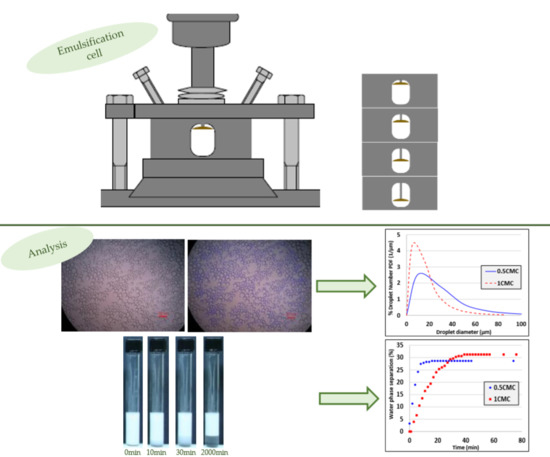
An Innovative Miniature Pulsating Emulsification Device: Flow Characterization and Measurement of Emulsion Stability
Abstract
:1. Introduction
2. Materials and Methods
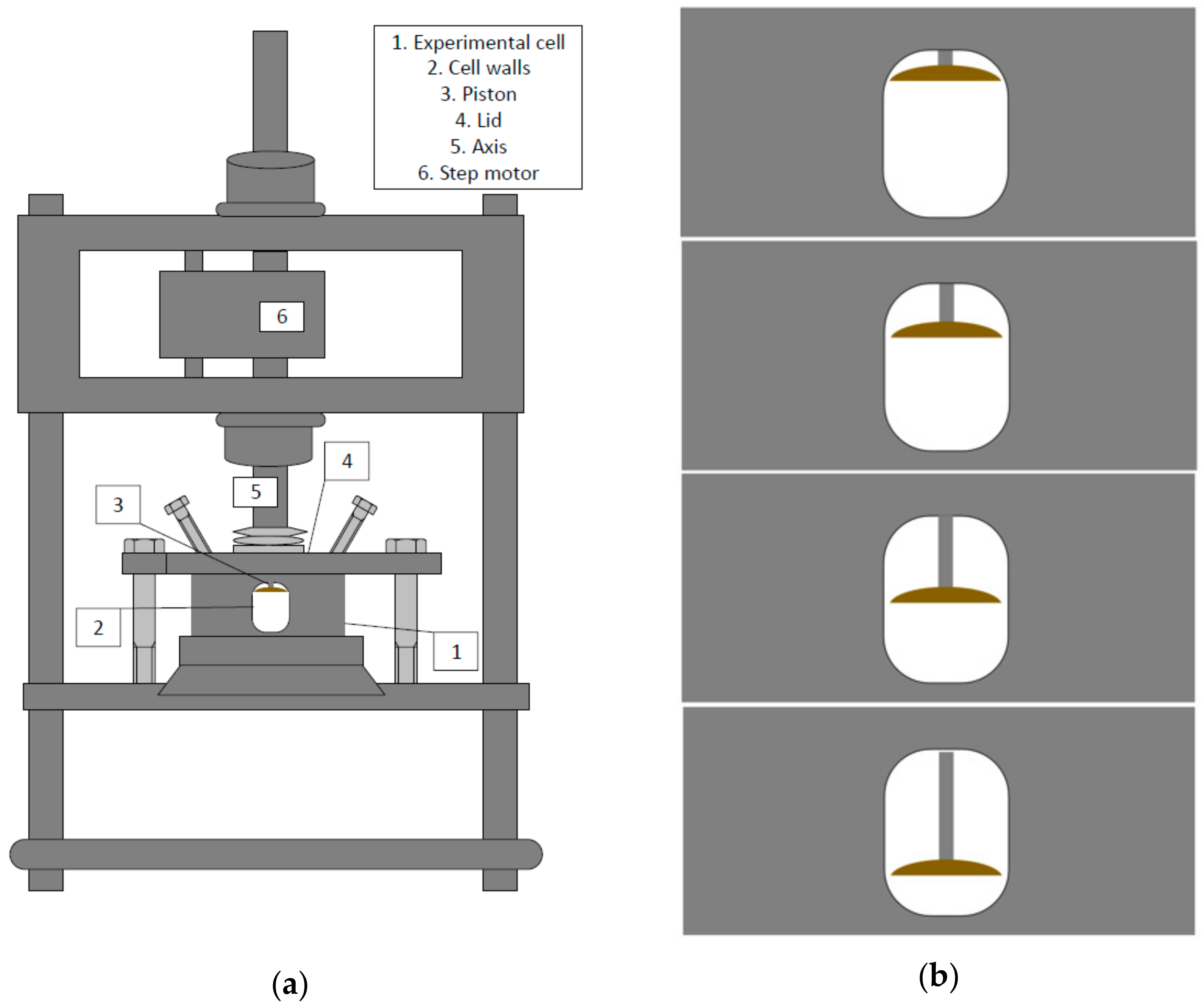
2.1. Miniature Pulsating Emulsification Device
