Delivery Systems for Hydroxytyrosol Supplementation: State of the Art
Abstract
1. Introduction
2. Hydroxytyrosol
2.1. General Information and Sources
2.2. Food Application and Health Effects
3. Delivery Systems
3.1. Emulsion-Based Systems
3.1.1. Colloidal Dispersions
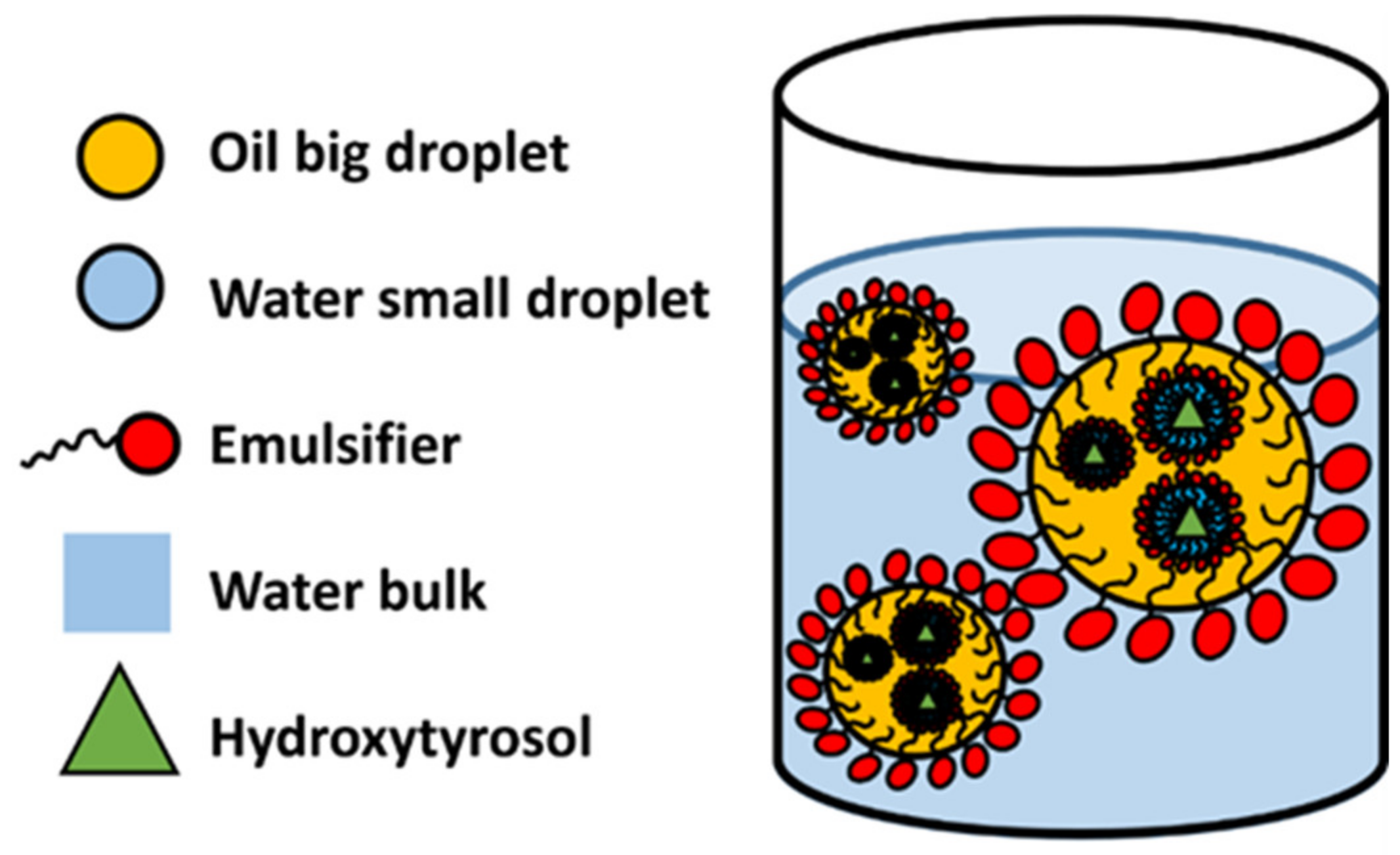
3.1.2. Double Emulsions
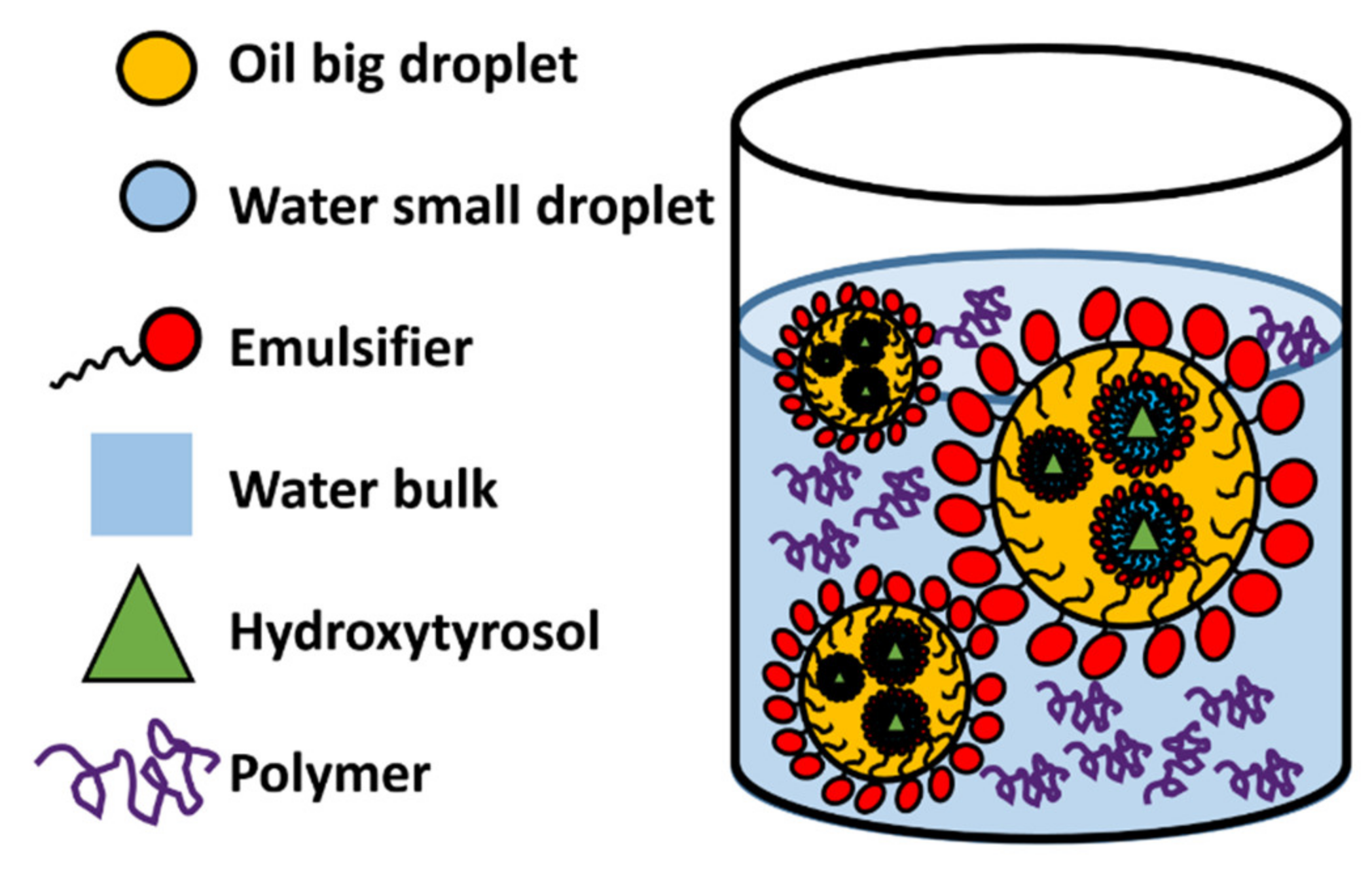
3.1.3. Gelled Double Emulsions
3.2. Encapsulated Systems
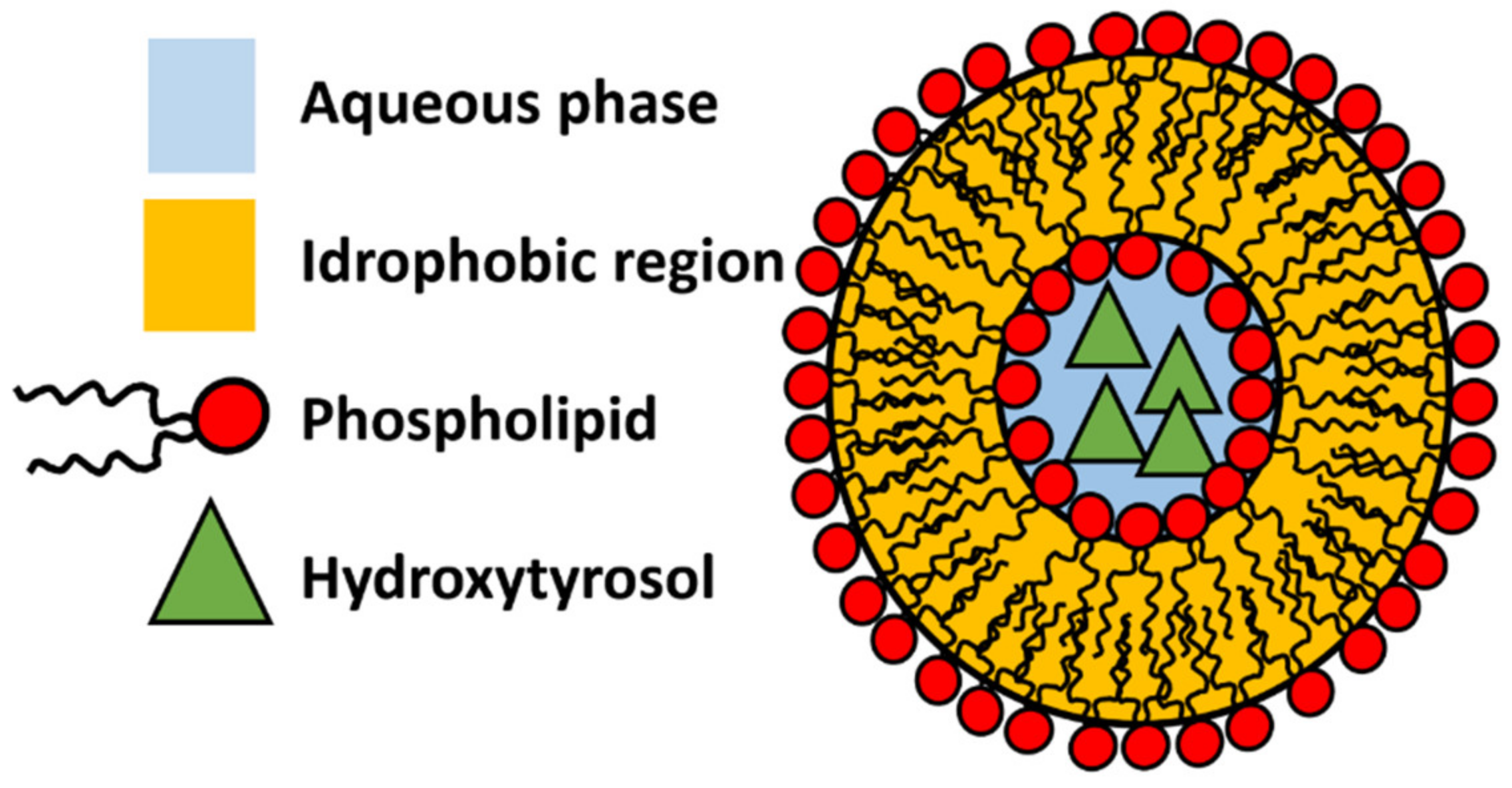
3.3. Phenolipids and Liposomes
3.4. Active Packaging
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wani, T.A.; Masoodi, F.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Nag, A. Antioxidant activity of various phenol extracts of olive-oil mill wastewaters. Acta Aliment. 2009, 38, 77–86. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Farre, M. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Achmon, Y.; Fishman, A. The antioxidant hydroxytyrosol: Biotechnological production challenges and opportunities. Appl. Microbiol. Biotechnol. 2015, 99, 1119–1130. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- De Leonardis, A.; Masino, F.; Macciola, V.; Montevecchi, G.; Antonelli, A.; Marconi, E. A study on acetification process to produce olive vinegar from oil mill wastewaters. Eur. Food Res. Technol. 2019, 245, 2123–2131. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Pegg, A. The application of natural hydrocolloids to foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Woodhead Publishing Series in Food Science: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2012; pp. 175–196. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of oleuropein degradation by olive leaf protein extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Arik, N.; Monteil, J.; Papadimitriou, V.; Leal-Calderon, F.; Xenakis, A. Microemulsion versus emulsion as effective carrier of hydroxytyrosol. Colloids Surf. B Biointerfaces 2016, 137, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Orenes-Piñero, E.; García-Carmona, F.; Sánchez-Ferrer, Á. A new process for obtaining hydroxytyrosol using transformed Escherichia coli whole cells with phenol hydroxylase gene from Geobacillus thermoglucosidasius. Food Chem. 2013, 139, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Tajima, K.; Munekata, M.; Keasling, J.D.; Lee, T.S. Engineering of L-tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol. Metab. Eng. 2012, 14, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mar, M.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Orozco-Solano, M.; Ferreiro-Vera, C.; Priego-Capote, F.; de Castro, M.L. Automated method for determination of olive oil phenols and metabolites in human plasma and application in intervention studies. J. Chromatogr. A 2012, 1258, 108–116. [Google Scholar] [CrossRef]
