between the solvents. The higher ionic content in the fiber prepared with 100 mM of CaCl2 decreases the chemical potential of the penetrating deionized water to a greater extent than in the fiber prepared with 50 mM of CaCl2. As a result, the driving force of swelling and the maximum degree of swelling for the fiber with higher calcium content is increased. This additional effect, which as mentioned above accounts for up to 50% of the increase in fiber thickness, is not observed in the swelling experiments with SMUF buffer (see Figure 2c) because of its high ionic strength of 80 mM. However, what is the reason that the untreated fibers in water do not swell more at 100 mM of CaCl2, but follow the general trend? It can be assumed here that, together with the dissolved casein fraction, a large part of the additional CaCl2 has also been released into the medium, so that the ion effect no longer has a decisive role in the swelling. In fact, comparable equilibrium swelling percentages are observed in the two aqueous solvents for the untreated fibers with slightly higher values, even for SMUF.
It can also be clearly seen that for all fibers in Figure 2, the swelling follows a sigmoidal curve. This swelling behavior is observed when deviations from the free diffusion of the solvent into the fiber occur. Thus, especially during swelling, there is solvent adhesion to the protein surfaces and changes in the structure and elastic properties of the gel network of the fiber. The initial delay in swelling with NaOH and water solvents is more pronounced for the fiber prepared with 100 mM of CaCl2 than for fibers with lower CaCl2 content. Fluorescence micrographs taken before TGase treatment show a more open, coarser fine structure for the fibers with 50 mM of CaCl2 than for those prepared with 100 mM of CaCl2 (See Supplementary Notes Figure S4). As a result of increased CaCl2 concentration, more p-CMs are incorporated into the fiber, which leads to a larger internal surface area. This additional protein surface has to be hydrated, which leads to a delay at the onset of swelling.
3.2. Influence of TGase Post-Treatment and the Plasticizer Glycerol on the Mechanical Stability of the Fibers
Untreated p-CMs fibers swell in distilled water without dissolving. However, the maximum increase in fiber thickness observed so far for these fibers was only about 50%, which is significantly lower than the values shown above for fibers with TGase treatment. This is in contradiction to the influence of cross-linkers on the swelling behavior of hydrogels. An increase in cross-links in the fiber network should result in reduced solvent uptake [33]. In order to understand the reasons for this discrepancy between theory and experimental findings, we investigated the mechanical properties of the fibers in more detail by means of tensile tests. In addition to the influence of TGase treatment, we also investigated the plasticizing effects on the elastic modulus. For this purpose, 1% and 10% glycerol were added to the initial solutions of CMs before the addition of the rennet enzyme. The elastic moduli determined from the stress–strain diagrams are summarized for all the samples investigated in Figure 4. The values of the elastic moduli are in the range of 1–4 GPa and thus in the same order of magnitude as those of fibers made of nylon, polyester, or acrylic [39]. The highest value of approximately 3.5 GPa was obtained for the fibers without added glycerol and TGase post-treatment The statistical ANOVA analysis showed that both depicted effects of glycerol and transglutaminase are significant with p-values of p = 0.0037 and p = 0.0014, respectively (compare Table S1, Supplementary Notes) However, there is no interaction between both effects (p = 0.69784). It can be clearly seen that for the untreated fiber, the median elastic modulus for fibers with 1% or 10% glycerol drops to about 2.9 GPa and 2.4 GPa, respectively. In contrast, the values for fibers containing glycerol, which were post-treated with TGase, are significantly smaller. The decrease of the elastic moduli shows that the stiffness of the fibers decreases after the addition of the plasticizer glycerol and that the network becomes more flexible. This behavior was also observed, for example, after the addition of glycerol to low-methoxyl pectin films [40]. On the other hand, the smaller values of elastic moduli for fibers post-treated with TGase compared to the untreated reference sample are unusual. For the fibers without added glycerol, the decrease amounts to about 20%.
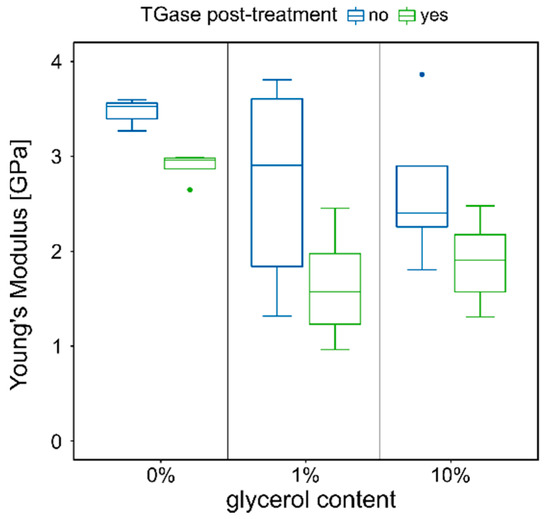
Figure 4.