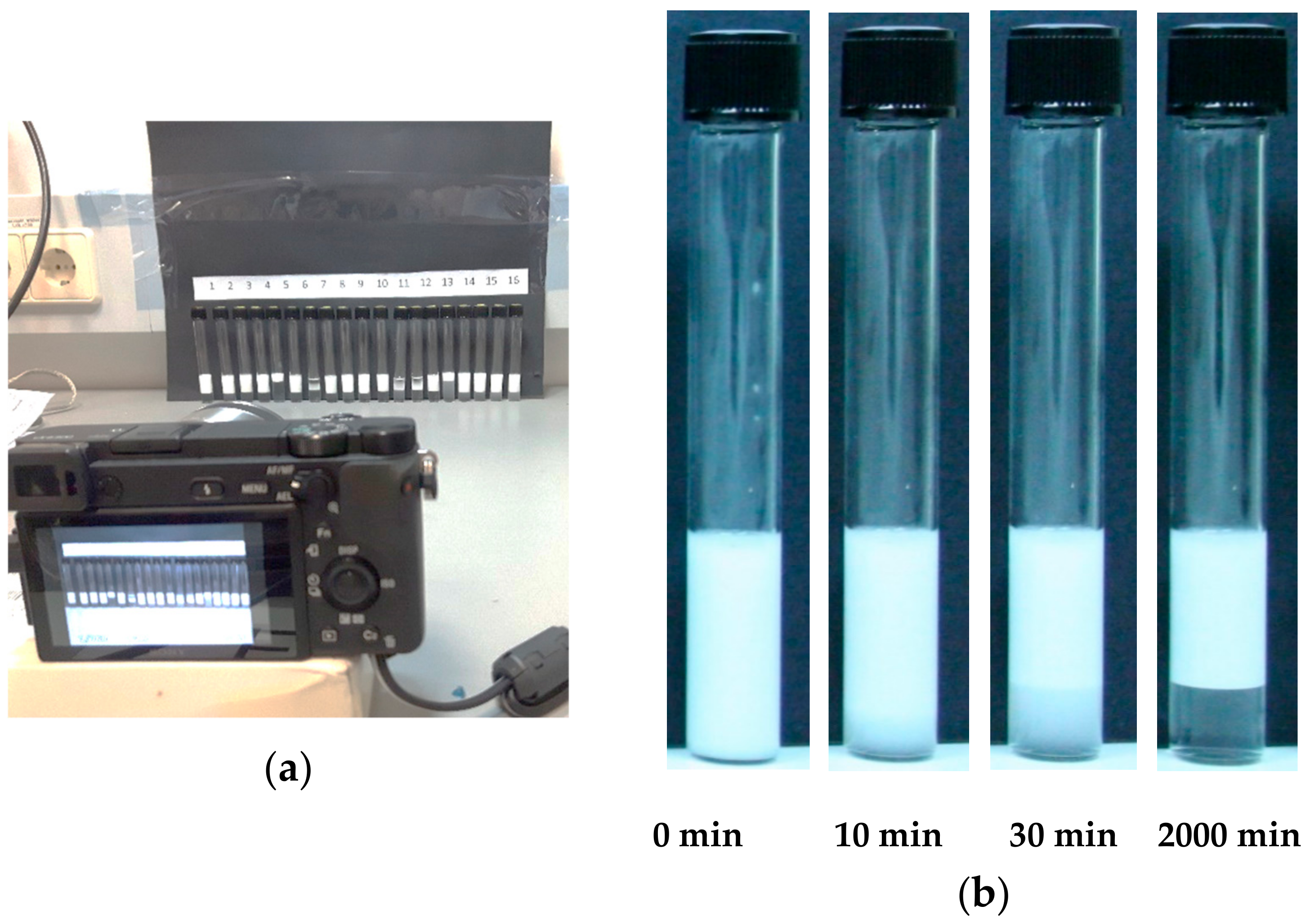
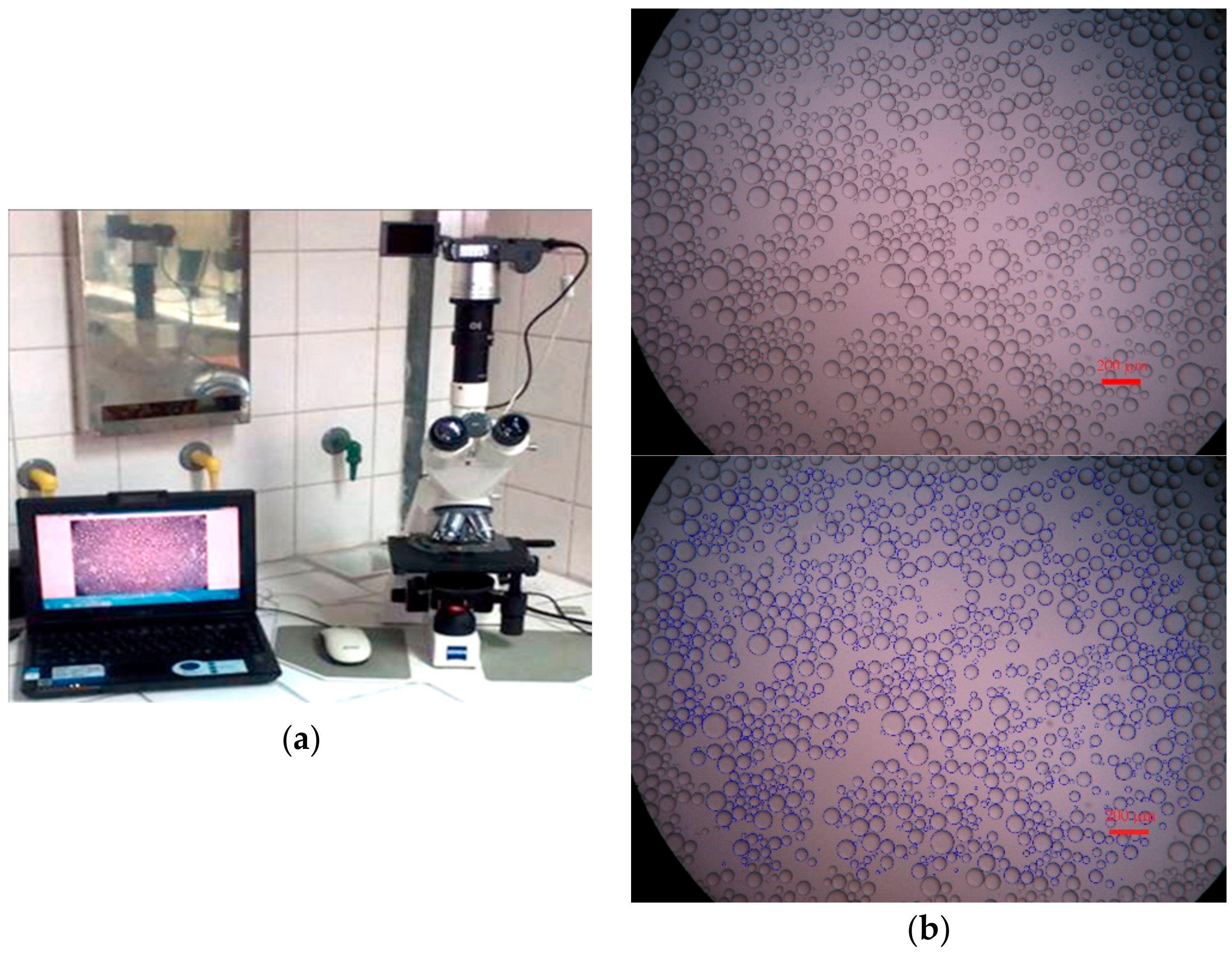
2.2. Experimental Procedure
2.3. Experimental Conditions
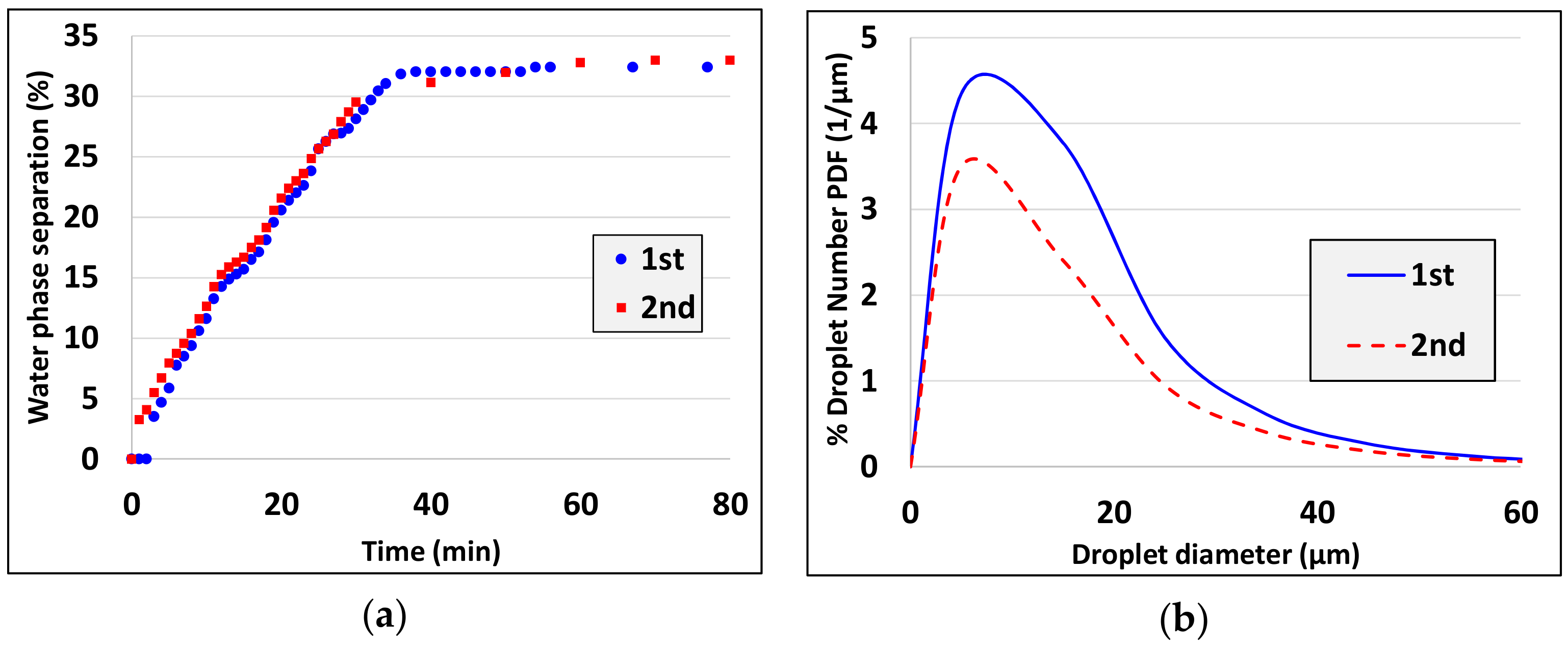
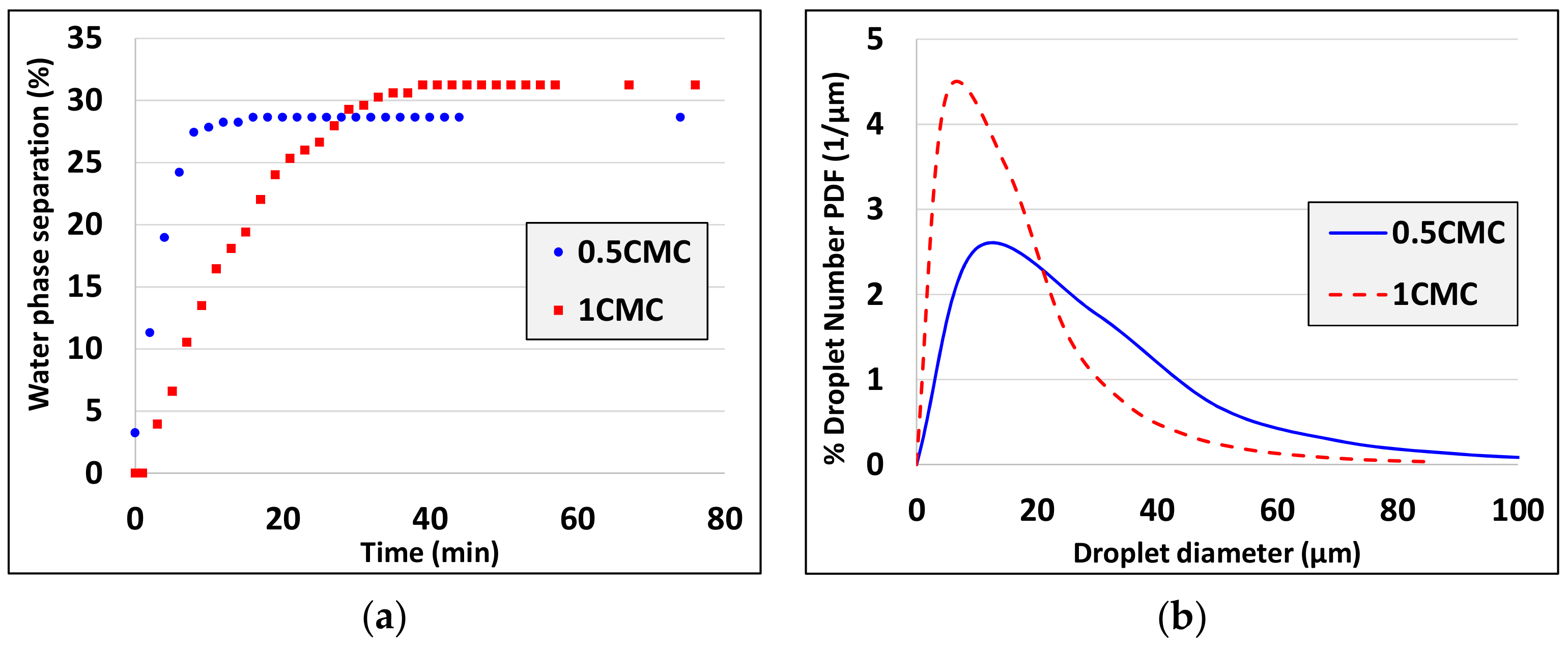
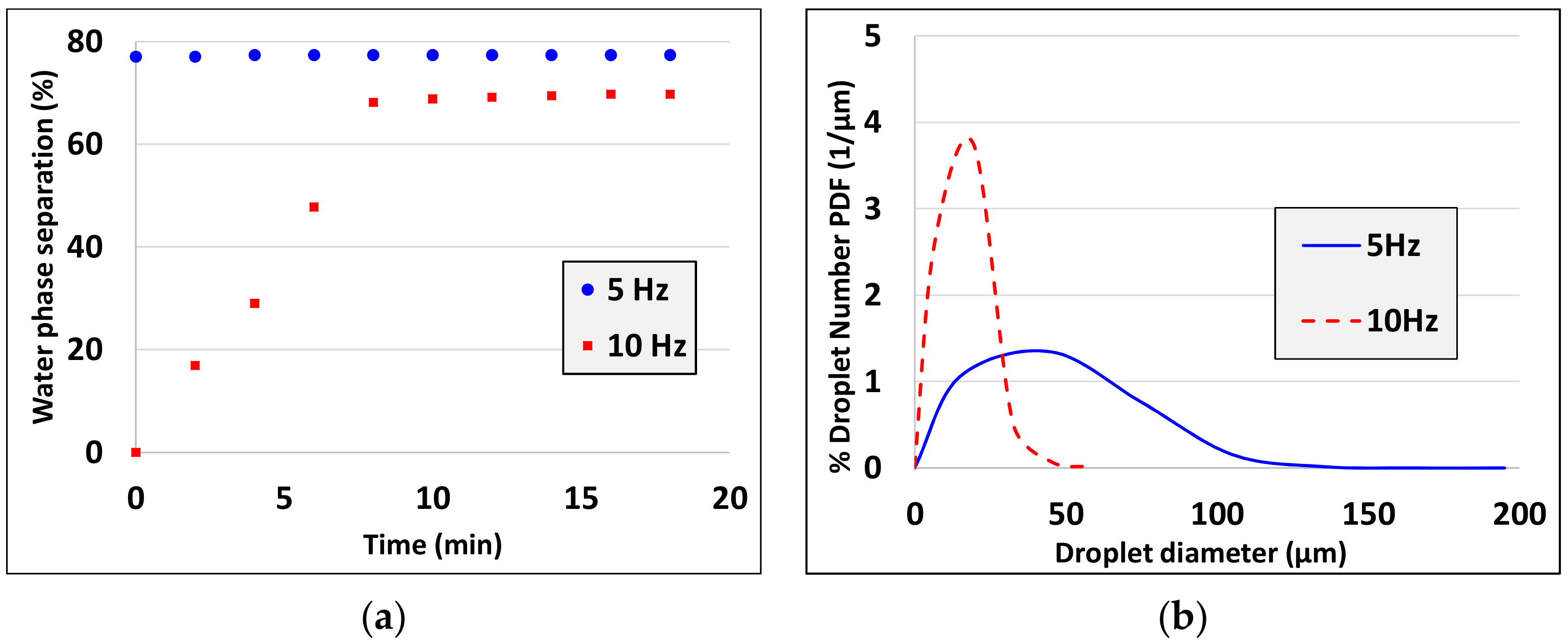
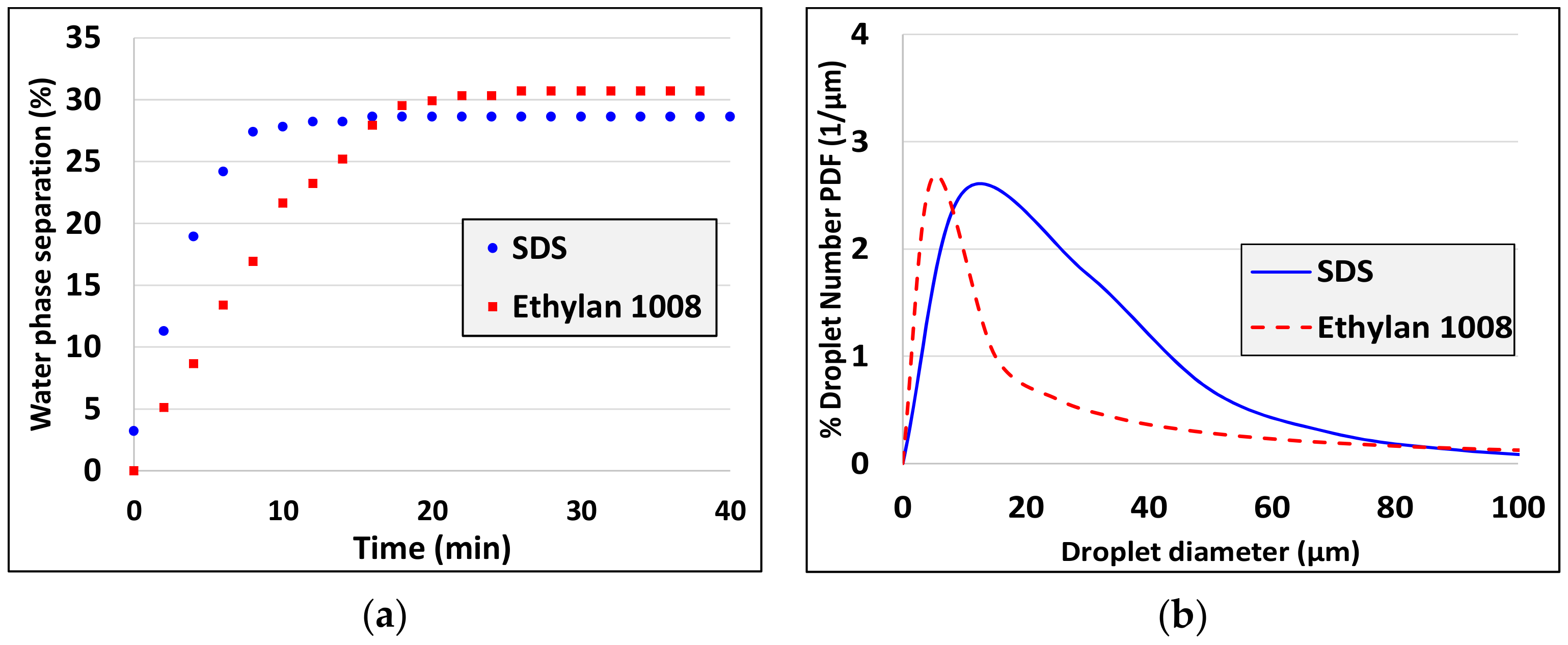
3. Experimental Results
