3.1. The Difference of g(r) in Binary and Unary Systems
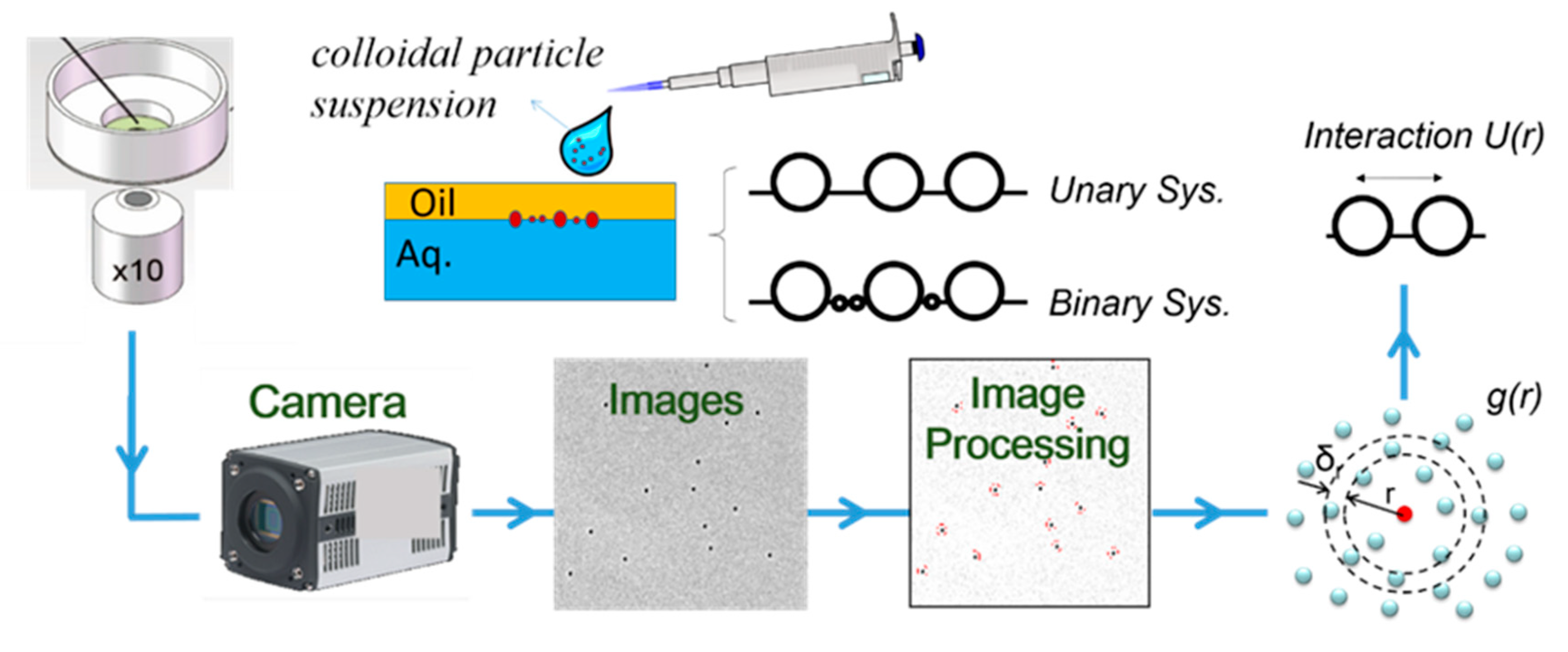
Figure 1 shows our approach to measure the interfacial interactions between particles of the unary system or binary mixtures trapped at the water–oil interface. We first created a colloid monolayer at an interface using a customized device. Using video microscopy, we then took a series of images for interfacial particles. Next, we used MATLAB to process images and segregate particle locations. From the location information, we further calculated parameters to characterize system properties, such as the
g(r) by reversible work theorem, from which we could derive the structure of interfacial system and extract the pair-wise potential (pair interaction)
U(r) between interfacial particles. We then measured in detail about the effects of density of small particles to the binary system property and compared the calculated results with the existing models.
