1. Introduction
Membrane filtration of oil-in-water emulsions is a cost-effective and selective way of separating water from oil. This is especially the case for stable emulsions, with droplets
m, which cannot be separated efficiently by, for instance, flotation or other gravity-driven processes [
1,
2,
3,
4,
5]. Membranes, however, suffer from fouling, which causes a decline in clean water production and an increase in energy consumption.
Membrane fouling has been studied extensively on a macroscopic level, mainly by correlating the flux decline of a filtration process with models [
6,
7,
8,
9]. The more microscopic interactions between particles or, in this case, oil droplets, and the membrane surface are harder to study in-situ. In the case of oil droplets on a membrane, the interaction between the oil droplets and the membrane surface will determine much of the fouling behavior. Droplets can deform, coalesce and spread out on the membrane surface. In contrast to hard particles, which form a dense but permeable cake layer on top of the membrane surface, oil droplets can also form a film of oil on the surface, with an even more detrimental effect on the flux [
5]. Direct observation of oil droplets on a membrane surface is therefore an important step towards better understanding membrane fouling by oil-in-water emulsions. In addition, understanding the interactions between oil droplets and the membrane surface is a first step towards better tailoring membrane filtration processes to their feed stream in the future.
Many different optical and spectroscopic techniques have been used to investigate the process of membrane fouling [
10]. Most of these techniques can be applied only in quite specific situations or systems, or require elaborate optical devices. Altmann and Rippeger used a laser triangulometer to measure the layer thickness of either diatomaceous earth or silica particles on a membrane during crossflow filtration [
11]. Based on their model, they concluded that smaller particles attach easier to the fouling layer than larger particles or aggregates, and that the latter are the only particles that can be detached from the layer once attached. They also concluded that by changing the operating parameters of the filtration process, the layer characteristics can be changed. Li et al. used direct observation through the membrane (DOTM) to study the deposition of either yeast or latex beads on the membrane surface [
