Aqueous Precipitate of Methanolic Extract of Bergenia ciliata Leaves Demonstrate Photoirradiation-Mediated Dual Property of Inhibition and Enhancement of Silver Nanoparticles Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
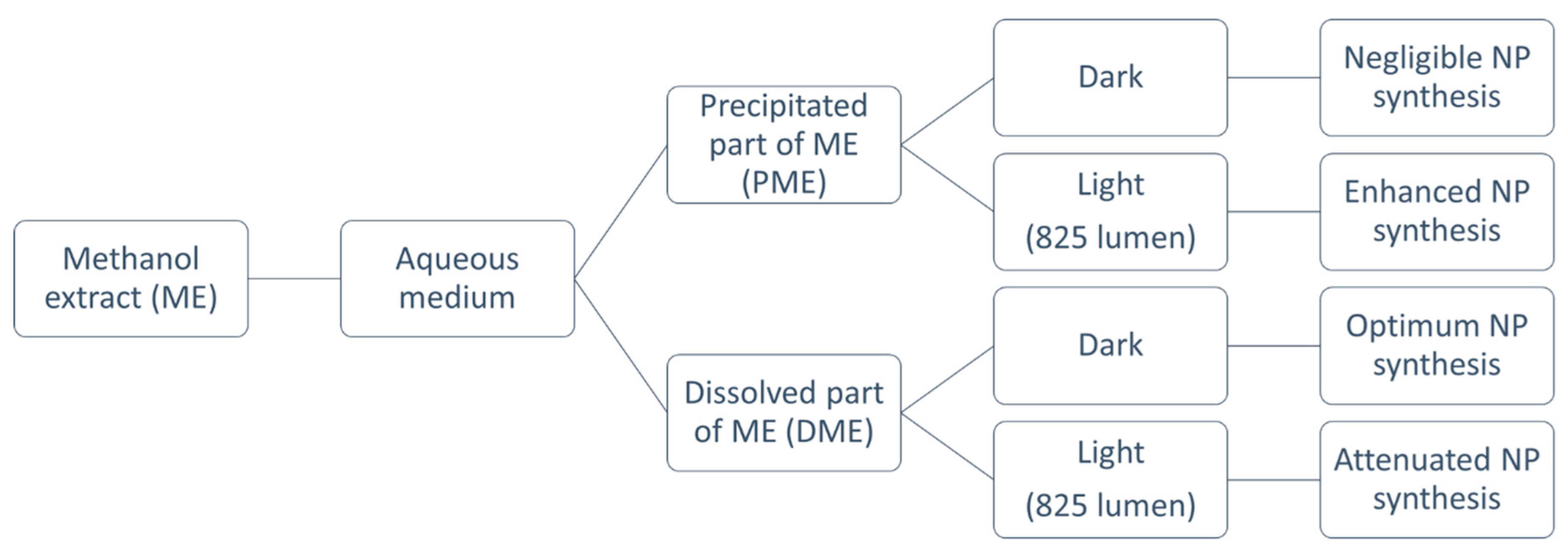
2.2. Extract Preparation
2.3. Estimation of the Total Phenolic Content of PME and DME
2.4. Ferric Ion Reducing Antioxidant Potential (FRAP) Assay of PME and DME
2.5. Gas Chromatography-Mass Spectroscopy (GCMS) Analysis of the Extracts
2.6. Green Synthesis of Silver Nanoparticles Under Different Light Conditions
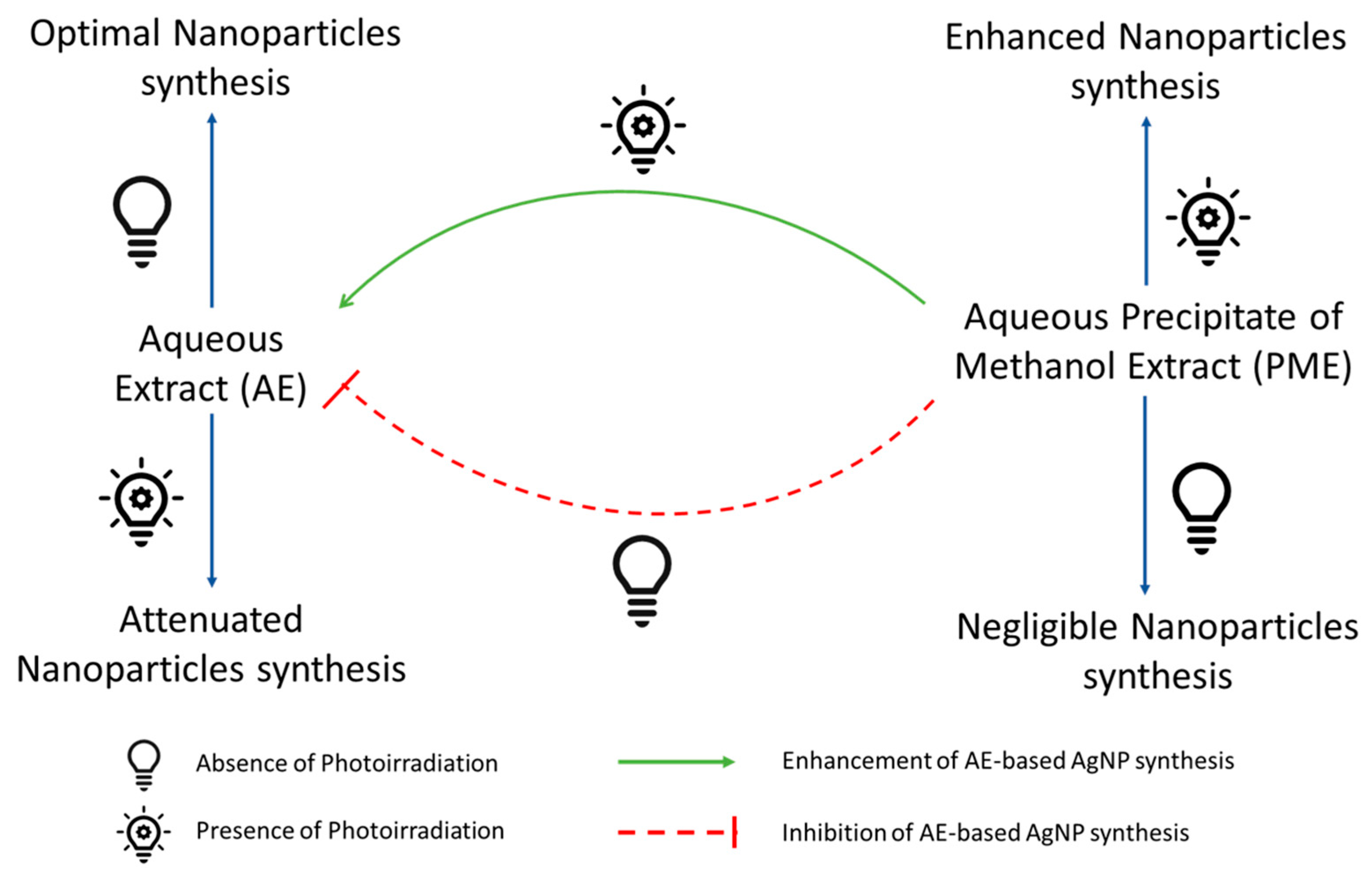
2.7. Impact of PME on Aqueous Extract-Based Silver Nanoparticles Synthesis
2.8. Physicochemical Characterization of the Silver Nanoparticles
2.8.1. Particle Size Analysis of the Silver Nanoparticles
2.8.2. Morphological Study of the Silver Nanoparticles
2.8.3. Percent Elemental Composition Analysis of the Silver Nanoparticles
2.8.4. Stability Studies of PME-AgNPs and DME-AgNPs
2.8.5. Statistical Analysis
3. Results
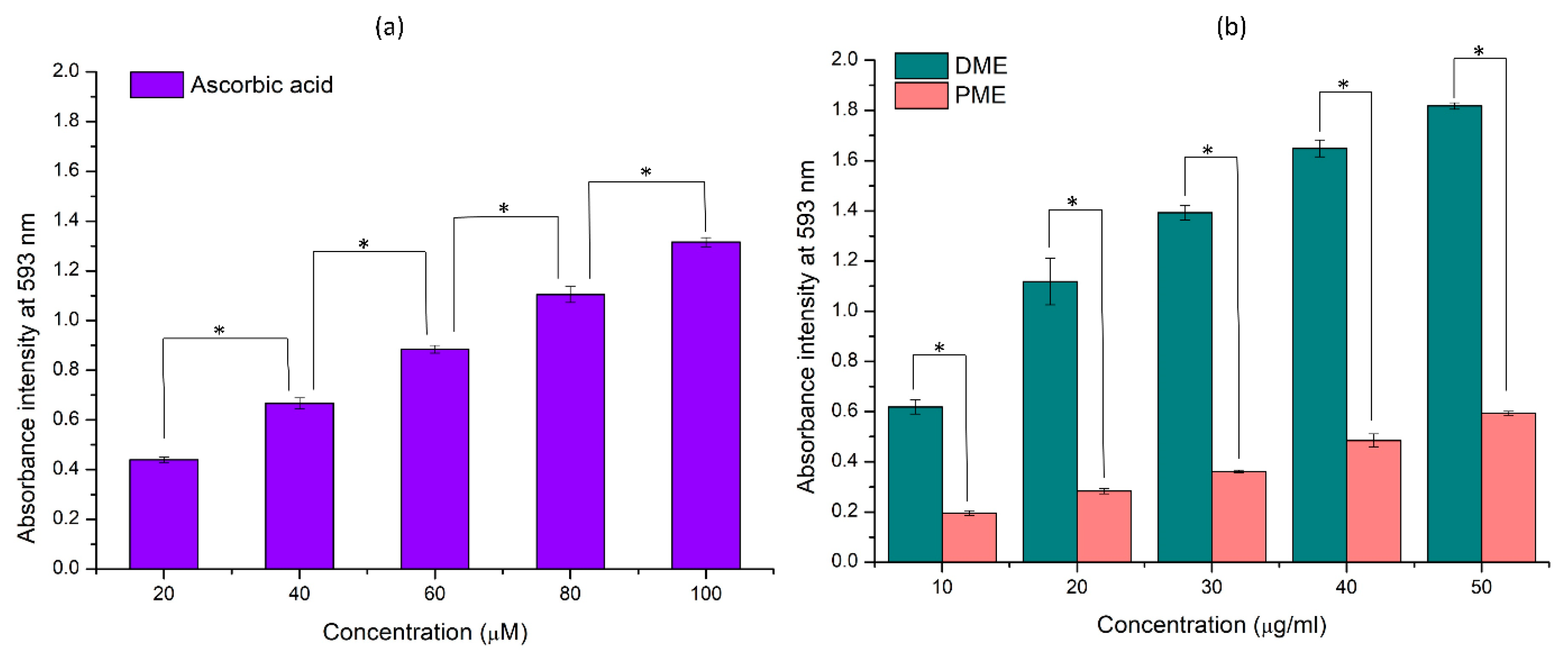
3.1. Total Phenolic Content and Ferric Reducing Antioxidant Power Assay
3.2. Gas Chromatography-Mass Spectroscopy Analysis of the Extracts
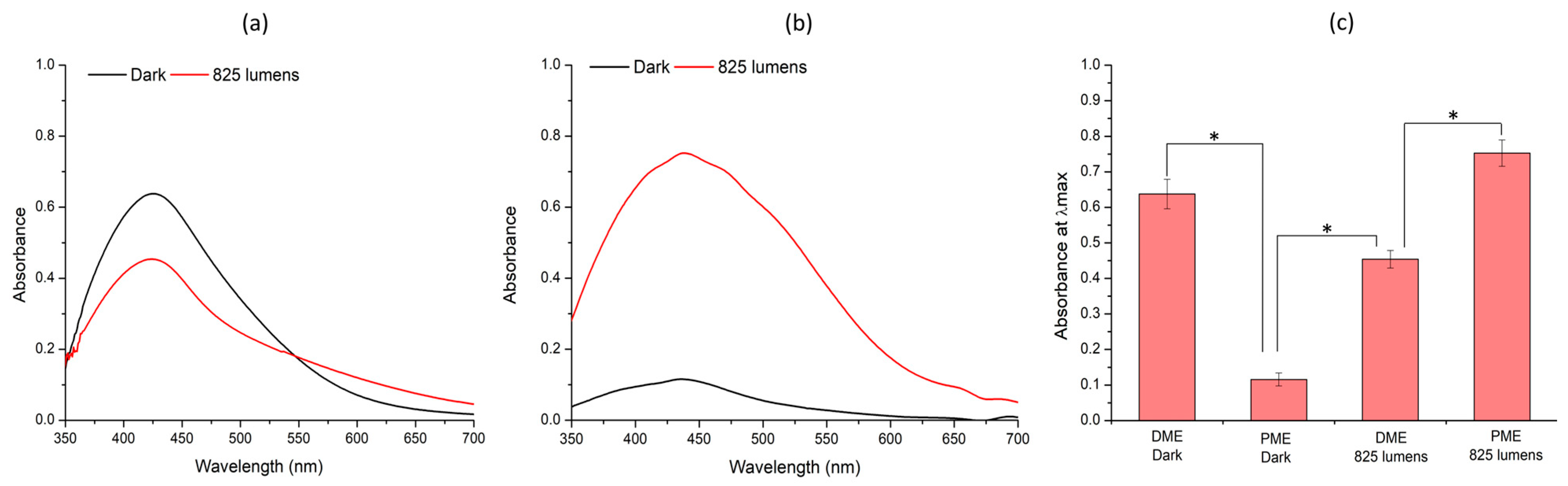
3.3. Green Synthesis of Silver Nanoparticles Under Different Light Conditions
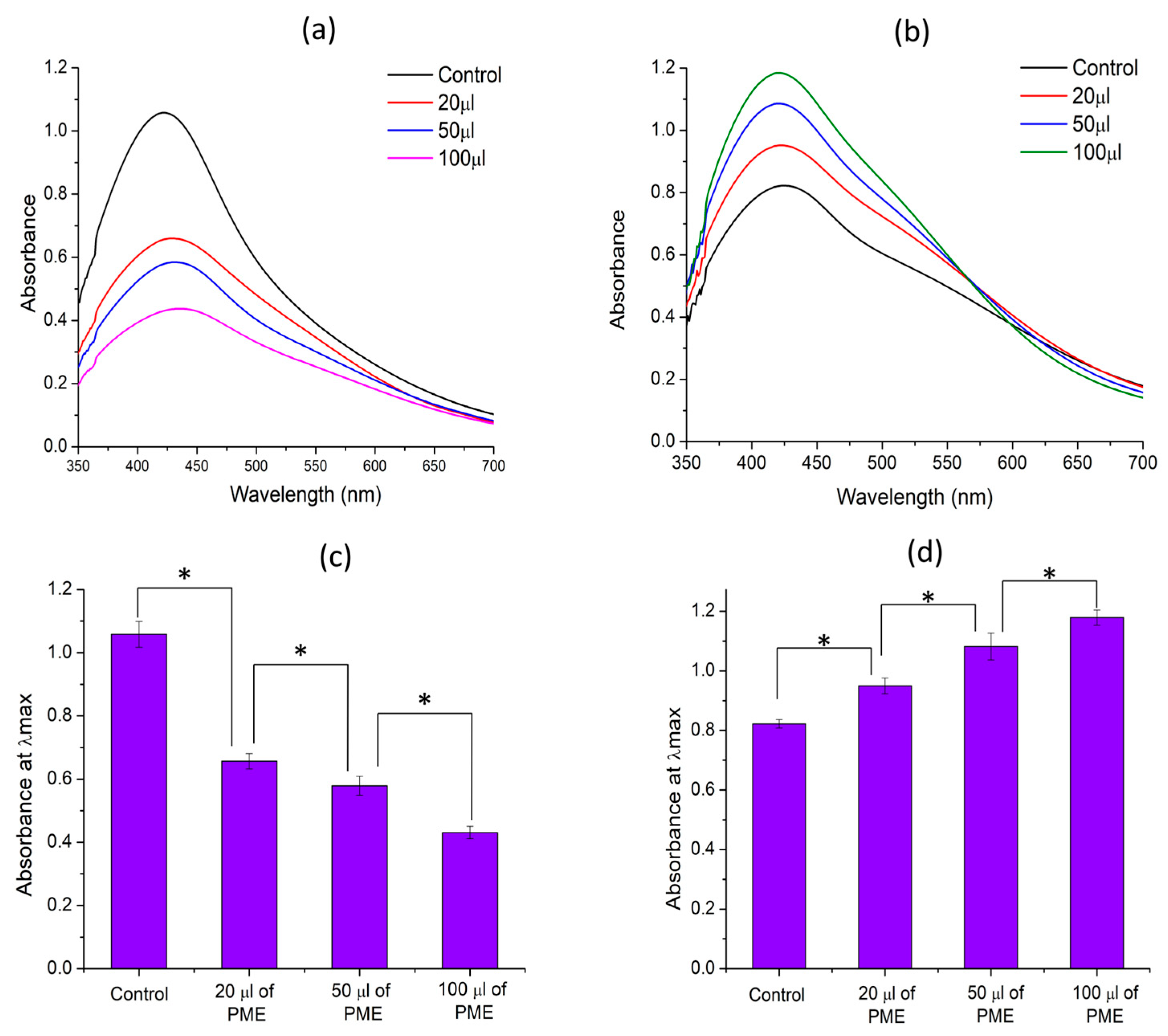
3.4. Impact of PME on Aqueous Extract-Based Silver Nanoparticles Synthesis