- Tirado, D.F.; de la Fuente, E.; Calvo, L. A selective extraction of hydroxytyrosol rich olive oil from alperujo. J. Food Eng. 2019, 263, 409–416. [Google Scholar] [CrossRef]
- Suarez, M.; Romero, M.-P.; Ramo, T.; Macia, A.; Motilva, M.-J. Methods for preparing phenolic extracts from olive cake for potential application as food antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef]
- Cornwell, D.G.; Ma, J. Nutritional benefit of olive oil: The biological effects of hydroxytyrosol and its arylating quinone adducts. J. Agric. Food Chem. 2008, 56, 8774–8786. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea Europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef] [PubMed]
- European Union Commission. Commission Regulation (EU) No 432/2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Communities 2020, 2, 1–136. [Google Scholar]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef]
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-based therapeutics and formulation strategies augmenting their efficiency to complement modern medicine: An overview. J. Funct. Foods 2014, 6, 82–99. [Google Scholar] [CrossRef]
- Visioli, F. Xenobiotics and human health: A new view of their pharma-nutritional role. Pharma Nutr. 2015, 3, 60–64. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and cytoprotection: A projection for clinical interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef] [PubMed]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Čepo, D.V. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT Food Sci. Technol. 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Larrosa, M.; Espín, J.C.; Tomás-Barberán, F.A. Antioxidant capacity of tomato juice functionalised with enzymatically synthesised hydroxytyrosol. J. Sci. Food Agric. 2003, 83, 658–666. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V. Olive biophenols as food supplements and additives. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 283–289. [Google Scholar]
- Grice, H. Safety evaluation of butylated hydroxyltoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem. Toxicol. 1986, 24, 1127–1130. [Google Scholar]
