Interaction of Polyoxometalates and Nanoparticles with Collector Surfaces—Focus on the Use of Streaming Current Measurements at Flat Surfaces
Abstract
1. Introduction
1.1. Background
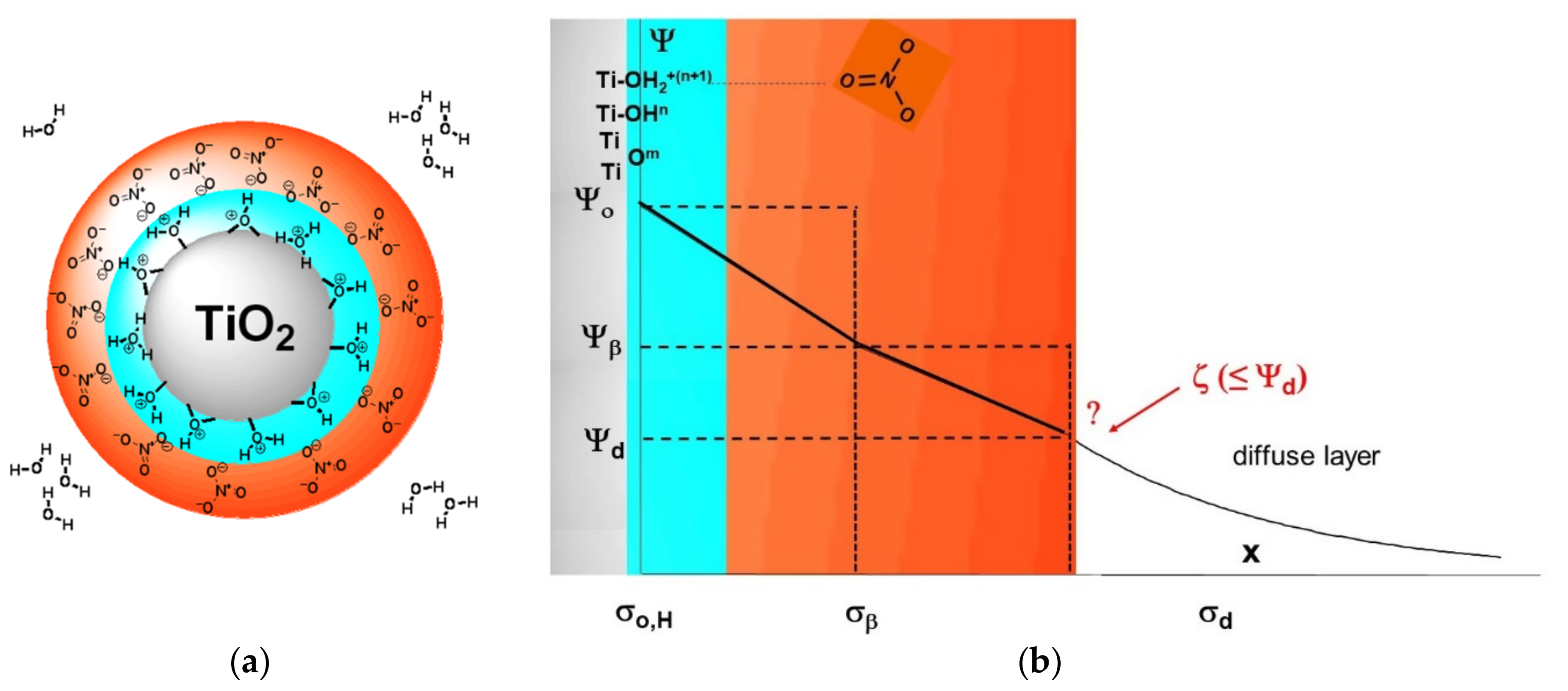
1.2. Short Survey of Oxide–Electrolyte Interfaces
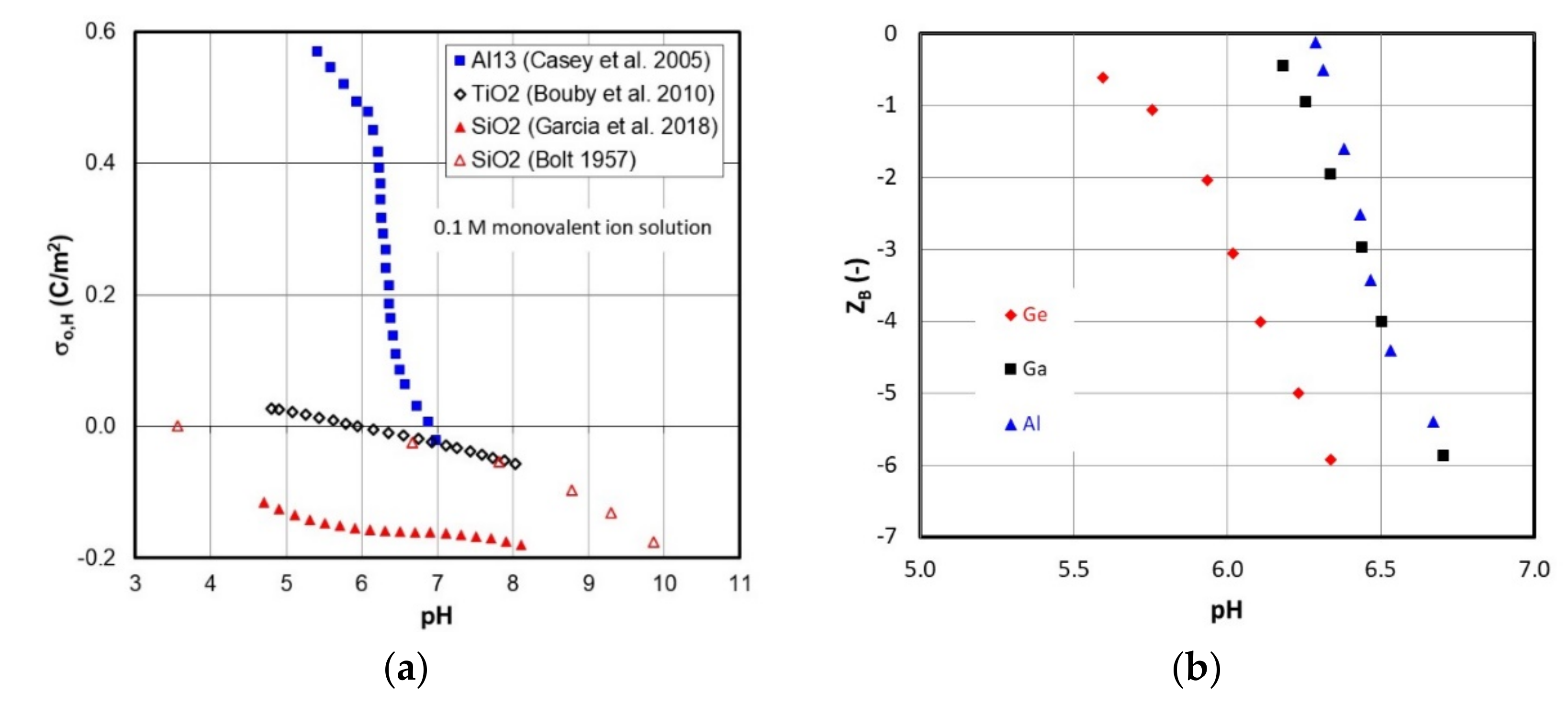
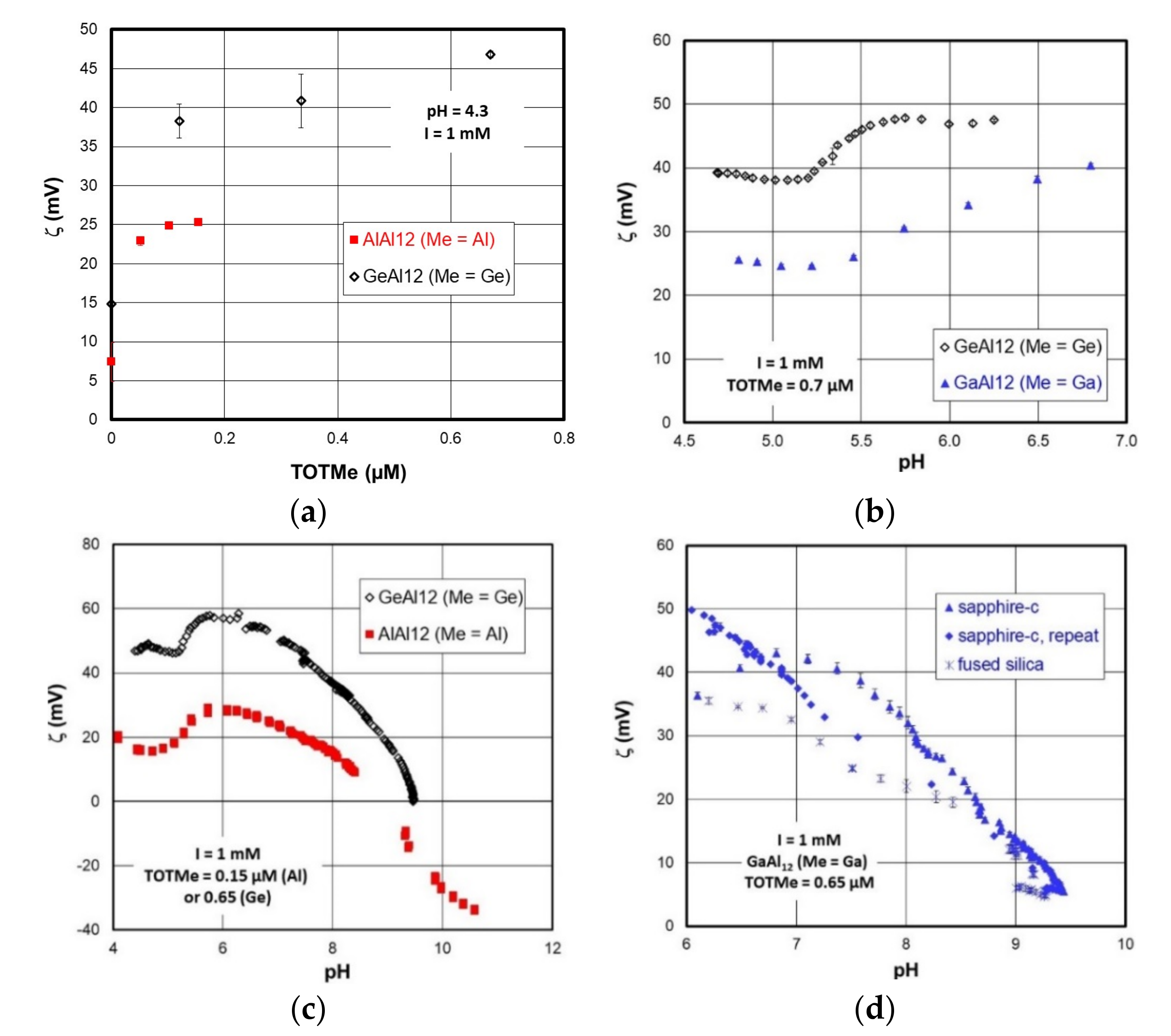
1.3. Short Survey of MeAl12-Keggin Acid–Base Chemistry
1.4. Corollary of Methods Applied and Systems Studied in the Present Work
1.5. Aims of the Study and Strategy
2. Materials and Methods
2.1. Solutions
2.2. Materials
2.2.1. Nanoparticles
TiO2 Nanoparticles
SiO2 Nanoparticles
2.2.2. Keggin-Ions
2.2.3. Collector Surfaces
2.3. Experimental Procedures and Methods
2.3.1. Electrophoretic Mobility Measurements
2.3.2. Determination of Zeta-Potential at Flat Surfaces
2.3.3. pH Measurements
2.3.4. Size Measurements
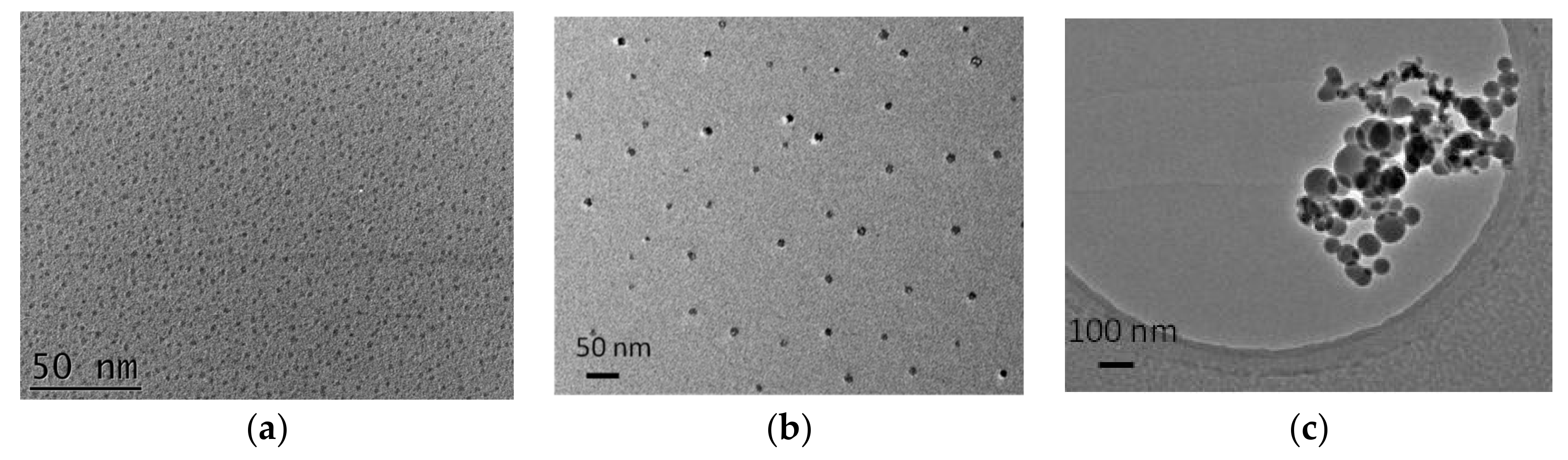
2.3.5. AFM and TEM Measurements
3. Results and Discussion
3.1. Determination of Zeta-Potentials of Flat Collector Surfaces Covered by POMs
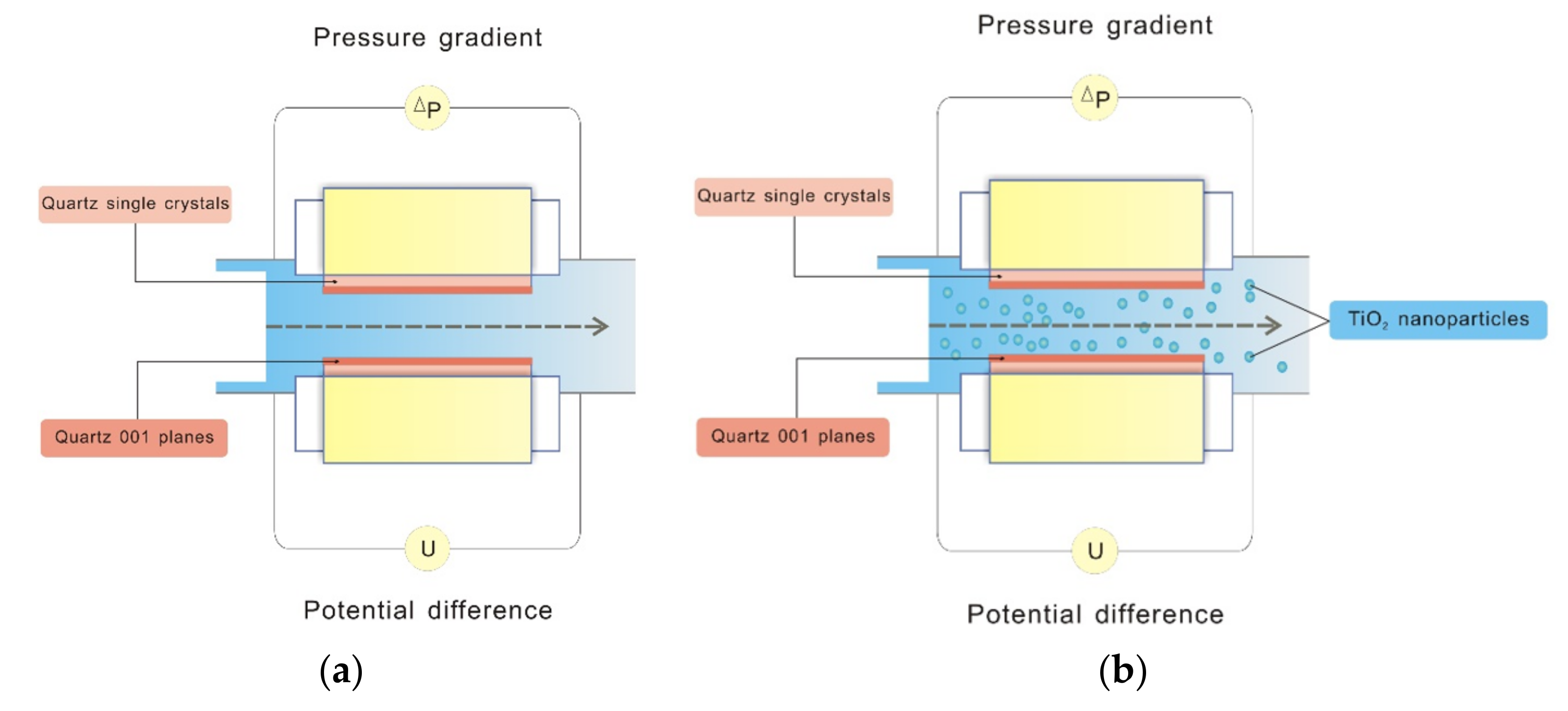
3.2. Determination of Zeta-Potentials via Streaming Current Measurements on Quartz (001) Single Crystals in the Presence of Aqueous Sample A TiO2 NPs
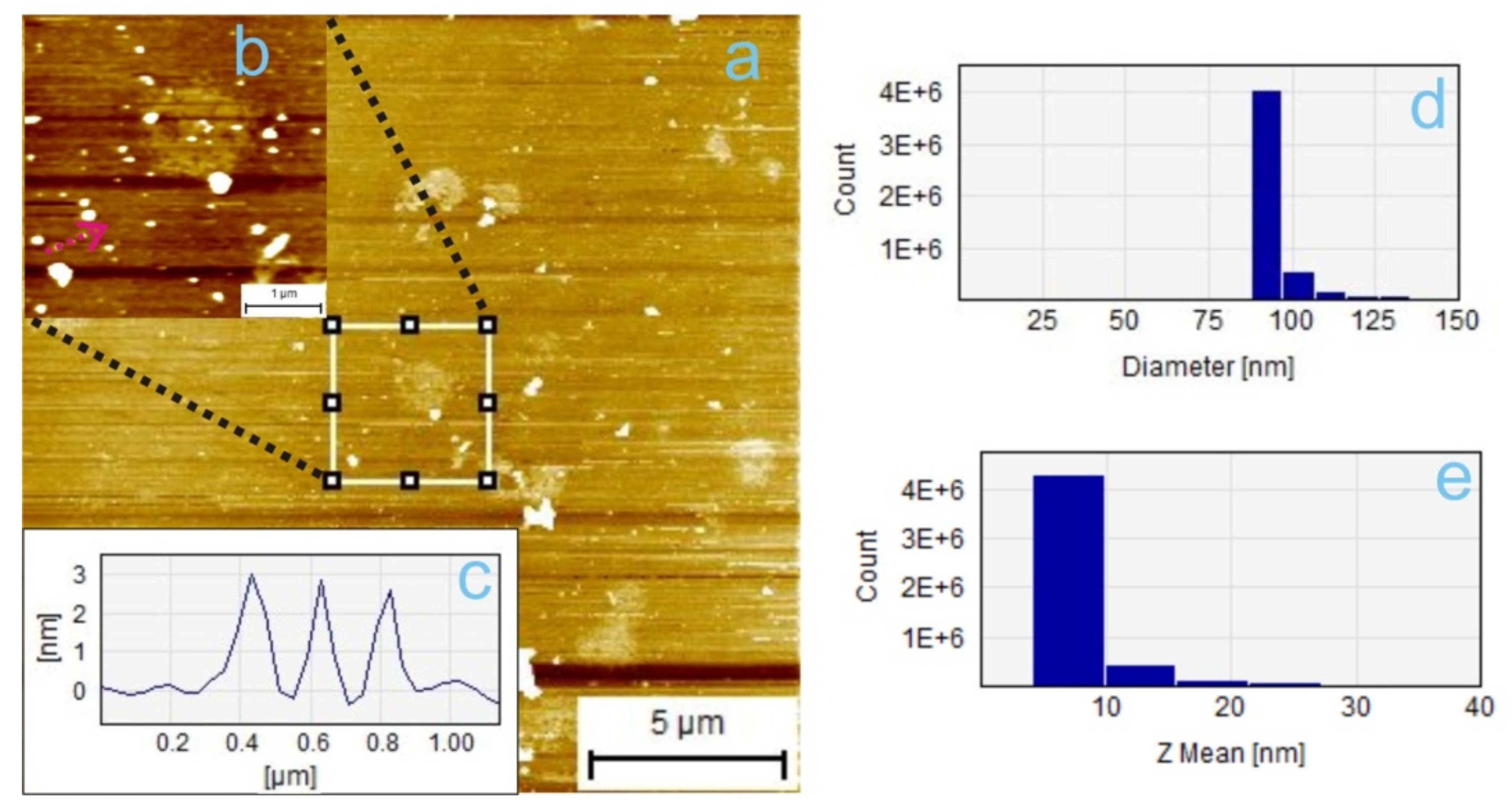
3.2.1. TiO2 Sample A Size Measurements
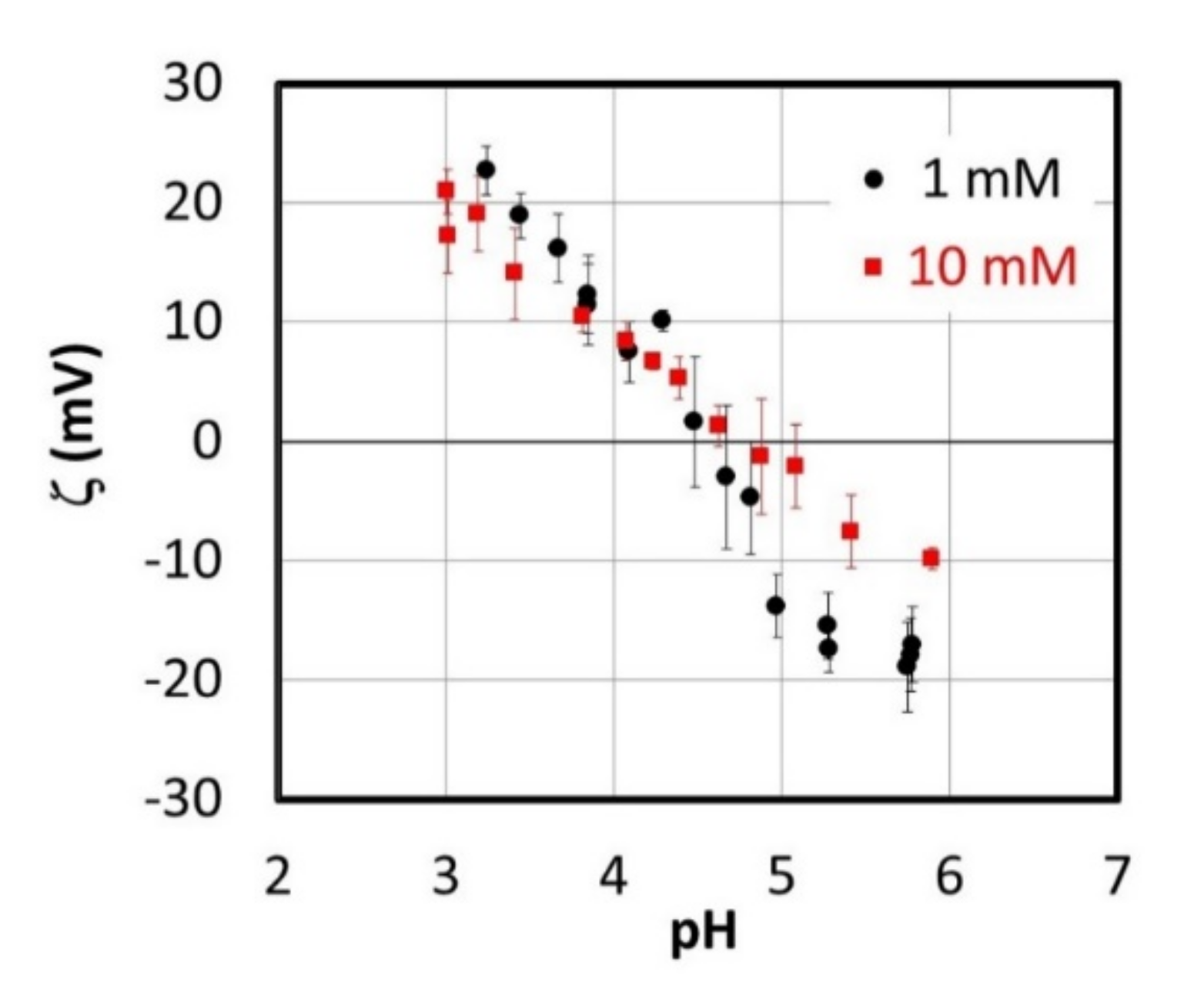
3.2.2. TiO2 Sample A Charging Characteristics
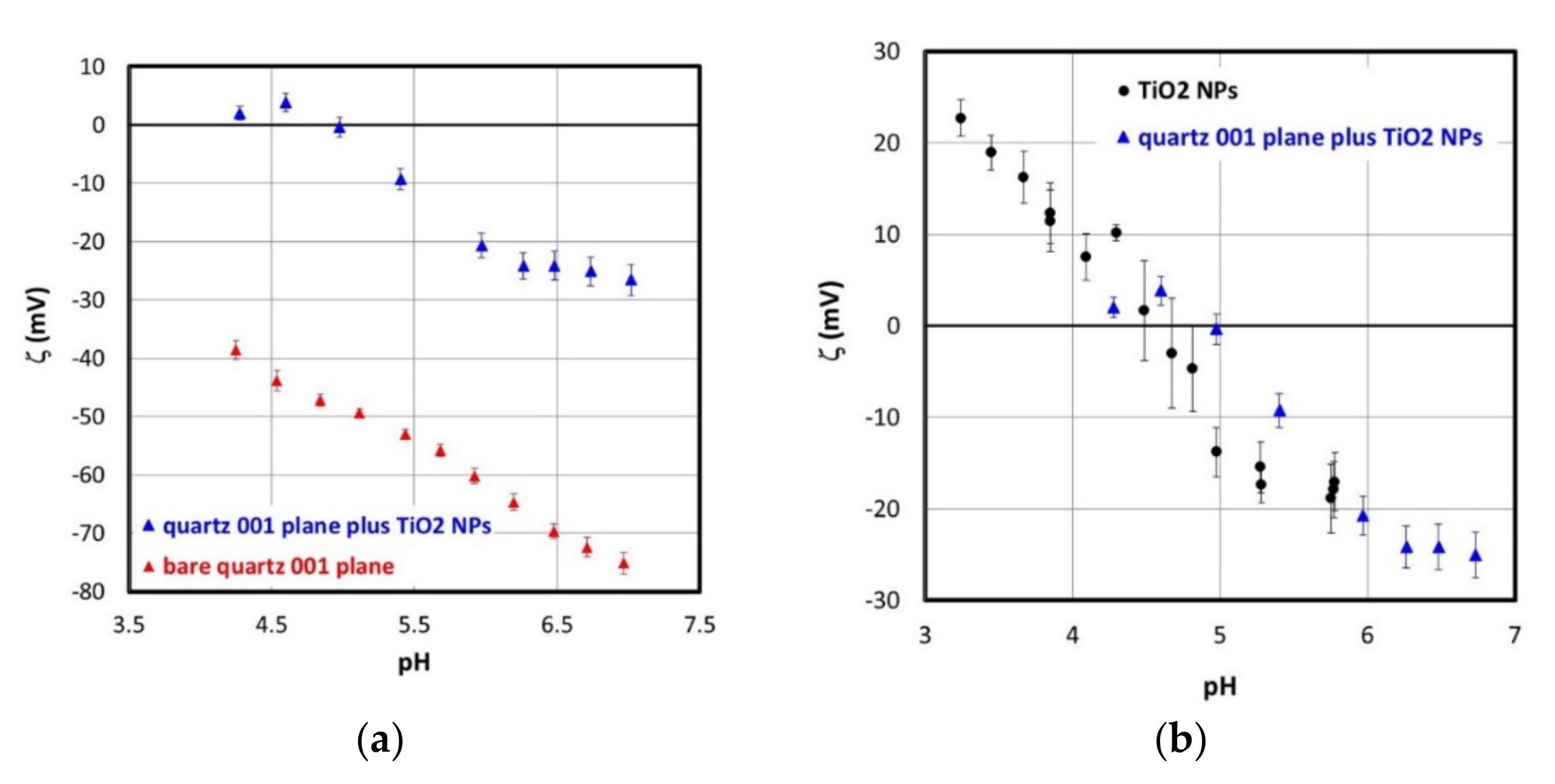
3.3. Interaction of Sample A TiO2 Nanoparticles with SiO2 Collector Particles
3.4. Additional Binary Systems Involving SiO2 and TiO2 Surfaces
3.4.1. Particle Charging Characteristics of TiO2 NPs (Samples B and C)
3.4.2. Sample B NP Interaction with Quartz Collector Surfaces
3.4.3. Sample C NPs in the Presence of Quartz Collector Surfaces
3.5. Interactions of SiO2 Nanoparticles with Flat Rutile Collector Surfaces
4. Conclusions and Summary
- The proposed approach is able to detect and collect very small NPs present in very small quantities.
- The collected NPs can subsequently (i.e., after, e.g., a streaming current experiment) be used for further studies.
- The interfacial behavior can be studied in more detail addressing for example interactions or remobilization under what we defined as “unfavorable” conditions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. The behavior and effects of nanoparticles in the environment. Environ. Pollut. 2009, 157, 1063–1064. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Schoenfisch, M.H. Silica Nanoparticle Phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [PubMed]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, Uptake, and Translocation of Engineered Nanomaterials in Vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.E.; Auffan, M.; Durenkamp, M.; McGrath, S.; Bottero, J.-Y.; Wiesner, M.R. Monte Carlo simulations of the transformation and removal of Ag, TiO2, and ZnO nanoparticles in wastewater treatment and land application of biosolids. Sci. Total Environ. 2015, 511, 535–543. [Google Scholar] [CrossRef]
- Schaumann, G.E.; Philippe, A.; Bundschuh, M.; Metreveli, G.; Klitzke, S.; Rakcheev, D.; Grün, A.; Kumahor, S.K.; Kühn, M.; Baumann, T.; et al. Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci. Total Environ. 2015, 535, 3–19. [Google Scholar] [CrossRef]
- Farkas, J.; Peter, H.; Gesielski, T.M.; Thomas, K.V.; Sommaruga, R.; Salvenmoser, W.; Weyhenmeyer, G.A.; Tranvik, L.J.; Jenssen, B.M. Impact of TiO2 nanoparticles on freshwater bacteria from three Swedish lakes. Sci. Total Environ. 2015, 535, 85–93. [Google Scholar] [CrossRef]
- Chen, L.; Sabatini, D.A.; Kibbey, T.C.G. Role of the Air–Water Interface in the Retention of TiO2 Nanoparticles in Porous Media during Primary Drainage. Environ. Sci. Technol. 2008, 42, 1916–1921. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.-H.; Zhang, H.; Zhao, L. UV Irradiation Induced Transformation of TiO2 Nanoparticles in Water: Aggregation and Photoreactivity. Environ. Sci. Technol. 2014, 48, 11962–11968. [Google Scholar] [CrossRef]
- Hu, J.; Shipley, H.J. Evaluation of desorption of Pb (II), Cu (II) and Zn (II) from titanium dioxide nanoparticles. Sci. Total Environ. 2012, 431, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Erhayem, M.; Sohn, M. Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter. Sci. Total Environ. 2014, 468, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Maczka, E.; Luetzenkirchen, J.; Kosmulski, M. The significance of the solid-to-liquid ratio in the electrokinetic studies of the effect of ionic surfactants on mineral oxides. J. Colloid Interface Sci. 2013, 393, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [PubMed]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Scheringr, M.; Bucheli, T.D.; Hungerbuhler, K. Critical Assessment of Models for Transport of Engineered Nanoparticles in Saturated Porous Media. Environ. Sci. Technol. 2014, 48, 12732–12741. [Google Scholar] [CrossRef]
- Darbha, G.K.; Fischer, C.; Michler, A.; Luetzenkirchen, J.; Schäfer, T.; Heberling, F.; Schild, D. Deposition of Latex Colloids at Rough Mineral. Surfaces: An Analogue Study Using Nanopatterned Surfaces. Langmuir 2012, 28, 6606–6617. [Google Scholar] [CrossRef]