Figure 2 shows
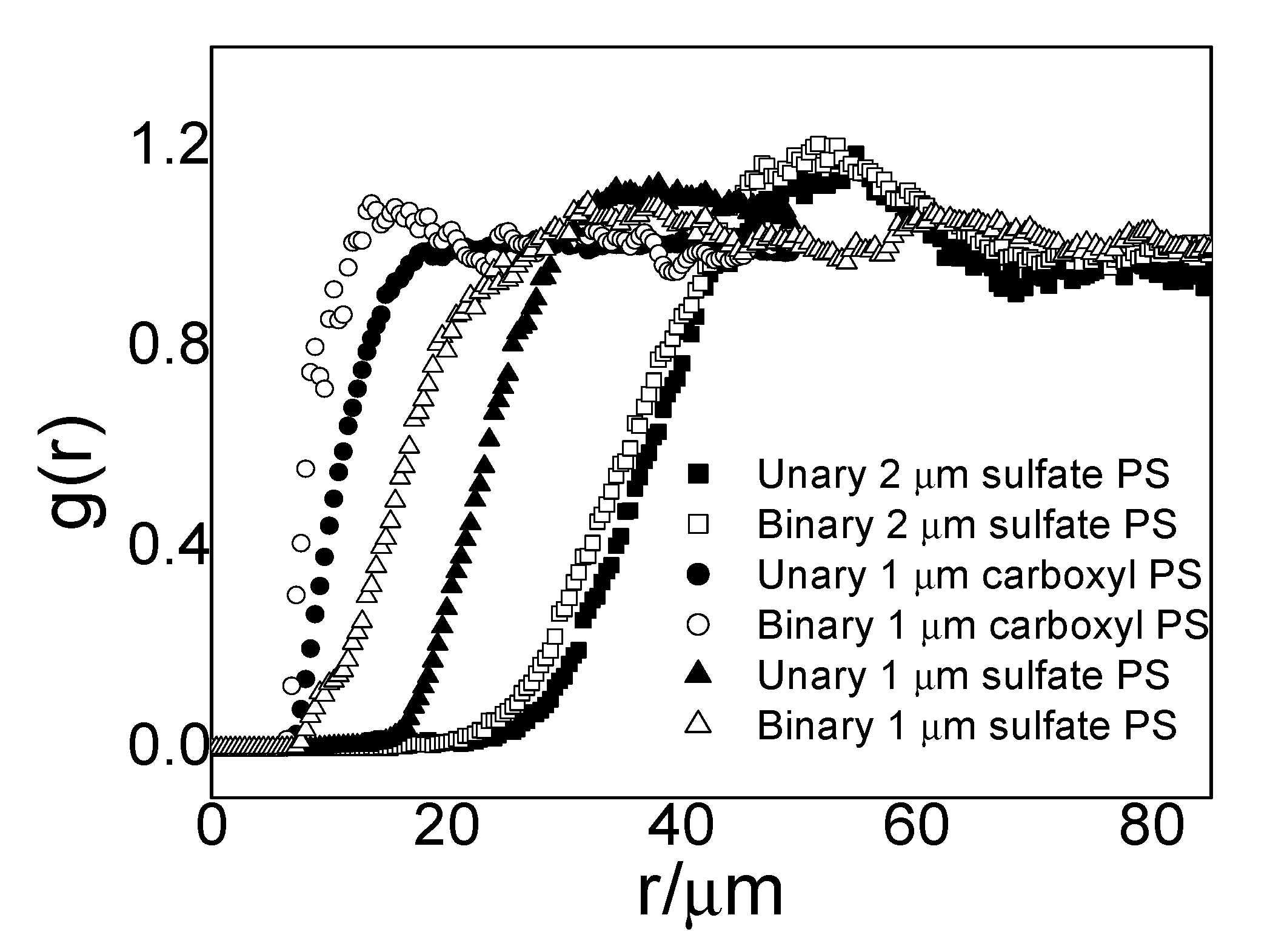
g(r) of large PS particles in unary and binary interfacial systems. The particle combinations of binary systems are: 2 µm sulfate PS with 0.21-µm sulfate PS, 1 µm carboxyl PS with 0.21-µm sulfate PS, and 1 µm sulfate PS with 0.21-µm sulfate PS. Since small and large particles are both negatively charged, they are repulsive. Intuitively, with the addition of small particles, the distance between large particles in a binary system should become longer due to the extra repulsion from small particles. To our surprise, large particles could approach each other at a closer distance in the presence of small particles, presumably due to some induced attractive force. This unexpected
g(r) result warranted further exploration; therefore, we conducted a more systematic study with only charged PS of the sulfate surface group. The sizes of large and small particles were 1 µm (zeta potential −66.44 mV) and 0.21-µm (zeta potential −84.78 mV), respectively, and the zeta potential shows that both PS particles are negatively charged.
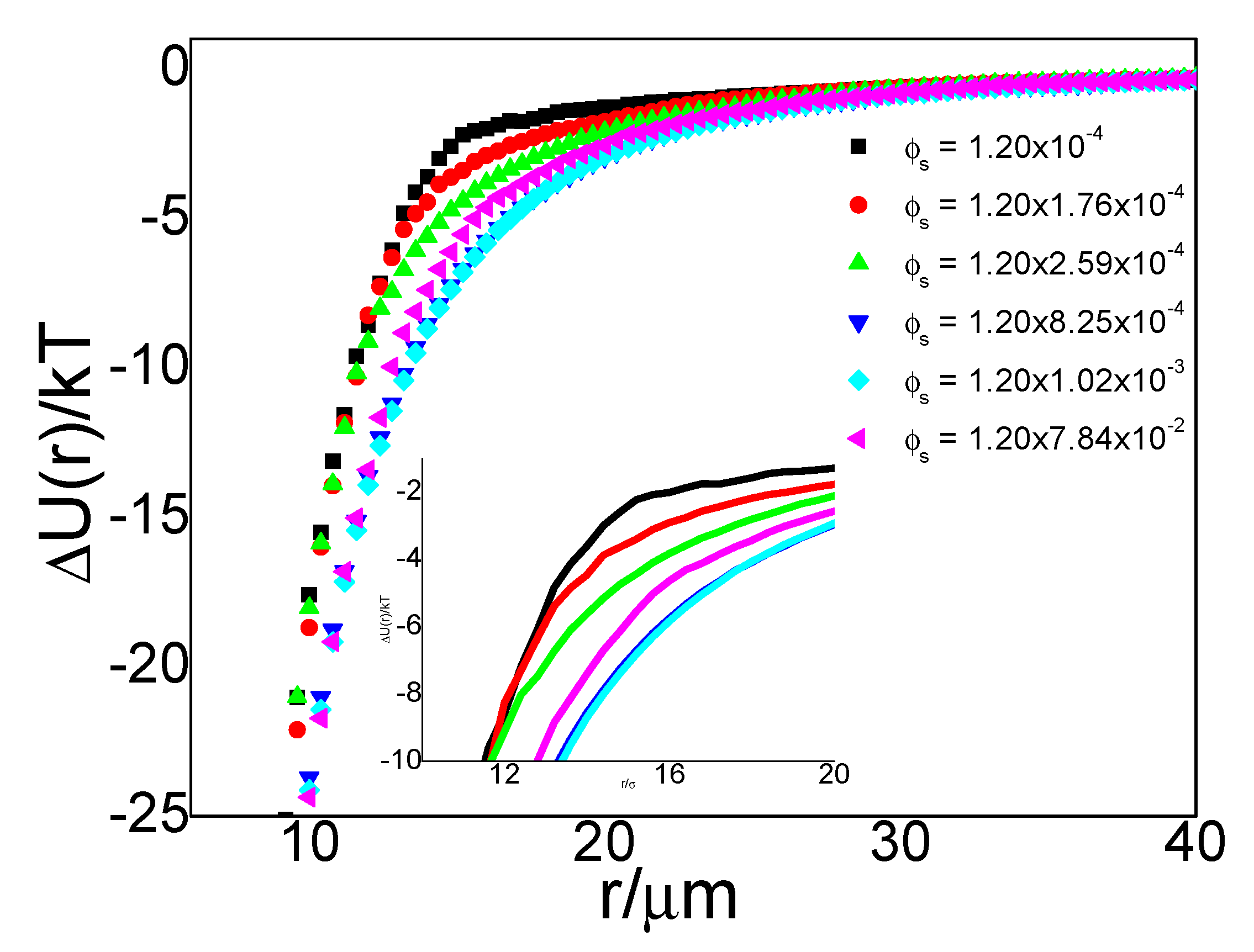