4. Characterization of Flow Field in the New Emulsification Device
4.1. Piston Plate of Uniform Height
4.2. Piston Plate of Non-Uniform Height
5. Discussion and Analysis of the Results
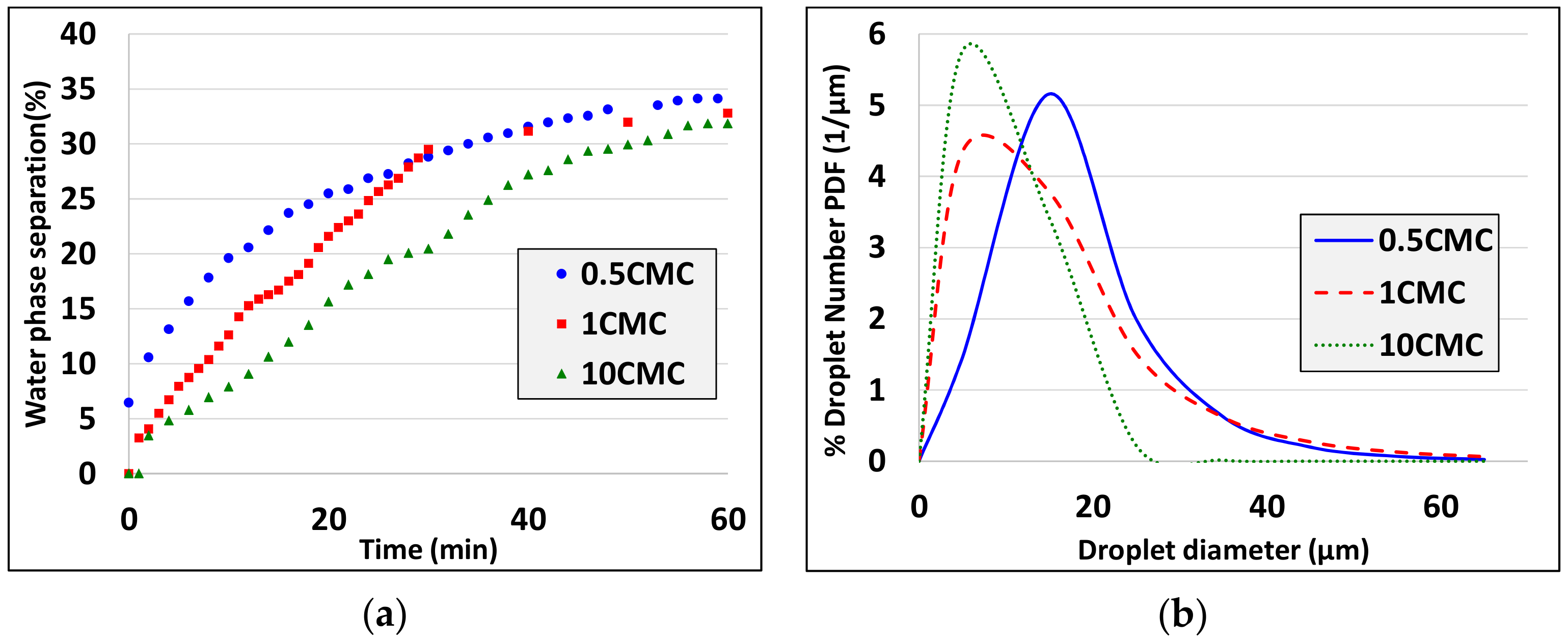
- Droplet size decreases as interfacial tension decreases (see experiments 