- Martin Cabanas, B.; Lützenkirchen, J.; Leclercq, S.; Barboux, P.; Lefevre, G. Surface charging patterns of stainless alloys—Effect of ageing in conditions of primary cooling circuit of pressurized water reactors. J. Nucl. Mater. 2012, 430, 150–155. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Zhang, W.; Pu, Z.; Jiang, L.; Chen, Y. Surface Interactions Affect the Toxicity of Engineered Metal. Oxide Nanoparticles toward Paramecium. Chem. Res. Toxicol. 2012, 25, 1675–1681. [Google Scholar] [CrossRef]
- Florez, L.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K.; Crespy, D.; Mailänder, V. How Shape Influences Uptake: Interactions of Anisotropic Polymer Nanoparticles and Human Mesenchymal Stem Cells. Small 2012, 8, 2222–2230. [Google Scholar] [CrossRef]
- Som, C.; Wick, P.; Krug, H.; Nowack, B. Environmental and health effects of nanomaterials in nanotextiles and facade coatings. Environ. Int. 2011, 37, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Güleryüz, H.; Filiatre, C.; Euvrard, M.; Buron, C.; Lakard, B. Novel strategy to prepare polyaniline—Modified SiO2/TiO2 composite particles. Synth. Met. 2013, 181, 104–109. [Google Scholar] [CrossRef]
- Dietrich, L.A.; Sahu, M.; Biswas, P.; Fein, J.B. Experimental study of TiO2 nanoparticle adhesion to silica and Fe(III) oxide-coated silica surfaces. Chem. Geol. 2012, 332, 148–156. [Google Scholar] [CrossRef]
- Bahadur, J.; Sen, D.; Mazumder, S.; Sastry, P.U.; Paul, B.; Bhatt, H.; Singh, S.G. One-Step Fabrication of Thermally Stable TiO2/SiO2 Nanocomposite Microspheres by Evaporation-Induced Self-Assembly. Langmuir 2012, 28, 11343–11353. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tian, B.; Zhang, J. Thermally stable SiO2-doped mesoporous anatase TiO2 with large surface area and excellent photocatalytic activity. J. Colloid Interface Sci. 2010, 344, 382–389. [Google Scholar] [CrossRef]
- Lutzenkirchen, J.; Behra, P. A new approach for modelling potential effects in cation adsorption onto binary (hydr)oxides. J. Contam. Hydrol. 1997, 26, 257–268. [Google Scholar] [CrossRef]
- De Faria, L.; Trasatti, S. Effect of composition on the point of zero charge of RuO2 + TiO2 mixed oxides. J. Electroanal. Chem. 1992, 340, 145–152. [Google Scholar] [CrossRef]
- Reymond, J.; Kolenda, F. Estimation of the point of zero charge of simple and mixed oxides by mass titration. Powder Technol. 1999, 103, 30–36. [Google Scholar] [CrossRef]
- Mustafa, S.; Dilara, B.; Nargis, K.; Naeem, A.; Shahida, P. Surface properties of the mixed oxides of iron and silica. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 273–282. [Google Scholar] [CrossRef]
