Effect of Polysorbates on Solids Wettability and Their Adsorption Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Contact Angle Measurements
3. Results and Discussion
3.1. Wetting and Surfactant Adsorption at the Solid-Water Interface
3.2. Gibbs Standard Free Energy of Adsorption at the Solid Water Interface
3.3. Work of Adhesion of Aqueous Solution of Tweens to the Solid Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the suitability of low hazard surfactants for the separation of phenols and carotenoids from red-flesh orange juice and olive mill wastewater using cloud point extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Weiszhár, Z.; Czúcz, J.; Révész, C.; Rosivall, L.; Szebeni, J.; Rozsnyay, Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm. Sci. 2012, 45, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Verma, G.; Aswal, V.K.; Date, A.A.; Mangal, A.; Nagarsenker, S.; Hassan, P.A. Tween 80−sodium deoxycholate mixed micelles: Structural characterization and application in doxorubicin delivery. J. Phys. Chem. B 2010, 114, 16414–16421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, G.; Liu, T.; Xu, L.; Zhou, Y. Foam and interfacial properties of Tween 20–bovine serum albumin systems. Colloids Surf. A 2013, 416, 23–31. [Google Scholar] [CrossRef]
- Ćirin, D.M.; Poša, M.M.; Krstonošić, V.S. Interactions between sodium cholate or sodium deoxycholate and nonionic surfactant (Tween 20 or Tween 60) in aqueous solution. Ind. Eng. Chem. Res. 2012, 51, 3670–3676. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Kumar, N.; Sagiri, S.S.; Pal, K.; Ray, S.S. Development of Span 80–Tween 80 based fluid-filled organogels as a matrix for drug delivery. J. Pharm. Biol. Sci. 2012, 4, 155–163. [Google Scholar]
- Sharma, S.C.; Warr, G.G. Phase behavior, self-assembly, and emulsification of Tween 80/water mixtures with limonene and perfluoromethyldecalin. Langmuir 2012, 28, 11707–11713. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.; Thomas, R.K.; Li, P.X.; Petkov, J.T.; Tucker, I.; Webster, J.R.P.; Terry, A.E. Adsorption at air–water and oil–water interfaces and self-assembly in aqueous solution of ethoxylatedpolysorbate nonionic surfactants. Langmuir 2015, 31, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huston, K.J.; Larson, R.G. Molecular dynamics simulations of structure -property relationships of Tween 80 surfactants in water and at interfaces. J. Phys. Chem. B 2014, 118, 12907–12918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhu, L.; Li, W.; Chen, B. Solubilization and biodegradation of phenanthrene in mixed anionic-nonionic surfactant solutions. Chemosphere 2005, 58, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, J.; Latowska, A.; Hupka, J. Micelle formation of Tween 20 nonionic surfactant in imidazolium ionic liquids. Colloids Surf. A 2015, 471, 26–37. [Google Scholar] [CrossRef]
- Hait, S.K.; Moulik, S.P. Determination of critical micelle concentration (CMC) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. J. Surfactants Deterg. 2001, 4, 303–309. [Google Scholar] [CrossRef]
- Khaledi, M.G. Micelles as separation media in high-performance liquid chromatography and high-performance capillary electrophoresis: Overview and perspective. J. Chromatogr. A 1997, 780, 3–40. [Google Scholar] [CrossRef]
- Glenn, K.M.; Moroze, S.; Bhattacharya, S.C.; Palepu, R.M. Effect of ethylene glycol on the thermodynamic and micellar properties of Tween 40, 60, and 80. J. Dispers. Sci. Technol. 2005, 26, 79–86. [Google Scholar] [CrossRef]
- Fua, J.; Cai, Z.; Gonga, Y.; O’Reilly, S.E.; Haoc, X.; Zhao, D. A new technique for determining critical micelle concentrations of surfactants and oil dispersants via UV absorbance of pyrene. Colloids Surf. A 2015, 484, 1–8. [Google Scholar] [CrossRef]
- Samanta, S.; Ghosh, P. Coalescence of bubbles and stability of foams in aqueous solutions of Tween surfactants. Chem. Eng. Res. Des. 2011, 89, 2344–2355. [Google Scholar] [CrossRef]
- Carnero Ruiz, C.; Molina-Bolıávar, J.A.; Aguiar, J.; MacIsaac, G.; Moroze, S.; Palepu, R. Effect of ethylene glycol on the thermodynamic and micellar properties of Tween 20. Colloid Polym. Sci. 2003, 281, 531–541. [Google Scholar] [CrossRef]
- Rehman, N.; Ullah, H.; Jan, A.K.; Khan, S.W.; Tariq, M. Surface and tehrmodynamic study of micellization of non ionic surfactant/diblock copolymer system as revealed by surface tension and conductivity. J. Mater. Environ. Sci. 2017, 8, 1161–1167. [Google Scholar]
- Sameer, H.; Kareemand, B.A. Surface properties of mixed adsorbed surfactants film of Tween 20 and Tween 80 on liquid—Air interfacial. Int. J. Sci. Res. 2015, 6, 936–939. [Google Scholar]
- Rosen, M. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley Interscience: New York, NY, USA, 2004. [Google Scholar]
- Cappelletti, G.; Ardizzone, S.; Meroni, D.; Soliveri, G.; Ceotto, M.; Biaggi, C.; Benaglia, M.; Raimondi, L. Wettability of bare and fluorinated silanes: A combined approach based on surface free energy evaluations and dipole moment calculations. J. Colloid Interface Sci. 2013, 389, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Riascos, I.; Cortés, F.B.; Hoyos, B.A. Chemical alteration of wettability of sandstones with polysorbate 80. Experimental and molecular dynamics study. Energy Fuels 2017, 31, 11918–11924. [Google Scholar] [CrossRef]
- Amani, A.; York, P.; de Waardc, H.; Anwar, J. Molecular dynamics simulation of a polysorbate 80 micelle in water. Soft Matter 2011, 7, 2900–2908. [Google Scholar] [CrossRef]
- Aizawa, H. Morphology of polysorbate 80 (Tween 80) micelles in aqueous 1,4-dioxane solutions. J. Appl. Crystallogr. 2009, 42, 592–596. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Krawczyk, J.; Jańczuk, B. Components and parameters of solid/surfactant layer surface tension. Colloids Surf. A 2017, 522, 461–469. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Jańczuk, B.; Longwic, R.; Sander, P. Adhesion of canola and diesel oils to some parts of diesel engine in the light of surface tension components and parameters of these substrates. Int. J. Adhes. Adhes. 2015, 60, 23–30. [Google Scholar] [CrossRef]
- Schrader, M.E. Spreadingpreassure in the Young equation and intermolecular force theory. Langmuir 1993, 9, 1959–1961. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Adsorption and aggregation properties of some polysorbates at different temperatures. J. Solut. Chem. (under review).
