Surface Forces between Nanomagnetite and Silica in Aqueous Ca2+ Solutions Studied with AFM Colloidal Probe Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
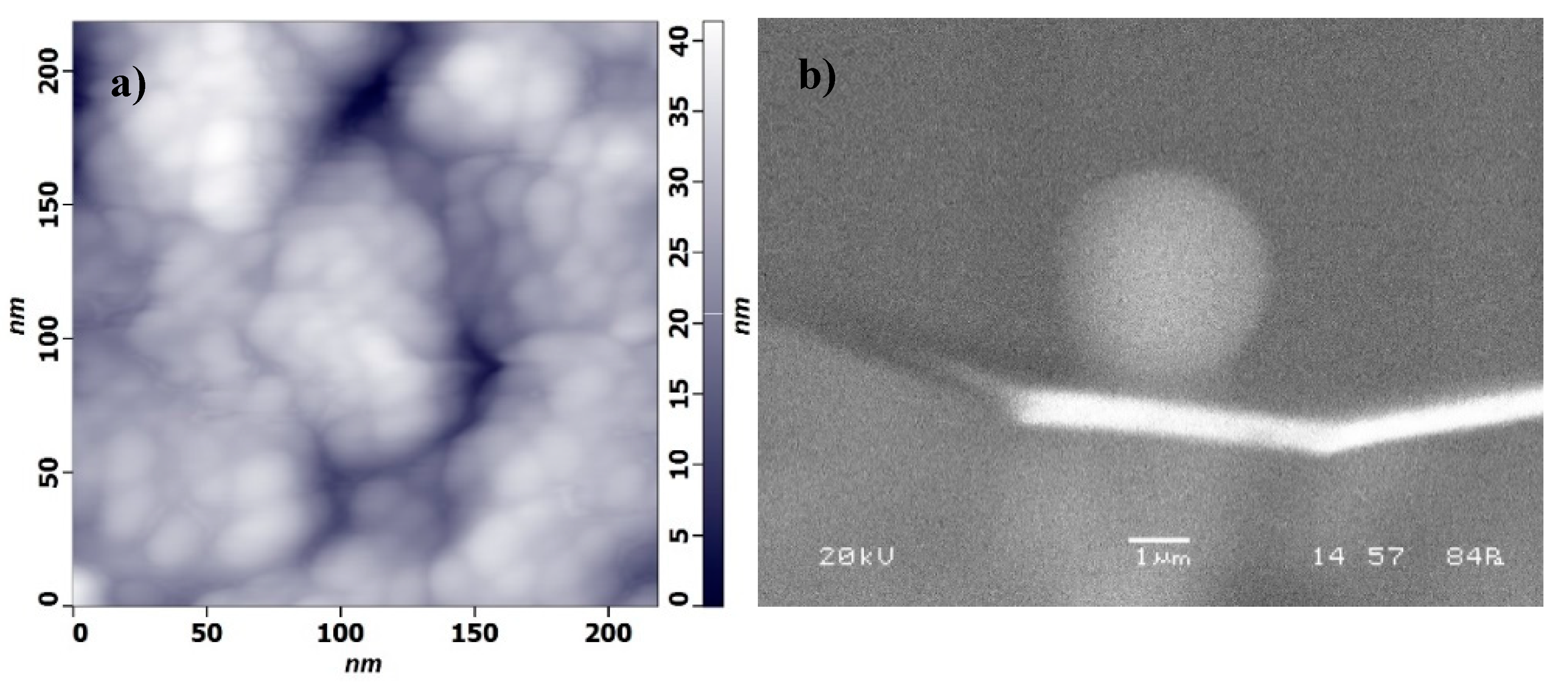
2.2. Substrate and Probe Preparation
2.3. Electrophoretic Mobility Measurement
2.4. Force Measurements with AFM
2.5. Adhesion Force Evaluation and Calculation
3. Results and Discussion
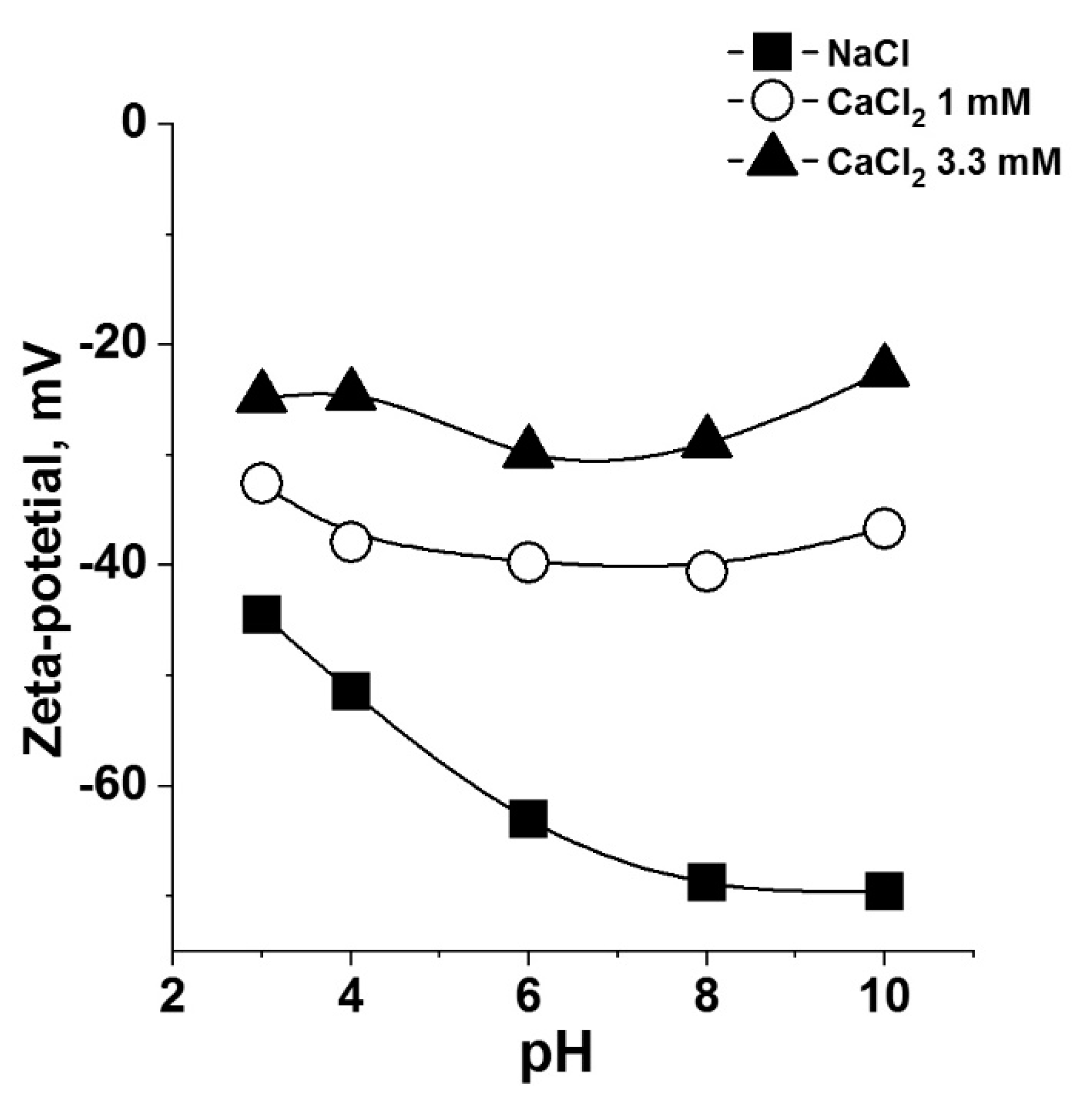
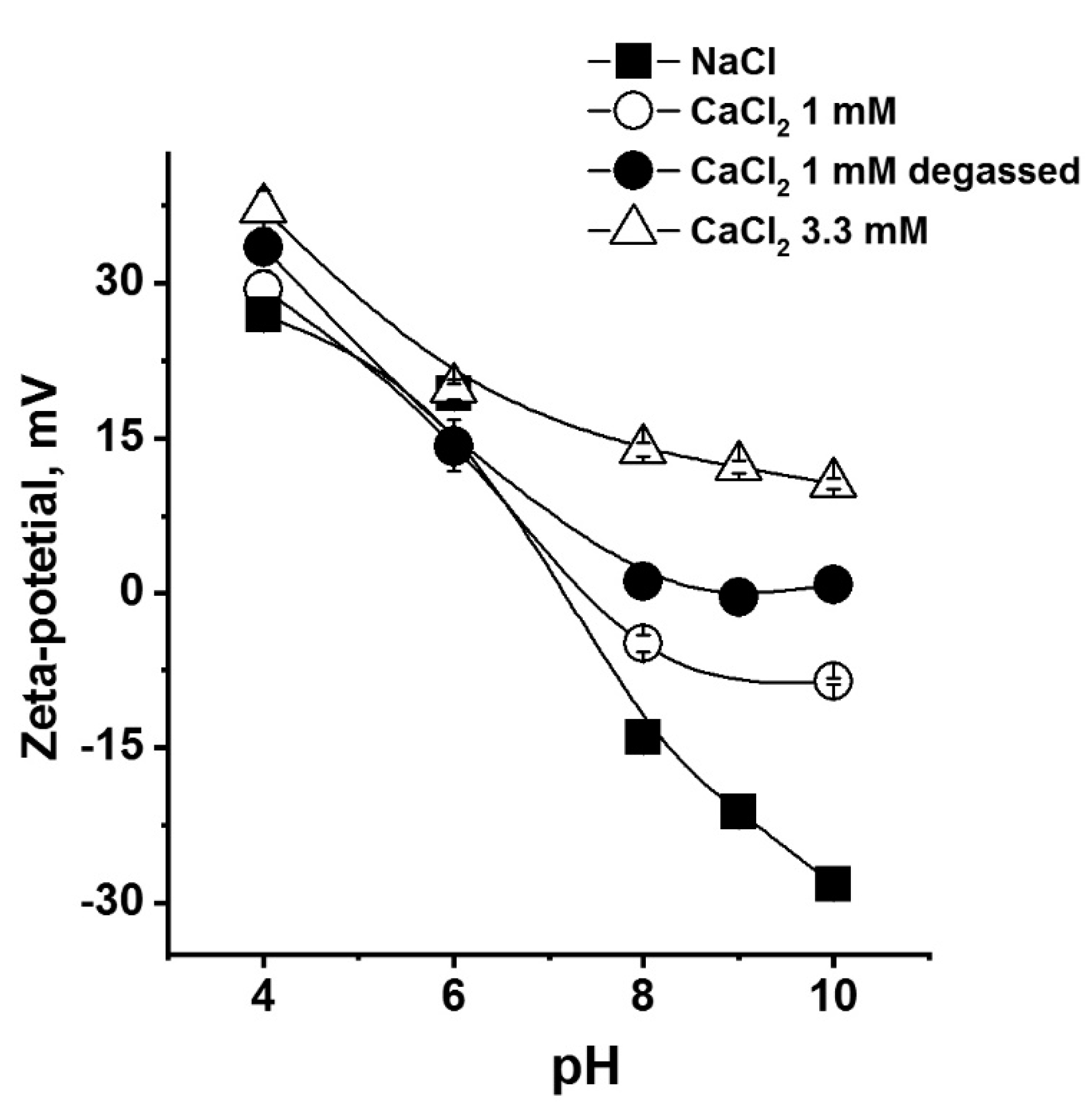
3.1. Effect of Calcium Concentration and pH on Zeta Potential
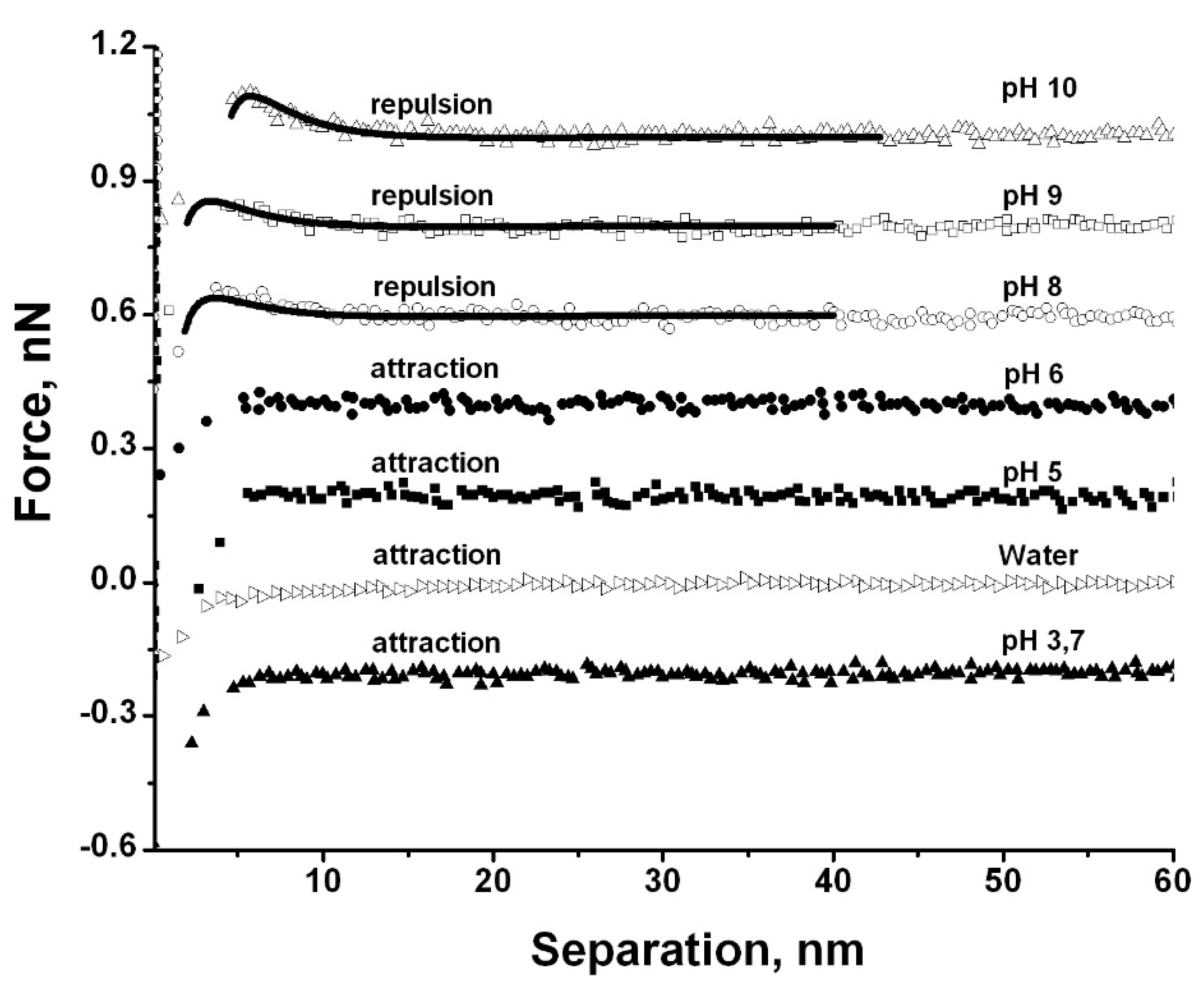
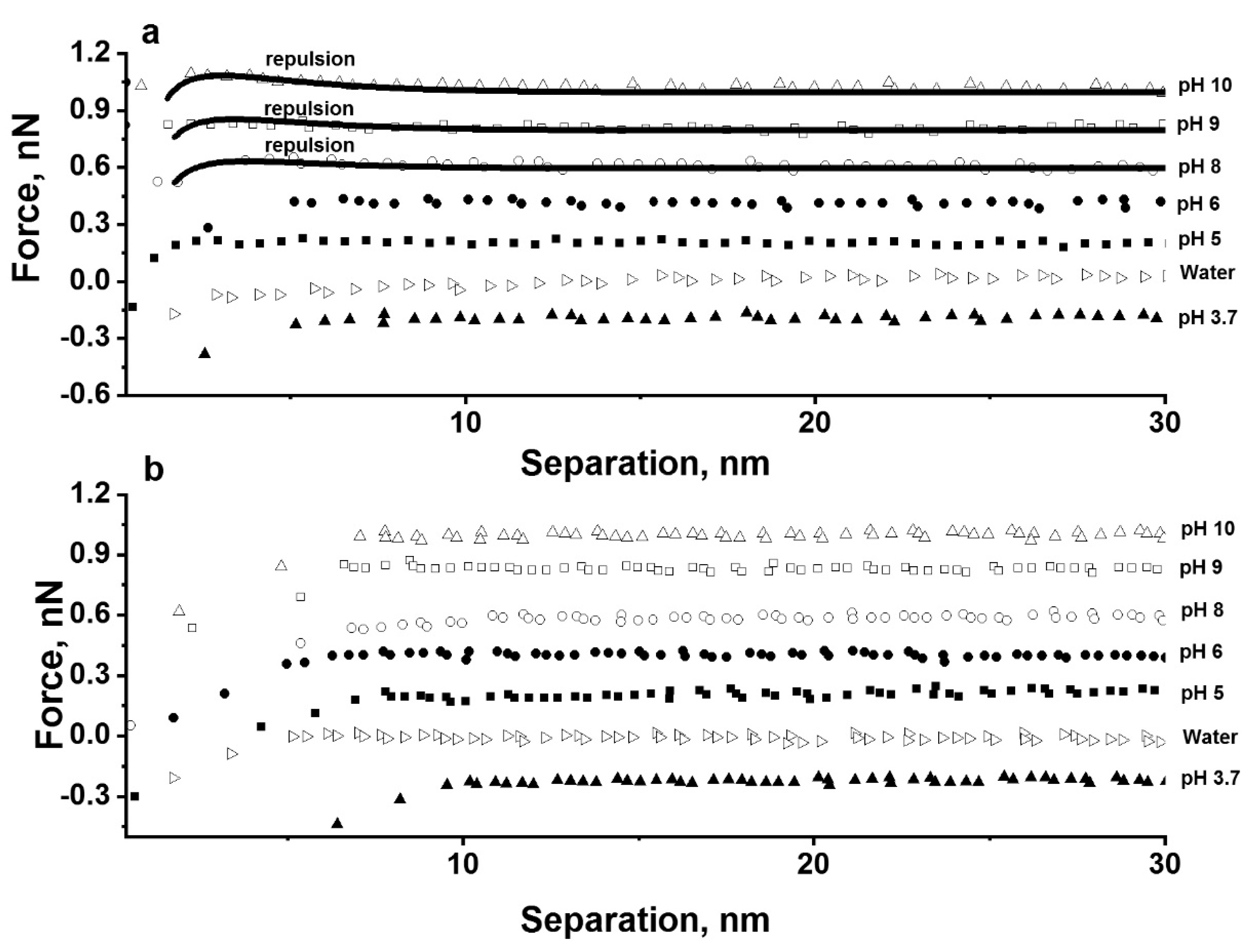
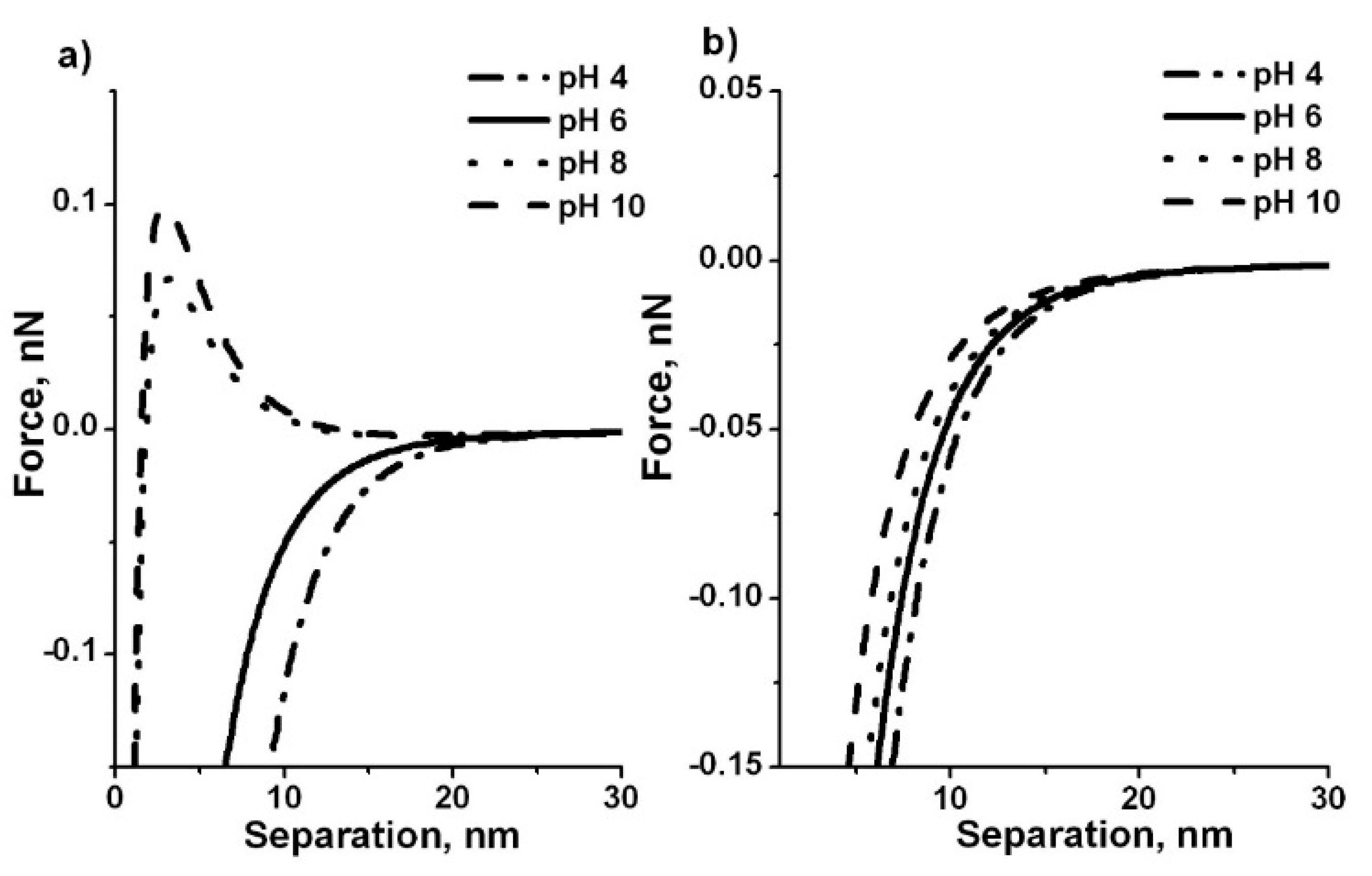
3.2. Effect of Ion Concentration and pH on Particle Interaction
3.3. Adhesion Forces between Nanomagnetite and Silica
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Kappl, M.; Butt, H.-J. The Colloidal Probe Technique and its Application to Adhesion Force Measurements. Part. Part. Syst. Charact. 2002, 19, 129–143. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Dobryden, I.; Yang, X.; Almqvist, N.; Holmgren, A.; Weber, H. An atomic force microscopy study of the interaction between magnetite particles: The effect of Ca2+ ions and pH. Powder Technol. 2013, 233. [Google Scholar] [CrossRef]