- Schwarz, J.; Driscoll, C.; Bhanot, A. The zero point of charge of silica—Alumina oxide suspensions. J. Colloid Interface Sci. 1984, 97, 55–61. [Google Scholar] [CrossRef]
- Koopal, L.K.; Dukhin, S.S. Modelling of the double layer and electrosorption of a patchwise heterogeneous surface on the basis off its homogeneous analogue 1. Non-interacting patches. Colloids Surf. A Physicochem. Eng. Asp. 1993, 73, 201–209. [Google Scholar] [CrossRef]
- Furrer, G.; Ludwig, C.; Schindler, P.W. On the Chemistry of the Keggin Al13 Polymer.1. Acid-Base Properties. J. Colloid Interface Sci. 1992, 149, 56–67. [Google Scholar] [CrossRef]
- Lee, A.P.; Furrer, G.; Casey, W.H. On the acid-base chemistry of the Keggin polymers: GaAl12 and GeAl12. J. Colloid Interface Sci. 2002, 250, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J. Synthèse de Nanoparticules D’Oxydes de fer et D’Aluminium pour L’Étude de L’Adsorption D’Entités Inorganiques Polycondensées. Conséquences sur la Stabilisation des Dispersions. Ph.D. Thesis, Universite Paris 6, Paris, France, 1998. [Google Scholar]
- Batista, C.A.S.; Larson, R.G.; Kotov, N.A. Nonadditivity of nanoparticle interactions. Science 2015, 350, 1242477. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; James, R.O.; Leckie, J.O. Surface ionization and complexation at the oxide/water interface: I. Computation of electrical double layer properties in simple electrolytes. J. Colloid Interface Sci. 1978, 63, 480–499. [Google Scholar] [CrossRef]
- Yates, D.E.; Levine, S.; Healy, T.W. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1974, 70, 1807–1818. [Google Scholar] [CrossRef]
- Darbha, G.K.; Fischer, C.; Luetzenkirchen, J.; Schäfer, T. Site-Specific Retention of Colloids at Rough Rock Surfaces. Environ. Sci. Technol. 2012, 46, 9378–9387. [Google Scholar] [CrossRef]
- Casey, W.H. Large Aqueous Aluminum Hydroxide Molecules. Chem. Rev. 2006, 106, 1–16. [Google Scholar] [CrossRef]
- Lützenkirchen, J.; Marsac, R.; Casey, W.H.; Furrer, G.; Kupcik, T.; Lindqvist-Reis, P. The Effect of Monovalent Electrolytes on the Deprotonation of MAl12 Keggin Ions. Aquat. Geochem. 2015, 21, 81–97, Erratum in 2015, 21, 555. [Google Scholar] [CrossRef]
- Bouby, M.; Lützenkirchen, J.; Dardenne, K.; Preocanin, T.; Denecke, M.A.; Klenze, R.; Geckeis, H. Sorption of Eu(III) onto titanium dioxide: Measurements and modeling. J. Colloid Interface Sci. 2010, 350, 551–561. [Google Scholar] [PubMed]
- Bolt, G.H. Determination of the Charge Density of Silica Sols. J. Phys. Chem. 1957, 61, 1166–1169. [Google Scholar] [CrossRef]