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Jańczuk, B. Components and parameters of liquids and some polymers surface tension at different temperature. Colloids Surf. A 2017, 529, 864–875. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Krawczyk, J.; Jańczuk, B. Activity and thermodynamic parameters of some surfactants adsorption at the water–air interface. Fluid Phase Equilibria 2012, 318, 25–33. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Krawczyk, J.; Jańczuk, B. Wettability, adhesion, adsorption and interface tension in the polymer/surfactant aqueous solution system. I. Critical surface tension of polymer wetting and its surface tension. Colloids Surf. A 2012, 402, 132–138. [Google Scholar] [CrossRef]
- Zisman, W.A. Contact Angle Wettability and Adhesion; In Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1964; Volume 43, pp. 1–51. [Google Scholar]
- Bernett, M.K.; Zisman, W.A. Relation of wettability by aqueous solutions to the surface constitution of low-energy solids. J. Phys. Chem. 1959, 63, 1241–1246. [Google Scholar] [CrossRef]
- Bernett, M.K.; Zisman, W.A. Wetting of low-energy solids by aqueous solutions of highly fluorinated acid and salts. J. Phys. Chem. 1959, 63, 1911–1916. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Van Oss, C.J.; Good, R.J. Surface tension and the solubility of polymers and biopolymers: The role of polar and apolar interfacial free energies. J. Macromol. Sci. 1989, 26, 1183–1203. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Spelt, J.K.; Li, D.; Neumann, A.W. The equation of state approach to interfacial tensions. In Modern Approaches to Wettability; Schrader, M.E., Loeb, G.L., Eds.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Li, D.; Neumann, A.W. Equation of state for interfacial tensions of solid-liquid systems. Adv. Colloid Interface Sci. 1992, 39, 299–345. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle interpretation in terms of solid surface tension. Colloids Surf. A 2000, 161, 31–48. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Krawczyk, J.; Jańczuk, B. Wettability, adhesion, adsorption and interface tension in the polymer/surfactant aqueous solution system: II. Work of adhesion and adsorption of surfactant at polymer–solution and solution–air interfaces. Colloids Surf. A 2012, 402, 139–145. [Google Scholar] [CrossRef]
- Joos, P. Thermodynamics of mixed monolayers. Bull. Soc. Chim. Belg. 1967, 76, 591–600. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- De Boer, H. The Dynamic Character of Adsorption; Oxford University: Oxford, UK, 1953. [Google Scholar]
- Mańko, D.; Zdziennicka, A.; Krawczyk, J.; Jańczuk, B. Wetting and adsorption properties of N-Octyl-β-D-Glucopyranoside and monorhamnolipid in the system polytetrafluoroethylene–solution–air. Colloids Surf. A 2015, 486, 114–123. [Google Scholar] [CrossRef]
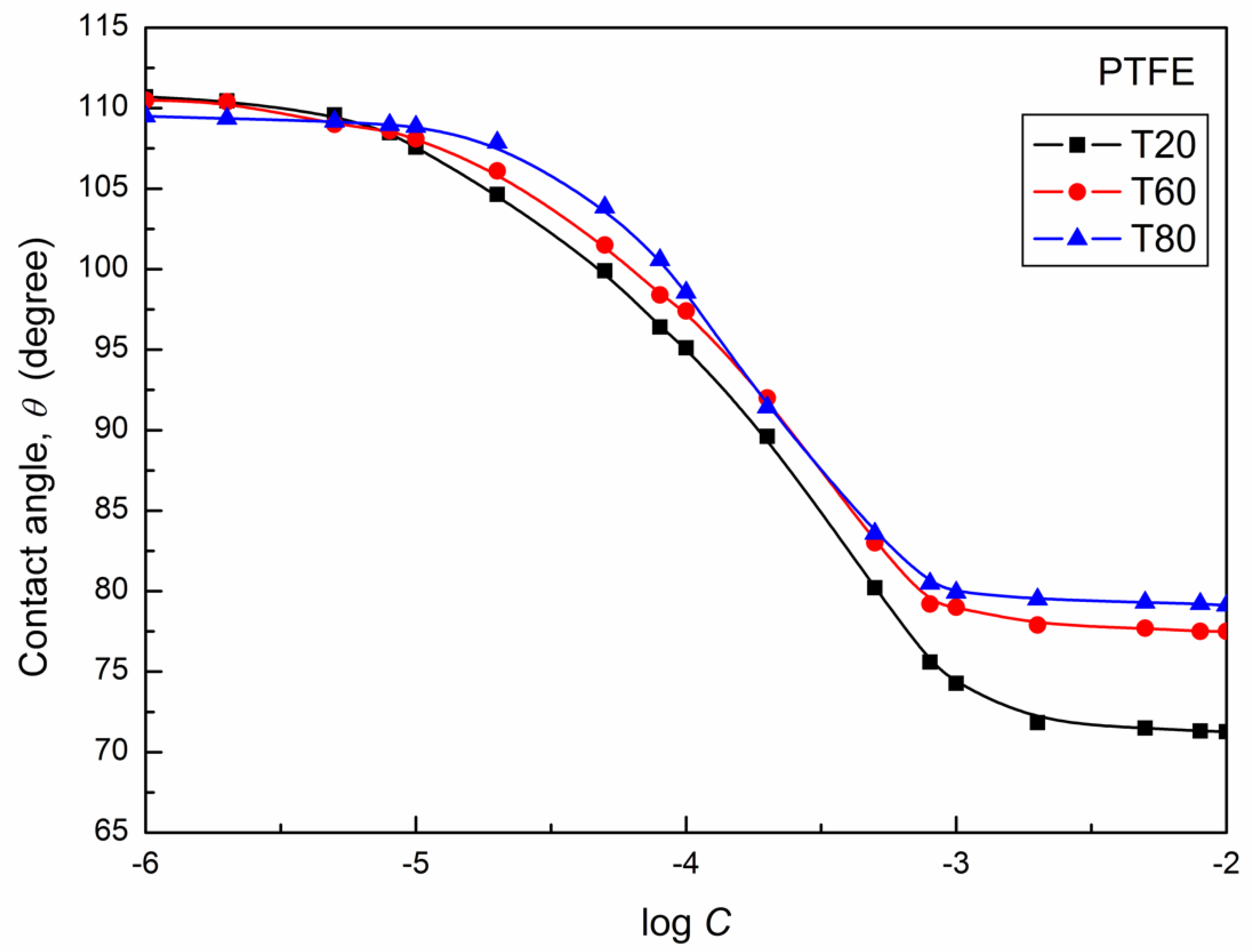
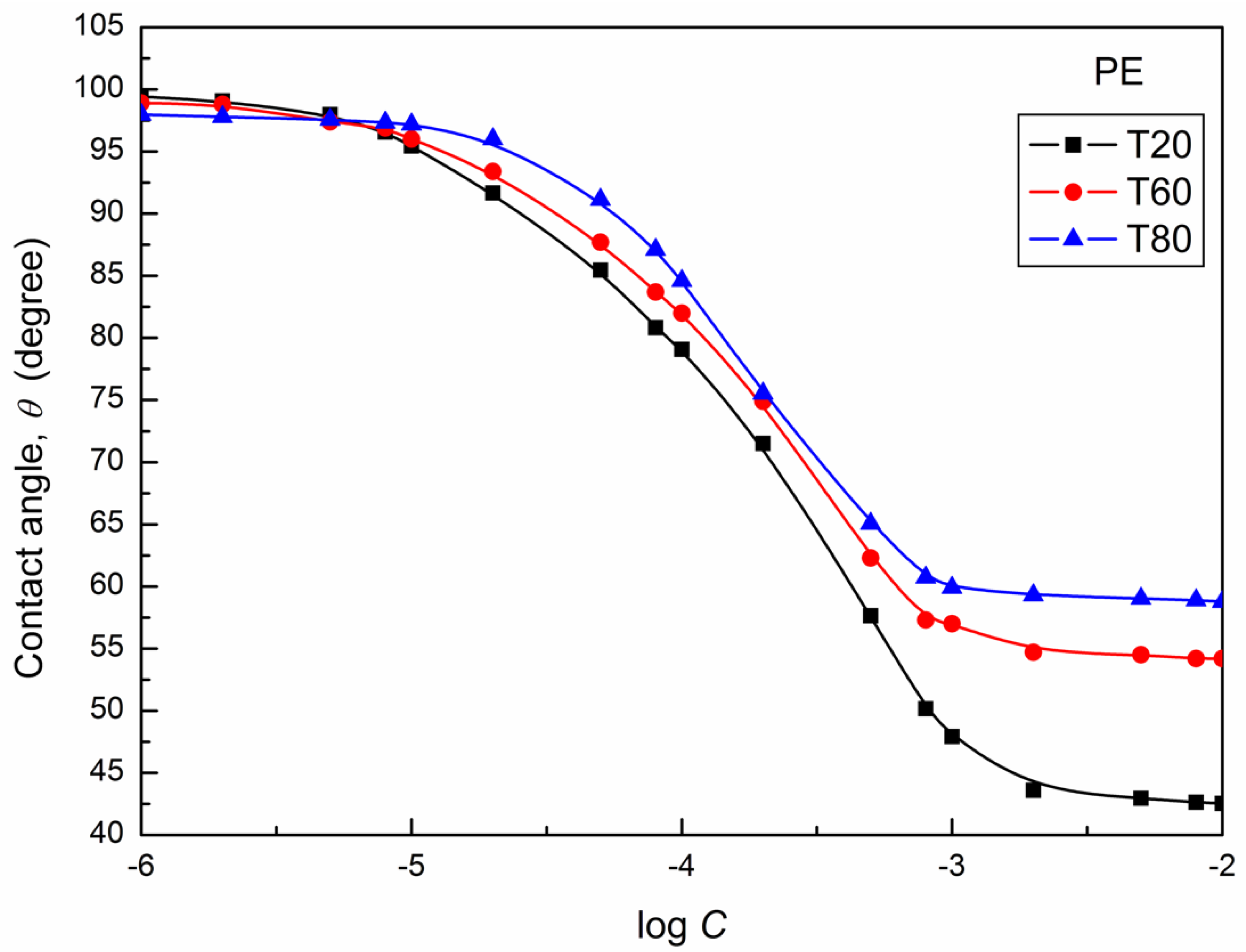
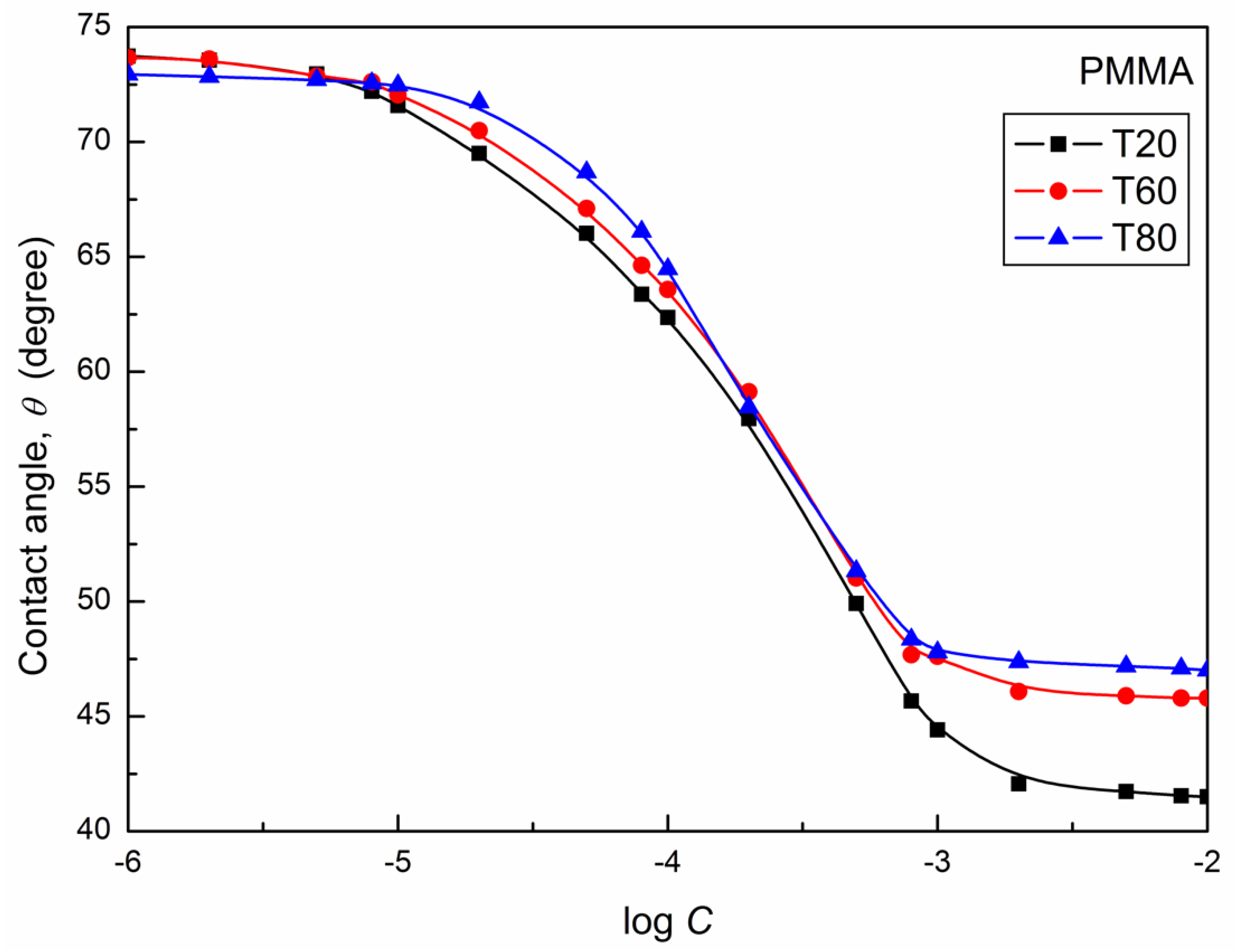
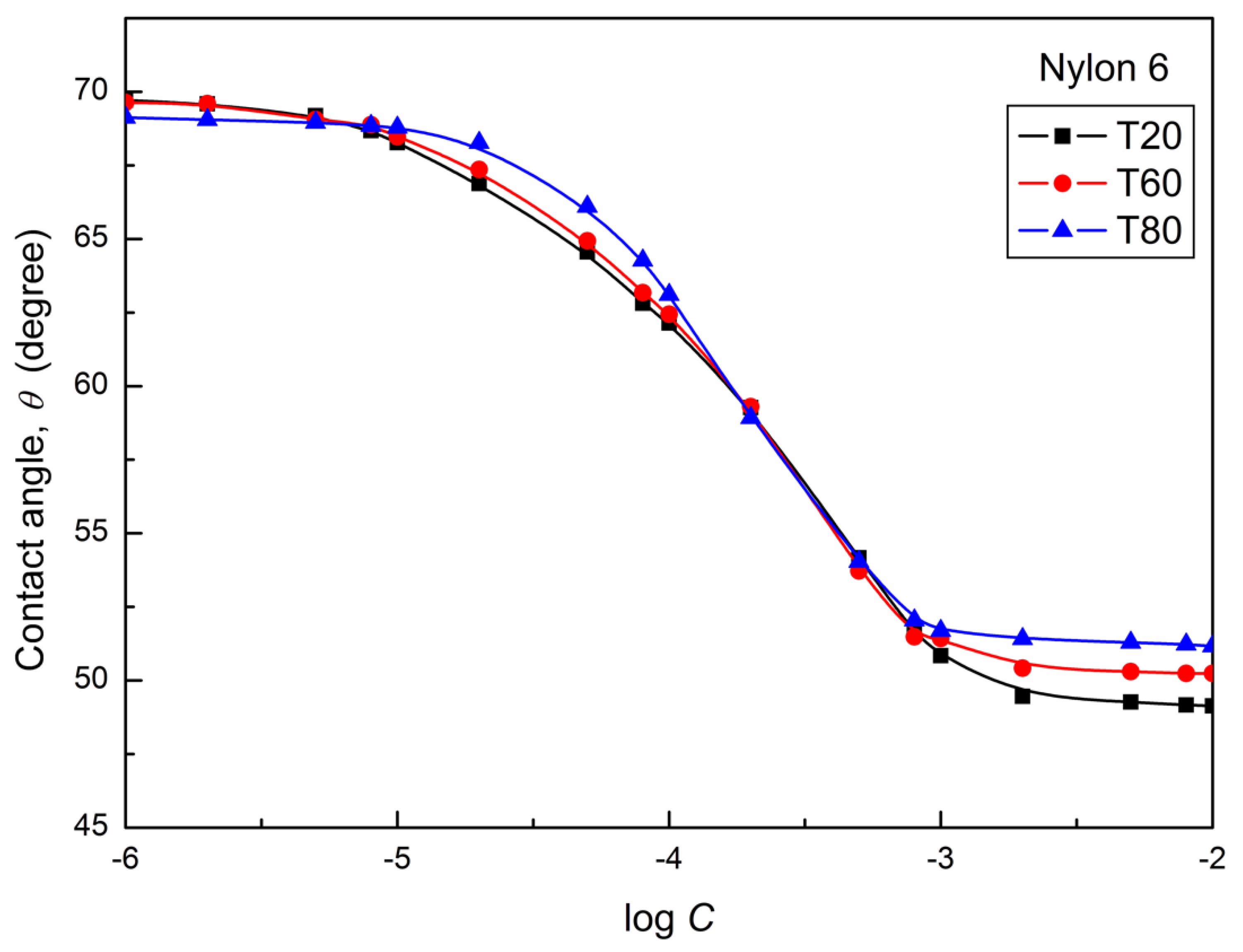
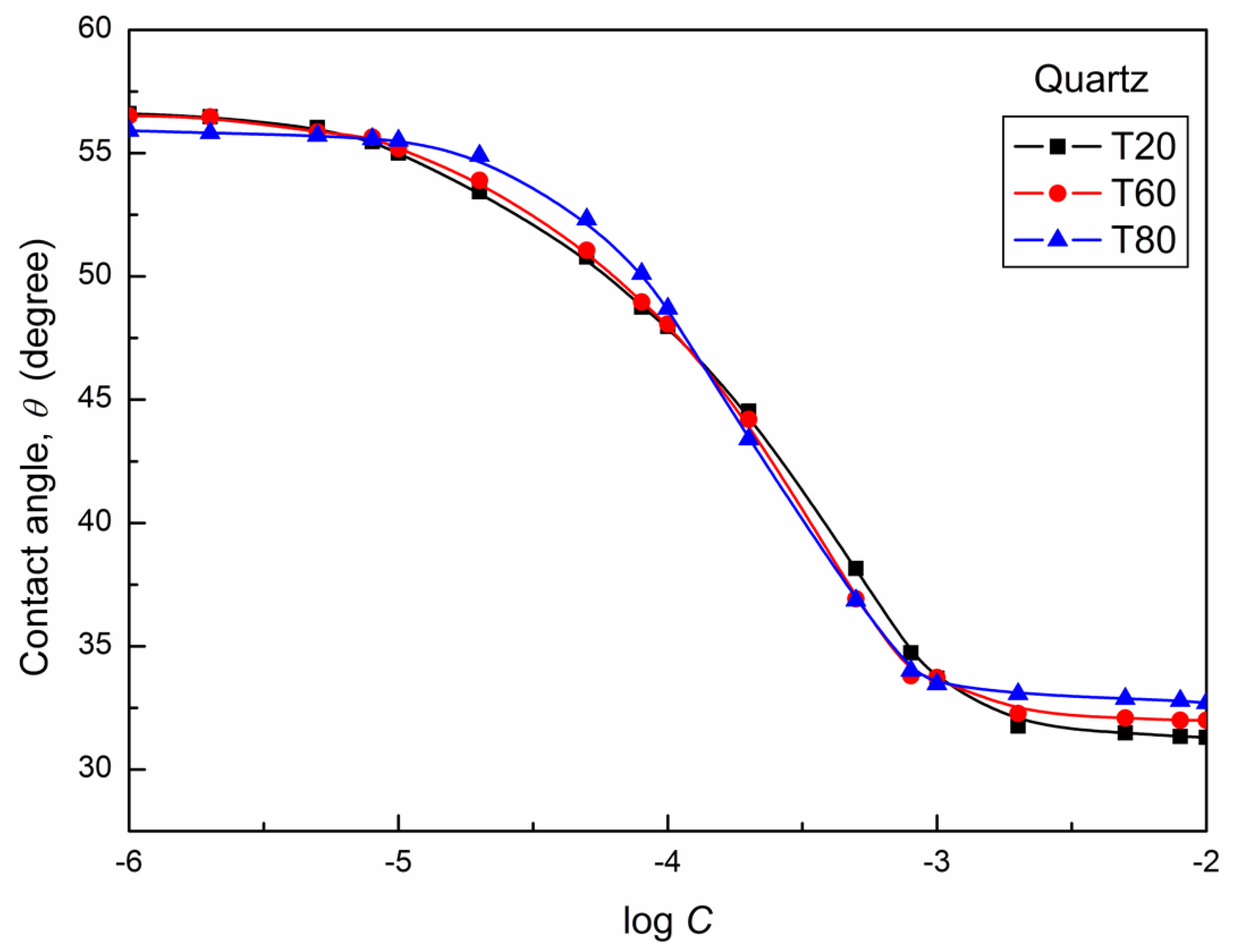
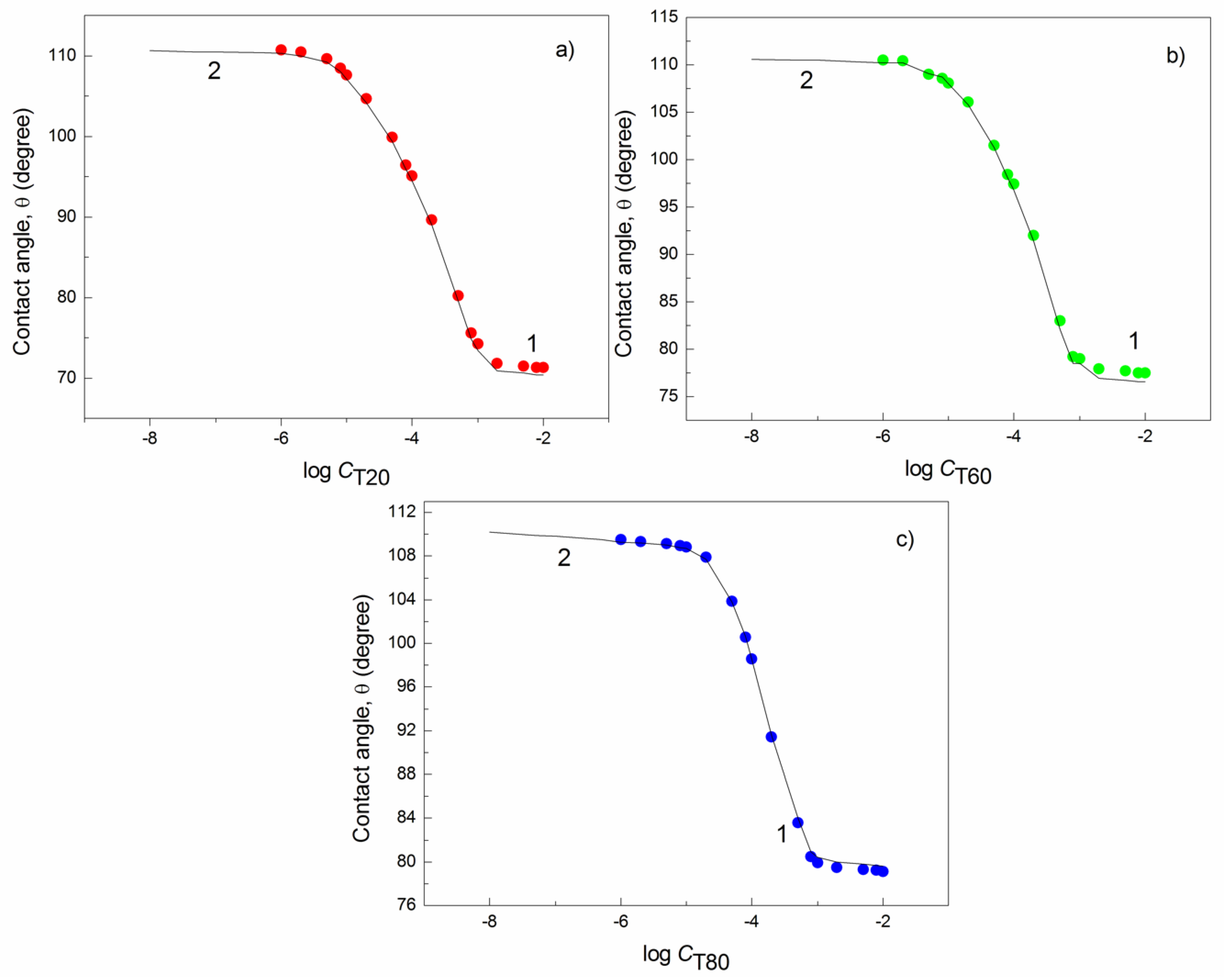
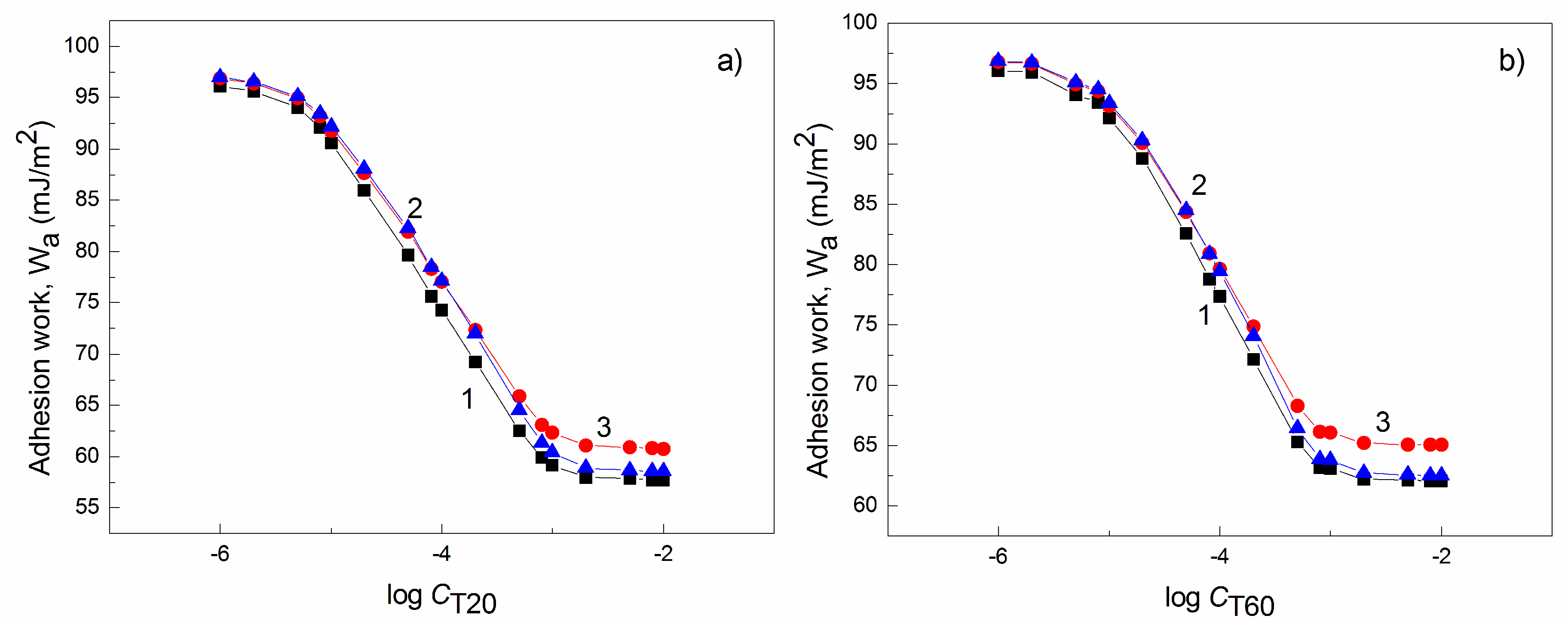
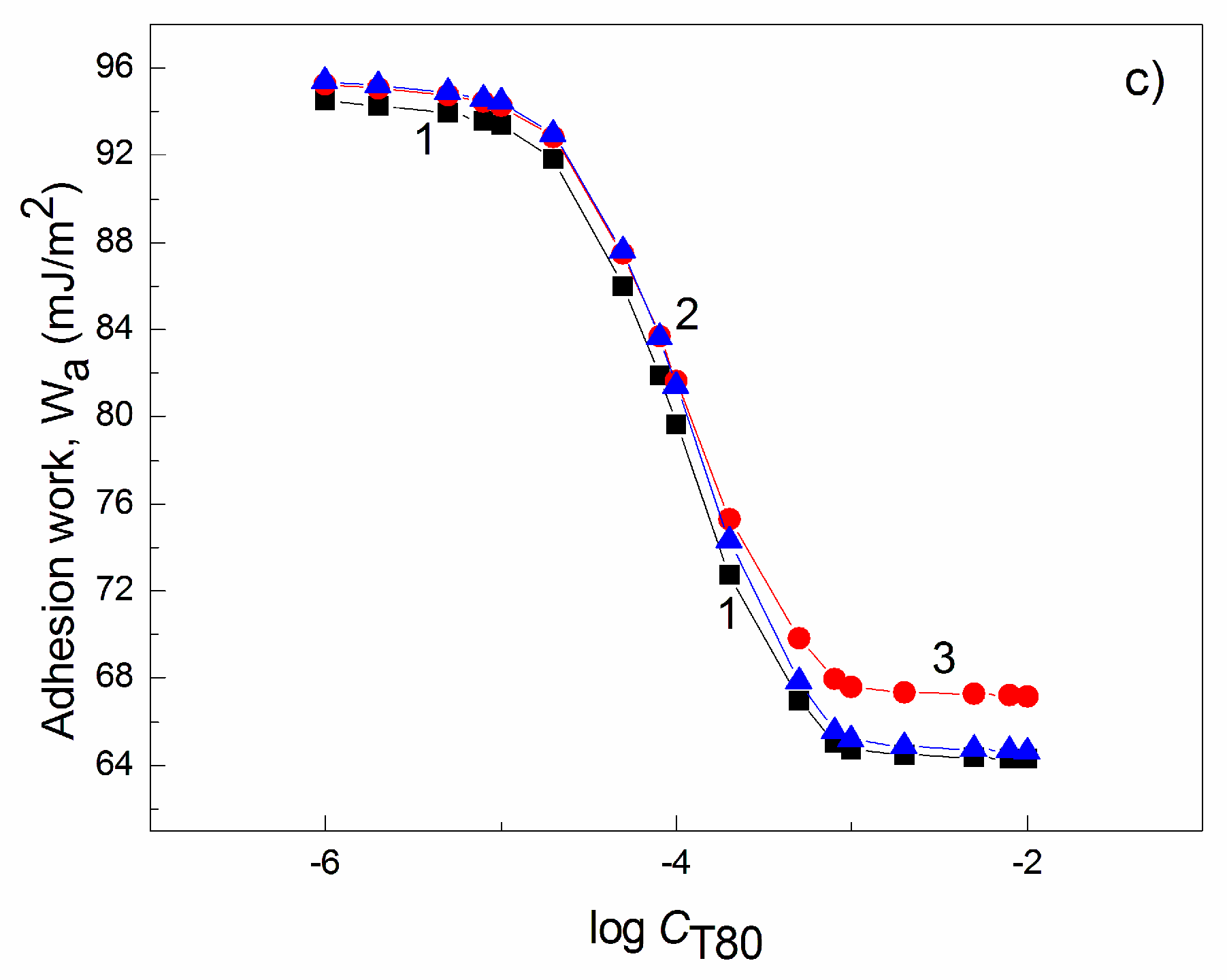
| W-A | PTFE-W | PE-W | PMMA-W | Nylon 6-W | Quartz-W | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | A | A | A | A | A | |||||||
| T20 | 2.79 a | 59.62 | 2.87 | 57.85 | 2.94 | 56.4 | 1.92 | 86.47 | 1.29 | 129.11 | 0.69 | 241.67 |
| 3.63 b | 45.74 | 3.73 | 44.5 | 3.83 | 43.40 | 2.53 | 65.70 | 1.74 | 95.20 | 0.97 | 172.05 | |
| = 2.45–4.84 c A = 34.30–67.64 c | = 2.55–2.27 c1 A = 65.19–73.09 c1 = 0.44–0.43 c2 A = 378.66–386.36 c2 = 0.37–0.36 c3 A = 443.85–459.45 c3 = 0.59 c4 A = 283.91 c4 | |||||||||||