- Dobryden, I.; Potapova, E.; Holmgren, A.; Weber, H.; Hedlund, J.; Almqvist, N. Force interactions between magnetite silica. and bentonite studied with atomic force microscopy. Phys. Chem. Miner. 2015, 42. [Google Scholar] [CrossRef]
- Champagne, P.-O.; Westwick, H.; Bouthillier, A.; Sawan, M. Colloidal stability of superparamagnetic iron oxide nanoparticles in the central nervous system: A review. Nanomedicine 2018, 13, 1385–1400. [Google Scholar] [CrossRef]
- Stevenson, M.C.; Beaudoin, S.P.; Corti, D.S. Toward an Improved Method for Determining the Hamaker Constant of Solid Materials Using Atomic Force Microscopy. I. Quasi-Static Analysis for Arbitrary Surface Roughness. J. Phys. Chem. C 2020, 124, 3014–3027. [Google Scholar] [CrossRef]
- Voogt, F.C.; Palstra, T.T.M.; Niesen, L.; Rogojanu, O.C.; James, M.A.; Hibma, T. Superparamagnetic behavior of structural domains in epitaxial ultrathin magnetite films. Phys. Rev. B 1998, 57, R8107–R8110. [Google Scholar] [CrossRef]
- Potapova, E.; Yang, X.; Westerstrand, M.; Grahn, M.; Holmgren, A.; Hedlund, J. Interfacial properties of natural magnetite particles compared with their synthetic analogue. Miner. Eng. 2012, 36–38, 187–194. [Google Scholar] [CrossRef][Green Version]
- Toikka, G.; Hayes, R.A.; Ralston, J.C. Adhesion of Iron Oxide to Silica Studied by Atomic Force Microscopy. J. Colloid Interface Sci. 1996, 180, 329–338. [Google Scholar] [CrossRef]
- Pensini, E.; Sleep, B.E.; Yip, C.M.; O’Carroll, D.M. Forces of interaction between fresh iron particles and iron oxide (magnetite): Effect of water chemistry and polymer coatings. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 104–110. [Google Scholar] [CrossRef]
- Pensini, E.; Sleep, B.E.; Yip, C.M.; O’Carroll, D.M. Forces of Interactions between Bare and Polymer-Coated Iron and Silica: Effect of pH. Ionic Strength. and Humic Acids. Environ. Sci. Technol. 2012, 46, 13401–13408. [Google Scholar] [CrossRef]