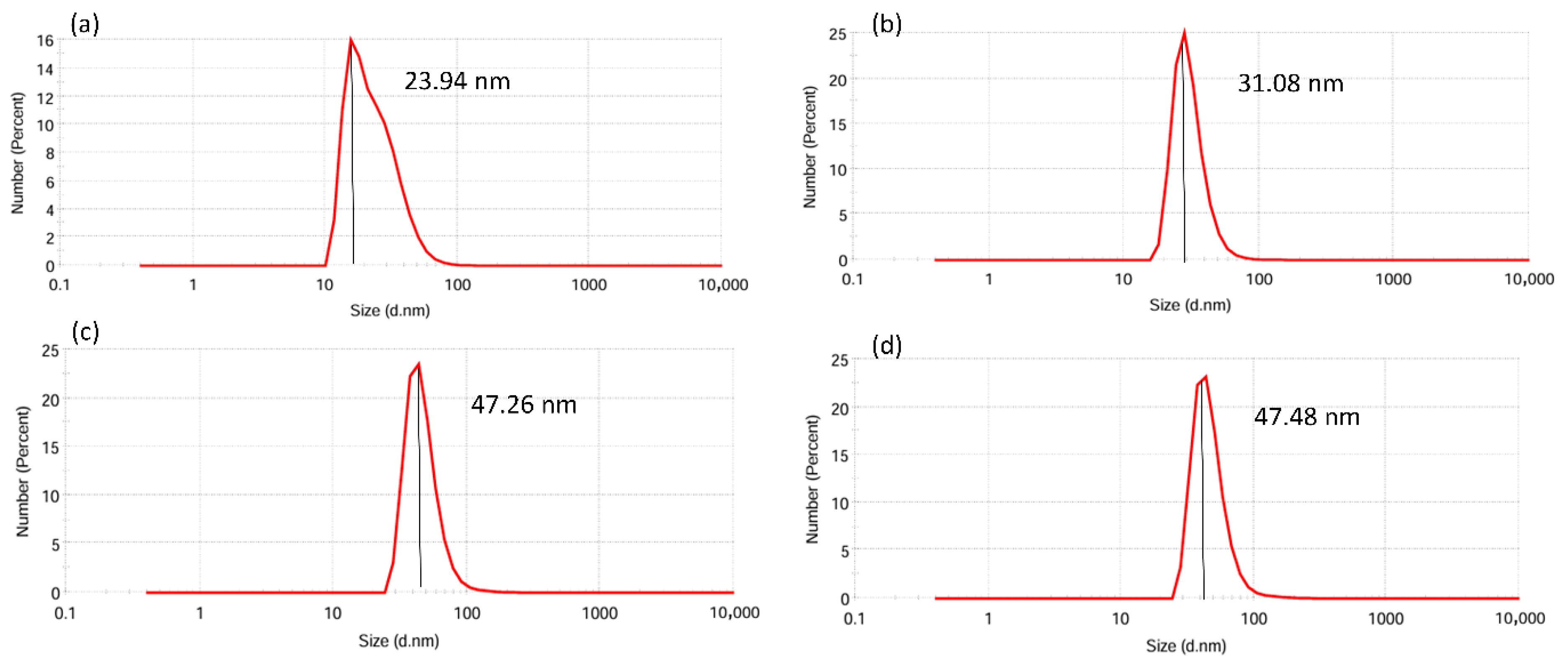
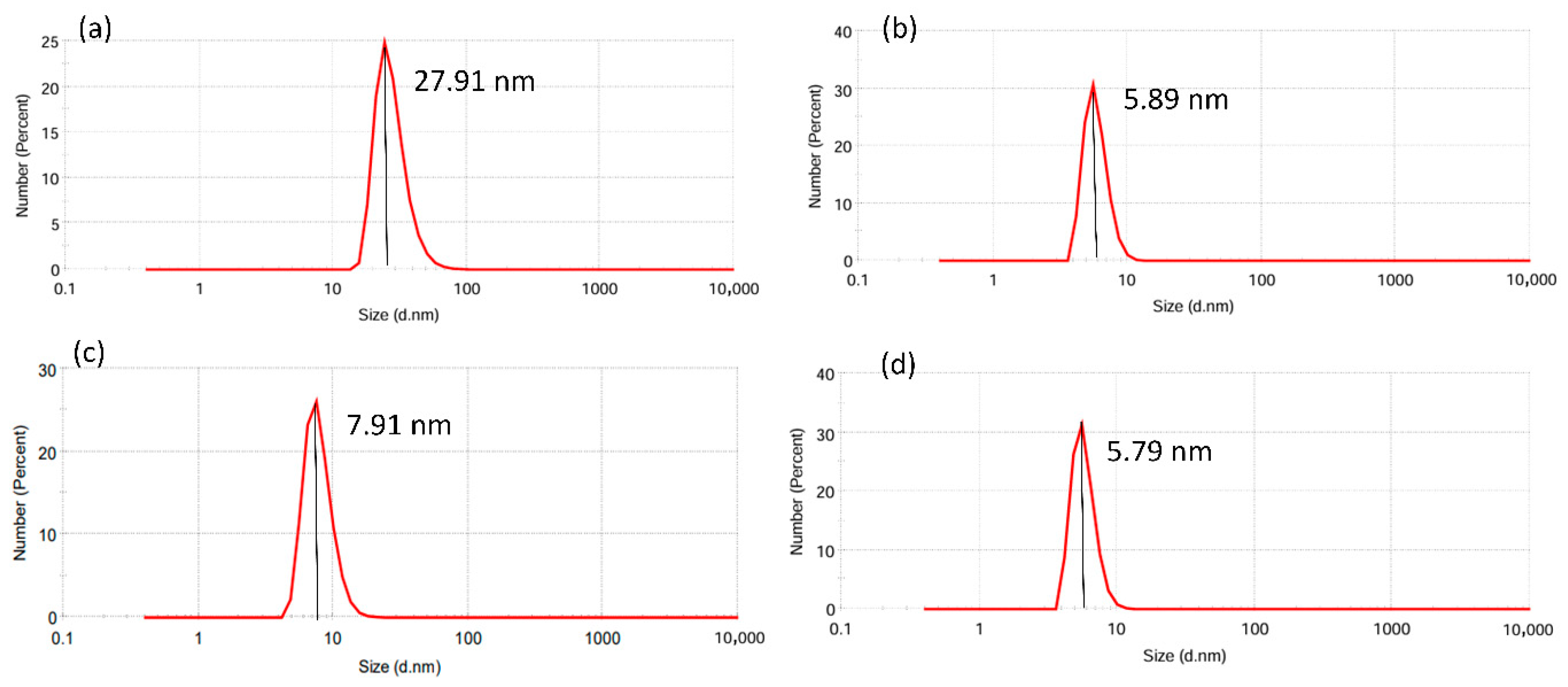
3.5. Particle Size Analysis of AgNPs
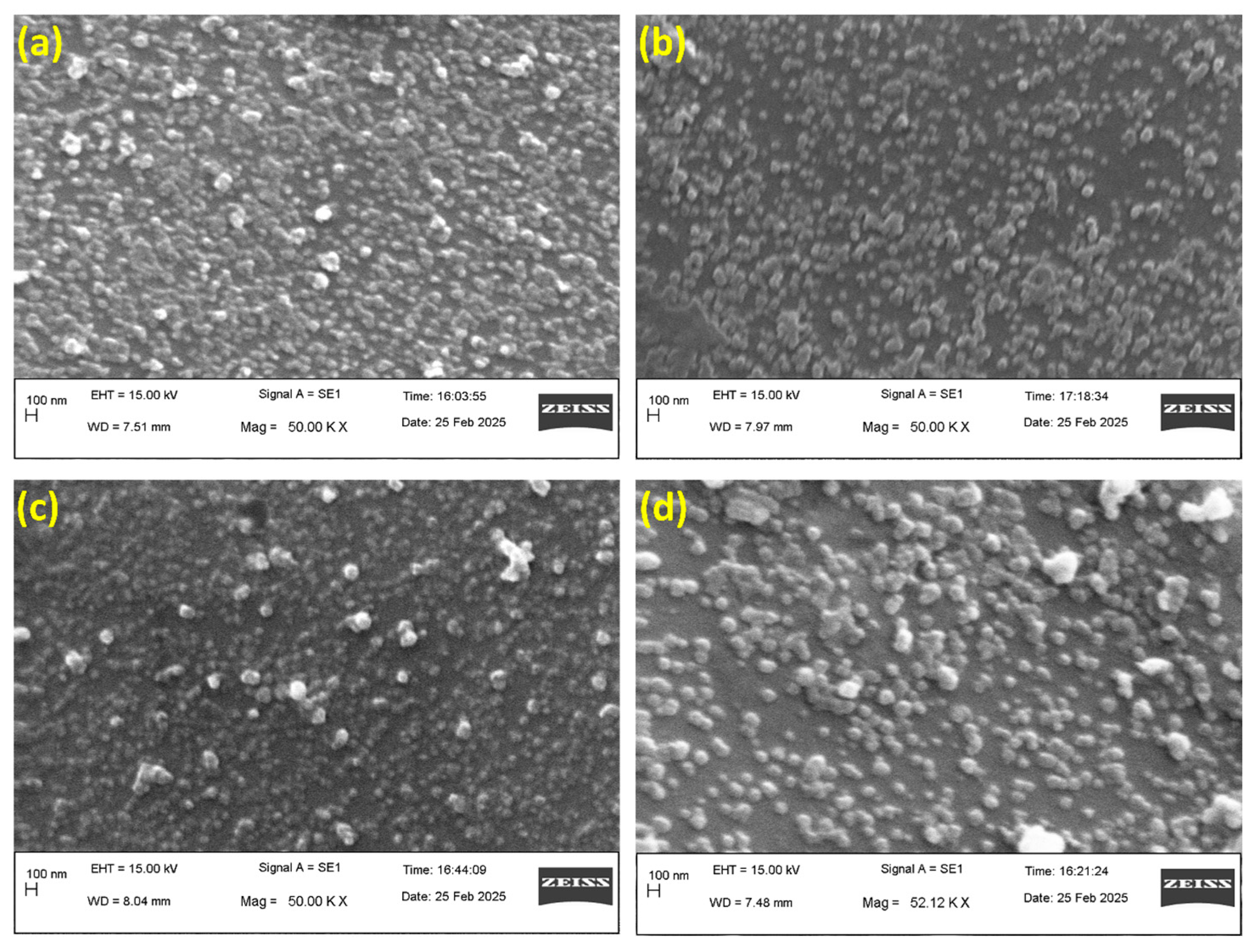
3.6. Morphological Study of the Silver Nanoparticles
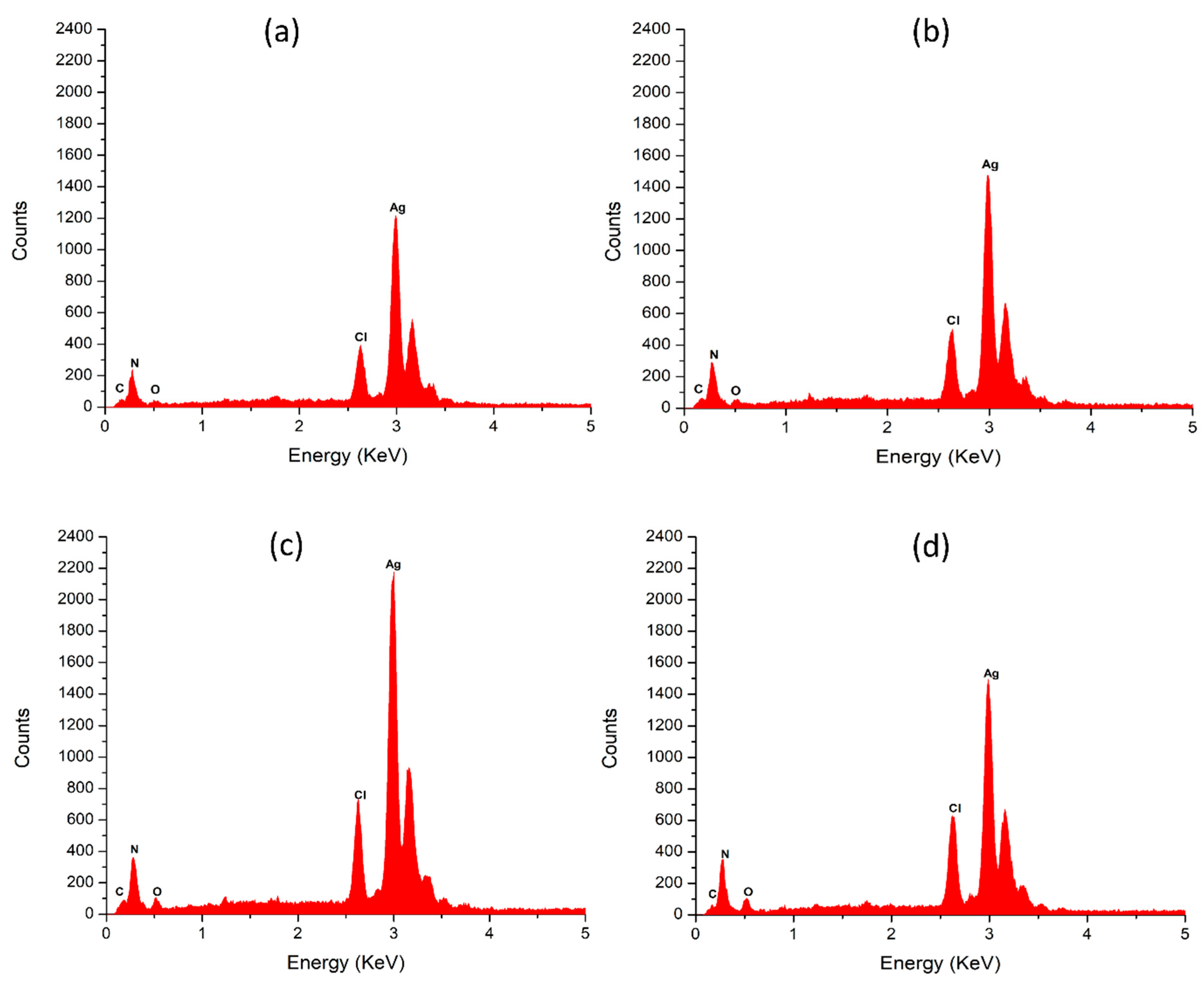
3.7. Elemental Analysis of the Silver Nanoparticles
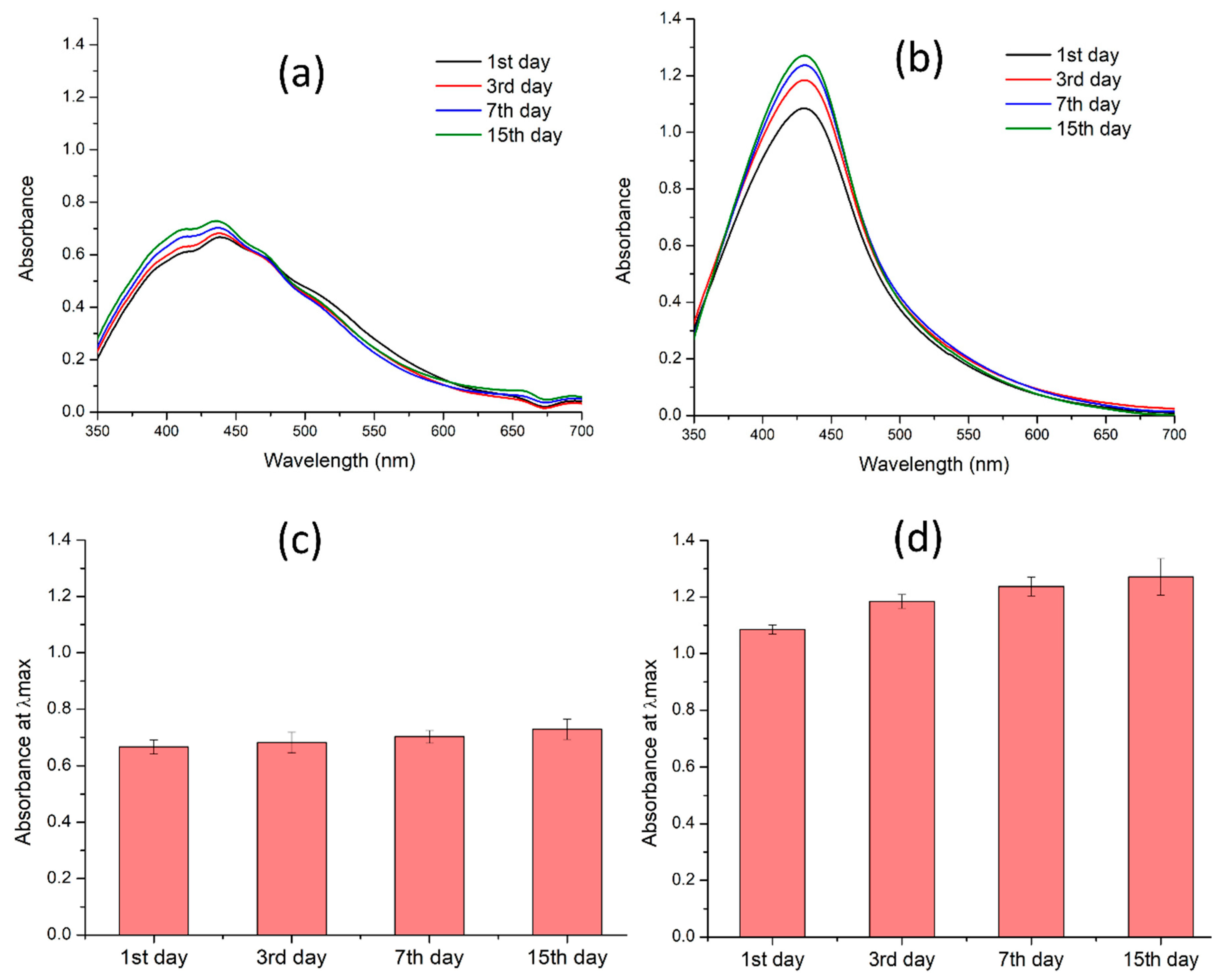
3.8. Stability Studies of Silver Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ME | Methanolic Extracts |
| AE | Aqueous Extracts |
| AgNP | Silver Nanoparticles |
| PME | Aqueous Precipitated ME |
| DME | Aqueous Dissolved ME |
| GCMS | Gas Chromatography-Mass Spectroscopy |
| BC | Bergenia ciliata |
| FRAP | Ferric Reducing Antioxidant Power |
| DLS | Dynamic Light Scattering |
| Poly-dispersive index | PDI |
| EDX | X-Ray Spectroscopy |
| SEM | Scanning Electron Microscopy |
References
- Ahmad, M.; Butt, M.A.; Zhang, G.; Sultana, S.; Tariq, A.; Zafar, M. Bergenia ciliata: A Comprehensive Review of Its Traditional Uses, Phytochemistry, Pharmacology and Safety. Biomed. Pharmacother. 2018, 97, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, N.; Singh, A. Bergenia ciliata—Phytochemistry and Pharmacology: A Review. Biomed. Mater. Devices 2024, 2, 891–904. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Jini, D.; Sharmila, S.; Anitha, A.; Pandian, M.; Rajapaksha, R.M.H. In Vitro and in Silico Studies of Silver Nanoparticles (AgNPs) from Allium Sativum against Diabetes. Sci. Rep. 2022, 12, 22109. [Google Scholar] [CrossRef]