| T60 | 3.00 | 55.38 | 2.94 | 56.45 | 2.97 | 55.88 | 2.08 | 79.82 | 1.454 | 114.19 | 0.86 | 193.96 |
| 3.61 | 45.99 | 3.73 | 44.57 | 3.69 | 45.03 | 2.69 | 61.81 | 1.93 | 85.98 | 1.17 | 141.66 | |
| = 2.45–4.84 c A = 34.30–67.64 c | = 1.720–1.54 c1 A = 96.55–108.15 c1 = 0.44–0.43 c2 A = 378.66–386.36 c2 = 0.35–0.34 c3 A = 475.22–494.50 c3 = 0.52 c4 A = 318.97 c4 | |||||||||||
| T80 | 3.94 | 42.14 | 3.41 | 48.65 | 3.35 | 49.58 | 2.40 | 69.21 | 1.67 | 99.42 | 1.03 | 161.04 |
| 4.04 | 41.10 | 4.14 | 40.10 | 4.06 | 40.89 | 3.48 | 47.76 | 2.51 | 66.17 | 1.60 | 103.83 | |
| = 2.45–4.84 c A = 34.30–67.64 c | = 1.73–1.55 c1 A = 96.05–107.32 c1 = 0.44–0.43 c2 A = 378.66–386.36 c2 = 0.35–0.34 c3 A = 474.72–493.68 c3 = 0.52 c4 A = 318.14 c4 | |||||||||||
| Surfactant | Equation | W-A | PTFE-W | PE-W | PMMA-W | Nylon 6-W | Quartz-W |