12]. The membrane in this case was an anodized aluminum membrane with see-through pores, which allowed them to place the microscope on the permeate size. They determined that the particle deposition on the membrane was largely dependent on a critical flux, which depends on the crossflow velocity. In addition, they discovered that initial deposition of particles causes more particles to deposit on the membrane. Mores et al. performed direct visual observation of yeast deposition on membranes in a specialized membrane cell and with colored yeast on a cellulose acetate and an anodized alumina membrane [
13]. By mounting the microscope on the feed side of the membrane, they could observe the yeast particles during filtration, but also during and after a backflush routine. Vanysacker et al. studied biofouling in a high throughput crossflow membrane filtration system by placing it in a confocal laser scanning microscope system [
14]. They combined this technique with SEM and optical microscopy to study the formation of a biofilm. By running multiple experiments simultaneously in this setup, they yielded reproducible results for statistical analysis. Fux and Ramon studied the behavior of surfactant-stabilized oil droplets on a membrane surface in the presence of a transmembrane flow [
15]. By studying the deformation of individual oil droplets on the membrane surface using a confocal microscope, they discovered that the transmembrane flow and the force exerted on the droplet determine the adhesion to the surface. To properly model this behavior, they proposed a modified capillary number. At higher forces and thus higher deformation, droplets are harder to remove, in some cases the adhesion is irreversible. Di et al. developed a microfluidic system in which a polyethersulfone membrane was mounted and subsequently fouled by latex microbeads [
16]. The whole system was placed in a confocal microscope, enabling them to acquire 3D time-sequenced images of the fouling layer. They observed that at increasing ionic strength, less latex particles deposited, but they formed more aggregates than at lower ionic strengths [
17].
In this chapter, we investigate the correlation between droplet adhesion on a model surface and fouling during crossflow membrane filtration. A recently developed flow-cell approach allows us to directly observe droplet adhesion to a model surface. The strength with which droplets are adhered to a surface can give an indication of the fouling potential of the oil droplets in an emulsion. Because the technique is relatively easy and fast, multiple parameters can be taken into account. In the case of produced water, which is a complex mixture of different components, this can prove useful to study the influence on fouling of each of those components. Membrane fouling by produced water, which fouls much more severely than other (less complex) oil-in-water emulsions, is still poorly studied, and this technique can contribute to extending our knowledge on the subject [