- Christenson, H.K. DLVO (Derjaguin–Landau–Verwey–Overbeek) theory and solvation forces between mica surfaces in polar and hydrogen-bonding liquids. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1984, 80, 1933–1946. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique. interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Trefalt, G.; Behrens, S.H.; Borkovec, M. Charge Regulation in the Electrical Double Layer: Ion Adsorption and Surface Interactions. Langmuir 2016, 32, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, F.J.M.; Oncsik, T.; Rodriguez-Valverde, M.A.; Maroni, P.; Cabrerizo-Vilchez, M. Specific Ion Effects and pH Dependence on the Interaction Forces between Polystyrene Particles. Langmuir 2016, 32, 11918–11927. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, Y.I.; Adler, J.J.; Ata, A.; Singh, R.K.; Moudgil, B.M. Adhesion between Nanoscale Rough Surfaces: II. Measurement and Comparison with Theory. J. Colloid Interface Sci. 2000, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gao, X.; Tian, R.; Li, H. Approach to Estimation of Hamaker Constant as Taking Hofmeister Effects into Account. J. Phys. Chem. C 2018, 122, 9432–9440. [Google Scholar] [CrossRef]
- Weimer, J.J. An uncertainty budget analysis on the Hamaker constant determined when fitting force-distance curves for a sphere-plate system. Measurement 2018, 118, 120–134. [Google Scholar] [CrossRef]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface energy and the contact of elastic solids. Proc. R Soc. Lond. A Math. Phys. Sci. 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Rumpf, H. Particle Technology; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Potapova, E.; Grahn, M.; Holmgren, A.; Hedlund, J. The effect of calcium ions and sodium silicate on the adsorption of a model anionic flotation collector on magnetite studied by ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2010, 345, 96–102. [Google Scholar] [CrossRef]
- Senden, T.J. Force microscopy and surface interactions. Curr. Opin. Colloid Interface Sci. 2001, 6, 95–101. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Bergström, L. Hamaker Constants of Iron Oxide Nanoparticles. Langmuir 2011, 27, 8659–8664. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–104. [Google Scholar] [CrossRef]