- Wichi, H. Enhanced tumor development of butylated hydroxyanisol (BHA) from the prospective of effect on fore stomach and oesophageal squamous epithlium. Food Chem. Toxicol. 1988, 26, 717–723. [Google Scholar]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf. B Biointerfaces 2018, 168, 163–168. [Google Scholar] [CrossRef]
- Cinelli, G.; Sbrocchi, G.; Iacovino, S.; Ambrosone, A.; Ceglie, A.; Lopez, F.; Cuomo, F. Red wine-enriched olive oil emulsions: Role of wine polyphenols in the oxidative stability. Colloids Interfaces 2019, 3, 59. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, 50–57. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Mitsou, E.; Yaghmur, A.; Xenakis, A.; Papadimitriou, V. Formulation and characterization of food-grade microemulsions as carriers of natural phenolic antioxidants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 130–136. [Google Scholar] [CrossRef]
- Lisete-Torres, P.; Losada, S.; Albuquerque, H.; Sánchez-Paz, V.; Paiva-Martins, F.; Bravo Díaz, C. Distribution of hydroxytyrosol and hydroxytyrosol acetate in olive oil emulsions and their antioxidant efficiency. J. Agric. Food Chem. 2012, 60, 7318–7325. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Fernández-Martín, F.; Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Olive oil based edible W/O/W emulsions stability as affected by addition of some acylglycerides. J. Food Eng. 2017, 196, 18–26. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F. Potential applications of multiple emulsions in the development of healthy and functional foods. Food Res. Int. 2013, 52, 64–74. [Google Scholar] [CrossRef]
- Freire, M.; Cofrades, S.; Serrano-Casas, V.; Pintado, T.; Jiménez, M.J.; Jimenez-Colmenero, F. Gelled double emulsions as delivery systems for hydroxytyrosol and n-3 fatty acids in healthy pork patties. J. Food Sci. Technol. 2017, 54, 3959–3968. [Google Scholar] [CrossRef]
- Cofrades, S.; Santos-López, J.A.; Freire, M.; Benedí, J.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. LWT-Food Sci. Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef]