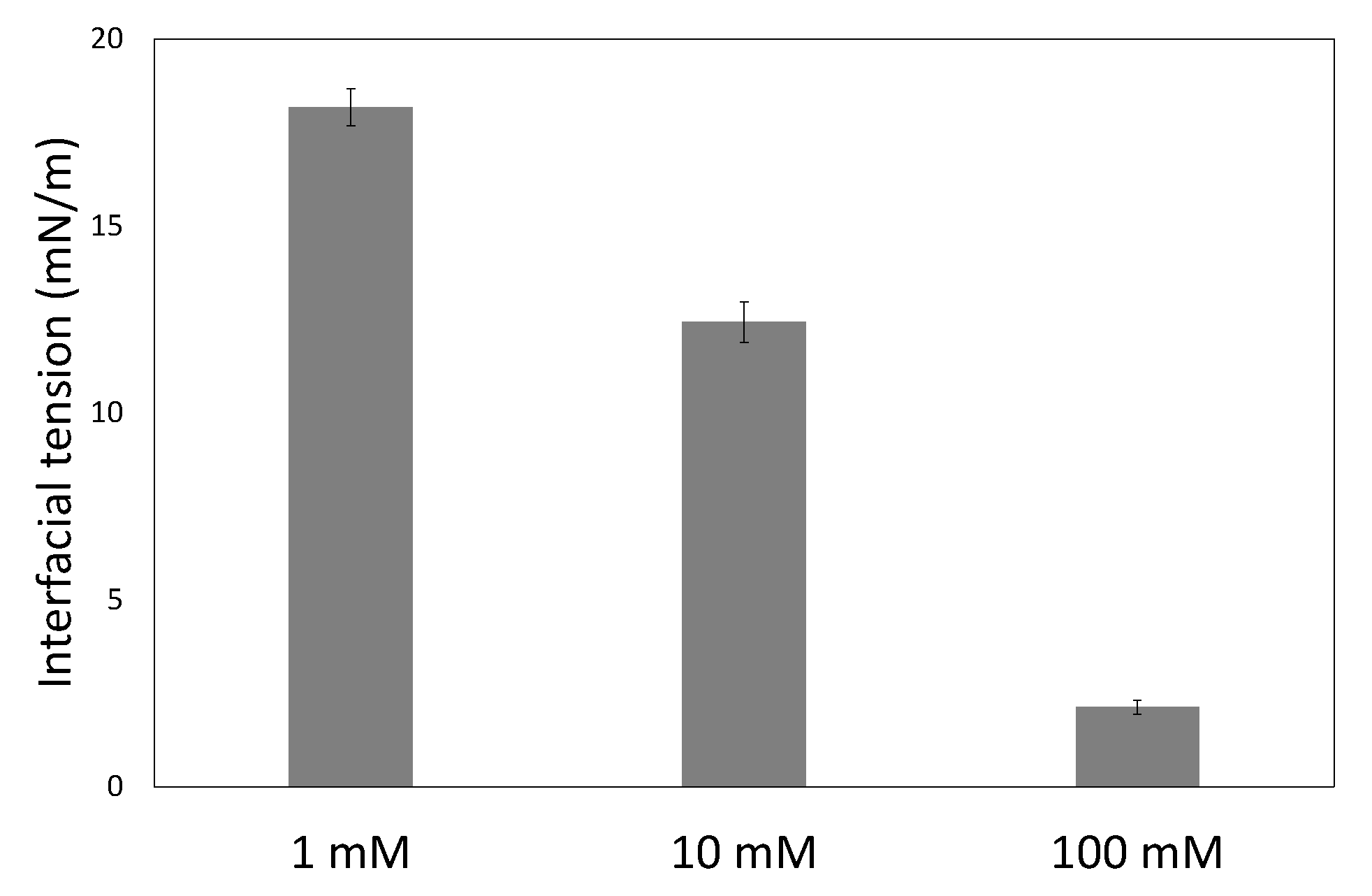
5]. For produced water, where a complex emulsion is often stabilized by charged surfactants, especially the ionic strength of the solution is expected to be key to oil droplet adhesion at the interface and subsequent cake layer build-up. Indeed in a recent study, the strength of adhesion of surfactant stabilized oil droplets was found to be strongly affected by the ionic strength, both on a hydrophobic and charged hydrophilic surface [
18].
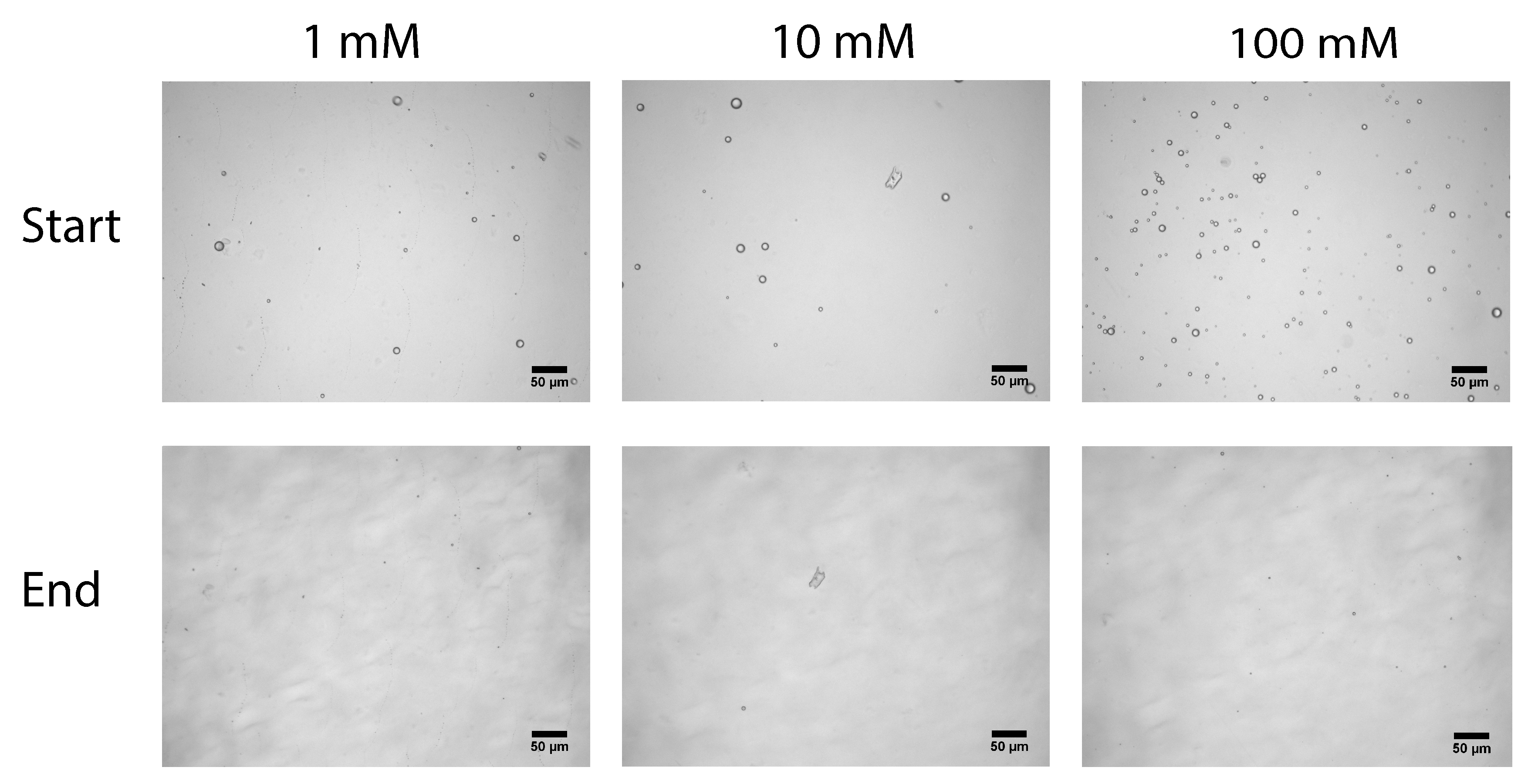
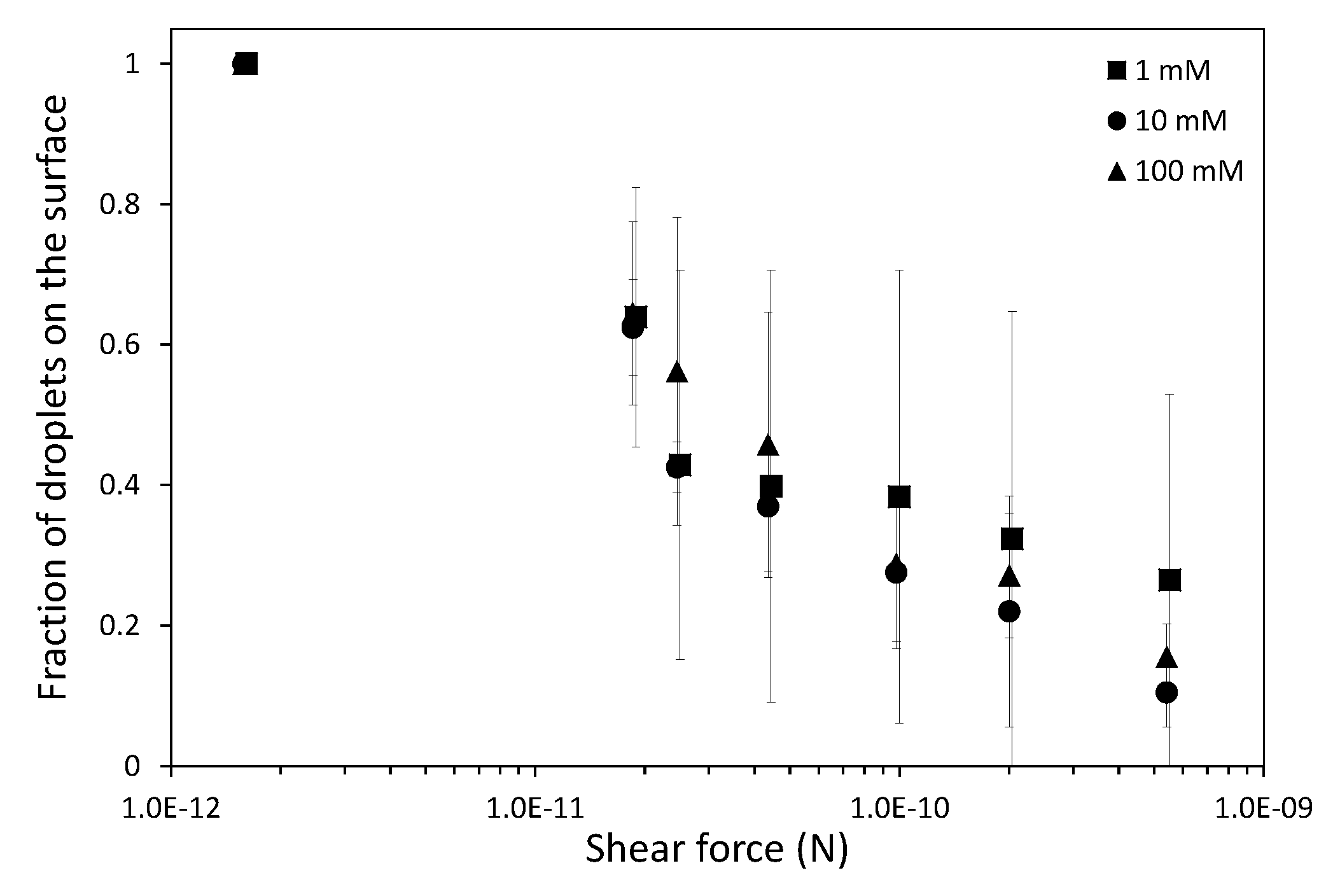
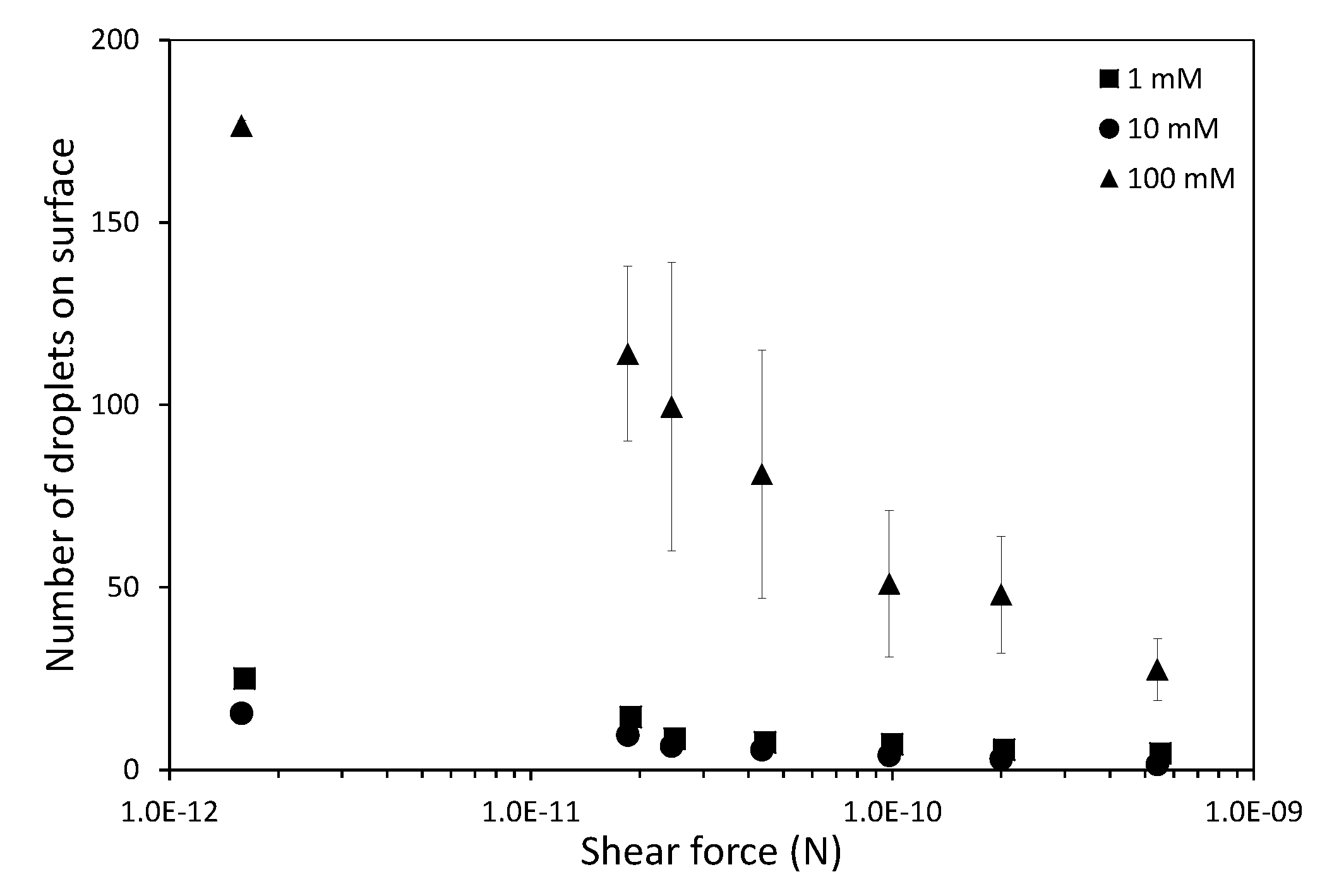
Although the flow cell has no permeation interface, the membrane and model surface are chemically identical (cellulose). The emulsion used for both flow cell and membrane filtration is also identical, with well defined characteristics. We directly observe the adhesion between the oil droplets in feed stream and the cellulose surface in the flow cell. From this, we know how much droplets stick to the surface, but by increasing the shear force we can also determine how strong the droplet-surface interactions are. In the membrane filtration experiments we observe the flux decline caused by fouling. The kinetics of this fouling can tell us how the fouling is formed and what the characteristics of the layer are.
2. Materials and Methods
To prepare the emulsions and flush solutions for all experiments, we used de-ionized (DI) water, sodium dodecyl sulfate (SDS, Sigma Aldrich 75746, Zwijndrecht, the Netherlands), n-hexadecane (Merck Schuchardt OHG 820633, Darmstadt, Germany), and sodium chloride (NaCl, Boom 51275, Meppel, The Netherlands). For the glass surface modification we used trichloromethylsilane (Sigma Aldrich M85301, Zwijndrecht, the Netherlands) and trimethylsilylcellulose (TMSC, Artecs bv, Hengelo, the Netherlands), prepared via the protocol in [
19], but without addition of the catalyst. The membranes we used were regenerated cellulose membranes on a polyethylene terephthalate (PET) support with a molecular weight cutoff of 500 kDa (Microdyn Nadir UC500, Wiesbaden, Germany). All chemicals were used without further purification steps.
2.1. Emulsion Preparation