1, 2 and experiments 10, 11, 12). There is a discrepancy to this rule comparing experiments 2 and 9 but there are two possible explanations: i) The differences are in the limit of resolution of interfacial tension and droplet diameter measurement techniques and ii) for such small differences not only the interfacial tension but also the interfacial elasticity (which differs for the two different surfactants) affects the droplet size.
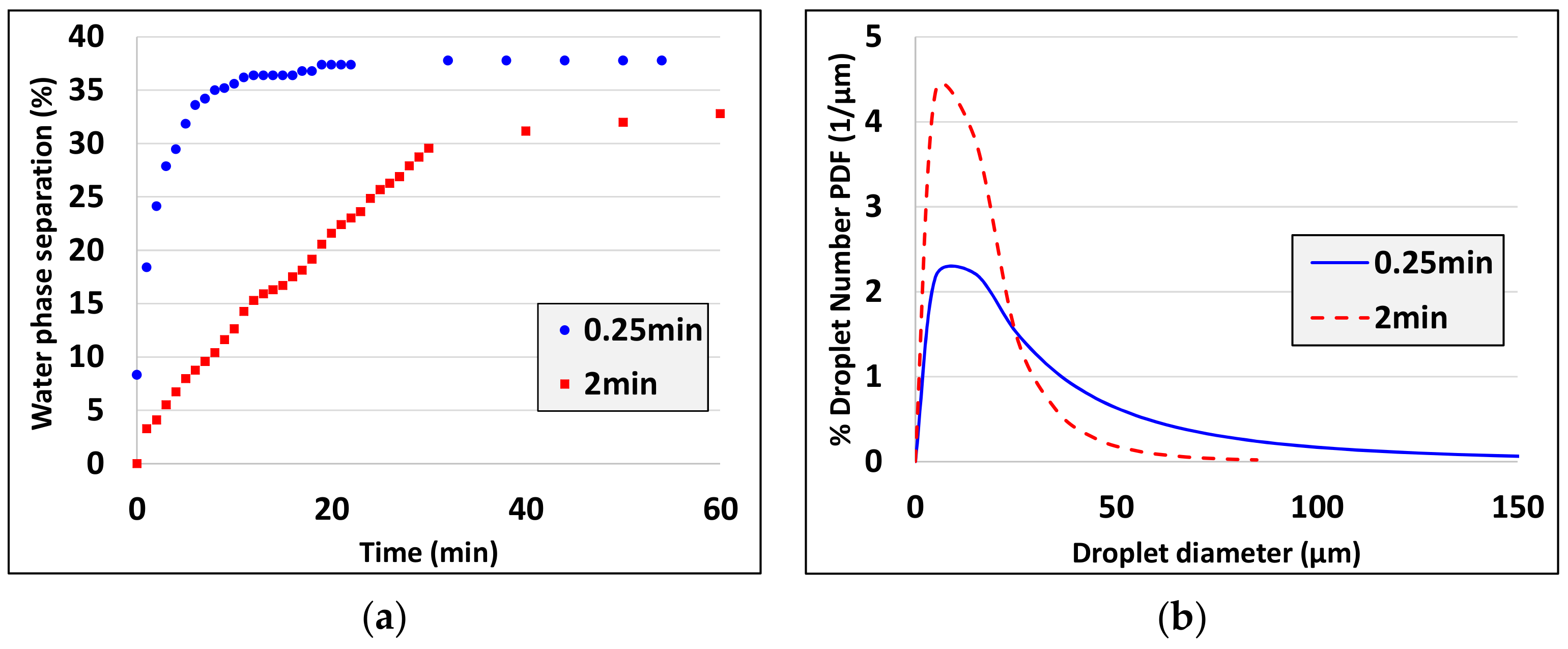
- Droplet size decreases as the actual emulsification time increases (see experiments 3, 4, experiments 5, 7 and experiments 10, 15).