It is worth mentioning that g(r) depends on the concentration of particles in the system. To identify the effect of small particle concentration, we kept concentrations of large particles almost the same in all test systems, and we used particle area fraction to characterize particle concentration. We controlled the area fractions ϕ (= Nσ2/A) of large particles to approximately 5 × 10−4 in different systems to ensure no structural effects in this concentration level of large particles, where N is the number of particles in an image, A is the total area of the image, and σ is the particle diameter. We compared the g(r) of large particles under different small particle concentrations from roughly 10−4 to 10−2 and obtained the following results.
We use
ϕL to denote the area fraction of large particles and
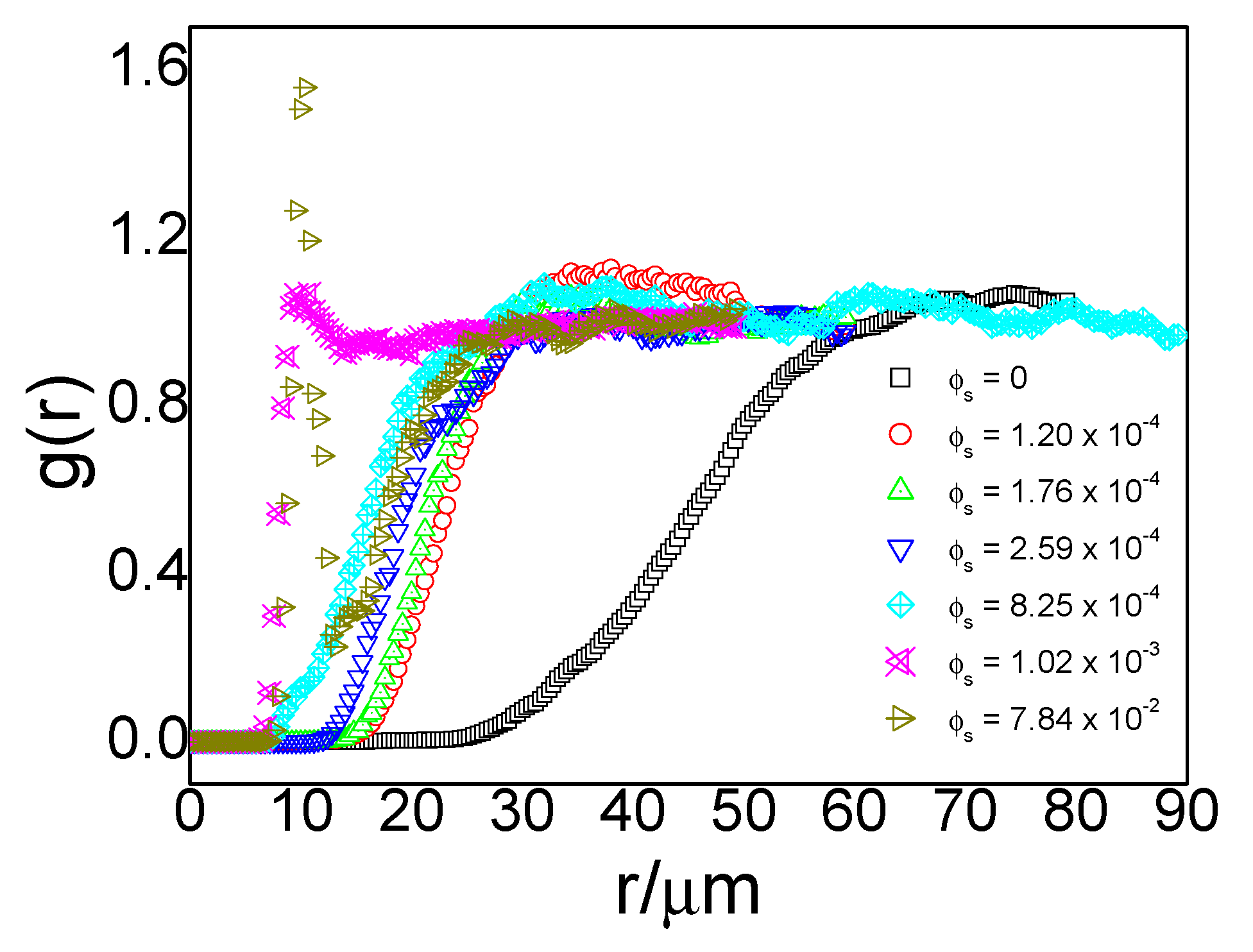
ϕs to denote the area fraction of small particles. For the system containing large particles only (i.e.,
ϕs = 0), its radial distribution function (black line in
Figure 3) demonstrated a typical gas-like structure with no peak in the
g(r) plot.
