Experimental Investigations of Behaviour of Biosurfactants in Brine Solutions Relevant to Hydrocarbon Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Critical Micelle Concentration Determination
2.3. Biosurfactants Solubility Tests
2.4. Biosurfactants Stability Tests
3. Results and Discussion
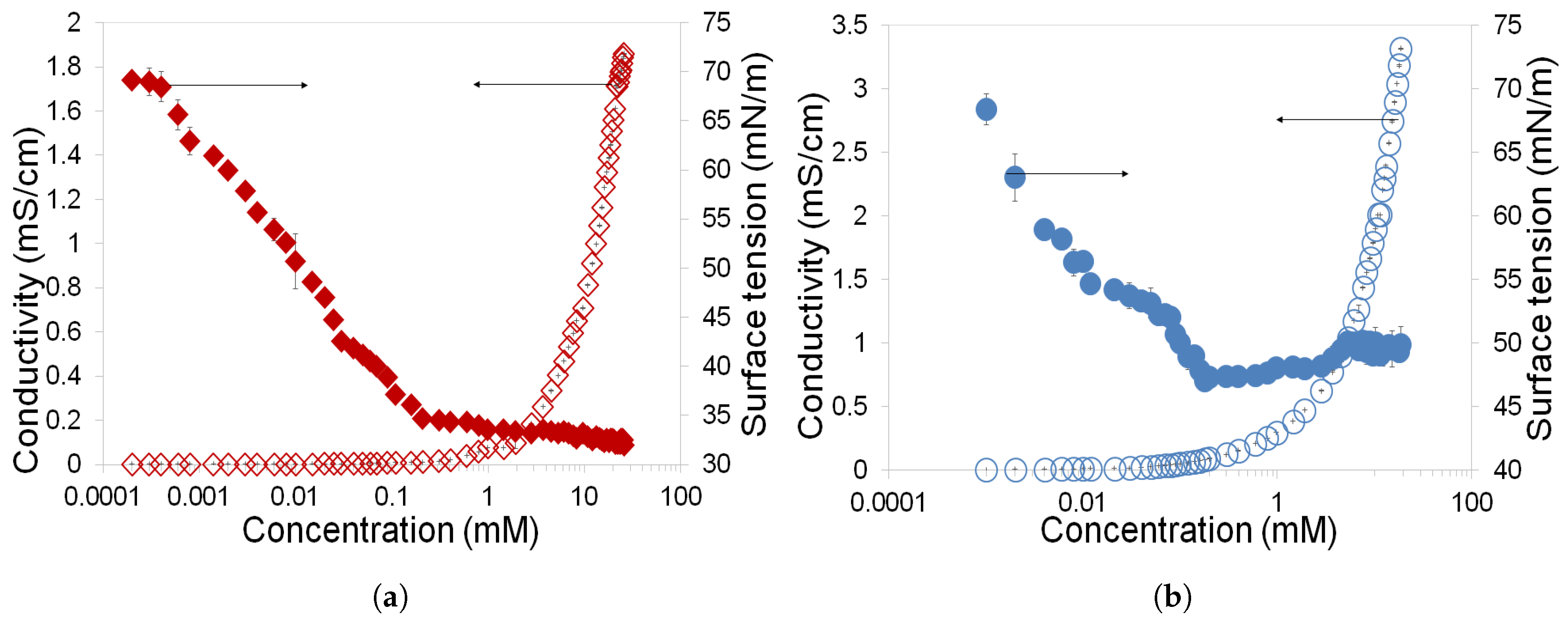
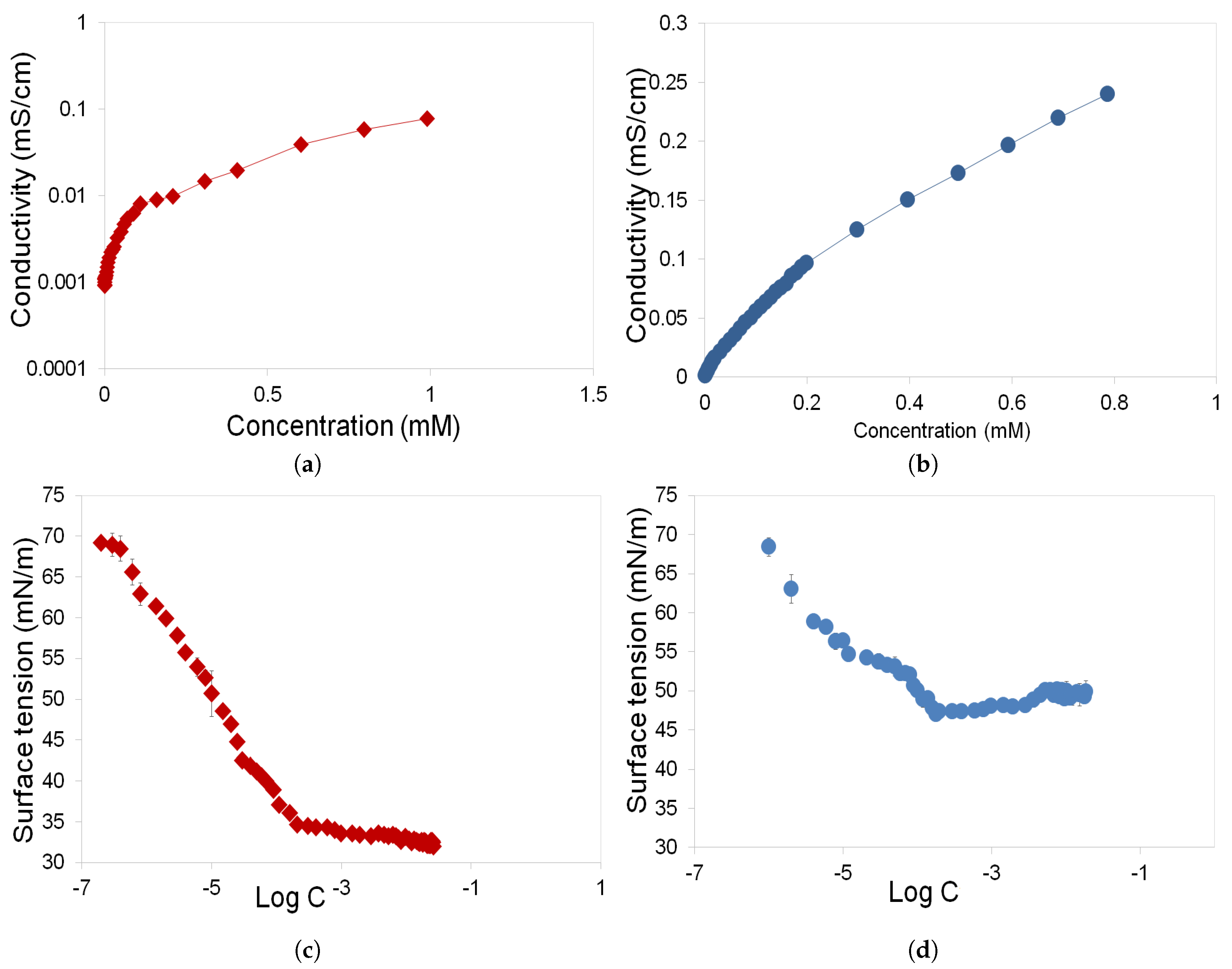