- Serunting, M.A.; Zulfikar, M.A.; Setyorini, D.A.; Rizki, W.O.S.; Kurniawan, R.; Setiyanto, H. Facile Sunlight-Irradiation Mediated Green Synthesis of Highly Stable Silver Nanoparticles Using Archidendron bubalinum Pods Extract for Antibacterial Activity Application. Case Stud. Chem. Environ. Eng. 2024, 10, 100811. [Google Scholar] [CrossRef]
- Filho, A.C.D.; de Jesus Soares, J.; Carriço, M.R.S.; Viçozi, G.P.; Flores, W.H.; Denardin, C.C.; Roehrs, R.; Denardin, E.L.G. Green Synthesis Silver Nanoparticles Bougainvillea glabra Choisy/LED Light with High Catalytic Activity in the Removal of Methylene Blue Aqueous Solution. Environ. Sci. Pollut. Res. 2023, 30, 36244–36258. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J. Formation of Naturally-Occurring Gold Nanoparticles by Photosensitive Surfactant Tween 80/85 under Light Irradiation: A New Origin in Aquatic Environment. J. Mol. Liq. 2024, 401, 124683. [Google Scholar] [CrossRef]
- Anjum, S.; Khan, A.K.; Qamar, A.; Fatima, N.; Drouet, S.; Renouard, S.; Blondeau, J.P.; Abbasi, B.H.; Hano, C.; Anjum, S.; et al. Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus Macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag–ZnO) Nanoparticles. Int. J. Mol. Sci. 2021, 22, 11294. [Google Scholar] [CrossRef]