The nearest approachable distance between particles, i.e., the onset of nonzero
g(r), was approximately 25 µm. Same as our previous qualitative results in the binary system shown in
Figure 2, narrowing between large particles in the presence of small particles appeared again in binary systems (colorful lines in
Figure 3). With an increase in the concentration of small particles, the narrowing effects became stronger. For a binary system whose small-particle area fractions were around 10
−4 (red line), the nearest approachable distance between large particles declined to approximately 15 µm. The
g(r) maintained a gas-like structure, indicating that a random distribution of interfacial large particles remained in the presence of small particles. When the small-particle concentration increased to approximately 10
−3 (aqua line), the particles could approach each other further down to less than 10 µm, and peaks began to appear in the
g(r) curve at a distance of 10 µm (cyan line). When the particle concentration further increased to 7.84 × 10
−2, the peak at approximately 10 µm grew steeper. Peaks in
g(r) curves of large particles only appeared in a system with a small-particle concentration higher than 10
−3; no well-defined peaks were found in the other four binary systems. A peak in
g(r) usually suggests an attraction at that distance.
3.2. U(r) of Particles of Different Concentrations
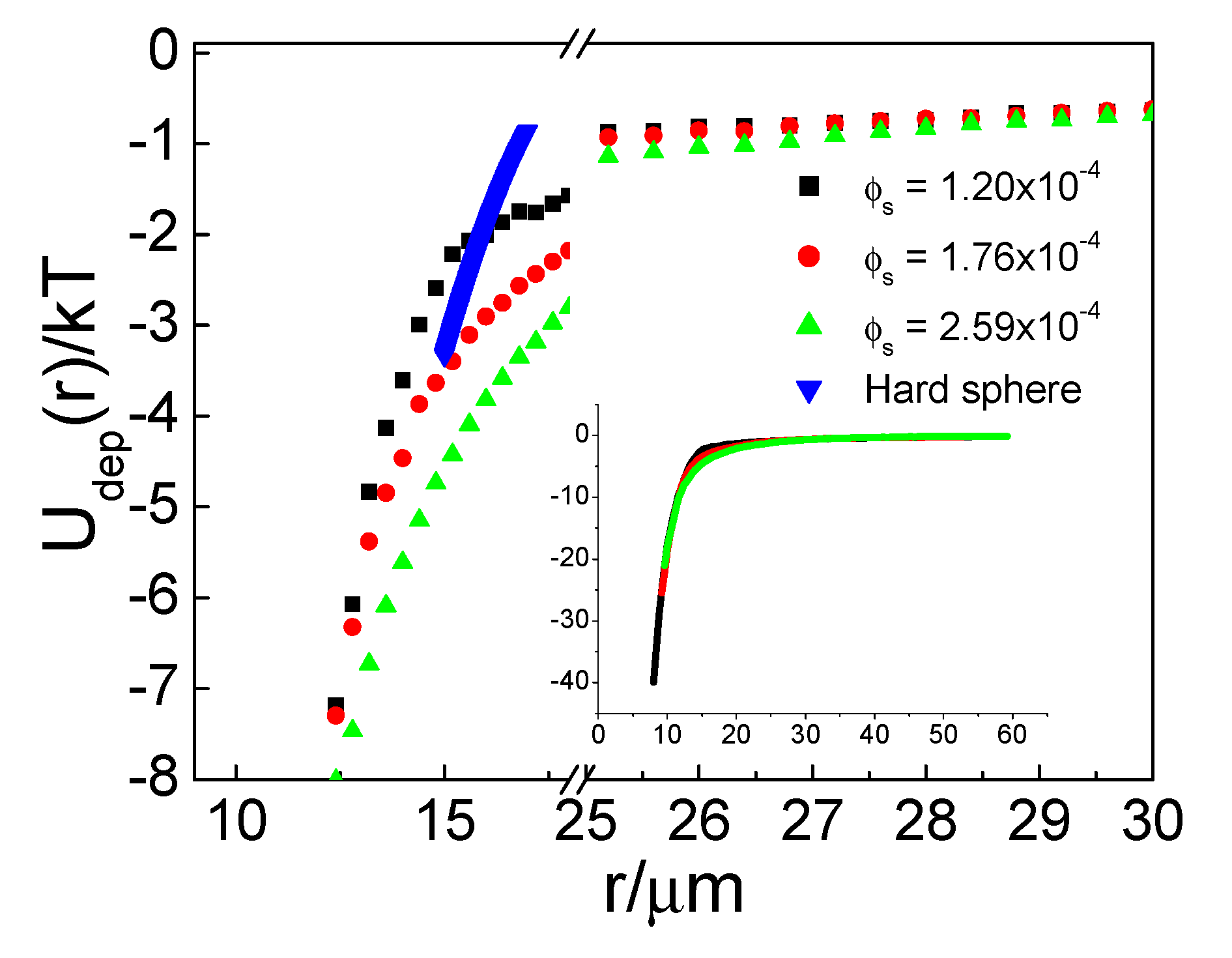
As we mentioned above, peaks in the PS particle curve indicated an attraction at that distance. To clarify this case, we compared the effective U(r) plot and treated small particles as the property of the medium to calculate the effective potential of large particles in the system using HNC approximation as shown in
Figure 4. In general, the large particles in our unary and binary systems exhibited repulsion to each other. The repulsive range was long in the unary system as well as in the binary system with low small-particle density, each at a distance larger than 10 µm.