3.1. Determination of CMC and Gibbs Free Energy of Rhamnolipids and Greenzyme
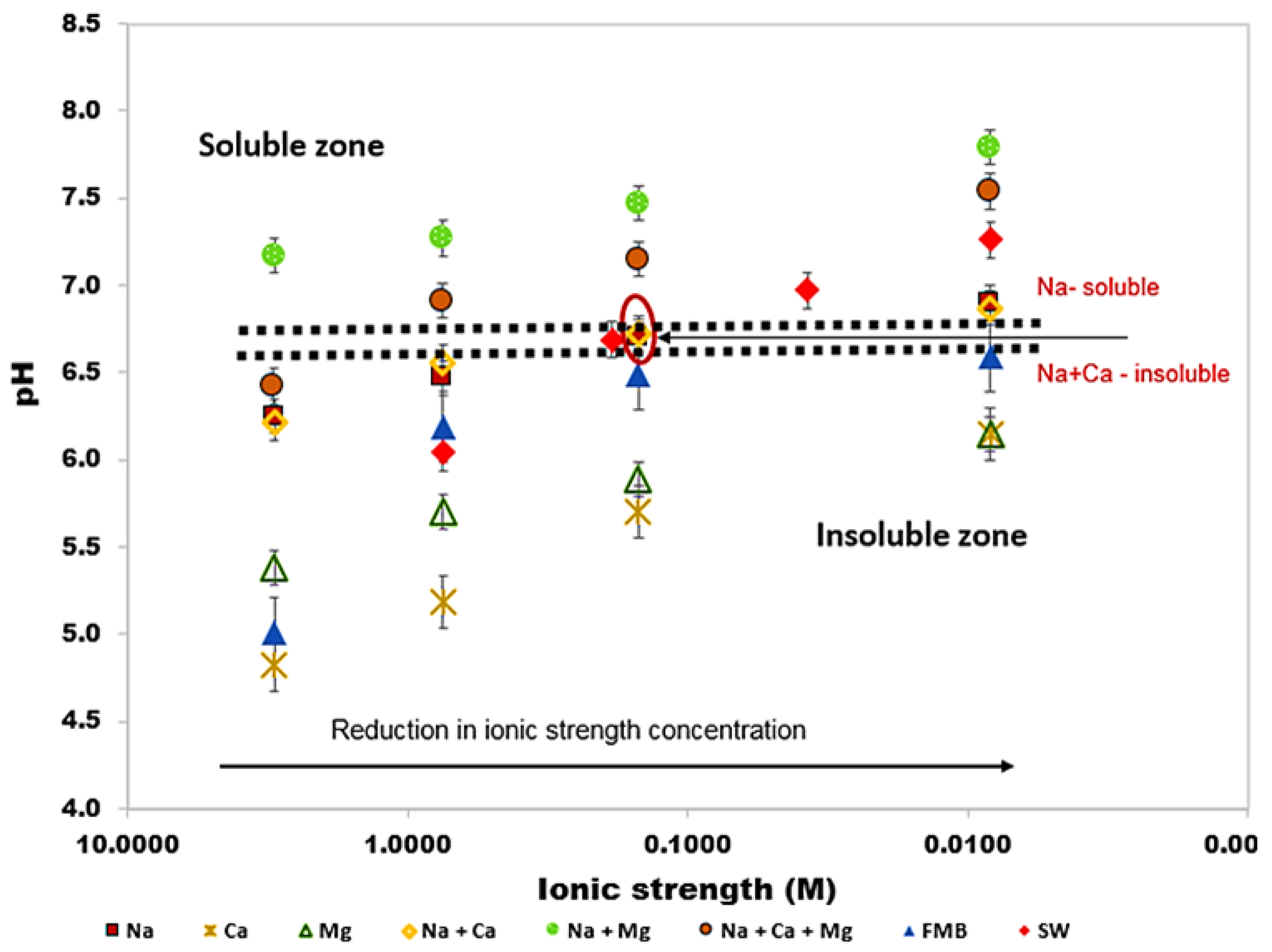
3.2. Effect of Brine Composition and Concentration on Biosurfactants Solubility
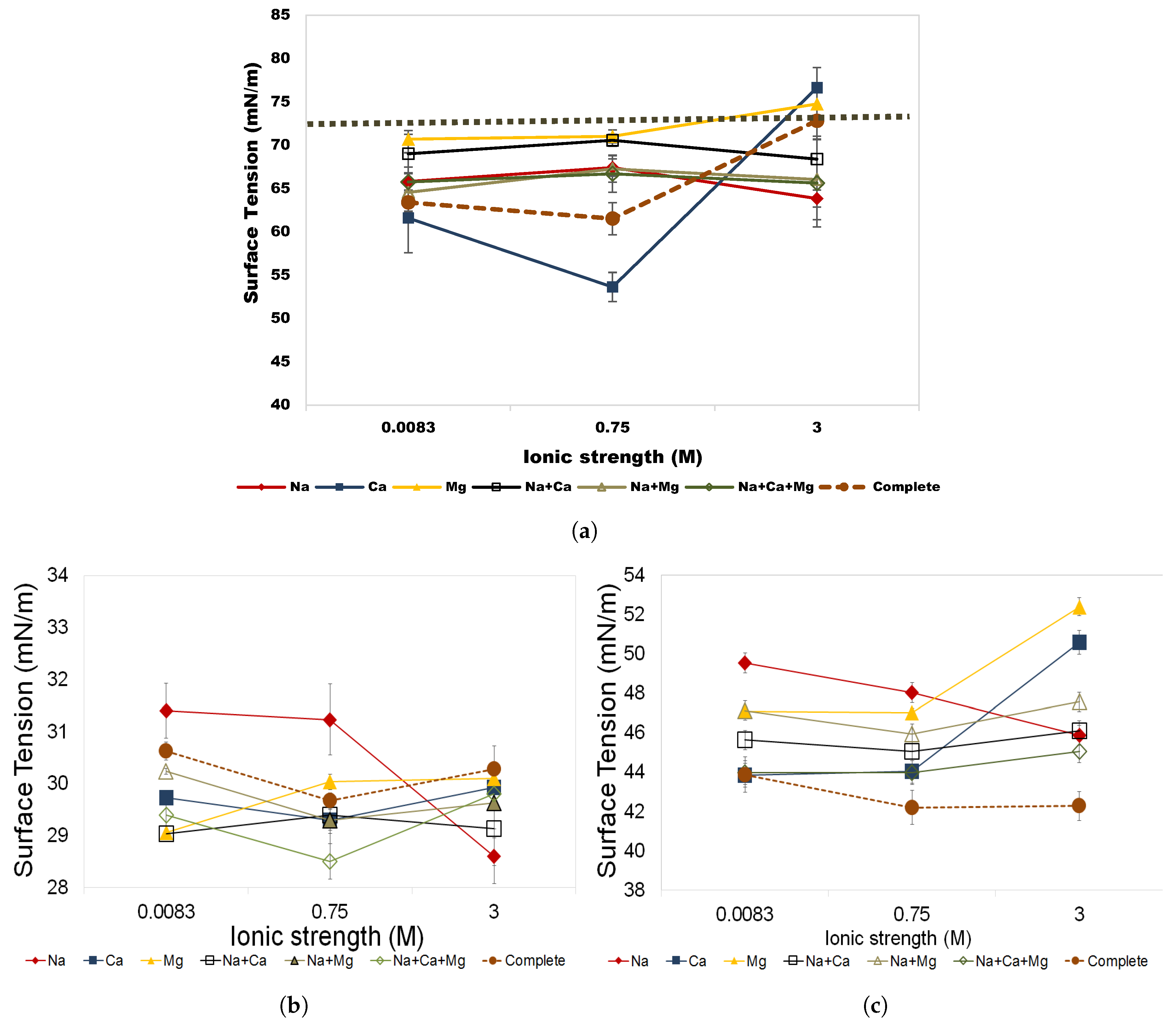
3.3. Aqueous Stability of Rhamnolipid and Greenzyme
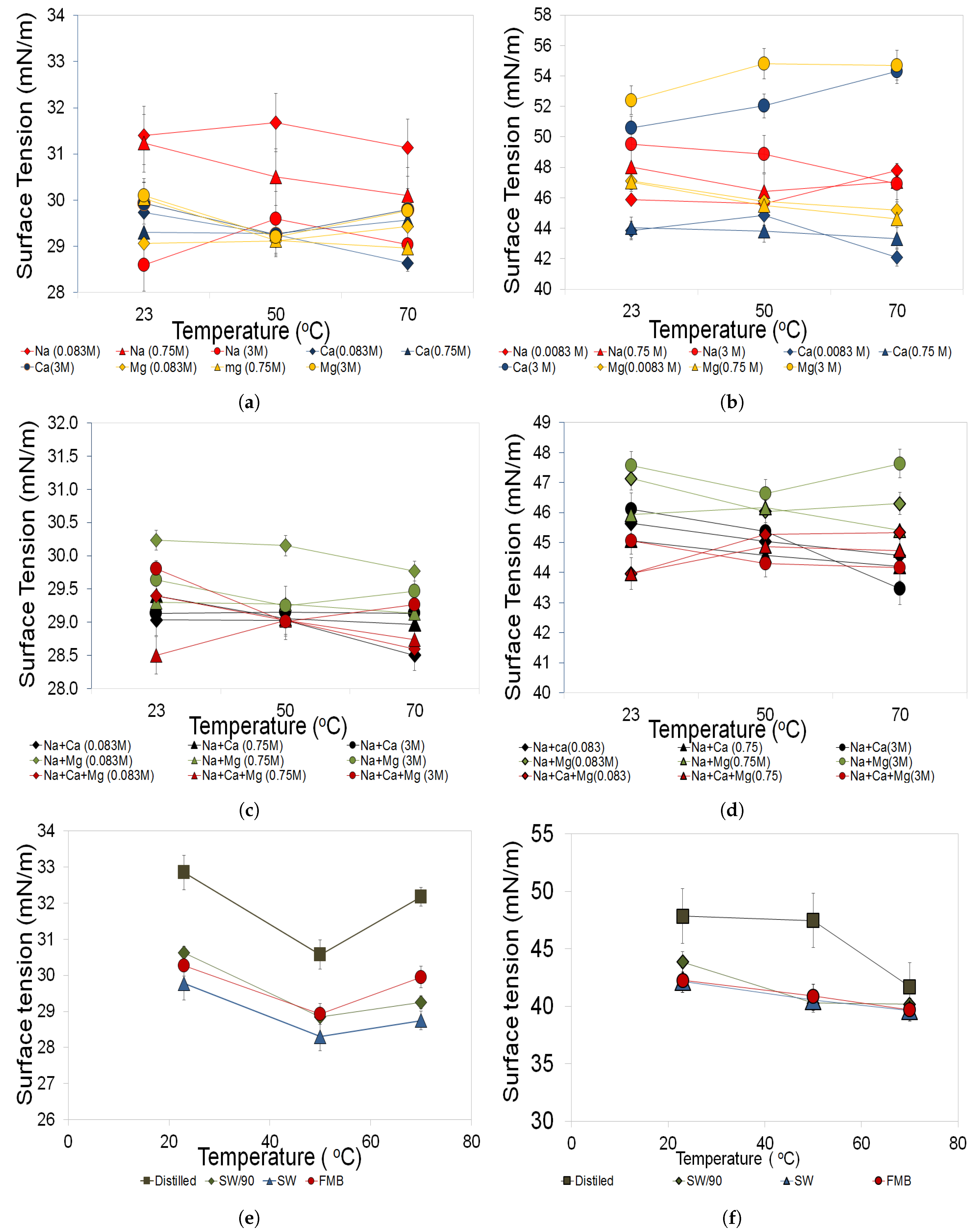
3.3.1. Salinity Effects
3.3.2. Temperature Effects
4. Conclusions
- Rhamnolipid and Greenzyme have demonstrated low CMC that is desirable of any surfactant. The surface tension of both biosurfactants was found to decrease with increasing concentration.
- Rhamnolipid demonstrated higher surface activity than Greenzyme while Greenzyme shows higher aggregation capacity that favours micelle formation than rhamnolipid.
- Greenzyme was found to be soluble in all brine solutions, while rhamnolipid solubility was found to be more dependent on brine composition than solution pH and ionic strength. Hence, biosurfactant aqueous behaviour should be carried out with relevant brine composition.
- The results of this study have also shown that Greenzyme can be used for EOR applications at salinity up to 3 M, typical for many hydrocarbon reservoirs while rhamnolipid cannot be used due to its insolubility. However, at low to medium salinity condition, both of them can be used for EOR investigations.
- Both biosurfactants demonstrated stable behavior in surface tension in solutions up to 3 M and temperatures up to 70 C, making these biosurfactants a suitable substitute for hazardous chemical surfactants used in chemical EOR.
