1. Introduction
Olive cultivation is a cornerstone of Jordan’s agricultural sector, with more than 12 million olive trees and 131 olive mills contributing significantly to the national economy [
1]; Olive mill wastewater (OMW), a major byproduct of olive oil production, is generated from washing, pressing, and centrifugation processes. OMW is highly problematic due to its dark color, acidic pH (3–6), pungent odor, and high concentrations of organic matter (notably polyphenols), suspended solids, and inorganic salts [
2]. These characteristics make OMW one of the most challenging agro-industrial effluents to treat, with severe ecological implications if discharged untreated.
Conventional OMW treatment methods—including thermal, physical, chemical, and biological approaches—each present specific advantages and limitations. Thermal and advanced oxidation processes achieve high organic load reduction but are energy-intensive and costly. Biological treatments, while environmentally friendly, are sensitive to pH fluctuations and the toxic nature of phenolic compounds, which inhibit microbial activity. Membrane filtration provides excellent separation efficiency but suffers from fouling and high maintenance costs. Adsorption techniques using natural or modified materials are simple and cost-effective; however, regeneration of spent adsorbents and disposal of concentrated sludge remain operational challenges. Conventional OMW treatment methods have shown varying degrees of success [
3,
4,
5,
6,
7]. In recent years, researchers have started looking into new ways to treat OMW pollution. One example is steam reforming of oxygen-rich compounds, which can lower the amount of organic pollutants while also producing hydrogen as a useful byproduct [
8]. This type of approach shows the shift toward treatment methods that not only clean wastewater but also recover valuable resources, making the process more sustainable and energy-efficient than traditional options. Recent advances include membrane technologies [
9] and nano-photocatalysts [
10]; however, their high cost, complexity, and scalability limit their wide adoption in resource-constrained regions. Although olive oil production and the associated wastewater pose challenges throughout the Mediterranean—including Spain, Greece, Italy, Tunisia, and Morocco [
11]—the predominant treatment methods in these countries rely on activated carbon and membrane filtration. In contrast, other approaches focus on natural adsorbents such as clay and date pit residues. Consequently, evaluating the cost-effectiveness and environmental sustainability of lime (CaO, which is produced by calcination of limestone at 1000 °C) and nano-limestone strategies highlights their promising potential for broader application across OMW-producing regions [
12,
13]. Previous studies employing natural adsorbents such as clay minerals and date-pit residues have demonstrated moderate removal efficiencies for olive mill wastewater pollutants. For instance, [
12] reported approximately 60–70% phenolic compound removal using acid-activated Jordanian bentonite, while [
13] achieved reductions in COD and phenols concentrations about 14% and 21%, respectively, with mixed clay and volcanic tuff adsorbents. Limestone pretreatment is essential to improve downstream treatment efficiency, typically involving pH adjustment, solid–liquid separation, and coagulation–flocculation to reduce organic load and suspended solids [
14]. Conversely, the treatment of OMW remains a major challenge for researchers, as it necessitates reducing its toxicity and eliminating harmful compounds and elements prior to environmental discharge [
15]. Al Bawab et al. reviewed several studies conducted in the Mediterranean region, emphasizing the wide range of OMW treatment approaches, including chemical, physical, and biological methods [
16,
17]. The integration of physical and chemical processes has been identified as an advanced strategy to improve OMW quality [
18,
19,
20].