- García, D.; Lützenkirchen, J.; Petrov, V.; Siebentritt, M.; Schild, D.; Lefèvre, G.; Rabung, T.; Altmaier, M.; Kalmykov, S.; Duro, L.; et al. Sorption of Eu(III) on quartz at high salt concentrations. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123610. [Google Scholar] [CrossRef]
- Lutzenkirchen, J.; Preočanin, T.; Kovačević, D.; Tomišić, V.; Lövgren, L.; Kallay, N. Potentiometric Titrations as a Tool for Surface Charge Determination. Croat. Chem. Acta 2012, 85, 391–417. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar]
- Scales, P.J.; Grieser, F.; Healy, T.W.; White, L.R.; Chan, D.Y. Electrokinetics of the Silica Solution Interface—A Flat-Plate Streaming Potential Study. Langmuir 1992, 8, 965–974. [Google Scholar] [CrossRef]
- Kallay, N.; Torbic, Z.; Golic, M.; Matijevic, E. Determination of the Isoelectric Points of Several Metals by an Adhesion Method. J. Phys. Chem. 1991, 95, 7028–7032. [Google Scholar] [CrossRef]
- Lin, X.Y.; Farhi, E.; Arribart, H. Determination of the Isoelectric Point of Planar Oxide Surfaces by a Particle Adhesion Method. J. Adhes. 1995, 51, 181–189. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Nattich, M.; Wasilewska, M.; Zaucha, M. Colloid particle and protein deposition—Electrokinetic studies. Adv. Colloid Interface Sci. 2011, 168, 3–28. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Zaucha, M.; Zembala, M. Zeta Potential Mica Cover. By Colloid Part: A Streaming Potential Study. Langmuir 2010, 26, 9368–9377. [Google Scholar]
- Nattich-Rak, M.; Adamczyk, Z.; Sadowska, M.; Morga, M.; Oćwieja, M. Hematite nanoparticle monolayers on mica: Characterization by colloid deposition. Colloids Surf. A-Physicochem. Eng. Asp. 2012, 412, 72–81. [Google Scholar] [CrossRef]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [PubMed]
- Lutzenkirchen, J.; Kupcik, T.; Fuss, M.; Walther, C.; Sarpola, A.; Sundman, O. Adsorption of Al-13-Keggin clusters to sapphire c-plane single crystals: Kinetic observations by streaming current measurements. Appl. Surf. Sci. 2010, 256, 5406–5411. [Google Scholar] [CrossRef]
- Kersting, A.B. Plutonium Transport in the Environment. Inorg. Chem. 2013, 52, 3533–3546. [Google Scholar] [CrossRef]
- Kersting, A.B.; Efurd, D.W.; Finnegan, D.L.; Rokop, D.J.; Smith, D.K.; Thompson, J.L. Migration of plutonium in ground water at the Nevada Test Site. Nature 1999, 397, 56–59. [Google Scholar]
- Altmaier, M.; Gaona, X.; Fanghänel, T. Recent Advances in Aqueous Actinide Chemistry and Thermodynamics. Chem. Rev. 2013, 113, 901–943. [Google Scholar] [CrossRef] [PubMed]