- This study has also shown that rhamnolipid and Greenzyme show highest surface tension and instability in NaCl solution that is commonly used in biosurfactant studies, while they were stable in multi-component brine solutions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myers, D. Surfactant surface activity. In Surfaces, Interfaces, and Colloids: Principles and Applications, 2nd ed.; Wiley-Vch: New York, NY, USA, 1999. [Google Scholar]
- Rosen, M.J. Adsorption of surface-active agents at interfaces: the electrical double layer. Surfactants Interfacial Phenom. 2004, 3, 34–104. [Google Scholar]
- Van Hamme, J.D.; Ajay, S.; Owen, P.W. Physiological aspects: Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Soberón-Chávez, G. Biosurfactants: From genes to applications. Springer Sci. Bus. Media 2010, 20, 2–3. [Google Scholar]
- Penfold, J.; Thomas, R.K.; Shen, H. Adsorption and self-assembly of biosurfactants studied by neutron reflectivity and small angle neutron scattering: Glycolipids, lipopeptides and proteins. Soft Matter 2012, 8, 578–591. [Google Scholar] [CrossRef]
- Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review. Bioresour. Technol. 1995, 51, 1–12. [Google Scholar] [CrossRef]
- Norde, W. Driving forces for protein adsorption at solid surfaces. Macromol. Symp. 1996, 103, 5–19. [Google Scholar] [CrossRef]
- Pornsunthorntawee, O.; Arttaweeporn, N.; Paisanjit, S.; Somboonthanate, P.; Abe, M.; Rujiravanit, R.; Chavadej, S. Isolation and comparison of biosurfactants produced by Bacillus subtilis PT2 and Pseudomonas aeruginosa SP4 for microbial surfactant-enhanced oil recovery. Biochem. Eng. J. 2008, 42, 172–179. [Google Scholar] [CrossRef]
- Bordoloi, N.K.; Konwar, B.K. Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf. B Biointerfaces 2008, 63, 73–82. [Google Scholar] [CrossRef]
- Wang, Y.; Kantzas, A.; Li, B.; Li, Z.; Wang, Q.; Zhao, M. New Agent for Formation-Damage Mitigation in Heavy-Oil Reservoir: Mechanism and Application. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 13–15 February 2008. [Google Scholar]
- Eskandari, S.; Rashedi, H.; Ziaie-Shirkolaee, Y.; Mazaheri-Assadi, M.; Jamshidi, E.; Bonakdarpour, B. Evaluation of oil recovery by rhamnolipid produced with isolated strain from Iranian oil wells. Ann. Microbial. 2009, 59, 573–577. [Google Scholar] [CrossRef]
- Nasiri, H.; Spildo, K.; Skauge, A. Use of enzymes to improve waterflood performance. In Proceedings of the International Symposium of the Society of Core Analysts, Noordwijk, The Netherlands, 27–30 September 2009; pp. 27–30. [Google Scholar]
- Wang, W. Experimental Study of Oil Displacement by the Bio-enzyme at the Third Type Reservoirs of Sabei Blocks. In Proceedings of the Power and Energy Engineering Conference (APPEEC), Chengdu, China, 28–31 March 2010; pp. 1–4. [Google Scholar]
- Amani, H.; Müller, M.M.; Syldatk, C.; Hausmann, R. Production of Microbial Rhamnolipid by Pseudomonas Aeruginosa MM1011 for Ex Situ Enhanced Oil Recovery. Appl. Biochem. Biotechnol. 2013, 170, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Amani, H. Study of enhanced oil recovery by rhamnolipids in a homogeneous 2D micromodel. J. Pet. Sci. Eng. 2015, 128, 212–219. [Google Scholar] [CrossRef]
- Ghojavand, H.; Vahabzadeh, F.; Roayaei, E.; Shahraki, A.K. Production and properties of a biosurfactant obtained from a member of the Bacillus subtilis group (PTCC 1696). J. Colloid Interface Sci. 2008, 324, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Al-Sulaimani, H.; Al-Wahaibi, Y.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Joshi, S.; Zargari, S. Optimization and partial characterization of biosurfactants produced by Bacillus species and their potential for ex-situ enhanced oil recovery. SPE J. 2011, 16, 672–682. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, X.; Bai, B.; Liang, X.; Shuler, P.J.; Goddard, W.A.; Tang, Y. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol. Bioeng. 2007, 98, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J.; Al-Makhmari, H.S.; Al-Sulaimani, H.S. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeterior. Biodegrad. 2013, 81, 141–146. [Google Scholar] [CrossRef]
