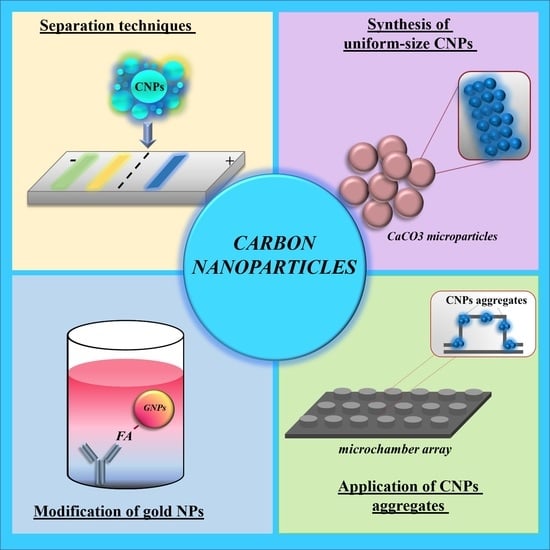
Carbon Nanoparticles and Materials on Their Basis
Abstract
:1. Introduction
2. Methods for Synthesis and Separation of CNPs
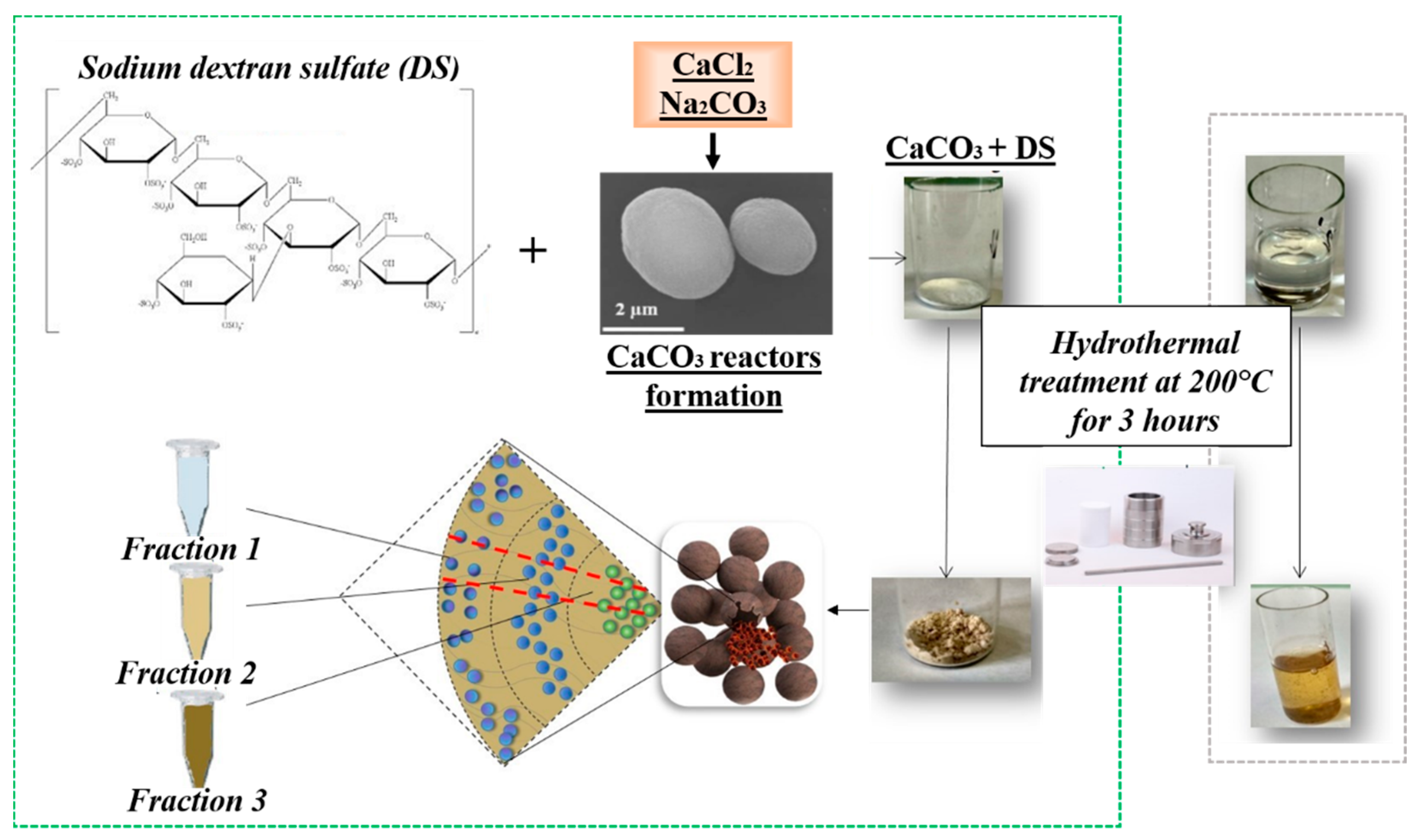
3. Synthesis of CNPs in Pores of CaCO3 Microparticles
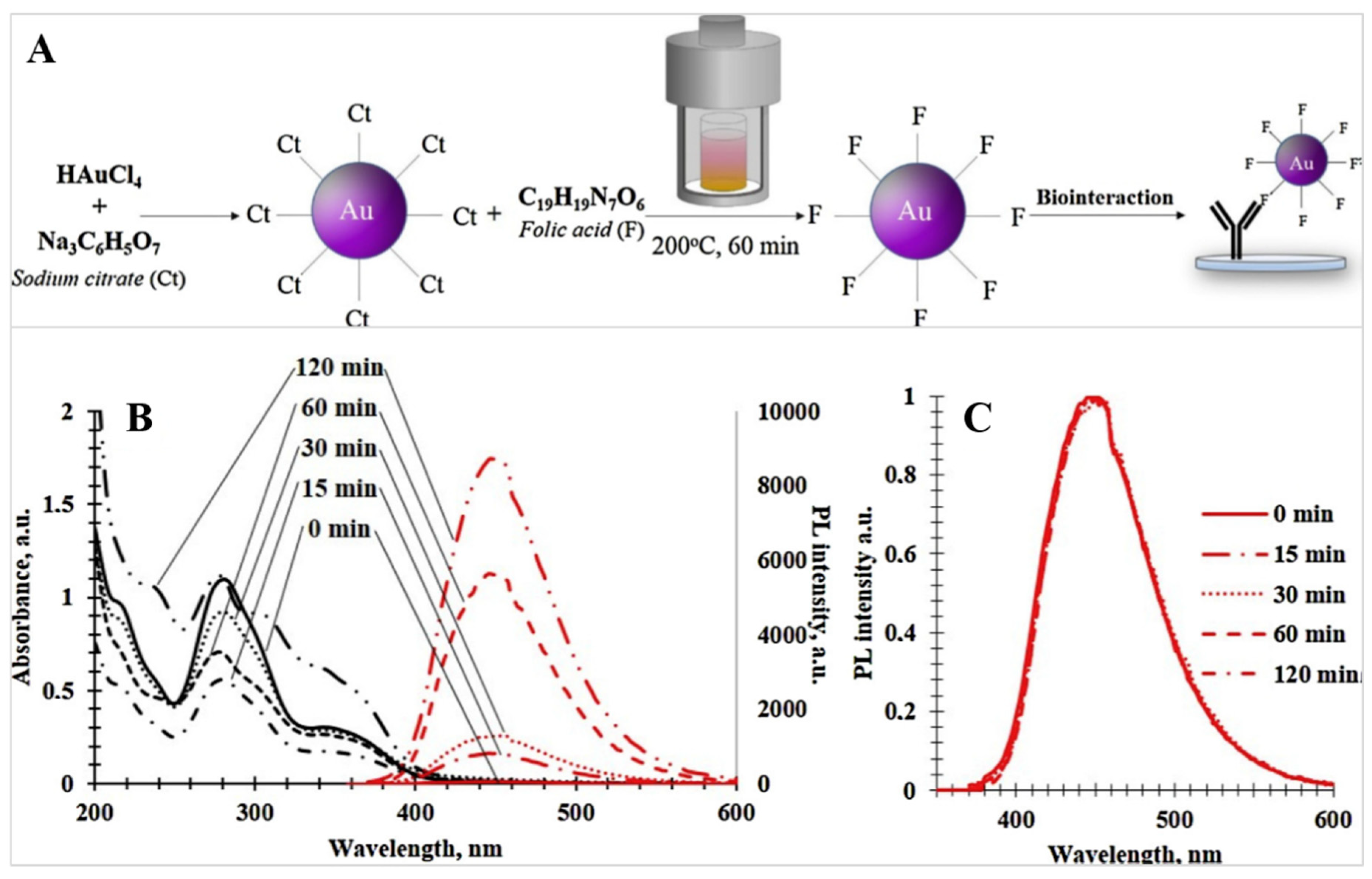
4. Application of Hydrothermal Treatment for Gold Nanoparticles Modification
5. Application of CNPs Aggregates for Laser-Induced Cargo Release
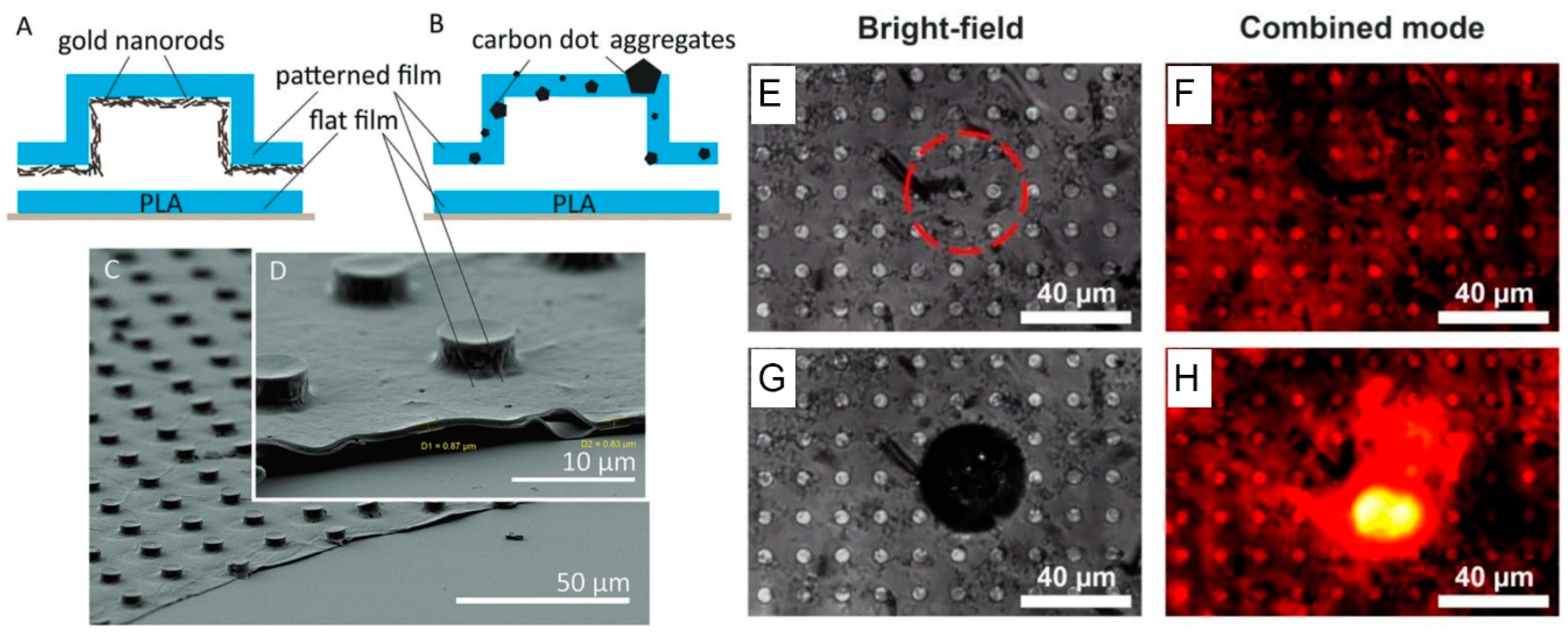
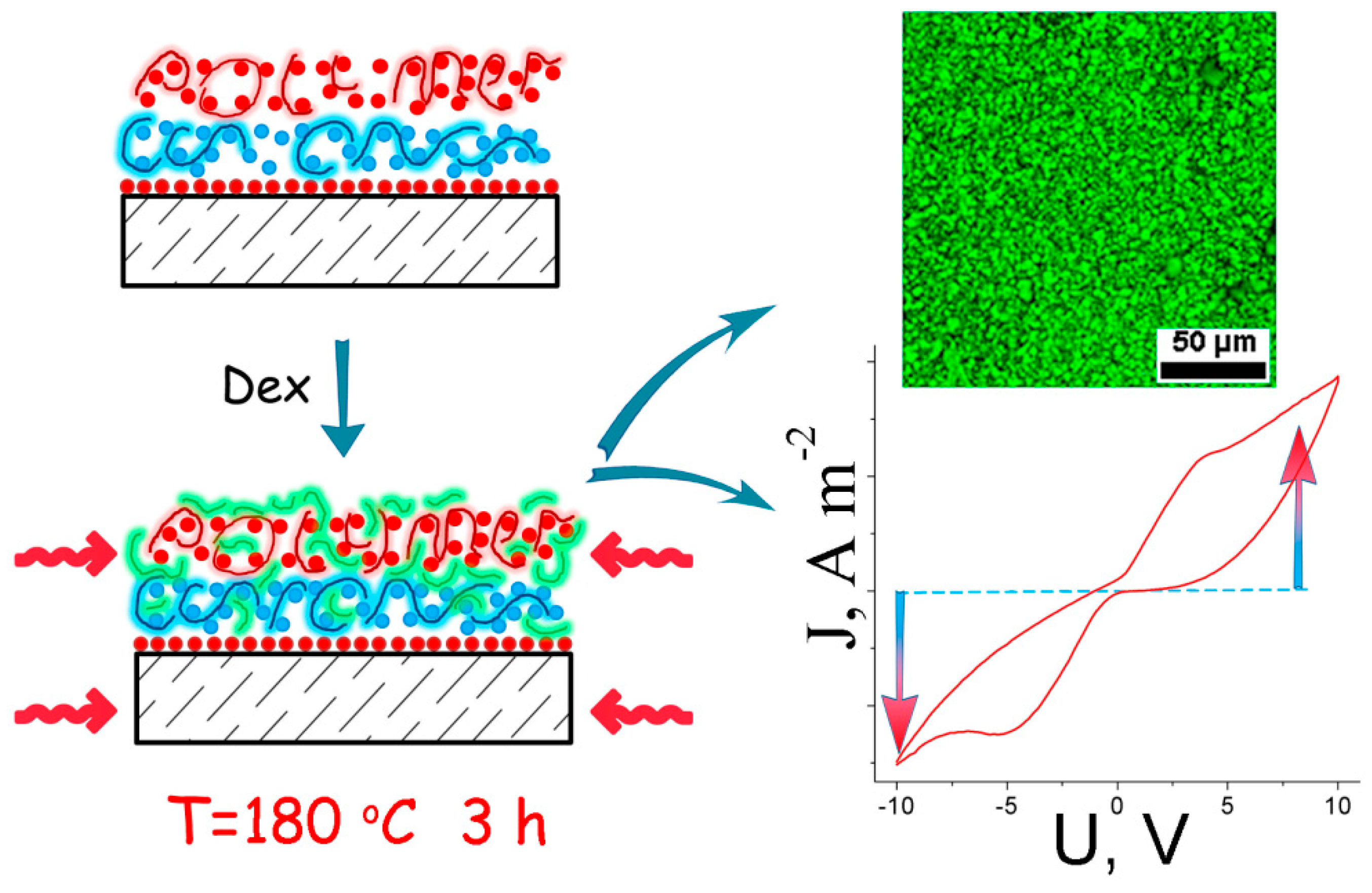
6. Application of in Situ Formed Carbon Nanostructures for Functionalization of Polymer-Based Materials
7. Conclusions
8. Future Perspectives
Funding
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sensors 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Reckmeier, C.J.; Schneider, J.; Susha, A.S.; Rogach, A.L. Luminescent colloidal carbon dots: Optical properties and effects of doping [Invited]. Opt. Express 2016, 24, A312–A340. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, I.Y.; Sapelkin, A.V.; Sukhorukov, G.B. Carbon nanodots: Mechanisms of photoluminescence and principles of application. Trends Anal. Chem. 2017, 90, 27–37. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Prikhozhdenko, E.S.; Sukhorukov, G.B.; Sapelkin, A.V.; Goryacheva, I.Y. Luminescent carbon nanoparticles: Synthesis, methods of investigation, applications. Russ. Chem. Rev. 2017, 86, 1157–1171. [Google Scholar] [CrossRef]
- Xiong, Y.; Schneider, J.; Ushakova, E.V.; Rogach, A.L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124–139. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Bakal, A.A.; Shpuntova, D.V.; Kostritskiy, A.Y.; Beloglazova, N.V.; De Saeger, S.; Sukhorukov, G.B.; Sapelkin, A.V.; Goryacheva, I.Y. Gel electrophoresis separation and origins of light emission in fluorophores prepared from citric acid and ethylenediamine. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ehrat, F.; Bhattacharyya, S.; Schneider, J.; Löf, A.; Wyrwich, R.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Tracking the source of carbon dot photoluminescence: Aromatic Domains versus molecular fluorophores. Nano Lett. 2017, 17, 7710–7716. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Sapelkin, A.V.; Sukhorukov, G.B.; Goryacheva, I.Y. Luminescent carbon nanoparticles separation and purification. Adv. Colloid Interface Sci. 2019, 274, 102043. [Google Scholar] [CrossRef]
- Vostrikova, A.V.; Prikhozhdenko, E.S.; Mayorova, O.A.; Goryacheva, I.Y.; Tarakina, N.V.; Sukhorukov, G.B.; Sapelkin, A.V. Thermal carbonization in nanoscale reactors: Controlled formation of carbon nanodots inside porous CaCO3 microparticles. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Liu, M.L.; Bin Chen, B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped carbon dots for sensing and bioimaging applications: A minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Ding, J.; Zhang, K.; Ding, L. Construction of biomass carbon dots based fluorescence sensors and their applications in chemical and biological analysis. TrAC Trends Anal. Chem. 2019, 118, 315–337. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Zhou, Y.; Fu, Y.; Guo, S.; Li, H.; Zhao, S.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots enhance the stability of CdS for visible-light-driven overall water splitting. Appl. Catal. B Environ. 2017, 216, 114–121. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, C.; Wang, L.; Guo, S.; Zhang, Y.; Li, H.; Huang, H.; Liu, Y.; Tang, J.; Kang, Z. Control strategy on two-/four-electron pathway of water splitting by multidoped carbon based catalysts. ACS Catal. 2017, 7, 1637–1645. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. A review on the effects of carbon dots in plant systems. Mater. Chem. Front. 2020, 4, 437–448. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Zheng, M.; Liu, Y.; Hu, C.; et al. Carbon dots as protective agent alleviating abiotic stresses on rice (Oryza sativa L.) through promoting nutrition assimilation and defense system. ACS Appl. Mater. Interfaces 2019, 12, 33575–33585. [Google Scholar] [CrossRef] [PubMed]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Bratashov, D.N.; Vostrikova, A.M.; Atkin, V.S.; Ermakov, A.V.; Khlebtsov, B.N.; Sapelkin, A.V.; Goryacheva, I.Y.; Sukhorukov, G.B. Carbon dot aggregates as an alternative to gold nanoparticles for the laser-induced opening of microchamber arrays. Soft Matter 2018, 14, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, B.; Zhu, A.; Ding, C.; Zhao, X.; Li, B.; Tian, Y. Carbon dot-based inorganic-organic nanosystem for two-photon imaging and biosensing of pH variation in living cells and tissues. Adv. Mater. 2012, 24, 5844–5848. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Su, R.; Zhang, M.; Zhang, R.; Li, W.; Wang, D.; Xu, M.; Fei, L.; Xu, Q. Highly fluorescent dual-emission red carbon dots and their applications in optoelectronic devices and water detection. New J. Chem. 2019, 43, 3050–3058. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Zhou, Y.; Liyanage, P.Y.; Devadoss, D.; Rios Guevara, L.R.; Cheng, L.; Graham, R.M.; Chand, H.S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood-brain barrier and inhibitors of β-amyloid. Nanoscale 2019, 11, 22387–22397. [Google Scholar] [CrossRef]
