CO2 Adsorption Property of Amine-Modified Amorphous TiO2 Nanoparticles with a High Surface Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Amorphous TiO2 Nanoparticles
2.2. Preparation of Amine-Modified TiO2 Nanoparticles
2.3. Charactarization
3. Results and Discussion
≡Ti–OH + HO–Ti≡ → ≡Ti–O–Ti≡ + H2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonenfant, D.; Mimeault, M.; Hausler, R. Determination of the structural features of distinct amines important for the absorption of CO2 and regeneration in aqueous solution. Ind. Eng. Chem. Res. 2003, 42, 3179–3184. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Song, C.F.; Kitamura, Y.; Li, S.H. Evaluation of Stirling cooler system for cryogenic CO2 capture. Appl. Energy 2012, 98, 491–501. [Google Scholar] [CrossRef]
- Wang, Q.A.; Luo, J.Z.; Zhong, Z.Y.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Hassan, S.M.N.; Douglas, P.L.; Croiset, E. Techno-economic study of CO2 capture from an existing cement plant using MEA scrubbing. Int. J. Green Energy 2007, 4, 197–220. [Google Scholar] [CrossRef]
- Ge, J.R.; Deng, K.J.; Cai, W.Q.; Yu, J.G.; Liu, X.Q.; Zhou, J.B. Effect of structure-directing agents on facile hydrothermal preparation of hierarchical gamma-Al2O3 and their adsorption performance toward Cr(VI) and CO2. J. Colloid Interface Sci. 2013, 401, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Kim, J.T.; Suh, J.K.; Lee, J.M.; Lee, C.H. Adsorption equilibria of CO2 on zeolite 13X and zeolite X/Activated carbon composite. J. Chem. Eng. Data 2002, 47, 1237–1242. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Cai, W.Q.; Tan, L.J.; Yu, J.G.; Jaroniec, M.; Liu, X.Q.; Cheng, B.; Verpoort, F. Synthesis of amino-functionalized mesoporous alumina with enhanced affinity towards Cr(VI) and CO2. Chem. Eng. J. 2014, 239, 207–215. [Google Scholar] [CrossRef]
- Le, Y.; Guo, D.P.; Cheng, B.; Yu, J.G. Amine-functionalized monodispersed porous silica microspheres with enhanced CO2 adsorption performance and good cyclic stability. J. Colloid Interface Sci. 2013, 408, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Leisen, J.; Foo, G.S.; Sievers, C.; Jones, C.W. Aminosilanes Grafted to Basic Alumina as CO2 Adsorbents-Role of Grafting Conditions on CO2 Adsorption Properties. ChemSusChem 2014, 7, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.D.; Bourrelly, S.; Llewellyn, P.L.; Carneiro, J.W.D.; Ronconi, C.M. Adsorption of CO2 on amine-functionalised MCM-41: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2015, 17, 11095–11102. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Wu, Z.B.; Chen, X.B.; Wang, H.Q.; Weng, X.L. Polyethyleneimine functionalized protonated titanate nanotubes as superior carbon dioxide adsorbents. J. Colloid Interface Sci. 2012, 386, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Ortiz, H.I.; Perera-Mercado, Y.; Mercado-Silva, J.A.; Olivares-Maldonado, Y.; Castruita, G.; Garcia-Cerda, L.A. Functionalization with amine-containing organosilane of mesoporous silica MCM-41 and MCM-48 obtained at room temperature. Ceram. Int. 2014, 40, 9701–9707. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhou, J.H.; Hu, J.; Liu, H.L. The effect of grafted amine group on the adsorption of CO2 in MCM-41: A molecular simulation. Catal. Today 2012, 194, 53–59. [Google Scholar] [CrossRef]
- Fujishima, K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, L.; Kang, B.; Wang, P.; Qiu, Y. Review of recent progress in solid-state dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 549–573. [Google Scholar] [CrossRef]
- Fan, J.J.; Zhao, L.; Yu, J.G.; Liu, G. The effect of calcination temperature on the microstructure and photocatalytic activity of TiO2-based composite nanotubes prepared by an in situ template dissolution method. Nanoscale 2012, 4, 6597–6603. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Dai, G.P.; Cheng, B. Effect of Crystallization Methods on Morphology and Photocatalytic Activity of Anodized TiO2 Nanotube Array Films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.; Joo, J.B.; Kim, W.; Yi, J. Influence of Aspect Ratio of TiO2 Nanorods on the Photocatalytic Decomposition of Formic Acid. J. Phys. Chem. C 2009, 113, 3050–3055. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zhang, L.Z.; Deng, K.J.; Chen, X.Y.; Zou, Z.G. Low temperature synthesis and photocatalytic activity of rutile TiO2 nanorod superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. Anatase TiO2 nanosheet: An ideal host structure for fast and efficient lithium insertion/extraction. Electrochem. Commun. 2009, 11, 2332–2335. [Google Scholar] [CrossRef]
- Hocevar, M.; Berginc, M.; Topic, M.; Krasovec, U.O. Sponge-like TiO2 layers for dye-sensitized solar cells. J. Sol-Gel Sci. Technol. 2010, 53, 647–654. [Google Scholar] [CrossRef]
- Jiang, G.D.; Huang, Q.L.; Kenarsari, S.D.; Hu, X.; Russell, A.G.; Fan, M.H.; Shen, X.D. A new mesoporous amine-TiO2 based pre-combustion CO2 capture technology. Appl. Energy 2015, 147, 214–223. [Google Scholar] [CrossRef]
- Liao, Y.S.; Cao, S.W.; Yuan, Y.P.; Gu, Q.; Zhang, Z.Y.; Xue, C. Efficient CO2 Capture and Photoreduction by Amine-Functionalized TiO2. Chem. Eur. J. 2014, 20, 10220–10222. [Google Scholar] [CrossRef] [PubMed]
- Kanarsari, S.D.; Fan, M.; Jiang, G.; Shen, X.; Lin, Y.; Hu, X. Use of Robust and Inexpensive Nanoporous TiO2 for Pre-combustion CO2 separation. Energy Fuels 2013, 27, 6938–6947. [Google Scholar] [CrossRef]
- Aquino, C.C.; Richner, G.; Kimling, M.C.; Chen, D.; Puxty, G.; Feron, P.H.M.; Caruso, R.A. Amine-Functionalized Titania-based Porous Structures for Carbon Dioxide Postcombustion Capture. J. Phys. Chem. C 2013, 117, 9747–9757. [Google Scholar] [CrossRef]