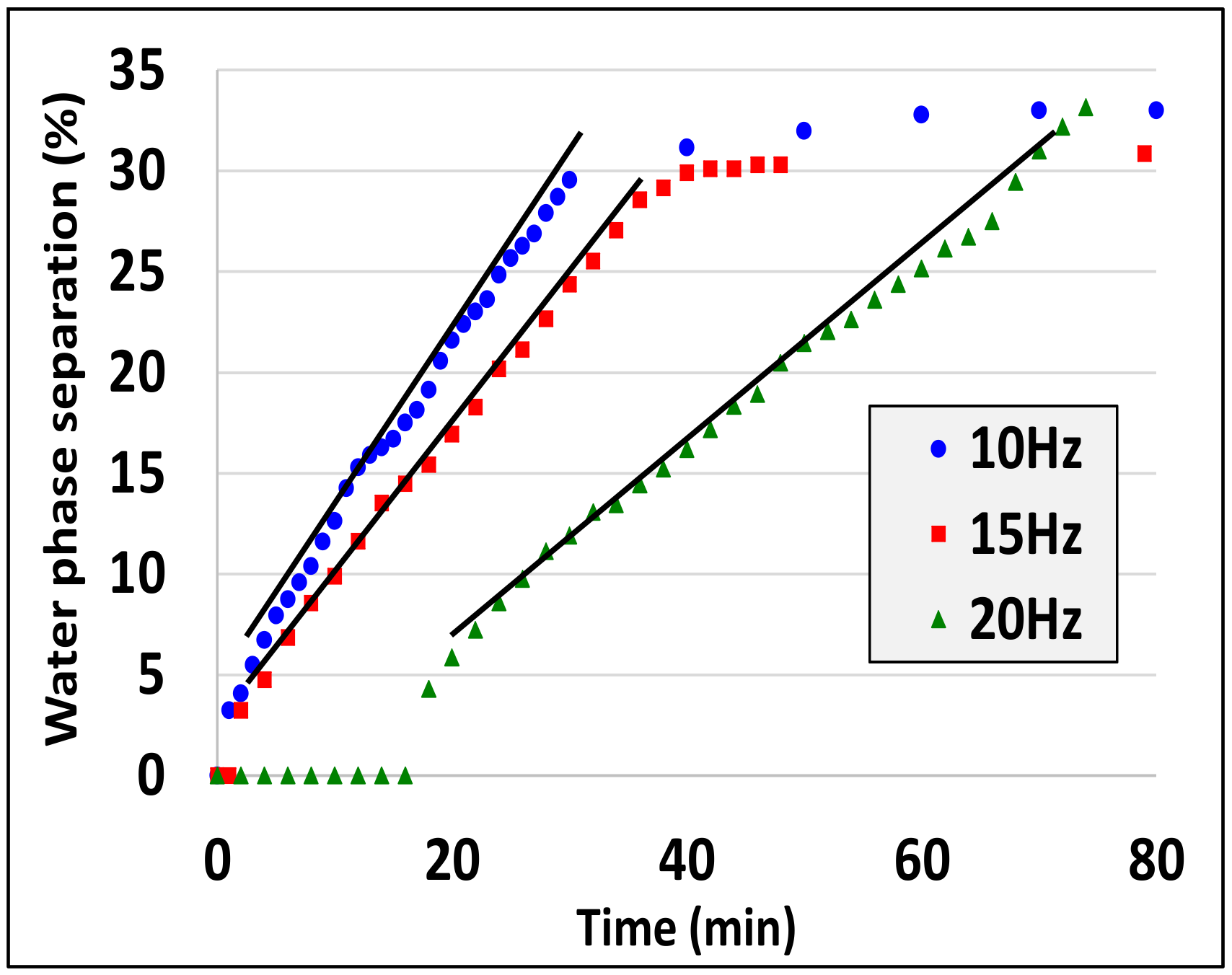
- Droplet size decreases as the characteristic shear rate increases (see experiments 5,6, experiments 7, 8 and experiments 10, 13, 14).
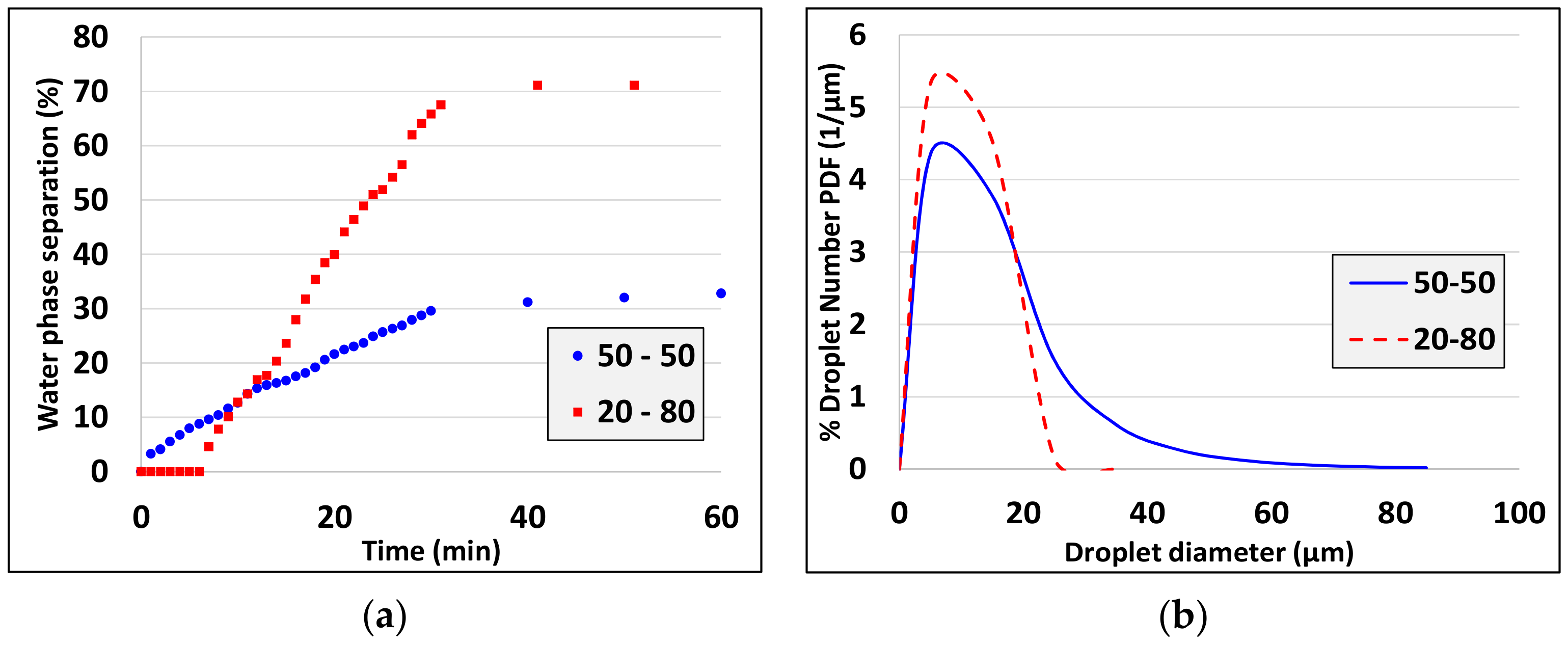
- Droplet size decreases as oil volume fraction decreases (see experiments 10, 16).