U(r) of unary particle system was consistent with our previous results [
25], attraction between particle was not detected in our system. Just like Kralchevsky et al. have discussed [
27,
28], long-range attraction between interfacial particles are caused by interface deformation resulting from interfacial particles’ weight, which is commonly found for interfacial system with particles larger than 5 µm [
27]. In our system, interfacial polystyrene particles were 1 µm and 0.21 µm, and particle density is only 1.05 g/cm
3. These small particles are not heavy enough to deform interface to give attraction. Our previous study with 1 µm polystyrene at oil–water interface has shown that contact angle is 100° ± 7 and there is no deformation of interface from confocal image [
25]. Contact angle of 0.21 µm polystyrene particle at oil–water interface is similar with 1 µm polystyrene [
29], this smaller and lighter particle also cannot deform the interface to bring attraction between large particles.
For the binary system, with the presence of small particles, the repulsion between large particles weakened; when the small-particle concentration increased to 1.02 × 10−3, an attractive well appeared at an approximate distance of 10 µm. At an even higher small-particle concentration of 7.84 × 10−2, an attractive well of −0.3 kBT was found.
3.3. Comparison with Published Work
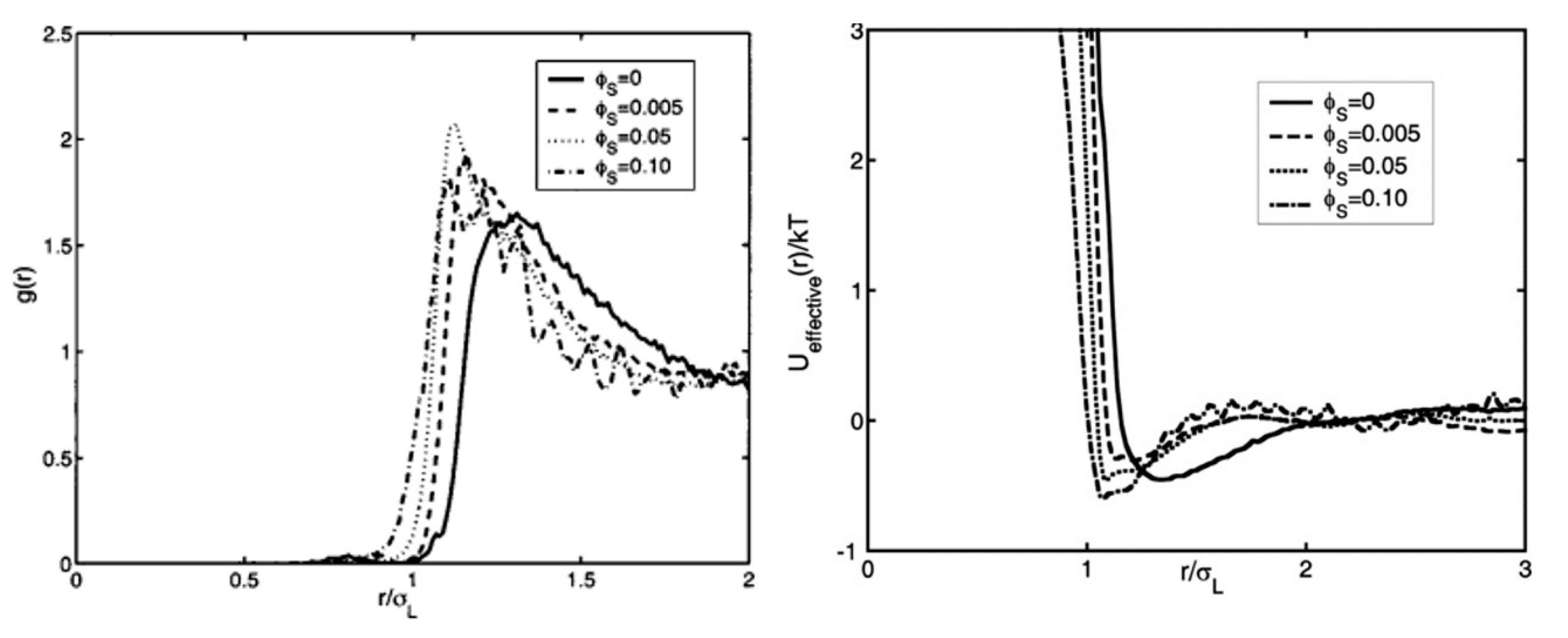
Cui et al. [
24] conducted a similar experimental study, where a suspension of silica (diameter σ
L = 1.58 µm) and polystyrene (diameter σ
S = 0.34 µm) was confined in a quasi-2D system by two plates, monolayer of large particle were sandwiched between this two plates. The resultant
g(r) of large particles are presented in
Figure A1, they use the large particles diameter σ
L as a measure to evaluate the separation distance
r between large particles. The area fraction (
Nσ2/
A) of large particles,
ϕL, at their confined layer was approximately 0.3, nearly 10 times our concentration. The concentration of small particles
ϕs in their quasi-two-dimensional system was the volume fraction of the suspension, which ranged from 0, 0.005, 0.05, to 0.1. Small particle concentrations in Cui’s system are at a higher level than our system.