|---|---|---|---|---|---|---|---|
| T20 | (11) | −37.16 | −37.33 | −37.33 | −36.55 | −37.67 | −37.45 |
| (13) | −39.68 | −40.83 | −40.83 | −40.03 | −41.32 | −41.23 | |
| (14) | −39.61 | −39.71 | −39.69 | −39.41 | −39.37 | −39.38 | |
| (14) | − | − | − | −29.88 | −25.17 | −12.79 | |
| (15) | − | −39.71 | −39.70 | −29.99 | −25.12 | −12.36 | |
| T60 | (11) | −35.99 | −37.49 | −36.47 | −36.83 | −36.15 | −36.34 |
| (13) | −38.06 | −39.77 | −39.41 | −39.81 | −39.37 | −39.55 | |
| (14) | −38.53 | −39.02 | −39.01 | −38.41 | −38.45 | −38.34 | |
| (14) | − | − | − | −30.36 | −26.91 | −18.87 | |
| (15) | − | −39.01 | −39.02 | −30.50 | −26.89 | −18.49 | |
| T80 | (11) | −32.32 | −34.32 | −34.62 | −33.95 | −32.79 | −33.23 |
| (13) | −33.28 | −35.57 | −35.36 | −37.03 | −37.50 | −36.47 | |
| (14) | −35.89 | −37.81 | −37.84 | −37.24 | −37.24 | −37.28 | |
| (14) | − | − | − | −30.76 | −27.93 | −22.07 | |
| (15) | − | −37.83 | −37.85 | −30.95 | −27.93 | −21.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Effect of Polysorbates on Solids Wettability and Their Adsorption Properties. Colloids Interfaces 2018, 2, 26. https://doi.org/10.3390/colloids2030026
Szymczyk K, Zdziennicka A, Jańczuk B. Effect of Polysorbates on Solids Wettability and Their Adsorption Properties. Colloids and Interfaces. 2018; 2(3):26. https://doi.org/10.3390/colloids2030026
Chicago/Turabian StyleSzymczyk, Katarzyna, Anna Zdziennicka, and Bronisław Jańczuk. 2018. "Effect of Polysorbates on Solids Wettability and Their Adsorption Properties" Colloids and Interfaces 2, no. 3: 26. https://doi.org/10.3390/colloids2030026
APA StyleSzymczyk, K., Zdziennicka, A., & Jańczuk, B. (2018). Effect of Polysorbates on Solids Wettability and Their Adsorption Properties. Colloids and Interfaces, 2(3), 26. https://doi.org/10.3390/colloids2030026