6. Conclusions
Author Contributions
Funding
Acknowledgments

Conflicts of Interest
References
- Chappat, M. Some applications of emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 57–77. [Google Scholar] [CrossRef]
- Ahmadi, P.; Asaadian, H.; Kord, S.; Khadivi, A. Investigation of the simultaneous chemicals influences to promote oil-in-water emulsions stability during enhanced oil recovery applications. J. Mol. Liq. 2019, 275, 57–70. [Google Scholar] [CrossRef]
- Al-Malah, K.I.; Azzam, M.O.J.; Omari, R.M. Emulsifying properties of BSA in different vegetable oil emulsions using conductivity technique. Food Hydrocoll. 2000, 14, 485–490. [Google Scholar] [CrossRef]
- Forgiarini, A.; Esquena, J.; Gonzalez, C.; Solans, C. Formation of Nano-emulsions by Low-Energy Emulsification Methods at Constant Temperature. Langmuir 2001, 17, 2076–2083. [Google Scholar] [CrossRef]
- Tadros, T.F. Applied Surfactants Principles and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Kazemzadeh, Y.; Ismail, I.; Rezvani, H.; Sharifi, M.; Riazi, M. Experimental investigation of stability of water in oil emulsions at reservoir conditions: Effect of ion type, ion concentration and system pressure. Fuel 2019, 243, 15–27. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Formation and Stability, 1st Ed. ed; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Agboola, S.O.; Singh, H.; Munro, P.A.; Dalgleish, D.G.; Singh, A.M. Destabilization of Oil-in-Water Emulsions Formed Using Highly Hydrolyzed Whey Proteins. J. Agric. Food Chem. 1998, 46, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tcholakova, S.; Denkov, D.N.; Lips, A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys. Chem. Chem. Phys. 2008, 10, 1597–1712. [Google Scholar] [CrossRef] [PubMed]
- Varka, E.M.; Karapantsios, T.D. Global versus local dynamics during destabilization of eco-friendly cosmetic emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2011, 391, 195–200. [Google Scholar] [CrossRef]
- Binks, P.B. Modern Aspects of Emulsion Science; Royal Society of Chemistry: Cambridge, UK, 1998. [Google Scholar]
- Friberg, S.; Larsson, K.; Sjoblom, J. Food Emulsions, 4th ed.; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Rosen, M.J. Surfactants and interfacial phenomena, 3rd ed.; John Wiley & Sons Inc.: New Jersey, NJ, USA, 2004. [Google Scholar]
- Schramm, L.L. Emulsions, Foams and Suspensions, Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Leal-Calderon, F.; Schmitt, V.; Bibette, J. Emulsion Science: Basic Principles, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Moinard-Checot, D.; Chevalier, Y.; Briancon, S.; Beney, L.; Fessi, H. Mechanism of nanocapsules formation by the emulsion-diffusion process. J. Colloid Interf. Sci. 2008, 317, 458–468. [Google Scholar] [CrossRef]
- Toledano, O.; Magdassi, S. Emulsification and Foaming Properties of Hydrophobically Modified Gelatin. J. Colloid Interf. Sci. 1998, 200, 235–240. [Google Scholar] [CrossRef]
- Williams, A.P.; Sayers, C.; Viebke, C.; Senan, C. Elucidation of the Emulsification Properties of Sugar Beet Pectin. J. Agric. Food Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Hartland, S. Dynamics of Emulsification and Demulsification of Water in Crude Oil Emulsions. Ind. Eng. Chem. Res. 1994, 33, 1271–1279. [Google Scholar] [CrossRef]
- Cabrera-Trujillo, M.A.; Filomena-Ambrosio, A.; Quintanilla-Carvajal, M.X.; Sotelo-Diaz, L.I. Stability of low-fat oil in water emulsions obtained by ultra turrax, rotor-stator and ultrasound homogenization methods. Int. J. Gastron. Food Sci. 2018, 13, 58–64. [Google Scholar] [CrossRef]
- Salerni, F.; Orsi, D.; Santini, E.; Liggieri, L.; Ravera, F.; Christofolini, L. Diffusing wave spectroscopy for investigating emulsions: II. Characterization of a paradigmatic oil-in-water emulsion. Coll. Surf. A 2019, 580, 123724. [Google Scholar] [CrossRef]
- Llamas, S.; Santini, E.; Liggieri, L.; Salerni, F.; Orsi, D.; Christofolini, L.; Ravera, F. Adsorption of Sodium Dodecyl Sulphate at Water-Dodecane Interface in Relation to the Oil in Water Emulsion Properties. Am. Chem. Soc. 2018, 34, 5978–5989. [Google Scholar]
- Ethylan 1008. Available online: https://www.nouryon.com/product/ethylan-1008-non-ionic-surfactant/ (accessed on 19 November 2019).
- Zaboulis, X.; Papara, M.; Chatziargyriou, A.; Karapantsios, T.D. Detection of densely dispersed spherical bubbles in digital images based on a template matching technique: Application to wet foams. Colloids Surf. A Physicochem. Eng. Asp. 2007, 309, 96–106. [Google Scholar] [CrossRef]
- Caserta, S.; Simeone, M.; Guido, S. Evolution of drop size distribution of polymer blends under shear flow by optical sectioning. Rheol. Acta 2004, 43, 491–501. [Google Scholar] [CrossRef]
- Caserta, S.; Sabetta, L.; Simeone, M.; Guido, S. Shear-induced coalescence in aqueous biopolymer mixtures. Chem. Engin. Sci. 2005, 60, 1019–1027. [Google Scholar] [CrossRef]
- Caserta, S.; Simeone, M.; Guido, S. A parameter investigation of shear-induced coalescence in semidilute PIB-PDMS polymer blends: Effects of shear rate, shear stress volume fraction and viscosity. Rheol. Acta 2006, 45, 505–512. [Google Scholar] [CrossRef]
- Ampatzidis, D.C.; Varka, E.M.; Karapantsios, T.D. Interfacial Activity of amino acid-based glycerol ether surfactants and their performance in stabilizing O/W cosmetic emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 176–183. [Google Scholar] [CrossRef]