- Romero, G.G.; Ruíz Ruíz, V.F.; Flores, A.B.; Toledo Jaldin, H.P.; Vilchis-Nestor, A.R.; Ávila-Márquez, D.M.; Contreras, D.R. Light-Driven Synthesis of Silver Nanoparticles Using Erythroxylum coca Extract for Catalytic Reduction of 4-Nitrophenol. Biocatal. Agric. Biotechnol. 2025, 64, 103492. [Google Scholar] [CrossRef]
- Gurung, S.; Sarmin, M.; Hoda, M. Implications of White Light-Emitting Diode-Based Photoirradiation on Green Synthesis of Silver Nanoparticles by Methanol- and Aqueous-Based Extracts of Bergenia ciliata Leaves. Nanomaterials 2024, 14, 1327. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Ozturk Sarikaya, S.B. Acethylcholinesterase Inhibitory Potential and Antioxidant Properties of Pyrogallol. J. Enzym. Inhib. Med. Chem. 2015, 30, 761–766. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural Characterization of N-Hexadecanoic Acid from the Leaves of Ipomoea eriocarpa and Its Antioxidant and Antibacterial Activities. Biomass Convers. Biorefin 2024, 14, 14547–14558. [Google Scholar] [CrossRef]
- Essien, E.E.; Thomas, P.S.; Ekanem, I.R.; Choudhary, M.I. Isolation and Characterization of 5-Hydroxymethylfurfural, Antiglycation, Antihyperglycaemic, Antioxidant, and Cytotoxic Effects of Garcinia kola Heckel Roots Extract and Fractions. S. Afr. J. Bot. 2021, 140, 62–67. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Zhao, Z.; Bai, B.; Sun, Z.; Cai, L.; Fu, Y.; Ma, Y.; Wang, Q.; Xi, G. Effect of Hydroxyl on Antioxidant Properties of 2,3-Dihydro-3,5-Dihydroxy-6-Methyl-4H-Pyran-4-One to Scavenge Free Radicals. RSC Adv. 2021, 11, 34456–34461. [Google Scholar] [CrossRef]