- Flaiz, L.; Freire, M.; Cofrades, S.; Mateos, R.; Weiss, J.; Jiménez-Colmenero, F.; Bou, R. Comparison of simple, double and gelled double emulsions as hydroxytyrosol and n-3 fatty acid delivery systems. Food Chem. 2016, 213, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 567–578. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Messia, M.C.; Sacco, P.; Lopez, F. Protective action of lemongrass essential oil on mucilage from chia (Salvia hispanica) seeds. Food Hydrocoll. 2020, 105, 105860. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Caballero, P.A.; Blanco, C.A.; Rosell, C.M. Functionality of different hydrocolloids on the quality and shelf-life of yellow layer cakes. Food Hydrocoll. 2007, 21, 167–173. [Google Scholar] [CrossRef]
- Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Technological characteristics of cold-set gelled double emulsion enriched with n-3 fatty acids: Effect of hydroxytyrosol addition and chilling storage. Food Res. Int. 2017, 100, 298–305. [Google Scholar] [CrossRef]
- Dickinson, E. Double emulsions stabilized by food biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Cofrades, S.; Bou, R.; Flaiz, L.; Garcimartín, A.; Benedí, J.; Mateos, R.; Sánchez-Muniz, F.J.; Olivero-David, R.; Jiménez-Colmenero, F. Bioaccessibility of hydroxytyrosol and n-3 fatty acids as affected by the delivery system: Simple, double and gelled double emulsions. J. Food Sci. Technol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.N.; Santos, L. Design of microparticles containing natural antioxidants: Preparation, characterization and controlled release studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Couvreur, P.; Dubernet, C.; Puisieux, F. Controlled drug delivery with nanoparticles: Current possibilities and future trends. Eur. J. Pharm. Biopharm. 1995, 41, 2–13. [Google Scholar]
- Patel, A.; Velikov, K.P. Colloidal delivery systems in foods: A general comparison with oral drug delivery. LWT-Food Sci. Technol. 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rodríguez-Juan, E.; González-Benjumea, A.; Fernández-Bolaños, J. Molecular interactions between 3,4-dihydroxyphenylglycol and pectin and antioxidant capacity of this complex in vitro. Carbohydr. Polym. 2018, 197, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocoll. 2012, 27, 316–323. [Google Scholar] [CrossRef]
- Malapert, A.; Reboul, E.; Tourbin, M.; Dangles, O.; Thiéry, A.; Ziarelli, F.; Tomao, V. Characterization of hydroxytyrosol-β-cyclodextrin complexes in solution and in the solid state, a potential bioactive ingredient. LWT-Food Sci. Technol. 2019, 102, 317–323. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Raval, J.P.; Naik, D.R.; Amin, K.A.; Patel, P.S. Controlled-release and antibacterial studies of doxycycline-loaded poly (ε-caprolactone) microspheres. J. Saudi Chem. Soc. 2014, 18, 566–573. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.S.; Cert, A.; Espartero, J.L. New lipophilic tyrosyl esters. Comparative antioxidant evaluation with hydroxytyrosyl esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Mateos, R.; Collantes de Teran, L.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic hydroxytyrosyl esters. antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, J.A.; Cermak, S.C.; Evans, E.O.; Compton, D.L.; Evangelista, R.; Berhow, M.A. Medium-chain alkyl esters of tyrosol and hydroxytyrosol antioxidants by cuphea oil transesterification. Eur. J. Lipid Sci. Technol. 2013, 115, 363–371. [Google Scholar] [CrossRef]
- Evans, K.O.; Laszlo, J.A.; Compton, D.L. Hydroxytyrosol and tyrosol esters partitioning into, location within, and effect on DOPC liposome bilayer behaviour. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.O.; Compton, D.L. Phosphatidyl-hydroxytyrosol and phosphatidyl-tyrosol bilayer properties. Chem. Phys. Lipids 2017, 202, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Comelles, F.; Alcantara, D.; Maldonado, O.S.; Curcuroze, M.; Parra, J.L.; Morales, J.C. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: A potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 8021–8026. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef]
- Evans, K.O.; Compton, D.L.; Appell, M. Determination of pH effects on phosphatidyl-hydroxytyrosol and phosphatidyl-tyrosol bilayer behavior. Methods Protoc. 2018, 1, 41. [Google Scholar] [CrossRef]
- Mallick, S.; Choi, J.S. Liposomes: Versatile and biocompatible nanovesicles for efficient biomolecules delivery. J. Nanosci. Nanotechnol. 2014, 14, 755–765. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. In Liposomes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 17–22. [Google Scholar]
- Yuan, J.-J.; Qin, F.G.; Tu, J.-L.; Li, B. Preparation, characterization, and antioxidant activity evaluation of liposomes containing water-soluble hydroxytyrosol from olive. Molecules 2017, 22, 870. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of antioxidant food packaging materials with controlled release properties. J. Food Eng. 2010, 96, 325–332. [Google Scholar] [CrossRef]
- Ludueña, L.; Vázquez, A.; Alvarez, V. Effect of lignocellulosic filler type and content on the behavior of polycaprolactone based eco-composites for packaging applications. Carbohydr. Polym. 2012, 87, 411–421. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Lagaron, J. Novel clay-based nanobiocomposites of biopolyesters with synergistic barrier to UV light, gas, and vapour. J. Appl. Polym. Sci. 2010, 118, 188–199. [Google Scholar] [CrossRef]