- Morga, M.; Adamczyk, Z.; Kosior, D.; Oćwieja, M. Hematite/silica nanoparticle bilayers on mica: AFM and electrokinetic characterization. Phys. Chem. Chem. Phys. 2018, 20, 15368–15379. [Google Scholar] [CrossRef] [PubMed]
- Morga, M.; Adamczyk, Z.; Basinska, T.; Komar, P.; Gosecka, M.; Żeliszewska, P.; Wasilewska, M. Spheroidal Microparticle Monolayers Characterized by Streaming Potential Measurements. Langmuir 2017, 33, 9916–9925. [Google Scholar] [CrossRef]
- Oćwieja, M.; Maciejewska-Prończuk, J.; Adamczyk, Z.; Roman, M. Formation of positively charged gold nanoparticle monolayers on silica sensors. J. Colloid Interface Sci. 2017, 501, 192–201. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Bonifacio, L.D.; Oreopoulos, J.; Yip, C.M.; Manners, I.; Ozin, G.A. Color from colorless nanomaterials: Bragg reflectors made of nanoparticles. J. Mater. Chem. 2009, 19, 3500–3506. [Google Scholar] [CrossRef]
- Makumire, S.; Chakravadhanula, V.S.; Köllisch, G.; Redel, E.; Shonhai, A. Immunomodulatory activity of zinc peroxide (ZnO2) and titanium dioxide (TiO2) nanoparticles and their effects on DNA and protein integrity. Toxicol. Lett. 2014, 227, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Špadina, M.; Plodinec, M.; Delač Marion, I.; Willinger, M.G.; Lützenkirchen, J.; Gafney, H.D.; Redel, E.A. Experimental and Theoretical Approach to Understanding the Surface Properties of One-Dimensional TiO2 Nanomaterials. J. Phys. Chem. C 2015, 119, 19729–19742, Correction in 2016, 120, 4150. [Google Scholar] [CrossRef]
- Vayssieres, L. On the thermodynamic stability of metal oxide nanoparticles in aqueous solutions. Int. J. Nanotechnol. 2005, 2, 411–439. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Sulpizi, M.; Lützenkirchen, J. Atypical titration curves for GaAl12 Keggin-ions explained by a joint experimental and simulation approach. J. Chem. Phys. 2018, 148, 222836. [Google Scholar] [CrossRef]
- Lutzenkirchen, J. Unpublished results
- Lützenkirchen, J.; Zimmermann, R.; Preočanin, T.; Filby, T.; Kupcik, T.; Küttner, D.; Abdelmonem, A.; Schild, D.; Rabung, T.; Plaschke, M.; et al. An attempt to explain bimodal behaviour of the sapphire c-plane electrolyte interface. Adv. Colloid Interface Sci. 2010, 157, 61–74. [Google Scholar]
- Brkljača, Z.; Namjesnik, D.; Lützenkirchen, J.; Předota, M.; Preočanin, T. Quartz/Aqueous Electrolyte Solution Interface: Molecular Dynamic Simulation and Interfacial Potential Measurements. J. Phys. Chem. C 2018, 122, 24025–24036. [Google Scholar] [CrossRef]
- Preočanin, T.; Namjesnik, D.; Brown, M.A.; Lützenkirchen, J. The relationship between inner surface potential and electrokinetic potential from an experimental and theoretical point of view. Environ. Chem. 2017, 14, 295–309. [Google Scholar] [CrossRef]