Adsorption has emerged as an attractive approach due to its simplicity, cost-effectiveness, and ability to significantly reduce phenolic compounds and COD [
21]. The ability of limestone (refers to the naturally occurring CaCO
3 mineral) to clean OMW is strongly linked to where it comes from and what it is made of. Because limestone forms in different environments—like seas, lakes, or areas rich in shells—its composition, texture, and level of impurities can vary. While calcium carbonate (CaCO
3) is usually the main component, some samples also include magnesium carbonate, silica, alumina, iron oxides, and small amounts of other elements. These differences can affect how well limestone adsorbs pollutants, how its surface interacts with contaminants, and how effective it is in coagulation and flocculation. Carbonate rocks account for roughly 20–25% of all sedimentary formations, with limestone representing the most abundant type [
22]. Natural adsorbents such as limestone, abundant in Jordan’s central regions [
23], offer further advantages of local availability and low cost. While limestone has been studied for heavy metal and nutrient adsorption [
24,
25], its application for real OMW treatment remains unexplored. Furthermore, calcined limestone (lime), composed primarily of CaO and Ca(OH)
2, is a proven coagulant in wastewater treatment [
26,
27], and could be synergistically integrated with limestone adsorption.
The conversion process, presented in Equation (1), describes the decomposition of solid calcium carbonate (CaCO
3) into solid calcium oxide (CaO) with the simultaneous release of carbon dioxide gas (CO
2).
In addition, lime is widely recognized as an effective coagulant due to its strong capacity to precipitate contaminants in wastewater. Owing to its chemical properties, lime demonstrates significant efficiency in removing diverse pollutants, thereby contributing to improved water quality [
27].
Several studies have explored the combination of treatment processes to enhance OMW remediation efficiency. For example, [
28] combined lime coagulation with biological treatment to reduce COD and phenolics, while [
29] applied sequential lime coagulation and filtration, achieving over 80% COD reduction. [
30] demonstrated that coupling coagulation–flocculation with nanoparticle adsorption significantly improved pollutant removal. These examples show that integrating coagulation–flocculation (pretreatment) with adsorption (polishing) can synergistically enhance OMW purification by combining bulk solids removal with surface adsorption of residual organics. Building on these findings, the present study introduces a dual-stage system uniting lime-based pretreatment and nano-limestone adsorption—evaluated through both batch and column configurations—to provide a cost-effective, scalable alternative for OMW management in resource-limited regions.
While natural adsorbents such as limestone, clays, biochar, and activated carbon have been studied for OMW remediation, these approaches often address adsorption and coagulation separately and rely on imported or synthetic materials with high operational costs. The novelty of the present work lies in the integrated use of lime and locally sourced nano-limestone composites derived from Jordanian carbonate waste to establish a dual-function treatment process that couple’s coagulation–flocculation pretreatment with subsequent adsorption. This combined strategy allows the assessment of synergistic effects between lime-induced aggregation and nanoscale adsorption, enhancing removal efficiency for both phenolic compounds and chemical oxygen demand (COD). Furthermore, by valorizing abundant native limestone resources, this study advances a cost-effective and sustainable pathway for agricultural wastewater management within a circular economy framework—an aspect that remains largely unexplored in previous Mediterranean studies.
2. Materials and Methods
2.1. Field of Study
The initial phase of this study focused on the collection of both limestone and olive mill wastewater (OMW) samples. A field excursion was carried out to an industrial zone located between the Swaqa and Al-Qatraneh areas (
Figure 1), which hosts three industries and several carbonate mines. Samples were obtained from the National Carbonates Company’s facility and mine. According to the factory owners, these samples were classified as waste limestone due to their failure to meet the required degree of whiteness. For the wastewater component, OMW samples were collected from different sites, specifically the Jordan Jarash Mountains Mill and the Zayy Automated Mill, to ensure representation from diverse production sources.
The primary aim of this project is to develop a cost-effective and environmentally sustainable method for removing organic and phenolic compounds from olive mill wastewater (OMW) using Jordanian industrial waste. The approach involves multiple stages, beginning with pretreatment via coagulation, flocculation, and sedimentation, followed by filtration using limestone of varying sizes and ratios. The treatment’s efficiency is evaluated through both batch and column experiments.
2.2. Materials
Olive mill wastewater (OMW) was collected during the 2023 harvest season from a three-phase olive oil mill in northern Jordan. The samples were stored at 4 °C and characterized prior to treatment and exhibited physicochemical properties consistent with those previously reported for Jordanian OMW [
18]. To ensure comparability with established datasets,
Table 1 presents the typical composition range of OMW in Jordan, which aligns closely with our measured values, confirming that seasonal variation did not significantly alter the wastewater characteristics.