To ensure all emulsions have the same characteristics, a stock emulsion was prepared under standard conditions, which was then diluted with carefully chosen concentrations to obtain the desired oil, salt and surfactant concentration for the membrane experiments and the flow cell. The stock emulsions were prepared by dissolving 463 mg/L SDS in 1 L of DI water in a Duran® bottle (Duran 21801545, Boom, Meppel, The Netherlands) by mixing with a dispersing mixer (IKA® T25 digital Ultra-Turrax with S25N 18G element, Boom, Meppel, The Netherlands) for 2 min at 14,000 rpm. Then, 2 g of n-hexadecane was injected near the mixer head and mixed for 10 min at 14,000 rpm. For the membrane filtration experiments, the stock emulsion was diluted to make up 20 L of emulsion with 100 mg/L hexadecane, 463 mg/L SDS and 1, 10 or 100 mM NaCl, which was then stored in a glass 20 L bottle.
For the flow cell, the stock emulsion was diluted to contain 1 g/L hexadecane, 463 mg/L SDS and 1, 10 or 100 mM NaCl. The concentration of hexadecane in the emulsions for the flow cell was chosen higher than the concentration of oil in the membrane filtration experiments to ensure enough droplets would be visible in the flow cell. Surfactant solutions used for rinsing and for applying shear were prepared the same as the emulsions, but without hexadecane. The concentrations of surfactant and NaCl were identical to the emulsion used in each experiment. After preparation, the surfactant solutions were degassed under vacuum and ultrasonic sonication for 15 min, followed by 15 min of only vacuum. The emulsions were not degassed, as this was not to be found necessary. Furthermore, surfactant solutions were also used for all contact angle and interfacial tension measurements. While in this work no zeta potentials where obtained of the emulsions, these SDS based emulsions are known to be strongly negatively charged (zeta potentials of −60 to −100 mV) for similar SDS concentrations and ionic strengths [
20].
2.2. Glass Preparation for Flow Cell
Microscope glass slides (VWR 631-1552, Boom, Meppel, The Netherlands) were washed with DI water and ethanol, dried in an oven and placed in a desiccator with 0.2 mL of trichloromethylsilane in a glass bottle. The glass slides were left overnight so the trichloromethylsilane could deposit on the glass slides to make them hydrophobic. A 20 mg/L solution of TMSC in chloroform was prepared by stirring overnight. To remove any solids, the solution was filtered over a steel filter with 15
m pores. The TMSC was spincoated on the hydrophobic glass slides by spinning at 2500 rpm for 20 s. The TMSC was then regenerated to cellulose by exposing the glass slides to a hydrochloric acid vapor by placing them in a desiccator above 37% hydrochloric acid for 15 min [
19]. After regeneration, the layer thickness was determined to be 115 ± 2 nm with a roughness of 30 ± 2 nm by ellipsometry.