- Konstandopoulos, A.; Kostoglou, M.; Vlachos, N.; Kladopoulou, E. Advances in the Science and Technology of Diesel Particulate Filter Simulation. Adv. Chem. Eng. 2007, 33, 213–275. [Google Scholar]
- Coulaloglou, C.A.; Tavlarides, L.L. Description of Interaction Processes in Agitated Liquid-Liquid Dispersions. Chem. Eng. Sci. 1977, 32, 1289. [Google Scholar] [CrossRef]
- Williams, A.; Janseen, M.J.J.; Prins, A. Behavior of droplets in simple shear flow in the presence of a protein emulsifier. Colloids Surf. A Physicochem. Eng. Asp. 1997, 125, 189–200. [Google Scholar] [CrossRef]
- Jansen, K.B.M.; Agterof, W.G.M.; Mellema, J. Droplet breakup in concentrated emulsions. J. Rheol. 2001, 45, 227. [Google Scholar] [CrossRef]
- Kostoglou, M.; Varka, E.M.; Kalogianni, E.P.; Karapantsios, T.D. Evolution of volume fractions and droplet sizes by analysis of electrical conductance curves during destabilization of oil-in-water emulsions. J. Colloid Interf. Sci. 2010, 349, 408–416. [Google Scholar] [CrossRef]
- Janssen, M.J.J.; Boon, A.; Agterof, M.W.G. Droplet break-up in simple shear flow in the presence of emulsifiers. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 141–148. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion Phenomena in High Viscosity Immiscible Fluid Systems and Application of Static Mixers as Dispersion Devices in Such Systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]





| Experimental parameters | |||||
| Oil: Water Ratio (%) | Stroke Frequency (Hz) | Surfactant Concentration (x CMC) | Nominal Emulsification Time (min) | ||
| Dodecane Millipore water SDS | 1 | 50:50 | 10 | 0.5 | 2 |
| 2 | 50:50 | 10 | 1 | 2 | |
| 3 | 50:50 | 10 | 1 | 0.5 | |
| 4 | 50:50 | 10 | 1 | 10 | |
| 5 | 20:80 | 5 | 10 | 0.5 | |
| 6 | 20:80 | 10 | 10 | 0.5 | |
| 7 | 20:80 | 5 | 10 | 2 | |
| 8 | 20:80 | 10 | 10 | 2 | |
| Dodecane Millipore water Ethylan 1008 | 9 | 50:50 | 10 | 0.5 | 2 |
| Experimental Parameters | |||||
|---|---|---|---|---|---|
| Oil: Water Ratio (%) | Stroke Frequency (Hz) | Surfactant Concentration (x CMC) | Nominal Emulsification Time (min) | ||
| Dodecane Millipore water SDS | 10 | 50:50 | 10 | 1 | 2 |
| 11 | 50:50 | 10 | 0.5 | 2 | |
| 12 | 50:50 | 10 | 10 | 2 | |
| 13 | 50:50 | 15 | 1 | 2 | |
| 14 | 50:50 | 20 | 1 | 2 | |
| 15 | 50:50 | 10 | 1 | 0.25 | |
| 16 | 20:80 | 10 | 1 | 2 | |
| Calculated Input Parameters | Emulsification Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic Shear Rate (1/s) | Actual Emulsification Time (min) | Interfacial Tension (mN/m) | Separation Front Velocity (mm/sec) | dav (μm) | dv (μm) | PI | ||
| Dodecane Millipore water SDS | 1 | 13600 | 0.706 | 17 | 0.0085 | 23.3 | 44.7 | 1.64 |
| 2 | 13600 | 0.706 | 9.8 | 0.0052 | 13.5 | 34.8 | 1.34 | |
| 3 | 13600 | 0.176 | 9.8 | 0.0078 | 16.2 | 43 | 1.49 | |
| 4 | 13600 | 3.52 | 9.8 | 0.0016 | 8.1 | 19.8 | 1.35 | |
| 5 | 6800 | 0.176 | 6.2 | too large | 45 | >45 | 1.26 | |
| 6 | 13600 | 0.176 | 6.2 | 0.0455 | 14.6 | 31.8 | 1.18 | |
| 7 | 6800 | 0.706 | 6.2 | too large | 39.4 | >39.4 | 1.26 | |
| 8 | 13600 | 0.706 | 6.2 | 0.058 | 10.9 | 36.1 | 1.33 | |
| Dodecane Millipore water Ethylan 1008 | 9 | 13600 | 0.706 | 9.2 | 0.0072 | 15.4 | 41.2 | 3.22 |
| Calculated Input Parameters | Emulsification Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic Shear Rate (1/s) | Actual Emulsification Time (min) | Interfacial Tension (mN/m) | Separation Front Velocity (mm/sec) | dav (μm) | dv (μm) | PI | ||
| Dodecane Millipore water SDS | 10 | 3920 | 3.72 | 9.8 | 0.0042 | 14.4 | 31.5 | 1.40 |
| 11 | 3920 | 3.72 | 17 | 0.005 | 17.3 | 34.5 | 1.20 | |
| 12 | 3920 | 3.72 | 6.2 | 0.0031 | 11.3 | 27 | 1.43 | |
| 13 | 5880 | 3.72 | 9.8 | 0.0038 | 12.1 | 30 | 1.38 | |
| 14 | 7840 | 3.72 | 9.8 | 0.0025 | 10.5 | 24.4 | 1.46 | |
| 15 | 3920 | 0.465 | 9.8 | 0.017 | 29.2 | 64 | 1.47 | |
| 16 | 3920 | 3.72 | 9.8 | 0.013 | 10.1 | 17.4 | 1.15 | |
| σ | d* (μm) | ||
|---|---|---|---|
| Dodecane Millipore water SDS | 1 | 0.53 | 24.69 |
| 2 | 0.58 | 13.73 | |
| 3 | 0.48 | 14.94 | |
| 4 | 0.83 | 9 | |
| 5 | - | - | |
| 6 | 0.19 | 14.53 | |
| 7 | - | - | |
| 8 | 0.6 | 11.9 | |
| Dodecane Millipore water Ethylan 1008 | 9 | 6.99 | 18.52 |
| σ | d* (μm) | ||
|---|---|---|---|
| Dodecane Millipore water SDS | 10 | 0.48 | 13.18 |
| 11 | 0.27 | 15.37 | |
| 12 | 0.16 | 9.43 | |
| 13 | 0.27 | 10.88 | |
| 14 | 0.15 | 9.16 | |
| 15 | 1.09 | 26.28 | |
| 16 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chondrou, A.P.; Evgenidis, S.P.; Kostoglou, M.; Karapantsios, T.D. An Innovative Miniature Pulsating Emulsification Device: Flow Characterization and Measurement of Emulsion Stability. Colloids Interfaces 2020, 4, 7. https://doi.org/10.3390/colloids4010007
Chondrou AP, Evgenidis SP, Kostoglou M, Karapantsios TD. An Innovative Miniature Pulsating Emulsification Device: Flow Characterization and Measurement of Emulsion Stability. Colloids and Interfaces. 2020; 4(1):7. https://doi.org/10.3390/colloids4010007
Chicago/Turabian StyleChondrou, Angeliki P., Sotiris P. Evgenidis, Margaritis Kostoglou, and Thodoris D. Karapantsios. 2020. "An Innovative Miniature Pulsating Emulsification Device: Flow Characterization and Measurement of Emulsion Stability" Colloids and Interfaces 4, no. 1: 7. https://doi.org/10.3390/colloids4010007
APA StyleChondrou, A. P., Evgenidis, S. P., Kostoglou, M., & Karapantsios, T. D. (2020). An Innovative Miniature Pulsating Emulsification Device: Flow Characterization and Measurement of Emulsion Stability. Colloids and Interfaces, 4(1), 7. https://doi.org/10.3390/colloids4010007