- Muller, K.; Lu, D.; Senanayake, S.D.; Starr, D.E. Monoethanolamine Adsorption on TiO2(110): Bonding, Structure, and Implications for Use as a Model Solid-Supported CO2 Capture Material. J. Phys. Chem. C 2014, 18, 1576–1586. [Google Scholar] [CrossRef]
- Kapica-Kozar, J.; Pirog, E.; Kusiak-Nejman, E.; Weobel, R.J.; Gesikiewicz-Puchalska, A.; Morawski, A.W.; Nakiewicz, U.; Michalkiewicz, B. Titanium dioxide modified with various amines used as sorbents of carbon dioxide. New J. Chem. 2017, 41, 1549–1557. [Google Scholar] [CrossRef]
- Ota, M.; Dwijaya, B.; Hirota, Y.; Uchida, Y.; Tanaka, S.; Nishiyama, N. Synthesis of Amorphous TiO2 Nanoparticles with a High Surface Area and Their Transformation to Li4Ti5O12 Nanoparticles. Chem. Lett. 2016, 45, 1285–1287. [Google Scholar] [CrossRef]
- Su, F.S.; Lu, C.Y.; Kuo, S.C.; Zeng, W.T. Adsorption of CO2 on Amine-Functionalized Y-Type Zeolites. Energy Fuels 2010, 24, 1441–1448. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Yao, W.Y.; Cen, W.L.; Wang, H.Q.; Weng, X.L.; Wu, Z.B. The effects of surface acidity on CO2 adsorption over amine functionalized protonated titanate nanotubes. RSC Adv. 2013, 3, 18803–18810. [Google Scholar] [CrossRef]

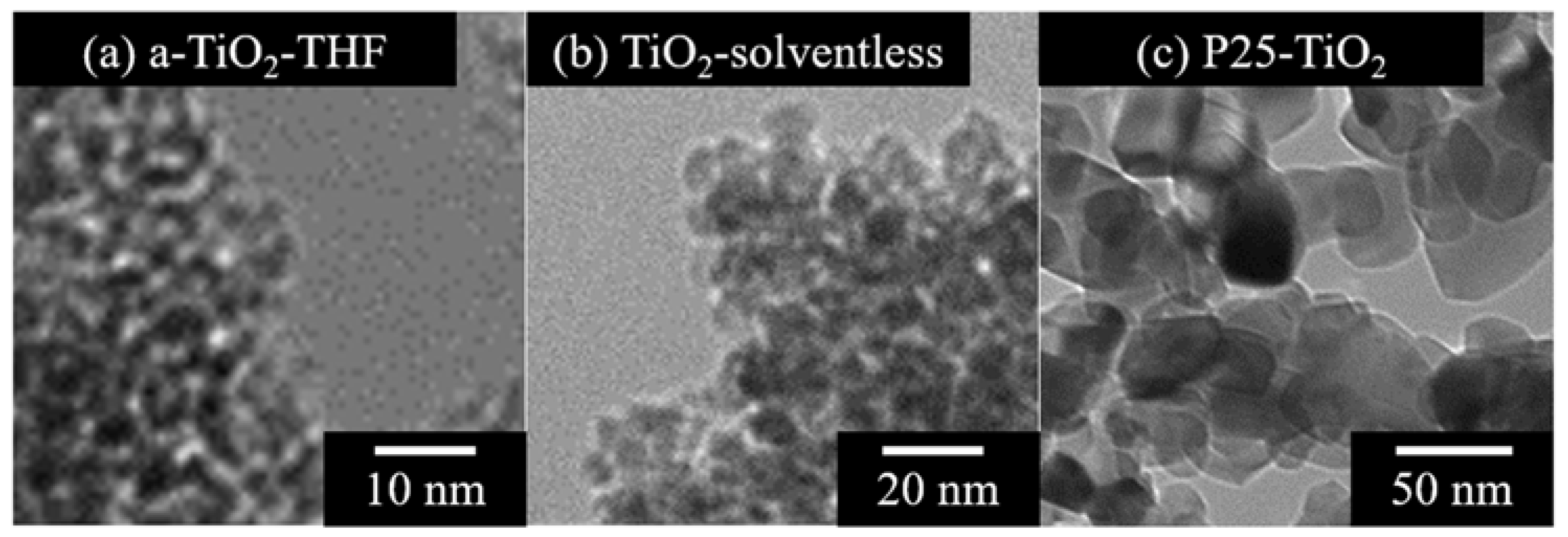
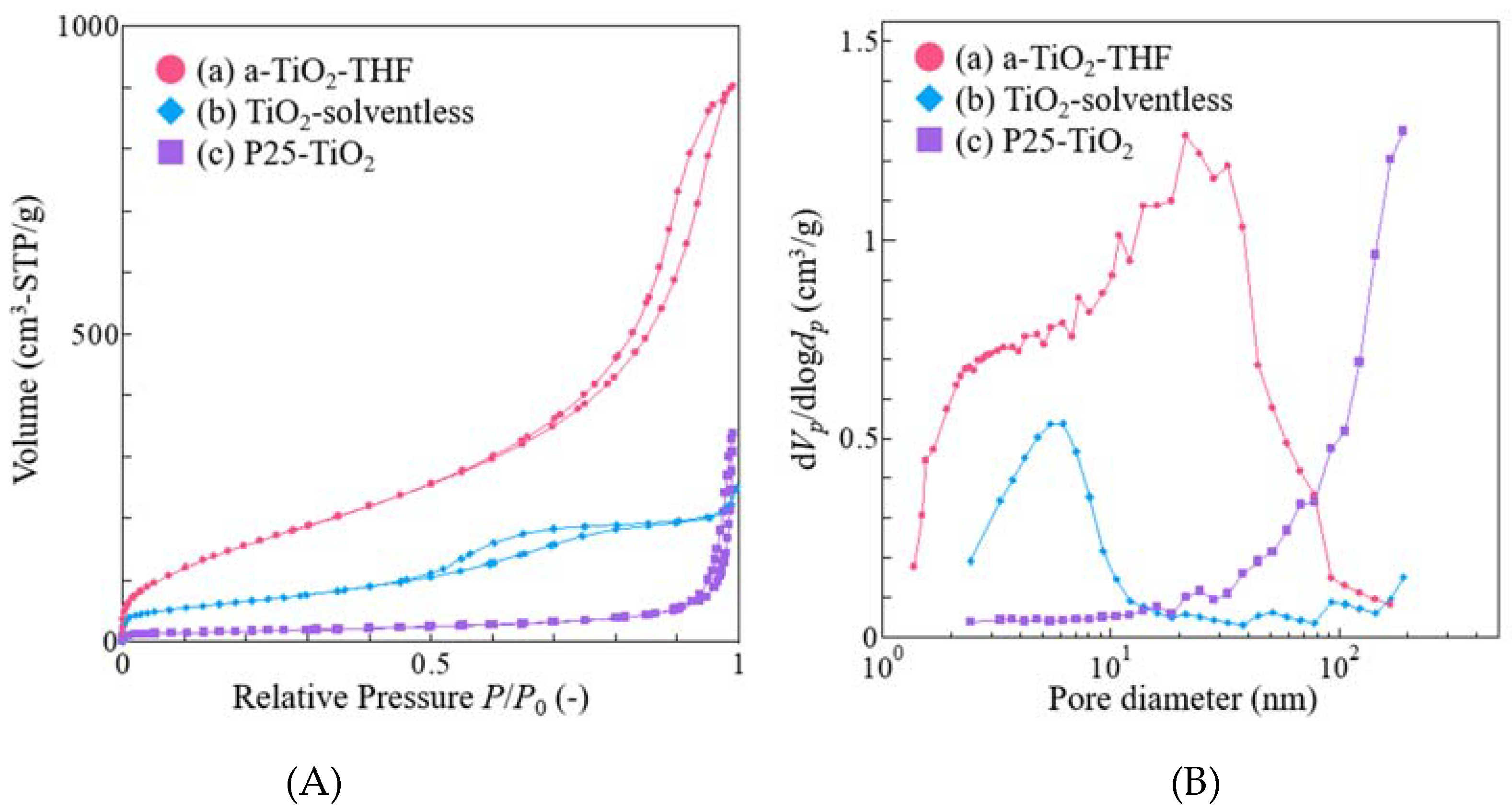
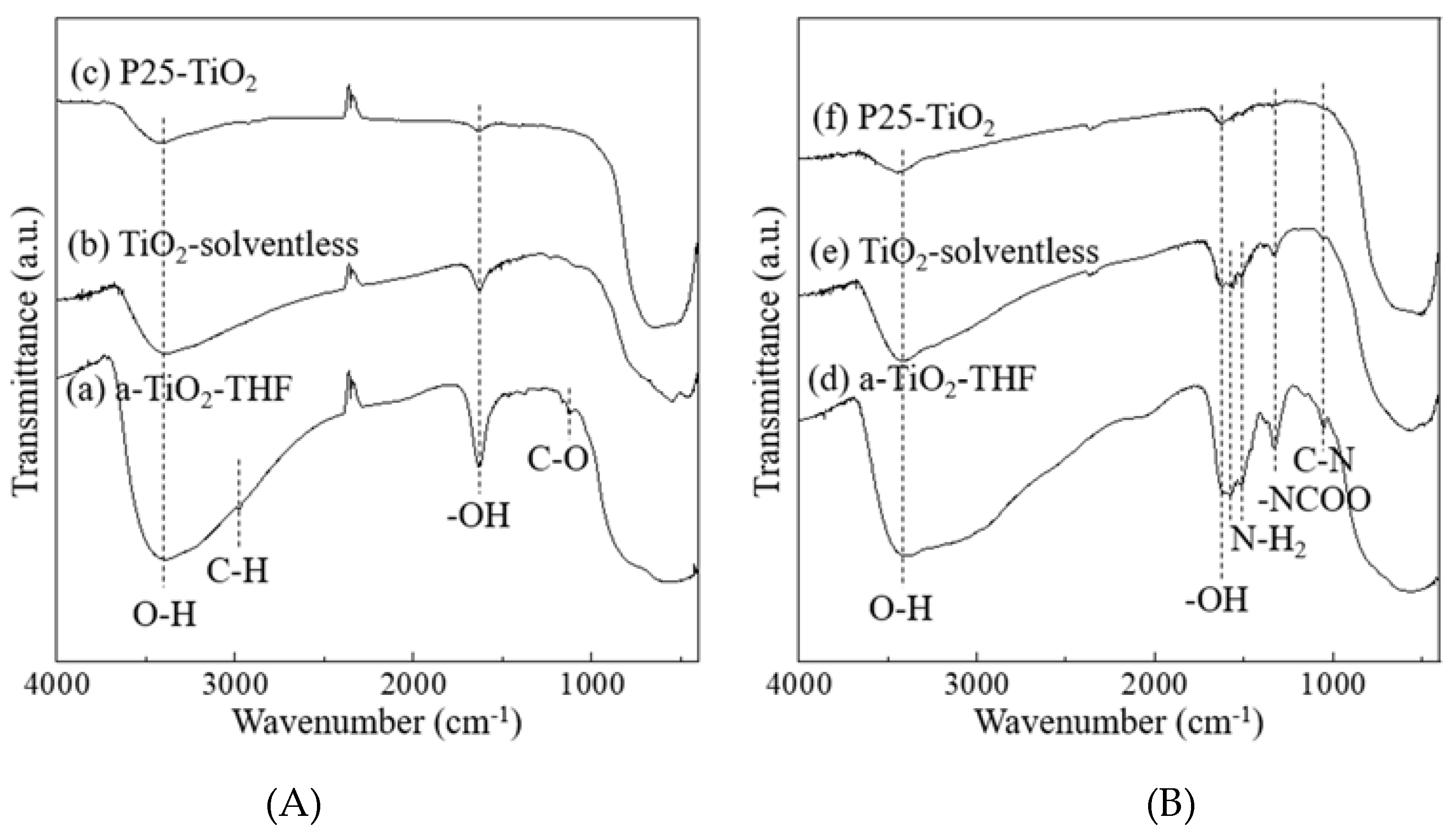
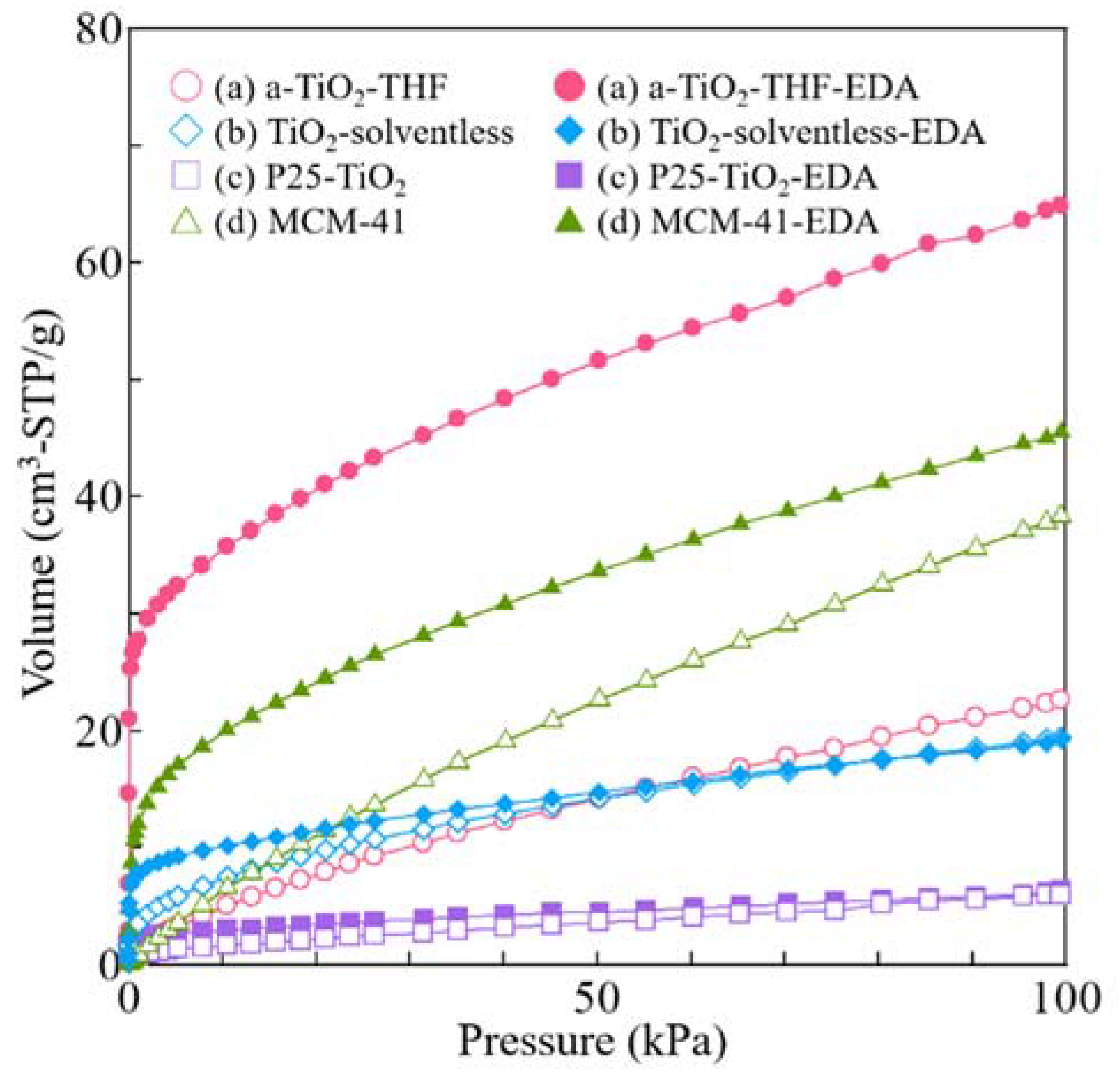