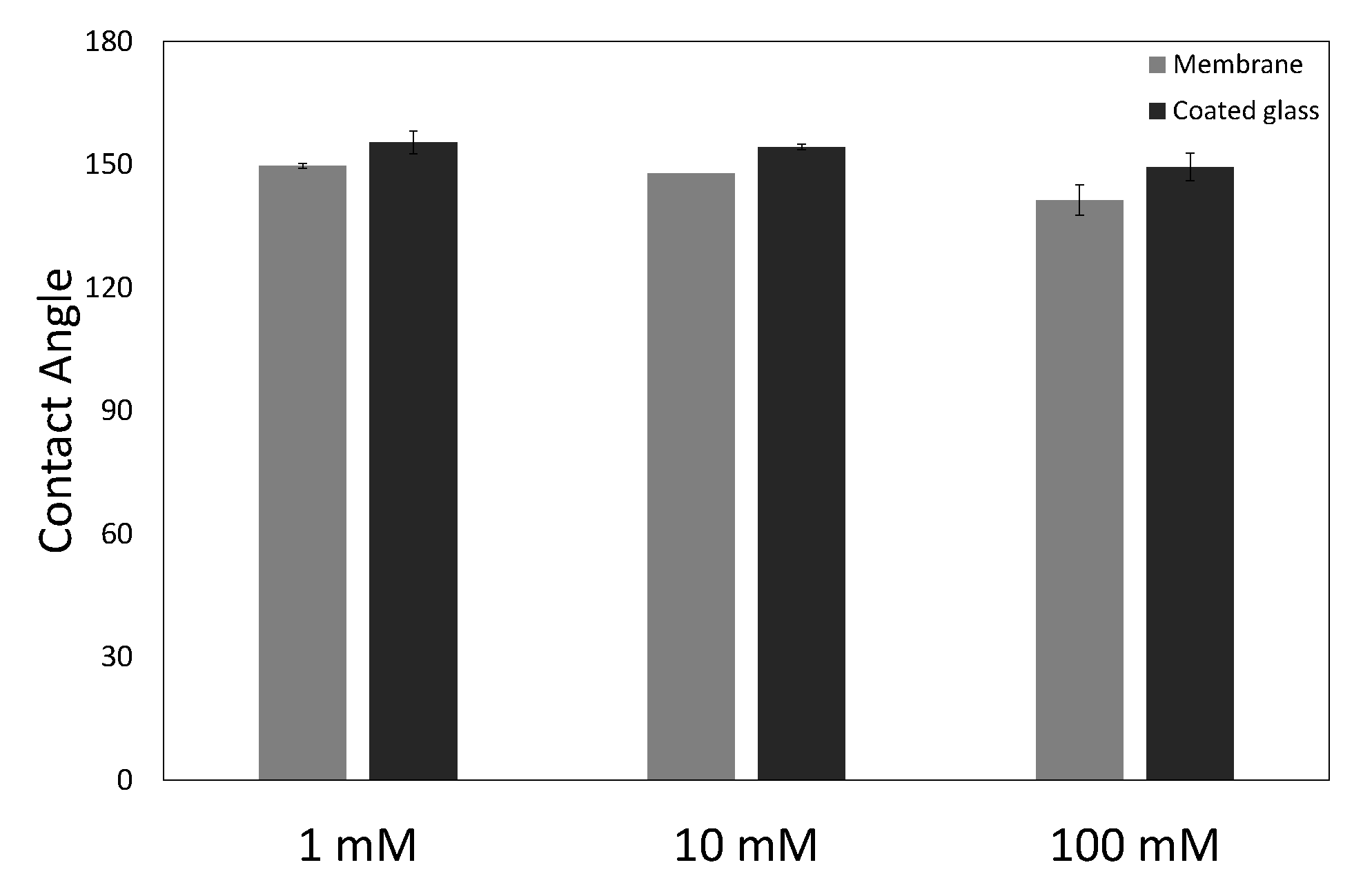
2.3. Contact Angle and Interfacial Tension Measurements
Measurements were performed on a contact angle and contour analysis instrument (Dataphysics, Filderstadt, Germany, OCA 35). The contact angle measurements were performed in captive bubble mode, where a droplet of hexadecane is captured under a cellulose coated glass slide or a piece of membrane in the aqueous solution with surfactant and salt. The interfacial tension measurements were performed with the pendant droplet technique, where a droplet of aqueous solution with surfactant and salt is suspended in hexadecane. Image analysis for both contact angle and interfacial tension measurements was performed with the software provided with the measuring instrument.
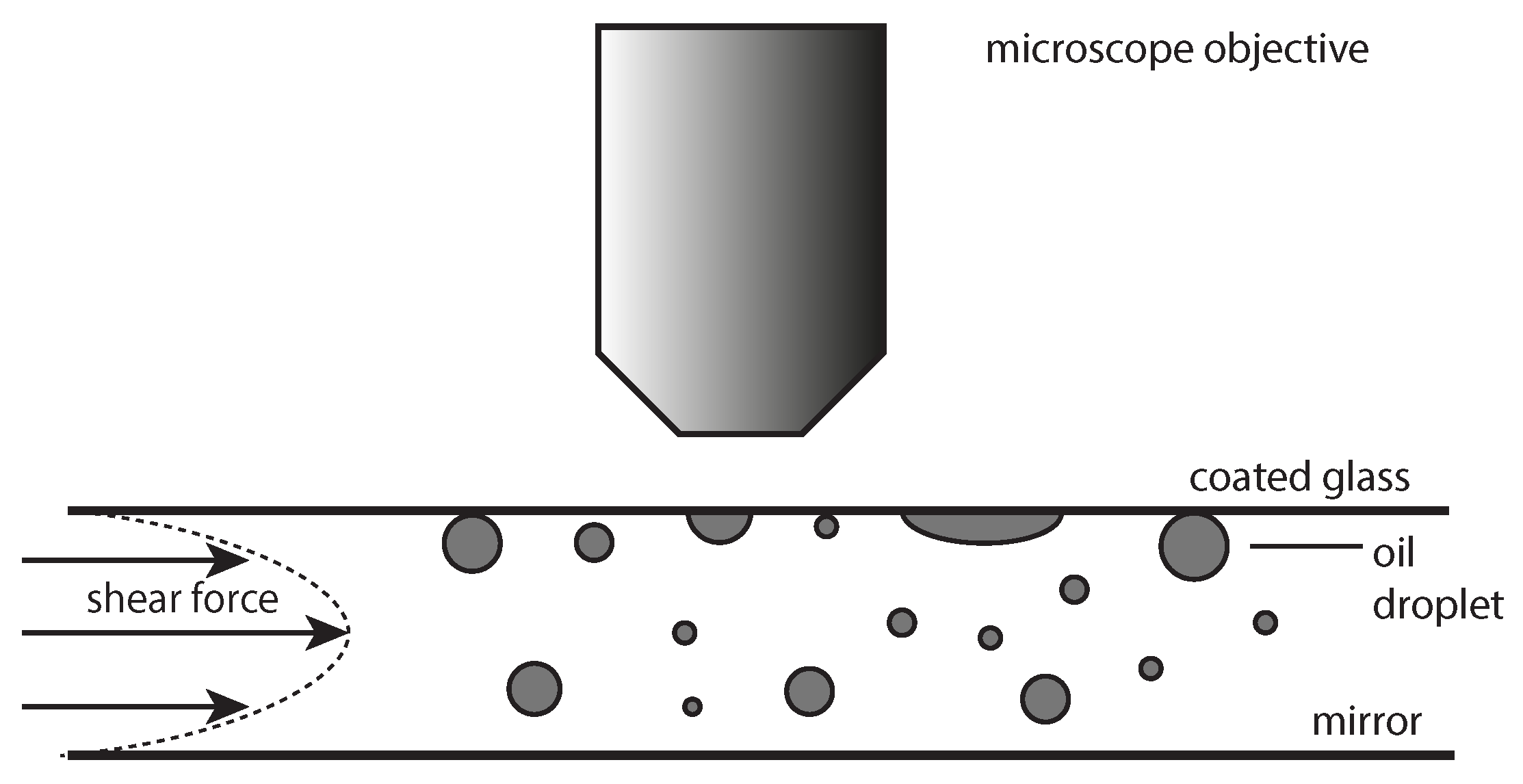
2.4. Flow Cell Setup and Operation
To measure the adhesion of oil droplets in the emulsion to the model cellulose surface, a flow cell setup was used. This flow cell has been used before in our previous work [
18]. The emulsion is fed into the flow chamber by a pulseless pump, and the droplets are observed by placing the flow cell under a microscope (
Figure 1). A detailed desciption of the flow cell, flow cell operation and image analysis can be found in [
18].
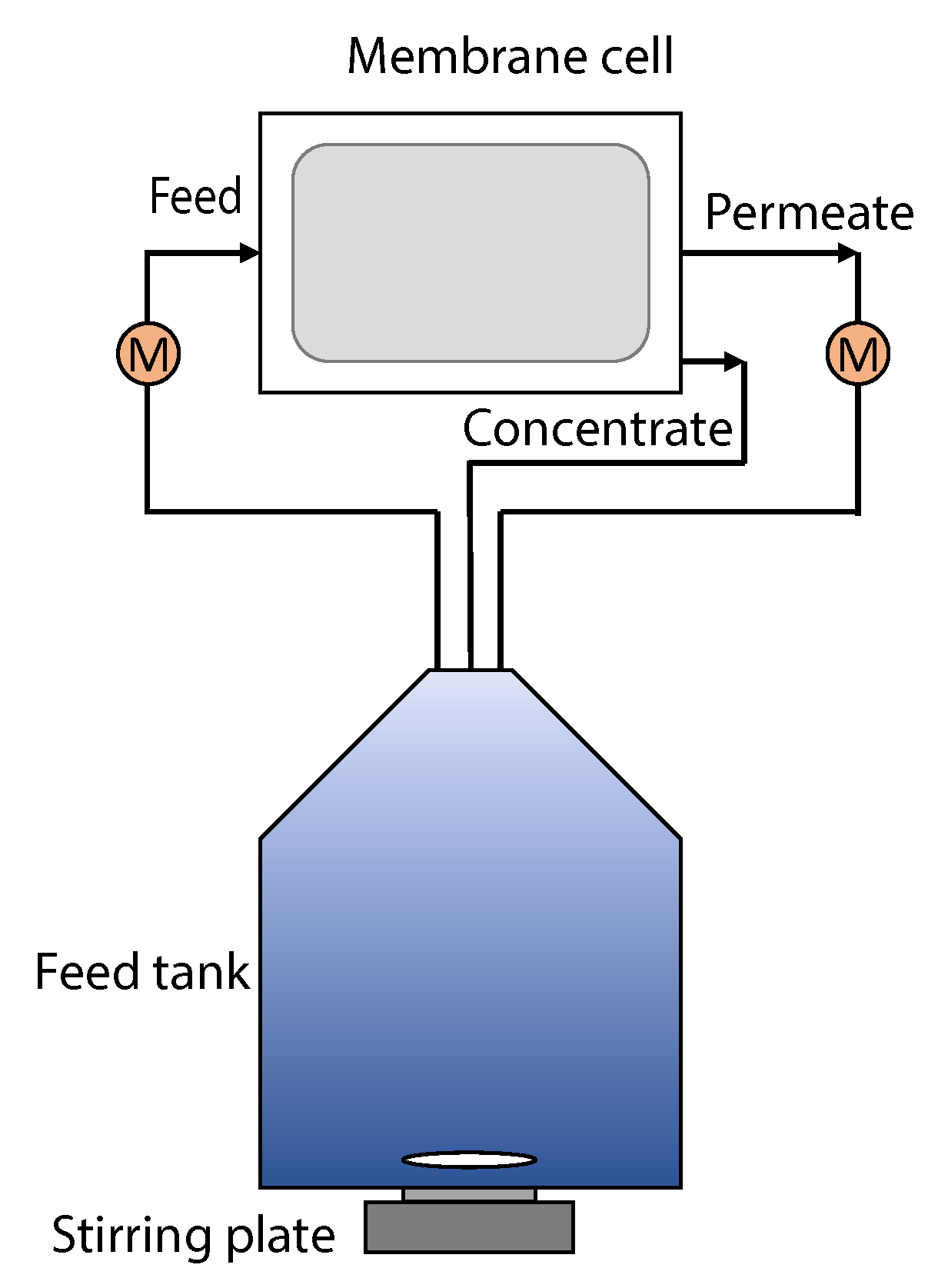
2.5. Membrane Filtration
The membrane filtration experiments were performed using an OSMO -inspector crossflow membrane filtration system built by Convergence (Enschede, the Netherlands) (
Figure 2). The membrane was mounted in a flat sheet crossflow membrane cell with an effective surface of 240 cm
, using a feed spacer with a thickness of 700
m, a filament angle of 90