In their results, well-defined initial peaks appeared in the
g(r) of large particles at the distance around 1–1.5 times large particle diameter. Peak in
g(r) appeared at a shorter distance with the increase in small-particle concentration
ϕs, and the nearest distance large particles could approach each other decrease accordingly with
ϕs increase. For their unary large particle system,
U(r) showed strong repulsion at around one particle diameter. In addition, the very short distance between confinement plates and large particles caused edge effects and resulted an attraction well right following repulsion branch in
U(r) profile [
24]. With
ϕs increase in their quasi-2D systems, repulsion branch part in the pair-wise potential
U(r) between large particles shifted to a shorter distance and the following attraction potential well became deeper (shown in
Figure A1). Also, the minimum of the attraction well shifted to smaller particle–particle separation. The attractive potential in their systems were approximately 0.3–0.8
kBT. Although attraction between large particles by confinement inducing edge effects is absent in our system because we have very dilute particles of gas-like structure at interface, our systems shared one common feature with their systems: introduction of small particle in system made the particle–particle distance between large particles smaller. Small particles in Cui’s quasi-2D system brought higher attraction between large particles while small particles in our decane-water interface system brought less repulsion between large particles. Note that Cui et al. reported nearly no measurable surface charge on particles; thus, the particles could essentially be considered an experimental hard sphere system. Volume exclusion between hard spheres naturally limits the nearest approachable separation between large particles to just around one large particle diameter, which accords with Cui’s results. In contrast, the approachable distance between large particles in our system was much longer than one large particle diameter for the highly charged interfacial particles, providing longer-range (more than 10 times the particle size) repulsion than the hard sphere model.
3.4. Depletion Interaction
To interpret the attractive force in a binary particle system, Cui et al. compared it with an entropic force called depletion. Experimental observation of flocculation between particles in a binary colloidal suspension [
30] was reported in 1925. Later, in 1958, Asakura and Oosawa [
31] provided a detailed illustration of the mechanism of this attractive interaction. Depletion originates from volume exclusion effects in colloidal systems of binary sizes; a schematic illustration is provided in
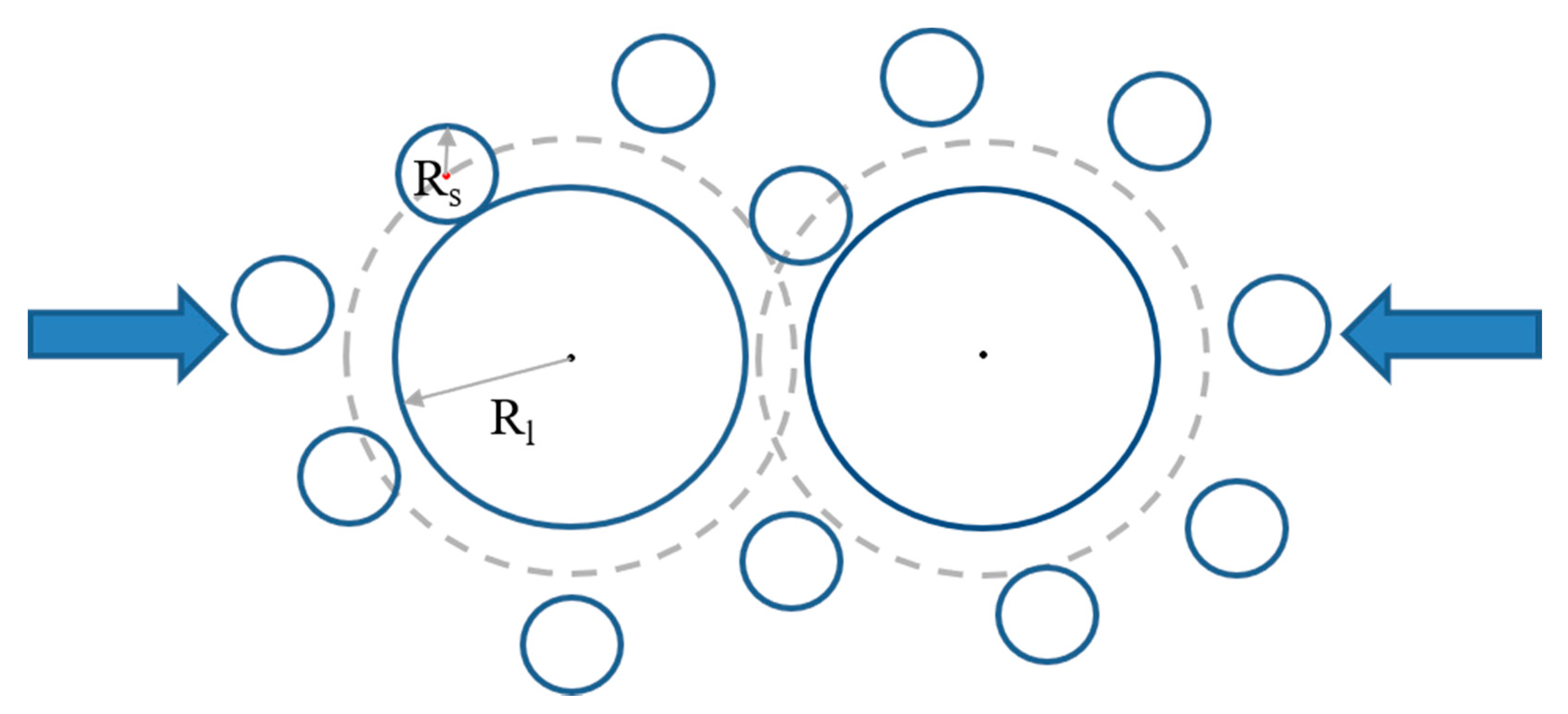
Figure 5. When considering the finite size of colloids and treating them as hard spheres, the small particles (depletants) are depleted from the large particle (with radius
Rl) surface at a distance of the small-particle radius
Rs; this zone is called the depletion layer. When two large particles get close and the surface-to-surface distance is less than the diameter (σ
S = 2
Rs) of the small particle, the exclusion of small particles will cause asymmetric distribution around one large particle, inducing an attraction. Depletion is an entropic interaction because the overlap of the depletion layer can provide the small particles more free space.