- Zhi, B.; Yao, X.X.; Cui, Y.; Orr, G.; Haynes, C.L. Synthesis, applications and potential photoluminescence mechanism of spectrally tunable carbon dots. Nanoscale 2019, 11, 20411–20428. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, Y.; Kwon, O.H.; Kim, B.S. Carbon dots: Bottom-up syntheses, properties, and light-harvesting applications. Chem. Asian J. 2018, 13, 586–598. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z. The formation mechanism and fluorophores of carbon dots synthesized: Via a bottom-up route. Mater. Chem. Front. 2020, 4, 400–420. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Prikhozhdenko, E.S.; Tarakina, N.V.; Sapelkin, A.V.; Sukhorukov, G.B.; Goryacheva, I.Y. Dispersion of optical and structural properties in gel column separated carbon nanoparticles. Carbon 2018, 127, 541–547. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein encapsulation via porous CaCO3 microparticles templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef]
- Casanova, H.; Higuita, L.P. Synthesis of calcium carbonate nanoparticles by reactive precipitation using a high pressure jet homogenizer. Chem. Eng. J. 2011, 175, 569–578. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Liu, X.; He, W.; Huang, Q.; Li, S.; Feng, Q. Calcium carbonate nanoparticles promote osteogenesis compared to adipogenesis in human bone-marrow mesenchymal stem cells. Prog. Nat. Sci. Mater. Int. 2018, 28, 598–608. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, W.; Xu, Z.P. Enhanced combination cancer therapy using lipid-calcium carbonate/phosphate nanoparticles as a targeted delivery platform. Nanomedicine 2019, 14, 77–92. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Zhang, J.; Cheng, J.; Wang, X. In situ formation of surface-functionalized ionic calcium carbonate nanoparticles with liquid-like behaviours and their electrical properties. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Vostrikova, A.M.; Kokorina, A.A.; Demina, P.A.; German, S.V.; Novoselova, M.V.; Tarakina, N.V.; Sukhorukov, G.B.; Goryacheva, I.Y. Fabrication and photoluminescent properties of Tb3+ doped carbon nanodots. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Yang, X.; Han, X.; Jiao, Y.; Wei, T.; Yang, D.; Xu, H.; Nie, G. Highly fluorescent chiral N-S-Doped carbon dots from cysteine: Affecting cellular energy metabolism. Angew. Chemie 2018, 130, 2401–2406. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon dots from sugars and ascorbic acid: Role of the precursors on morphology, properties, toxicity, and drug uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, W.; Liu, X.; Zhang, Y.; Bai, Y. Hydrothermal synthesis and photoluminescent mechanistic investigation of highly fluorescent nitrogen doped carbon dots from amino acids. Mater. Res. Bull. 2017, 89, 26–32. [Google Scholar] [CrossRef]
- Vostrikova, A.M.; Kokorina, A.A.; Mitrophanova, A.N.; Sindeeva, O.A.; Sapelkin, A.V.; Sukhorukov, G.B.; Goryacheva, I.Y. One step hydrothermal functionalization of gold nanoparticles with folic acid. Colloids Surf. B Biointerfaces 2019, 181, 533–538. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van Der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Man, S.M.; Tonoyan, A.; Low, H.Y.; Sukhorukov, G.B. Adhesion of polyelectrolyte multilayers: Sealing and transfer of microchamber arrays. Langmuir 2012, 28, 5678–5686. [Google Scholar] [CrossRef]
- Ke, H.; Wang, J.; Dai, Z.; Jin, Y.; Qu, E.; Xing, Z.; Guo, C.; Yue, X.; Liu, J. Gold-nanoshelled microcapsules: A theranostic agent for ultrasound contrast imaging and photothermal therapy. Angew. Chemie. Int. Ed. 2011, 50, 3017–3021. [Google Scholar] [CrossRef]