and a maze size of 2.5 × 2.5 mm. The volume and density of the feed and permeate streams were measured by Bronckhorst M15 mass flow meters. The 20 L glass feed bottle was constantly stirred to prevent creaming of the feed. Both concentrate and permeate were recycled to the feed bottle to ensure a consistent feed quality. Before mounting the membrane, it was soaked in DI water overnight to remove production chemicals and glycerine from the membrane. Then, the clean water flux was measured under experimental conditions because the membrane sheets provided were not uniform (
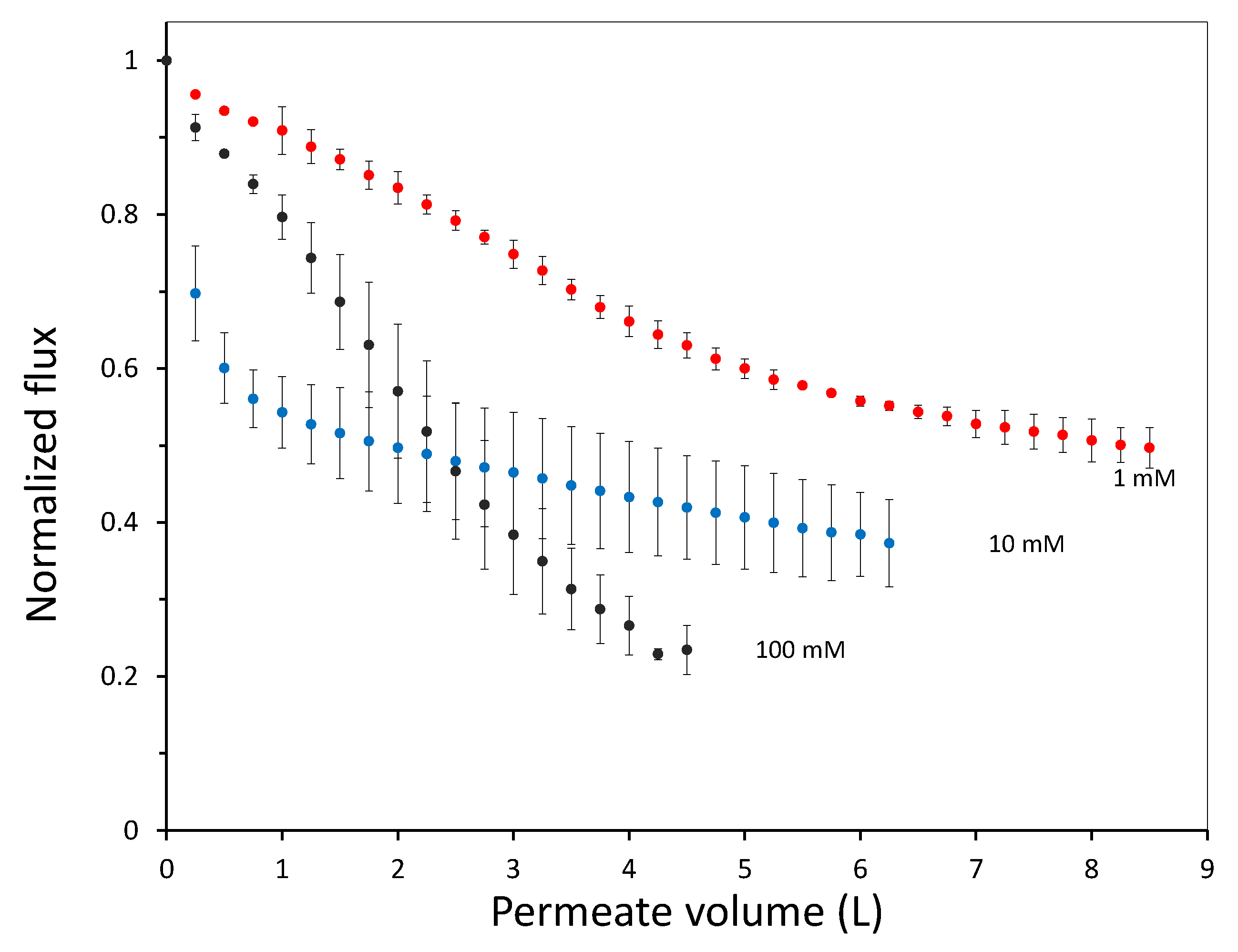
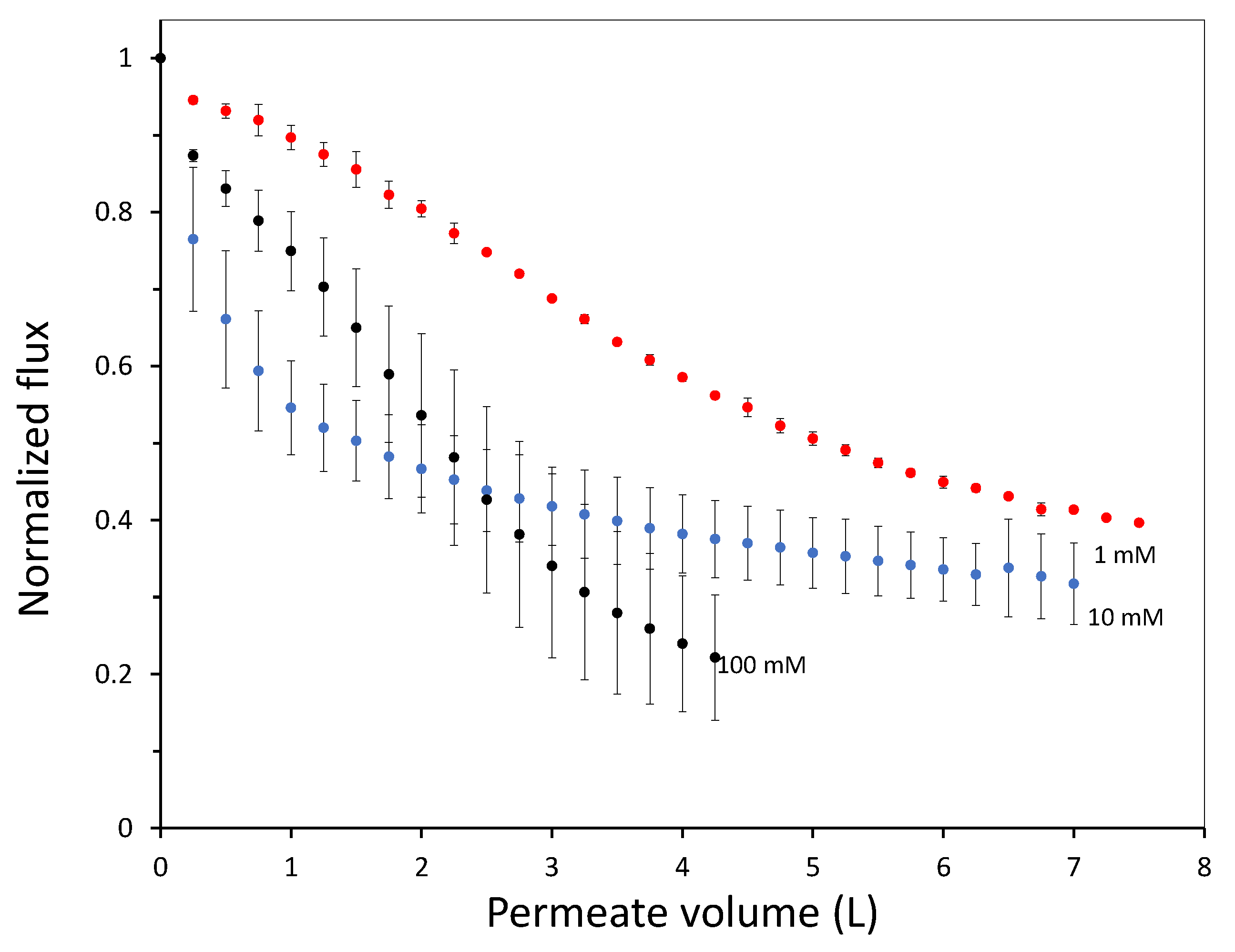
Supplementary Information 1). A membrane filtration experiment consisted of filtering for 3 h at a transmembrane pressure (TMP) of 1 bar and a flow rate of 48 kg/h, which corresponds to a crossflow velocity of 0.2 m/s. The permeate flux was constantly monitored. To clean the membrane, the cell was flushed with DI water for 1 h without applied pressure, then a backflush with DI water of 3 min at 0.2 bar, and then another flush. After the cleaning, the clean water flux was measured again to determine the flux recovery.
The amount of oil in the permeate was determined by liquid-liquid extraction with hexane followed by HPLC. The extraction protocol can be found in
Supplementary Information 2. To check the stability of the emulsion during the experiment, emulsion characteristics where checked before and after the experiment on a number of occasions. As the emulsions used in this study are quite stable, only small changes where observed. Typically the oil concentration decreased by 10–20% while also the average droplet size decreased by 10–20%. The decrease in oil concentration was taken into account when calculating the end of experiment oil retention.
4. Conclusions