As depletion is caused by uneven surroundings of small particles around a large particle, the interaction range
r is 2
Rl <
r < 2(
Rl + Rs) when the depletion layer outside the large particle overlaps. The interaction
Uo(r) is determined by the overlapped volume and concentration of small particles
ϕs. A calculation by the first order of small-particle concentration was provided by Asakura and Oosawa [
31,
32] as below when the depletant concentration is low:
Similarly, for the 2D case, we can replace the overlap volume term with the overlap area; the depletion between the hard disk is
The depletion interaction range depends on the sizes of large and small particles, and the depth depends on the concentration of small particles. The interaction range of depletion matched the total effective potential range of 1–1.5 large particle size in the system from Cui et al.; however, in our system, this range was far below the measured result.
3.5. Structure of Binary Interfacial Particle System
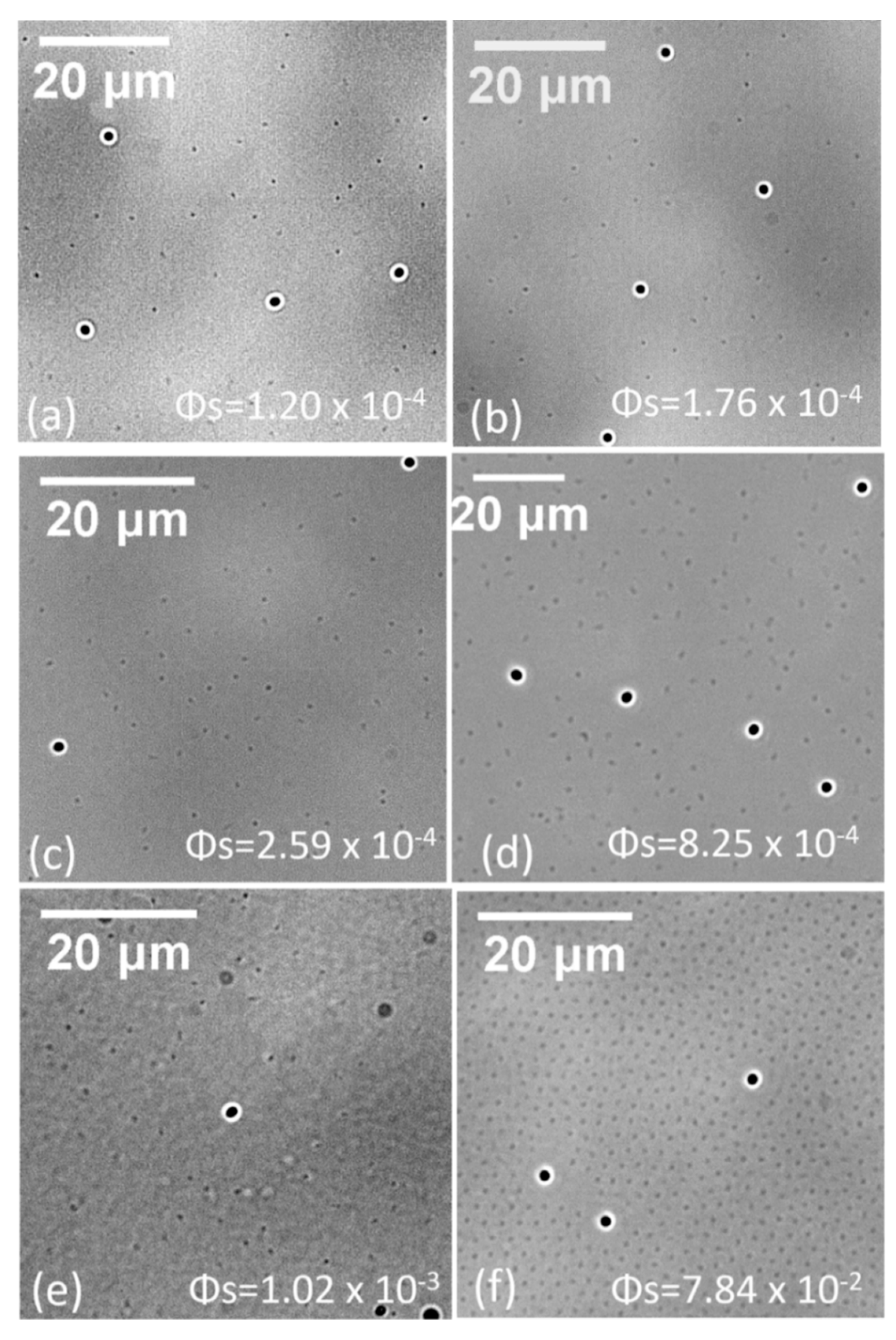
The exclusion of particles to a distance far longer than their own sizes in our system appears clearly in the bright-field image (
Figure 6). The black dots are large particles, and the shallow grey dots are small particles. Small particles were randomly distributed around large particles, except for system (f) with small-particle area fraction of 7.84 × 10
−2. In system (f), the
g(r) showed a high peak at an approximate distance of 10 µm, whereas other distances displayed a distribution of large particles nearly identical to that in the system with lower small-particle densities (systems a–c). The interfacial structure in system (f) was mosaic under a high particle density: large particles, either individual or particle pairs, were surrounded by hexagonally ordered small particles. For large particle pairs, the separation between them was maintained around 10 µm, despite extreme crowding in the surroundings, concurring with the distance where the
g(r) peak appeared.