- Janusz, W.; Patkowski, J.; Chibowski, S. Competitive adsorption of Ca2+ and Zn(II) ions at monodispersed SiO2/electrolyte solution interface. J. Colloid Interface Sci. 2003, 266, 259–268. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Studies on Bitumen−Silica Interaction in Aqueous Solutions by Atomic Force Microscopy. Langmuir 2003, 19, 3911–3920. [Google Scholar] [CrossRef]
- Potapova, E.; Yang, X.; Grahn, M.; Holmgren, A.; Forsmo, S.; Fredriksson, A.; Hedlund, J. The effect of calcium ions. sodium silicate and surfactant on charge and wettability of magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 79–86. [Google Scholar] [CrossRef]
- Zeltner, W.A.; Anderson, M.A. Surface charge development at the goethite/aqueous solution interface: Effects of CO2 adsorption. Langmuir 1988, 4, 469–474. [Google Scholar] [CrossRef]
- Potapova, E.; Jolsterå, R.; Holmgren, A.; Grahn, M. In-situ spectroscopic study of surfactant adsorption onto hematite from binary mixtures and the effect of inorganic ions. Surf. Interface Anal. 2014, 46, 1110–1114. [Google Scholar] [CrossRef]
- Burnham, N.A.; Chen, X.; Hodges, C.S.; Matei, G.A.; Thoreson, E.; Roberts, C.J.; Davies, M.C.; Tendler, S.J.B. Comparison of calibration methods for atomic-force microscopy cantilevers. Nanotechnology 2002, 14, 1–6. [Google Scholar] [CrossRef]
- Liang, Y.; Hilal, N.; Langston, P.; Starov, V. Interaction forces between colloidal particles in liquid: Theory and experiment. Adv. Colloid Interface Sci. 2007, 134–135, 151–166. [Google Scholar] [CrossRef]
- Baldessari, F. Electrokinetics in nanochannels: Part I. Electric double layer overlap and channel-to-well equilibrium. J. Colloid Interface Sci. 2008, 325, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Creuzet, F.; Arribart, H. Atomic force microscopy for local characterization of surface acid-base properties. J. Phys. Chem. 1993, 97, 7272–7276. [Google Scholar] [CrossRef]
- Sokolov, I.Y.; Ong, Q.K.; Shodiev, H.; Chechik, N.; James, D.; Oliver, M. AFM study of forces between silica. silicon nitride and polyurethane pads. J. Colloid Interface Sci. 2006, 300, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.; Bauer, K.; Niinimaki, J.; Peuker, U.A. Validity of the Rumpf and the Rabinovich adhesion force models for alumina substrates with nanoscale roughness. Powder Technol. 