- Ermakov, A.; Lim, S.H.; Gorelik, S.; Kauling, A.P.; de Oliveira, R.V.B.; Castro Neto, A.H.; Glukhovskoy, E.; Gorin, D.A.; Sukhorukov, G.B.; Kiryukhin, M.V. Polyelectrolyte–Graphene oxide multilayer composites for array of microchambers which are mechanically robust and responsive to NIR light. Macromol. Rapid Commun. 2019, 40, 1–7. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, J.; Zhang, Z.; Cao, Y.; Li, J.; Cao, S. PEGylated graphene oxide-capped gold nanorods/silica nanoparticles as multifunctional drug delivery platform with enhanced near-infrared responsiveness. Mater. Sci. Eng. C 2019, 104, 109889. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Kudryavtseva, V.L.; Demina, P.; Verkhovskii, R.; Zhang, J.; Lengert, E.; Sapelkin, A.; Goryacheva, I.Y.; Sukhorukov, G. Site-specific release of reactive oxygen species from ordered arrays of microchambers based on polylactic acid and carbon nanodots. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef]
- Sharma, S.; Shriwastava, S.; Kumar, S.; Bhatt, K.; Tripathi, C.C. Alternative transparent conducting electrode materials for flexible optoelectronic devices. Opto Electron. Rev. 2018, 26, 223–235. [Google Scholar] [CrossRef]
- Liu, L.; Ye, D.; Dong, R.; Chen, D.; Li, S.; Cao, K.; Cheng, G.; Chen, S.; Huang, W. Work function-tunable graphene-polymer composite electrodes for organic light-emitting diodes. ACS Appl. Energy Mater. 2020, 3, 4068–4077. [Google Scholar] [CrossRef]
- Gupta, A.; Sardana, S.; Dalal, J.; Lather, S.; Maan, A.S.; Tripathi, R.; Punia, R.; Singh, K.; Ohlan, A. Nanostructured Polyaniline/Graphene/Fe2O3 composites hydrogel as a high-performance flexible supercapacitor electrode material. ACS Appl. Energy Mater. 2020, 3, 6434–6446. [Google Scholar] [CrossRef]
- Kumar, D.S.; Kumar, B.J.; Mahesh, H.M. Polyelectrolyte layer-by-layer spin assembly of aqueous CdTe quantum dot multilayered thin films. J. Alloys Compd. 2018, 735, 2558–2566. [Google Scholar] [CrossRef]