- Proudfoot, J.M.; Croft, K.D.; Puddey, I.B.; Beilin, L.J. The Role of Copper Reduction by α-Tocopherol in Low-Density Lipoprotein Oxidation. Free Radic. Biol. Med. 1997, 23, 720–728. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Hu, J.; Zhuiykov, S. Wafer-Scale Two-Dimensional Au-TiO2 Bilayer Films for Photocatalytic Degradation of Palmitic Acid under UV and Visible Light Illumination. Mater. Res. Bull. 2017, 95, 380–391. [Google Scholar] [CrossRef]
- Shrestha, M.; Luo, M.; Li, Y.; Xiang, B.; Xiong, W.; Grassian, V.H. Let There Be Light: Stability of Palmitic Acid Monolayers at the Air/Salt Water Interface in the Presence and Absence of Simulated Solar Light and a Photosensitizer. Chem. Sci. 2018, 9, 5716–5723. [Google Scholar] [CrossRef]
- Gunawardana, N.; Ke, C.Y.; Huang, C.L.; Yang, C.H. One-Pot Synthesis of near-Infrared Absorbing Silver Nanoprisms via a Simple Photochemical Method Using an Ultraviolet-C Light Source. Opt. Mater. 2024, 148, 114932. [Google Scholar] [CrossRef]
- Urbina, I.; Herrera, L.J.M.; Etcheverry, M.E.; Villa, C.; Sepúlveda, R.D.M.; Garavaglia, L.; Arce, V.; Tebaldi, M.C. Shape Modification of Silver Nano Triangles under Light Irradiation. Photonics Nanostruct 2025, 67, 101470. [Google Scholar] [CrossRef]