2013, 246, 545–552. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobryden, I.; Mensi, E.; Holmgren, A.; Almqvist, N. Surface Forces between Nanomagnetite and Silica in Aqueous Ca2+ Solutions Studied with AFM Colloidal Probe Method. Colloids Interfaces 2020, 4, 41. https://doi.org/10.3390/colloids4030041
Dobryden I, Mensi E, Holmgren A, Almqvist N. Surface Forces between Nanomagnetite and Silica in Aqueous Ca2+ Solutions Studied with AFM Colloidal Probe Method. Colloids and Interfaces. 2020; 4(3):41. https://doi.org/10.3390/colloids4030041
Chicago/Turabian StyleDobryden, Illia, Elizaveta Mensi, Allan Holmgren, and Nils Almqvist. 2020. "Surface Forces between Nanomagnetite and Silica in Aqueous Ca2+ Solutions Studied with AFM Colloidal Probe Method" Colloids and Interfaces 4, no. 3: 41. https://doi.org/10.3390/colloids4030041
APA StyleDobryden, I., Mensi, E., Holmgren, A., & Almqvist, N. (2020). Surface Forces between Nanomagnetite and Silica in Aqueous Ca2+ Solutions Studied with AFM Colloidal Probe Method. Colloids and Interfaces, 4(3), 41. https://doi.org/10.3390/colloids4030041