We know that for charged interfacial particles, particles interact with each other by soft electrostatic potentials via both aqueous phase and oil phase. Charge clouds from particle’s surface group dissociation in aqueous phase provide Coulombic electrostatic potential between interfacial particle, and this Coulombic potential is closely with the ionic environment, which can be characterized by Debye length
κ−1 in aqueous phase [
33,
34,
35]. The counterpart electrostatic potential originates from charge on particle surface in the oil part [
36,
37], and range of electrostatic interaction via oil phase is much longer than the range of electrostatic interaction in aqueous phase due to oil’s much lower dielectric constant. At near range when
κr < 10 (
r is the distance between particles), screened Coulombic repulsion dominated, while at far range
κr ≥ 10, the counterpart electrostatic interaction dominated [
35,
38]. However, the charge on particle surface in oil part is unstable and resultant electrostatic interaction from oil phase decay with time to vanish [
25,
39]. In this work, we allow sufficient time for the charge on particle surface oil phase to vanish, thus we can skip discussion about electrostatic interaction from oil phase.
Because we used water as the aqueous sub-phase, the Debye length κ−1 was approximately 1 µm in this ultra-low ionic medium. Thus, the screened Coulombic repulsion dominated in a range less than 10 µm in our systems, according with that large particles kept a distance around 10 µm in particle pairs under extreme uneven distribution of surrounding small particles in system (f). The individual particles in system (f) were nearly frozen by the surrounding small particles and maintained their initial separation when they were deposited at the interface. A depletion layer outside the large particles with an approximate length of 1 µm are apparent in the bright-field image(f). The freezing by small particles resulted in the g(r) in system (f) exhibiting a high peak at 10 µm; at the other separation, the particle distribution was nearly identical to the dilute systems (a–c).
For systems (d–e), the concentrations of small particles were not high enough to freeze them; thus, only the statistical result of narrowing between the large particles is shown. At a low probability, the large particles were squeezed in the range of 10 µm where direct Coulombic repulsion between large particles dominated. For systems (a–c), the overall particle density was low, and substantial free space existed for small and large particles. Narrowing between the randomly distributed large particles was due to an increase in overall interfacial particles; as such, the g(r) of large particles continued to show a gas-like structure.
Previous reports have revealed that, for a 2D particle system with low area fraction [
40] (less than 0.20), localization for the soft and hard disk was indistinguishable. The bright-field image of the binary system showed that the effective particle sizes that induced exclusion were beyond their actual size due to the negative surface charge. The particles were randomly distributed like gas in binary systems, with small-particle area fraction around 10
−4 and showed nearly no compressive exclusion as seen in the bright image. These systems (a–c) were hence considered to be in a dilute situation. A detailed discussion follows to compare the soft particle with an effective hard disk of a radius of the onset of their
g(r). For the system with high density, since the area fraction 10
−2 was within the area fraction limit of Foster et al.’s findings [
40], the large particles acted like non-compressive hard particles of 10 µm in diameter.
3.7. Experimental Results Vs. Theoretical Prediction
As noted earlier, the influencing range of a small particle’s size effects at the interface in our system were far more than their physical size. Since for a small-area fraction, the hard particles and soft particles could be mapped to each other, we first used hard sphere mapping [
40] to see the effects for a system with no crowding effects (systems a–c). Treating particles as hard sphere, we chose an effective diameter size of 15 µm (around the onset of
g(r)) for large particles and a small-particle diameter of approximately 2 µm. We theoretically calculate the hard sphere depletion interaction
Udep(r) and compare it with our experimental results, treating
∆U(r) as experimental
Udep(r) (
Figure 8). It is obvious that, even after adjusting the effective size to be much larger than the physical size of particles according to the interfacial particle image in
Figure 6, the theoretical predicted attraction is still far below the experimental results in both range and depth.
Considering the stringent 2D confinement of oil–water interface of our system, we similarly compared our experimental results with the theoretical hard disk model. For the hard disk concentration, we calculated the average of the three dilute systems (systems a–c) as 0.007; results appear in
Figure 9. The theoretical predicted results caused by the hard disk were even weaker than a hard sphere system. The range and depth of the theoretical prediction were each smaller than the experimental measured result. And the experimentally measured attraction was approximately 3 orders of magnitude stronger than the hard disk prediction.
Although there has reported successful theoretical prediction for mapping between soft potential and hard potential in the ultra-dilute system [
40], substantial divergence of experimentally measured results of soft interacting particles from hard particles were found in our results.
3.8. Electrostatic-Enhanced Interactions
Despite a report [
41] suggesting that the softness of the potential can enhance depletion-like attraction between particles, few reported experimental results have supported this finding. Experiments [
42] using a binary system of polystyrene particles measuring 100 nm and 25 nm in a water suspension with laser-light scattering have been reported. They found a depletion-like attraction enhanced by electrostatic repulsion, because of the enhanced excluded volume by the repulsion between the particle and the depletant. In low-electrolyte suspension, the interaction range was defined as σ
L + σ
s + 2
κ−1. However, the interaction range detected in our experiment (approximately 10 µm) was much longer than the incorporation of the long Debye length (1 + 0.21 + 2 = 3.21 µm). Additionally, the overall effective interaction between the large particles in their binary system was always attractive even under an ultra-low small particle concentration (10
−5). In our case, the overall effective interaction in the system remained repulsive. We identified an attraction enhanced by the particle charge, but more quantitative research remains to be done to elucidate the ultra-long range and depth of attraction with a minuscule concentration of small particles.