- Lützenkirchen, J. Specific Ion. Effects at Two Single-Crystal Planes of Sapphire. Langmuir 2013, 29, 7726–7734. [Google Scholar]
- Lützenkirchen, J.; Franks, G.V.; Plaschke, M.; Zimmermann, R.; Heberling, F.; Abdelmonem, A.; Darbha, G.K.; Schild, D.; Filby, A.; Eng, P.; et al. The surface chemistry of sapphire-c: A literature review and a study on various factors influencing its IEP. Adv. Colloid Interface Sci. 2018, 251, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, C.; Waychunas, G.A.; Shen, Y.R. Structures and charging of alpha-alumina (0001)/water interfaces studied by sum-frequency vibrational spectroscopy. J. Am. Chem. Soc. 2008, 130, 7686–7694. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H. A surface structural model for ferrihydrite I: Sites related to primary charge, molar mass, and mass density. Geochim. Cosmochim. Acta 2009, 73, 4423–4436. [Google Scholar] [CrossRef]
- Preocanin, T.; Selmani, A.; Lindqvist-Reis, P.; Heberling, F.; Kallay, N.; Lützenkirchen, J. Surface charge at Teflon/aqueous solution of potassium chloride interfaces. Colloids Surf. A-Physicochem. Eng. Asp. 2012, 412, 120–128. [Google Scholar] [CrossRef]
- Fatisson, J.; Domingos, R.F.; Wilkinson, K.J.; Tufenkji, N. Deposition of TiO2 Nanoparticles onto Silica Measured Using a Quartz Crystal Microbalance with Dissipation Monitoring. Langmuir 2009, 25, 6062–6069. [Google Scholar] [CrossRef]
- Wilhelm, P.; Stephan, D. On-line tracking of the coating of nanoscaled silica with titania nanoparticles via zeta-potential measurements. J. Colloid Interface Sci. 2006, 293, 88–92. [Google Scholar] [CrossRef]
- Hintz, W.; Kleinschmidt, S.; Yordanova-Bineva, V.; Tomas, J. Surface-modification of Silica-particles by nano-scaled Titania-particles via Sol.-Gel-Process. In Proceedings of the World Congress on Particle Technology 6, WCTP6 2010, Nürnberg, Germany, 24–29 April 2010. [Google Scholar]
- Bullard, J.W.; Cima, M.J. Orientation dependence of the isoelectric point of TiO2 (rutile) surfaces. Langmuir 2006, 22, 10264–10271. [Google Scholar] [CrossRef]
- Schmidt, M.; Wilson, R.E.; Lee, S.S.; Soderholm, L.; Fenter, P. Adsorption of Plutonium Oxide Nanoparticles. Langmuir 2012, 28, 2620–2627. [Google Scholar] [CrossRef]
- Altmaier, M.; Neck, V.; Lützenkirchen, J.; Fanghänel, T. Solubility of plutonium in MgCl2 and CaCl2 solutions in contact with metallic iron. Radiochim. Acta 2009, 97, 187–192. [Google Scholar] [CrossRef]