- Özdemir, G.; Peker, S.; Helvaci, S.S. Effect of pH on the surface and interfacial behavior of rhamnolipids R1 and R2. Colloids Surf. A Physicochem. Eng. Asp. 2004, 234, 135–143. [Google Scholar] [CrossRef]
- Mańko, D.; Zdziennicka, A.; Jańczuk, B. Thermodynamic properties of rhamnolipid micellization and adsorption. Colloids Surf. B Biointerfaces 2014, 119, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Khusainova, A.; Shapiro, A.A.; Stenby, E.H.; Woodley, J.M. Wettability Improvement with Enzymes: Application to Enhanced Oil Recovery under Conditions of the North Sea Reservoirs. Grad. Sch. Yearb. 2012 2014, 119, 125. [Google Scholar]
- Udoh, T.; Akanji, L.; Vinogradov, J. Experimental Investigation of Potential of Combined Controlled Salinity and Bio-Surfactant CSBS in Enhanced Oil Recovery EOR Processes. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 6–8 August 2018. [Google Scholar] [CrossRef]
- Chen, G. Rhamnolipid biosurfactant behavior in solutions. J. Biomater. Sci. Polym. Ed. 2004, 15, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Demissie, H.; Duraisamy, R. Effects of electrolytes on the surface and micellar characteristics of Sodium dodecyl sulphate surfactant solution. J. Sci. Innov. Res. 2016, 5, 208–214. [Google Scholar]
- Szymczyk, K.; Jańczuk, B. The adsorption at solution-air interface and volumetric properties of mixtures of cationic and nonionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2007, 293, 39–50. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery; Gulf Professional Publishing: Burlington, MA, USA, 2011. [Google Scholar]
- Bâldea, I. Molecular Electronics: An Experimental and Theoretical Approach; Pan Stanford Publishing, 8 Temasek Boulevard: Singapore, 2016. [Google Scholar]
- Attwood, D.; Florence, A.T. FASTtrack Physical Pharmacy; Pharmaceutical Press: London, UK, 2012. [Google Scholar]
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; Garcia, F.; Manresa, A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas a eruginosa AT10 from soybean oil refinery wastes. Langmuir 2001, 17, 1367–1371. [Google Scholar] [CrossRef]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I. Solution self-assembly and adsorption at the air-water interface of the monorhamnose and dirhamnose rhamnolipids and their mixtures. Langmuir 2010, 26, 18281–18292. [Google Scholar] [CrossRef]
- Dyke, M.I.V.; Couture, P.; Brauer, M.; Lee, H.; Trevors, J.T. Pseudomonas aeruginosa UG2 rhamnolipid biosurfactants: Structural characterization and their use in removing hydrophobic compounds from soil. Can. J. Microbiol. 1993, 39, 1071–1078. [Google Scholar] [CrossRef]
- Rahman, P.K.S.M.; Gakpe, E. Production, characterisation and applications of biosurfactants-Review. Biotechnology 2008, 7, 360–370. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Abassi, H.; Ahmadkhaniha, R.; Roostaazad, R.; Masoomi, F.; Zahiri, H.S.; Ahmadian, G.; Vali, H.; Noghabi, K.A. Structural characterization of a rhamnolipid-type biosurfactant produced by Pseudomonas aeruginosa MR01: Enhancement of di-rhamnolipid proportion using gamma irradiation. Colloids Surf. B Biointerfaces 2010, 81, 397–405. [Google Scholar] [CrossRef]