| SBET (m2/g) | V (cm3/g) | Amount of Loaded Amine (wt %) | Amount of Loaded Amine (mg/m2) | |
|---|---|---|---|---|
| a-TiO2-THF | 617 | 1.582 | - | - |
| a-TiO2-THF-EDA | 472 | 1.134 | 15.1 | 0.245 |
| TiO2-solventless | 241 | 0.356 | - | - |
| TiO2-solventless-EDA | 201 | 0.330 | 4.7 | 0.197 |
| P25-TiO2 | 63 | 0.486 | - | - |
| P25-TiO2-EDA | 40 | 0.502 | 1.1 | 0.175 |
| MCM-41 | 978 | 0.504 | - | - |
| MCM-41-EDA | 360 | 0.148 | 12.2 | 0.125 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, M.; Hirota, Y.; Uchida, Y.; Nishiyama, N. CO2 Adsorption Property of Amine-Modified Amorphous TiO2 Nanoparticles with a High Surface Area. Colloids Interfaces 2018, 2, 25. https://doi.org/10.3390/colloids2030025
Ota M, Hirota Y, Uchida Y, Nishiyama N. CO2 Adsorption Property of Amine-Modified Amorphous TiO2 Nanoparticles with a High Surface Area. Colloids and Interfaces. 2018; 2(3):25. https://doi.org/10.3390/colloids2030025
Chicago/Turabian StyleOta, Misaki, Yuichiro Hirota, Yoshiaki Uchida, and Norikazu Nishiyama. 2018. "CO2 Adsorption Property of Amine-Modified Amorphous TiO2 Nanoparticles with a High Surface Area" Colloids and Interfaces 2, no. 3: 25. https://doi.org/10.3390/colloids2030025
APA StyleOta, M., Hirota, Y., Uchida, Y., & Nishiyama, N. (2018). CO2 Adsorption Property of Amine-Modified Amorphous TiO2 Nanoparticles with a High Surface Area. Colloids and Interfaces, 2(3), 25. https://doi.org/10.3390/colloids2030025