- Nifontova, G.; Krivenkov, V.; Zvaigzne, M.; Samokhvalov, P.; Efimov, A.E.; Agapova, O.I.; Agapov, I.I.; Korostylev, E.; Zarubin, S.; Karaulov, A.; et al. Controlling charge transfer from quantum dots to polyelectrolyte layers extends prospective applications of magneto-optical microcapsules. ACS Appl. Mater. Interfaces 2020, 12, 35882–35894. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Prikhozhdenko, E.S.; Demina, P.A.; Gorbachev, I.A.; Vostrikova, A.M.; Sapelkin, A.V.; Goryacheva, I.Y.; Sukhorukov, G.B. Composite multilayer films based on polyelectrolytes and in situ-formed carbon nanostructures with enhanced photoluminescence and conductivity properties. J. Appl. Polym. Sci. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhorukov, G.B. In situ synthesis of fluorescent Carbon Dots/Polyelectrolyte Nanocomposite microcapsules with reduced permeability and ultrasound sensitivity. ACS Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokorina, A.A.; Ermakov, A.V.; Abramova, A.M.; Goryacheva, I.Y.; Sukhorukov, G.B. Carbon Nanoparticles and Materials on Their Basis. Colloids Interfaces 2020, 4, 42. https://doi.org/10.3390/colloids4040042
Kokorina AA, Ermakov AV, Abramova AM, Goryacheva IY, Sukhorukov GB. Carbon Nanoparticles and Materials on Their Basis. Colloids and Interfaces. 2020; 4(4):42. https://doi.org/10.3390/colloids4040042
Chicago/Turabian StyleKokorina, Alina A., Alexey V. Ermakov, Anna M. Abramova, Irina Yu. Goryacheva, and Gleb B. Sukhorukov. 2020. "Carbon Nanoparticles and Materials on Their Basis" Colloids and Interfaces 4, no. 4: 42. https://doi.org/10.3390/colloids4040042
APA StyleKokorina, A. A., Ermakov, A. V., Abramova, A. M., Goryacheva, I. Y., & Sukhorukov, G. B. (2020). Carbon Nanoparticles and Materials on Their Basis. Colloids and Interfaces, 4(4), 42. https://doi.org/10.3390/colloids4040042