- Corzo, C.; Meindl, C.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. Novel Approach for Overcoming the Stability Challenges of Lipid-Based Excipients. Part 3: Application of Polyglycerol Esters of Fatty Acids for the next Generation of Solid Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2020, 152, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Palomba, R.; Di Francesco, M.; Di Francesco, V.; Piccardi, F.; Catelani, T.; Ferreira, M.; Palange, A.L.; Decuzzi, P. Boosting Nanomedicine Performance by Conditioning Macrophages with Methyl Palmitate Nanoparticles. Mater. Horiz. 2021, 8, 2726–2741. [Google Scholar] [CrossRef] [PubMed]
| Methanol Extract | Precipitated Methanolic Extract | Dissolved Methanolic Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| PubChem ID (Class of Compound) | Area % | Metal Reducing Potential | PubChem ID (Class of Compound) | Area % | Metal Reducing Potential | PubChem ID (Class of Compound) | Area % | Metal Reducing Potential |
| 1057 (phenol) | 32.75 | [12] | 1057 (phenol) | 19.34 | [12] | 1057 (phenol) | 27.14 | [12] |
| 1176 (urea) | 1.42 | -- | 1176 (urea) | 0.49 | -- | 1176 (urea) | 2.75 | -- |
| 139694 (pyridine) | 1.29 | -- | 139694 (pyridine) | 0.44 | -- | 139694 (pyridine) | 2.42 | -- |
| 91694353 (fatty acid ester) | 8.42 | -- | 91694353 (fatty acid ester) | 3.32 | -- | -- | -- | -- |
| 985 (fatty acid) | 6.65 | [13] | 985 (fatty acid) | 13.34 | [13] | -- | -- | -- |
| 5352845 (fatty alcohol) | 1.68 | -- | 5352845 (fatty alcohol) | 2.28 | -- | -- | -- | -- |
| 533672 (fatty acid ester) | 0.71 | -- | 533672 (fatty acid ester) | 1.23 | -- | -- | -- | -- |