- Neck, V.; Altmaier, M.; Rabung, T.; Lützenkirchen, J.; Fanghänel, T. Thermodynamics of trivalent actinides and neodymium in NaCl, MgCl2, and CaCl2 solutions: Solubility, hydrolysis, and ternary Ca-M(III)-OH complexes. Pure Appl. Chem. 2009, 81, 1555–1568. [Google Scholar] [CrossRef]
- Lützenkirchen, J. Surface complexation models of adsorption: A critical survey in the context of experimental data. Surfactant Sci. Ser. 2002, 107, 631–710. [Google Scholar]
- Abbas, Z.; Labbez, C.; Nordholm, S.; Ahlberg, E. Size-dependent surface charging of nanoparticles. J. Phys. Chem. C 2008, 112, 5715–5723. [Google Scholar] [CrossRef]
- Vayssieres, L. On the Effect of Nanoparticle Size on Water-Oxide Interfacial Chemistry. J. Phys. Chem. C 2009, 113, 4733–4736. [Google Scholar] [CrossRef]




| TiO2 NP A | TiO2 NP B | TiO2 NP C | SiO2 NP A | SiO2 NP B | |
|---|---|---|---|---|---|
| Source/Reference | Synthesized [64] | Synthesized [61] | Synthesized [63] | LUDOX TMA Sigma Aldrich | Aerosil OX 50 Degussa |
| Primary particle size | <5 nm | 4–8 nm | 50–300 nm (diameter) 500–300 nm (length) | 22 nm | 40 nm |
| Size from measurements in suspension | 60 nm | 30–60 nm | NA | 40 nm | 450 nm |
| IEP (electrophoresis) | 4.4–5.1 | 6.1 | 3.6 | <3 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lützenkirchen, J.; Darbha, G.K.; Chakravadhanula, V.S.K.; Redel, E.; Selmani, A.; Vayssières, L. Interaction of Polyoxometalates and Nanoparticles with Collector Surfaces—Focus on the Use of Streaming Current Measurements at Flat Surfaces. Colloids Interfaces 2020, 4, 39. https://doi.org/10.3390/colloids4030039
Lützenkirchen J, Darbha GK, Chakravadhanula VSK, Redel E, Selmani A, Vayssières L. Interaction of Polyoxometalates and Nanoparticles with Collector Surfaces—Focus on the Use of Streaming Current Measurements at Flat Surfaces. Colloids and Interfaces. 2020; 4(3):39. https://doi.org/10.3390/colloids4030039
Chicago/Turabian StyleLützenkirchen, Johannes, Gopala Krishna Darbha, Venkata Sai Kiran Chakravadhanula, Engelbert Redel, Atiđa Selmani, and Lionel Vayssières. 2020. "Interaction of Polyoxometalates and Nanoparticles with Collector Surfaces—Focus on the Use of Streaming Current Measurements at Flat Surfaces" Colloids and Interfaces 4, no. 3: 39. https://doi.org/10.3390/colloids4030039
APA StyleLützenkirchen, J., Darbha, G. K., Chakravadhanula, V. S. K., Redel, E., Selmani, A., & Vayssières, L. (2020). Interaction of Polyoxometalates and Nanoparticles with Collector Surfaces—Focus on the Use of Streaming Current Measurements at Flat Surfaces. Colloids and Interfaces, 4(3), 39. https://doi.org/10.3390/colloids4030039