Mechanical properties of p-CMs fibers post-treated with TGase and spun without or with 1% and 10% glycerol.
The values for the elastic modulus of the TGase-treated fibers drop strongly with increasing glycerol content from approximately 2.9 GPa to 1.6 GPa and 1.9 GPa, respectively. SEM images show minor structural changes at the level of the fiber network, which could explain this drastic reduction. Figure 5 shows the surface structure of the fibers with 1% (a) and 10% (b) glycerol, with corresponding magnifications below. The images over the larger fiber regions in the top row show a porous structure with a horizontal structure that becomes less pronounced with increasing glycerol content. The reason for this preferential direction in the structure is µm-strong strands aligned in the fiber direction (see magnifications below). Further material is irregularly incorporated into these stacked fiber bundles in the form of spherical aggregates. In the images of the glycerol-containing fibers, this irregularly incorporated material combines to form a continuum, while the horizontal orientation of the fibers becomes less and less pronounced. With increasing glycerol content, the structure also becomes more refined and branched, and an irregular fine structure in the sub-µm range is finally visible in fibers with 10% glycerol. In general, polyols transform protein conformations so that more open network structures are formed [41,42]. This could also be the reason why the very compact gel particles, as depicted in Figure 5(a2,b2), become more and more branched and expanded with increasing glycerol content. The loss of the long-range aligned structure decreases the stiffness and increases the flexibility of the fiber network and explains the observed reduction in elastic moduli. A decrease in elastic modulus with increasing glycerol content was also found in other studies on caseinate films [41].
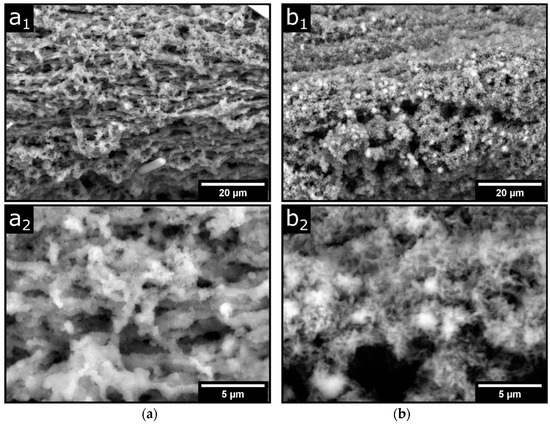
Figure 5.
Scanning electron micrographs of dried p-CMs fibers post-treated with TGase and spun with (a) 1% and (b) 10% glycerol in 50 mM CaCl2. (a1,b1): ×2000 magnification, (a2,b2): ×8000 magnification.
4. Conclusions
Starting from a cooled p-CMs solution, a two-substance nozzle can be used to reproducibly produce fibers that change to a brittle, glassy state after drying. Sigmoidal swelling curves of the fibers indicate deviations from Fick’s swelling behavior in deionized water and SMUF buffer, which are due to strong interactions between solvent and the protein surfaces, enhanced swelling after the transition to the rubbery state, and elastic effects of the fiber network. With NaOH and HCl as solvents, dissociation of the stabilizing salt bridges within the fiber network additionally occurs due to the extreme pH change. Furthermore, protein–protein contacts are weakened by electrostatic repulsion, as polypeptide chains become negatively charged in NaOH and positively charged in HCl. Nevertheless, the fibers remain intact throughout the swelling process without being dissociated, which can be attributed, in particular, in NaOH, to the stabilization by TGase treatment (see also Supplementary Material Figures S1 and S3). Fibers have a lower elastic modulus after TGase treatment and, contrary to theory, exhibit significantly higher equilibrium swelling values in all solvents. At this point, we can only speculate about the reason, but it could have to do with a higher volume fraction of casein, which is bound in the fiber by TGases or the finer network structure due to the smaller gel particles. We want to examine this in future studies by using other complementary high-resolution techniques, such as confocal laser scan microscopy. However, a similar decrease in elastic modulus is also observed in fibers made with the plasticizer glycerol. In these cases, the SEM images show that the fibers have a fine structure in the sub-µm range, which increases the internal surface area enormously. The TGase treatment would also increase the internal surface area of the fiber by binding a higher volume fraction of casein. This increases the solvent absorption capacity and could also improve the flexibility of the fiber in a similar way as with glycerol. The study gives the first indications of the influence of a treatment with a cross-linking enzyme on the swelling behavior and the mechanical stability of the fibers. However, since our results are based on indirect measurements, future studies should provide direct evidence that cross-linking has occurred.