Table 1 compiles representative values from published studies [
12,
13,
18]. These reports collectively show that regional and seasonal variations are minor, supporting the use of these ranges as a national reference baseline.
Locally sourced limestone was obtained from central Jordan, mechanically crushed, and sieved into two distinct size fractions. The microscale-limestone (Micro-Ls, refers to ground limestone particles with mean diameters in the micrometer range 1–200 μm, prepared through mechanical grinding) fraction was obtained after manual crushing and sieving through a 5000–3000 mesh stainless-steel screen. To prepare nano-limestone (Nano-LS), the finer fraction was subjected to planetary ball milling (Retsch PM100, Haan, Germany) for 3 h at 400 rpm using tungsten carbide balls (10:1 ball-to-powder ratio), followed by air drying at 80 °C for 12 h. The resultant powder was stored in airtight containers to prevent moisture uptake before use. Lime (CaO) was prepared by calcination of limestone at 1000 °C for 3 h in a muffle furnace.
All reagents were of analytical grade and used without further purification. De-ionized water was employed throughout. Hydrochloric acid (HCl) (37%, VWR, Radnor, PA, USA), Ferric chloride (FeCl3) (AR grade, Fischer Chemicals, Guangzhou, Guangdong Province, China), polymeric quaternary amine (Superfloc C 577, Kemira, Helsinki, Uusimaa, Finland), Sodium nitrate (NaNO3) (Hopkin & Williams Ltd., Wolverhampton, West Midlands, UK), Sodium hydroxide (NaOH) (AR grade, Alpha Chemika, Mumbai, Maharashtra, India), Hexane (95%, ACS, Washington, DC, USA), Ethyl acetate (99.5%, Fischer Chemical, India), Methanol (HPLC grade, ACS, Washington, DC, USA), Sodium carbonate (Na2CO3) (Fischer Chemicals, Guangzhou, Guangdong Province, China), Folin reagent (Merck KGaA, Darmstadt, Hesse, Germany), COD kits (Lovibond Vario, Lovibond House, England, UK), and Gallic acid (99%, Xilong, China).