| 214694 (phenol) | 1.68 | -- | 214694 (phenol) | 0.6 | -- | -- | -- | -- |
| 91695431 (fatty acid ester) | 0.96 | -- | 91695431 (fatty acid ester) | 2.78 | -- | 293713 (thiopene) | 1.14 | -- |
| 135453913 (imidazone) | 0.38 | -- | 135453913 (imidazone) | 0.22 | -- | -- | -- | -- |
| 237332 (furan) | 3.06 | [14] | 135443984 (amine) | 0.51 | -- | 135443984 (amine) | 1.08 | -- |
| 24466 (pyran) | 2.68 | [15] | 8181 (fatty acid ester) | 0.43 | -- | 8181 (fatty acid ester) | 7.85 | -- |
| 6420230 (thiopene) | 0.54 | -- | -- | -- | -- | 6420230 (thiopene) | 0.62 | -- |
| 293713 (thiopene) | 0.4 | -- | 14985 (vitamin E) | 4.67 | [16] | -- | -- | -- |
| Extract Type | Light Conditions | Size (d. nm ± SD) | PDI |
|---|---|---|---|
| DME | Dark | 31.08 ± 10.70 | 0.293 |
| 825 lm | 47.48 ± 17.40 | 0.339 | |
| PME | Dark | 23.94 ± 11.19 | 0.230 |
| 825 lm | 47.26 ± 15.80 | 0.208 |
| Extract Type | Light Conditions | PME (µL) | Size (d. nm ± SD) | PDI |
|---|---|---|---|---|
| Aqueous extract (AE) | Dark | 20 | 27.91 ± 8.749 | 0.336 |
| 50 | 5.898 ± 1.197 | 0.438 | ||
| 825 lm | 20 | 7.919 ± 2.051 | 0.452 | |
| 50 | 5.790 ± 1.144 | 0.466 |
| Extract Type | Light Conditions | Silver (% ± SD) | Carbon (% ± SD) | Oxygen (% ± SD) | Nitrogen (% ± SD) |
|---|---|---|---|---|---|
| PME | Dark | 68.01 ± 2.314 | 19.86 ± 1.075 | 4.05 ± 0.734 | 1.28 ± 0.676 |
| 825 lm | 69.46 ± 1.195 | 19.66 ± 0.608 | 3.47 ± 0.820 | 1.28 ± 0.395 | |