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/colloids6020017/s1, Figure S1: Microscopy images of fibers with (top) and without (bottom) TGase treatment at different times after NaOH addition. The untreated fibers completely disintegrate after about 60s whereas the treated fibers remain intact after a rapid swelling; Figure S2: Swelling behavior in SMUF of fibers with and without TGase post treatment; Figure S3: Swelling behavior of CM Fibers in 1M NaOH. Top: Fiber post treated in deactivated TGase. The fibers completely dissolve after about 20 s. The inset in the first and last image shows that the fiber has completely dissolved after NaOH exposure. Bottom: Fiber post treated in TGase. The fiber exhibits a strong swelling but remains its structural integrity after NaOH exposure; Figure S4: Confocal micrographs of fibers fabricated in (a) 100 mM Cacl2 and (b) 50 mM CaCl2 + 50mM MgCl2, swollen in water; Figure S5: Exemplary stress strain curves. The Young’s modulus is obtained via a linear fit between 0.3 and 1% strain (interval indicated by dashed lines); Table S1: Full dataset for the ANOVA analysis with mean and standard deviation of the elastic moduli; Table S2: Results of the ANOVA analysis
Author Contributions
Conceptualization, S.T. and R.G.; methodology, S.T. and R.G.; validation, S.T. and R.G.; formal analysis, R.G. and S.T.; investigation, S.T.; resources, R.G.; writing—original draft preparation, R.G. and S.T.; writing—review and editing, R.G. and S.T.; visualization, S.T. and R.G.; supervision, R.G.; project administration, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This article is partly funded by the Exploratory Research Space of RWTH Aachen University (project BioTrans012).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully thank our master students, especially Timon Kratzenberg for their contribution to the swelling experiments and fiber fabrication. We also thank Karin Faensen (AVT.CVT) for her support with the electron microscopy analysis and the AVT.CVT-SMALL group for the support with the confocal fluorescence experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, J.; Su, J.; Ma, C.; Göstl, R.; Herrmann, A.; Liu, K.; Zhang, H. Fabrication and Mechanical Properties of Engineered Protein-Based Adhesives and Fibers. Adv. Mater. 2020, 32, e1906360. [Google Scholar] [CrossRef] [PubMed]
- Visser, J. Dry Spinning of Milk Proteins. In Food Structure; Elsevier: Amsterdam, The Netherlands, 1988; pp. 197–218. ISBN 9781855733961. [Google Scholar]
- Cui, L.; Reddy, N.; Xu, H.; Fan, X.; Yang, Y. Enzyme-modified casein fibers and their potential application in drug delivery. Fibers Polym. 2017, 18, 900–906. [Google Scholar] [CrossRef]
- Brooks, M.M. Regenerated protein fibres: A preliminary review. In Handbook of Textile Fibre Structure; Elsevier: Amsterdam, The Netherlands, 2009; pp. 234–265. ISBN 9781845697303. [Google Scholar]
- Yang, Y.; Reddy, N. Properties and potential medical applications of regenerated casein fibers crosslinked with citric acid. Int. J. Biol. Macromol. 2012, 51, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Sun, J.; Gu, R.; Ma, C.; Liu, K. Biological fibers based on naturally sourced proteins: Mechanical investigation and applications. Mater. Today Adv. 2020, 8, 100095. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Li, B.; Yang, C.; Shen, J.; Wang, N.; Gu, R.; Wang, D.; Chen, D.; Hu, H.; et al. Robust Biological Fibers Based on Widely Available Proteins: Facile Fabrication and Suturing Application. Small 2020, 16, e1907598. [Google Scholar] [CrossRef] [PubMed]
- Thill, S.; Schmidt, T.; Wöll, D.; Gebhardt, R. A regenerated fiber from rennet-treated casein micelles. Colloid Polym. Sci. 2021, 15, 201. [Google Scholar] [CrossRef]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef] [Green Version]
- Holt, C.; Carver, J.A. Quantitative multivalent binding model of the structure, size distribution and composition of the casein micelles of cow milk. Int. Dairy J. 2022, 126, 105292. [Google Scholar] [CrossRef]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef]
- Bouchoux, A.; Gésan-Guiziou, G.; Pérez, J.; Cabane, B. How to squeeze a sponge: Casein micelles under osmotic stress, a SAXS study. Biophys. J. 2010, 99, 3754–3762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, D.J.; Oommen, B.S. Supramolecular structure of the casein micelle. J. Dairy Sci. 2008, 91, 1709–1721. [Google Scholar] [CrossRef] [Green Version]