- Peltzer, M.; Navarro, R.; López, J.; Jiménez, A. Evaluation of the melt stabilization performance of hydroxytyrosol (3,4-dihydroxy-phenylethanol) in polypropylene. Polym. Degrad. Stab. 2010, 95, 1636–1641. [Google Scholar] [CrossRef]
- Peltzer, M.; Jiménez, A.; Matisová-Rychlá, L.; Rychlý, J. Use of isothermal and nonisothermal chemiluminescence measurements for comparison of stabilizing efficiency of hydroxytyrosol (3,4-dihydroxy-phenylethanol), α-tocopherol and irganox® 1076 in polypropylene. J. Appl. Polym. Sci. 2011, 121, 3393–3399. [Google Scholar] [CrossRef]
- Beltrán, A.; Valente, A.J.; Jiménez, A.; Garrigós, M.C. Characterization of poly (ε-caprolactone)-based nanocomposites containing hydroxytyrosol for active food packaging. J. Agric. Food Chem. 2014, 62, 2244–2252. [Google Scholar]
- Fortunati, E.; Luzi, F.; Dugo, L.; Fanali, C.; Tripodo, G.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Effect of hydroxytyrosol methyl carbonate on the thermal, migration and antioxidant properties of PVA-based films for active food packaging. Polym. Int. 2016, 65, 872–882. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Fanali, C.; Dugo, L.; Belluomo, M.G.; Torre, L.; Kenny, J.M.; Santi, L.; Bernini, R. Hydroxytyrosol as active ingredient in poly (vinyl alcohol) films for food packaging applications. J. Renew. Mater. 2017, 5, 81–95. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leonardis, A.; Macciola, V.; Iacovino, S. Delivery Systems for Hydroxytyrosol Supplementation: State of the Art. Colloids Interfaces 2020, 4, 25. https://doi.org/10.3390/colloids4020025
De Leonardis A, Macciola V, Iacovino S. Delivery Systems for Hydroxytyrosol Supplementation: State of the Art. Colloids and Interfaces. 2020; 4(2):25. https://doi.org/10.3390/colloids4020025
Chicago/Turabian StyleDe Leonardis, Antonella, Vincenzo Macciola, and Silvio Iacovino. 2020. "Delivery Systems for Hydroxytyrosol Supplementation: State of the Art" Colloids and Interfaces 4, no. 2: 25. https://doi.org/10.3390/colloids4020025
APA StyleDe Leonardis, A., Macciola, V., & Iacovino, S. (2020). Delivery Systems for Hydroxytyrosol Supplementation: State of the Art. Colloids and Interfaces, 4(2), 25. https://doi.org/10.3390/colloids4020025