In this work we have used a unique combinatorial approach to study membrane fouling by surfactant stabilized oil droplets at different ionic strengths. We do not only study fouling by looking at indirect measurements, such as flux decline of the membrane and oil permeation, but also more directly by observing oil droplet adhesion on a chemically identical surface using a flow cell approach. On a weakly negative cellulose surface the force of adhesion between the surface and SDS stabilized oil droplets is found to be independent on the ionic strength. The low degree of charge, and thus low charge repulsion between surface and droplet means that the addition of ions has no significant effect on the strength of adhesion. In all cases, the attached droplets can nearly completely be removed from the cellulose surface using shear forces. Still the number of droplets that adhere to the surface before applying shear is clearly affected by the ionic strength, with many more droplets adhering especially at the high ionic strength of 100 mM NaCl. The weak negative charge on the surface does seem to provide a kinetic barrier that slows down droplet adhesion, but this effect disappears at higher ionic strength leading to many more adhered droplets. Membrane fouling on cellulose membranes was subsequently studied over time-scales of a few hours. Here we can distinguish two regimes, initially the rate of flux decline is coupled to droplets adhering to the membrane surface, while for longer times the build up of a cake layer will dominate the flux decline. At the start of the experiment, the flux decline is higher at 100 mM than at 1 mM, which can be coupled to the higher droplet adhesion expected at high salt concentration on the basis of the flow-cell results. However, at 10 mM the flux