- Dalgleish, D.G. On the structural models of bovine casein micelles—Review and possible improvements. Soft Matter 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Huppertz, T.; Gazi, I.; Luyten, H.; Nieuwenhuijse, H.; Alting, A.; Schokker, E. Hydration of casein micelles and caseinates: Implications for casein micelle structure. Int. Dairy J. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- Horne, D.S. Casein structure, self-assembly and gelation. Curr. Opin. Colloid Interface Sci. 2002, 7, 456–461. [Google Scholar] [CrossRef]
- Horne, D.S.; Lucey, J.A. Rennet-Induced Coagulation of Milk. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–143. ISBN 9780124170124. [Google Scholar]
- Mezzenga, R.; Schurtenberger, P.; Burbidge, A.; Michel, M. Understanding foods as soft materials. Nat. Mater. 2005, 4, 729–740. [Google Scholar] [CrossRef]
- Thill, S.; Schmidt, T.; Wöll, D.; Gebhardt, R. Single particle tracking as a new tool to characterise the rennet coagulation process. Int. Dairy J. 2020, 104659. [Google Scholar] [CrossRef]
- Liu, D.Z.; Weeks, M.G.; Dunstan, D.E.; Martin, G.J.O. Temperature-dependent dynamics of bovine casein micelles in the range 10–40 °C. Food Chem. 2013, 141, 4081–4086. [Google Scholar] [CrossRef]
- Koutina, G.; Knudsen, J.C.; Andersen, U.; Skibsted, L.H. Temperature effect on calcium and phosphorus equilibria in relation to gel formation during acidification of skim milk. Int. Dairy J. 2014, 36, 65–73. [Google Scholar] [CrossRef]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Vaia, B.; Smiddy, M.A.; Kelly, A.L.; Huppertz, T. Solvent-mediated disruption of bovine casein micelles at alkaline pH. J. Agric. Food Chem. 2006, 54, 8288–8293. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.; Stöckermann, M.; Gebhardt, R. Influence of pH on the stability and structure of single casein microparticles. Food Hydrocoll. 2020, 105, 105741. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P.; Gebhardt, R.; Roth, S.V.; Metwalli, E.; Doster, W. Effect of calcium concentration on the structure of casein micelles in thin films. Biophys. J. 2007, 93, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, R.; Burghammer, M.; Riekel, C.; Roth, S.V.; Müller-Buschbaum, P. Structural changes of casein micelles in a calcium gradient film. Macromol. Biosci. 2008, 8, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhardt, R.; Takeda, N.; Kulozik, U.; Doster, W. Structure and stabilizing interactions of casein micelles probed by high-pressure light scattering and FTIR. J. Phys. Chem. B 2011, 115, 2349–2359. [Google Scholar] [CrossRef]
- Smiddy, M.A.; Martin, J.-E.G.H.; Kelly, A.L.; De Kruif, C.G.; Huppertz, T. Stability of Casein Micelles Cross-Linked by Transglutaminase. J. Dairy Sci. 2006, 89, 1906–1914. [Google Scholar] [CrossRef] [Green Version]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll. 2009, 23, 1670–1677. [Google Scholar] [CrossRef]
- Huppertz, T.; Smiddy, M.A.; de Kruif, C.G. Biocompatible micro-gel particles from cross-linked casein micelles. Biomacromolecules 2007, 8, 1300–1305. [Google Scholar] [CrossRef]
- Kruif, C.d.; Anema, S.G.; Zhu, C.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Aktaş, D.K.; Evingür, G.A.; Pekcan, Ö. A fluorescence study on swelling of hydrogels (PAAm) at various cross-linker contents. Adv. Polym. Technol. 2009, 28, 215–223. [Google Scholar] [CrossRef]
- De Kee, D.; Liu, Q.; Hinestroza, J. Viscoelastic (Non-Fickian) Diffusion. Can. J. Chem. Eng. 2005, 83, 913–929. [Google Scholar] [CrossRef]
- Kipcak, A.S.; Ismail, O.; Doymaz, I.; Piskin, S. Modeling and Investigation of the Swelling Kinetics of Acrylamide-Sodium Acrylate Hydrogel. J. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Saraydın, D.; Karadag, E.; Işıkver, Y.; Şahiner, N.; Güven, O. The Influence of Preparation Methods on the Swelling and Network Properties of Acrylamide Hydrogels with Crosslinkers. J. Macromol. Sci. Part A 2004, 41, 419–431. [Google Scholar] [CrossRef]
- Dumpler, J. Heat Stability of Concentrated Milk Systems; Springer: Wiesbaden, Germany, 2018; ISBN 978-3-658-19695-0. [Google Scholar]
- Ercili Cura, D.; Lille, M.; Partanen, R.; Kruus, K.; Buchert, J.; Lantto, R. Effect of Trichoderma reesei tyrosinase on rheology and microstructure of acidified milk gels. Int. Dairy J. 2010, 20, 830–837. [Google Scholar] [CrossRef]
- Ashby, M.F.; Jones, D.R.H. An Introduction to Their Properties & Applications, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2003; ISBN 0750630817. [Google Scholar]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ali, M.A.; Dias, G.J. Effect of cross-linking on microstructure and physical performance of casein protein. Biomacromolecules 2009, 10, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid–beeswax mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).