| DME | Dark | 58.11 ± 3.114 | 25.79 ± 1.949 | 10.06 ± 2.124 | 2.24 ± 2.352 |
| 825 lm | 72.43 ± 1.930 | 15.21 ± 1.887 | 5.69 ± 0.558 | 1.18 ± 0.183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gurung, S.; Sarmin, M.; Hoda, M. Aqueous Precipitate of Methanolic Extract of Bergenia ciliata Leaves Demonstrate Photoirradiation-Mediated Dual Property of Inhibition and Enhancement of Silver Nanoparticles Synthesis. Colloids Interfaces 2026, 10, 5. https://doi.org/10.3390/colloids10010005
Gurung S, Sarmin M, Hoda M. Aqueous Precipitate of Methanolic Extract of Bergenia ciliata Leaves Demonstrate Photoirradiation-Mediated Dual Property of Inhibition and Enhancement of Silver Nanoparticles Synthesis. Colloids and Interfaces. 2026; 10(1):5. https://doi.org/10.3390/colloids10010005
Chicago/Turabian StyleGurung, Sourav, Monalisha Sarmin, and Muddasarul Hoda. 2026. "Aqueous Precipitate of Methanolic Extract of Bergenia ciliata Leaves Demonstrate Photoirradiation-Mediated Dual Property of Inhibition and Enhancement of Silver Nanoparticles Synthesis" Colloids and Interfaces 10, no. 1: 5. https://doi.org/10.3390/colloids10010005
APA StyleGurung, S., Sarmin, M., & Hoda, M. (2026). Aqueous Precipitate of Methanolic Extract of Bergenia ciliata Leaves Demonstrate Photoirradiation-Mediated Dual Property of Inhibition and Enhancement of Silver Nanoparticles Synthesis. Colloids and Interfaces, 10(1), 5. https://doi.org/10.3390/colloids10010005