decline is even stronger than for 100 mM, indicating that at 10 mM another fouling mechanism is occurring, most likely pore blocking. For the flux decline at the later stages of the experiment, there is a clear trend, with a greater flux decline at increasing ionic strength. The porosity of the cake layer plays a key role in the flux reduction. At low ionic strength, oil droplets repel each other strongly and an open, more permeable, cake layer is formed. However at higher ionic strength, a screening of charge interactions leads to a lower porosity and thereby a lower flux. Flux recovery after a forward flush and backwash is high at all ionic strengths, in line with the flow cell observation that droplets can easily be sheared off. The flux recovery is highest at high ionic strength, possibly due to the more compact cake layer, or due to the much higher amount of surfactant molecules at the interface of the oil droplets. Naturally, in more complex oil water emulsions, adhesion and fouling mechanisms could change. The approach developed here, utilizing a combination of flow-cell and membrane experiments, could also yield valuable information when applied to these more realistic emulsions. Overall this work highlights the key role that ionic strength plays in the fouling of membranes by emulsions stabilized with an anionic surfactant. Moreover the work demonstrates the potential of our combinatorial approach, where insights from a flow-cell, where droplets adhesion can be directly observed, are combined with standard membrane fouling experiments. While the flow cell approach will not show all intricacies of membrane fouling (no pores, no permeation), it provides valuable insights that help in the interpretation of the more indirect flux decline data.