- Butt, H.; Graf, K.; Kappl, M. Thermodynamics of Interfaces. In Physics and Chemistry of Interfaces; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 26–41. ISBN 9783527602315. [Google Scholar]
- Xia, J. Protein-Based Surfactants: Synthesis: Physicochemical Properties, and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2001; Volume 101. [Google Scholar]
- Ren, Z.H. Mechanism of the salt effect on micellization of an aminosulfonate amphoteric surfactant. Ind. Eng. Chem. Res. 2015, 54, 9683–9688. [Google Scholar] [CrossRef]
- Piazza, R. Interactions and phase transitions in protein solutions. Curr. Opin. Colloid Interface Sci. 2000, 5, 38–43. [Google Scholar] [CrossRef]
- Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 1986, 25, 267–340. [Google Scholar] [CrossRef]
- Ishigami, Y.; Gama, Y.; Ishii, F.; Choi, Y.K. Colloid chemical effect of polar head moieties of a rhamnolipid-type biosurfactant. Langmuir 1993, 9, 1634–1636. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalid, Z.M.; Khan, M.S.; Banat, I.M.; Rehman, A.; Naeem, A.; Saddique, M.T. Surface properties and sub-surface aggregate assimilation of rhamnolipid surfactants in different aqueous systems. Biotechnol. Lett. 2010, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Mehrnia, M.R.; Sarrafzadeh, M.H.; Haghighi, M.; Soudi, M.R. Scale up and application of biosurfactant from bacillus subtilis in enhanced oil recovery. Appl. Biochem. Biotechnol. 2010, 162, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Howes, T.; Adhikari, B.; Bhandari, B. Investigation of Relationship between Surface Tension of Feed Solution Containing Various Proteins and Surface Composition and Morphology of Powder Particles. Drying Technol. 2012, 30, 1548–1562. [Google Scholar] [CrossRef]
- Raheb, J.; Hajipour, M.J. The Stable Rhamnolipid Biosurfactant Production in Genetically Engineered Pseudomonas Strain Reduced Energy Consumption in Biodesulfurization. Energy Sources Part A Recovery Util. Environ. Effects 2011, 33, 2113–2121. [Google Scholar] [CrossRef]
- Stubenrauch, C. Sugar surfactants—Aggregation, interfacial, and adsorption phenomena. Curr. Opin. Colloid Interface Sci. 2001, 6, 160–170. [Google Scholar] [CrossRef]

| Ions | FBM (M) | SW (M) | NaCl (M) | CaCl (M) | MgCl (M) | Na+Ca (M) | Na+Mg (M) | Na+Ca+Mg (M) |
|---|---|---|---|---|---|---|---|---|
| Na | 1.463 | 0.550 | 3.000 | - | - | 1.200 | 2.500 | 1.103 |
| Ca | 0.420 | 0.014 | - | 1.000 | - | 0.600 | - | 0.559 |
| Mg | 0.091 | 0.045 | - | - | 1.000 | - | 0.167 | 0.074 |
| Cl | 2.485 | 0.620 | 3.000 | 2.000 | 2.000 | 2.400 | 2.835 | 2.367 |
| SO | 0.002 | 0.024 | - | - | - | - | - | - |
| Ionic strength | 3.000 | 0.750 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| pH | 5.01 | 6.04 | 6.37 | 4.82 | 5.41 | 6.21 | 7.17 | 6.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udoh, T.; Vinogradov, J. Experimental Investigations of Behaviour of Biosurfactants in Brine Solutions Relevant to Hydrocarbon Reservoirs. Colloids Interfaces 2019, 3, 24. https://doi.org/10.3390/colloids3010024
Udoh T, Vinogradov J. Experimental Investigations of Behaviour of Biosurfactants in Brine Solutions Relevant to Hydrocarbon Reservoirs. Colloids and Interfaces. 2019; 3(1):24. https://doi.org/10.3390/colloids3010024
Chicago/Turabian StyleUdoh, Tinuola, and Jan Vinogradov. 2019. "Experimental Investigations of Behaviour of Biosurfactants in Brine Solutions Relevant to Hydrocarbon Reservoirs" Colloids and Interfaces 3, no. 1: 24. https://doi.org/10.3390/colloids3010024
APA StyleUdoh, T., & Vinogradov, J. (2019). Experimental Investigations of Behaviour of Biosurfactants in Brine Solutions Relevant to Hydrocarbon Reservoirs. Colloids and Interfaces, 3(1), 24. https://doi.org/10.3390/colloids3010024