2.3. Material Characterization
The size and surface morphology of limestone and lime particles were examined by Scanning Electron Microscopy (SEM) (Precisa 410AM FR, JEOL, Tokyo, Japan), confirming the transition from irregular micro-grains to uniform submicron and nanoscale particles. The crystalline structure was verified by X-ray diffraction (XRD) (MAXIMA, Shimadzu, Kyoto, Japan). Specific surface area and porosity were measured using the Brunauer–Emmett–Teller (BET) nitrogen adsorption method with a Quantachrome NOVA 2200 analyzer (Quantachrome Instruments, Boynton Beach, FL, USA). Although TEM analysis was not performed, SEM results confirmed the nanoscale dimensions of the prepared materials. Total phenolic content (TPC) was measured using the Folin–Ciocalteau colorimetric method [
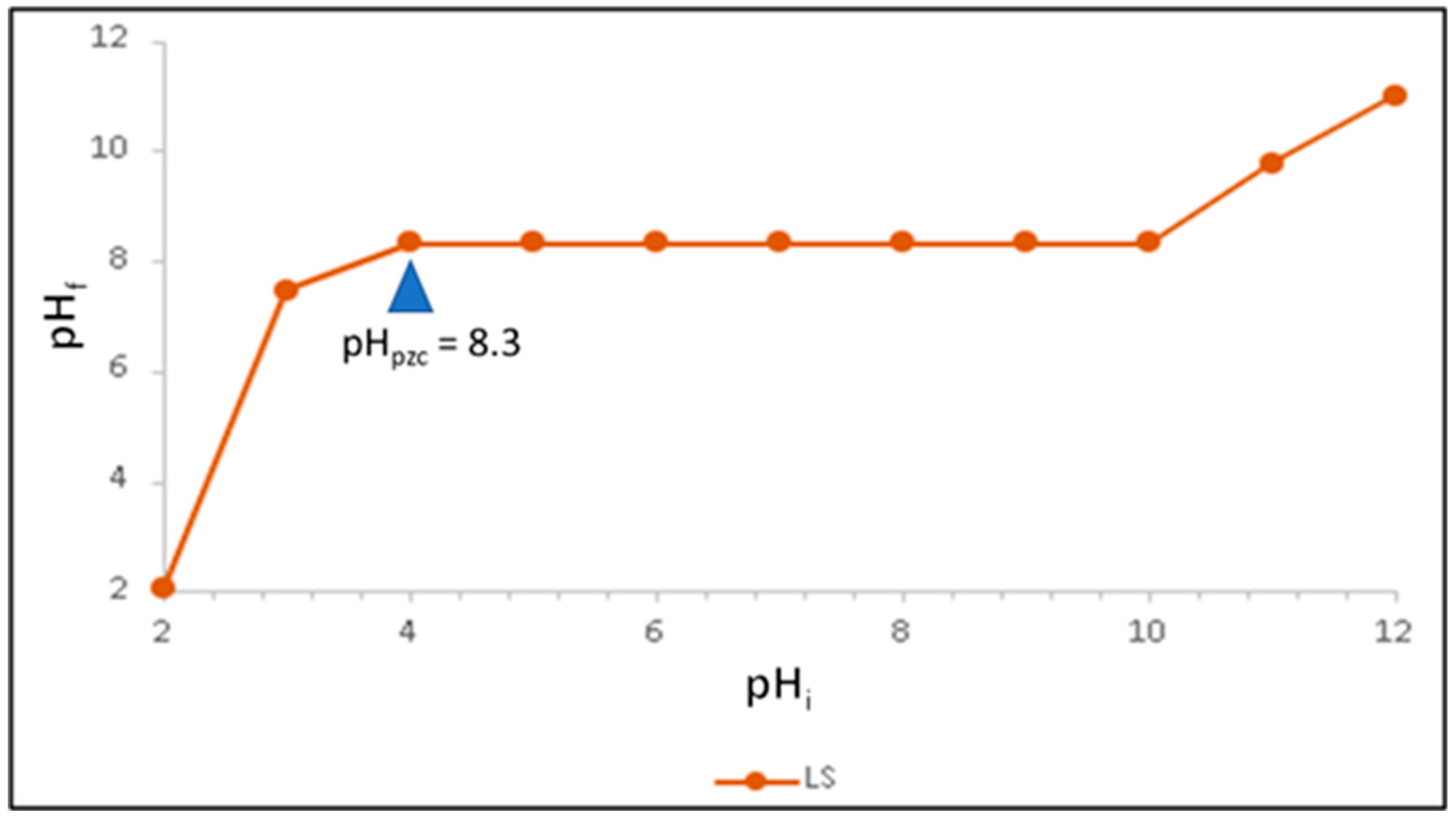
31]. Limestone was converted to lime in a Muffle Furnace (Witeg, Wertheim, Germany), and the OMW pretreatment process was carried out using a Jar Test apparatus (VELP SCIENTIFICA JLT6, Usmate, Italy). Point of zero charge (pHₚzc) was estimated by the pH drift method [
32] to evaluate surface charge behavior.
2.4. Preparation of Lime
Limestone samples were calcined to produce lime through CO
2 elimination. Experiments were conducted by varying crucible sizes (100 × 80, 80 × 50, and 40 × 40 mm), limestone weights (1, 2, and 3 g), and calcination durations (1, 2, and 3 h). The crucible size and sample mass were adjusted to maintain uniform heating and prevent incomplete calcination or material loss. XRD analysis confirmed that all batches achieved full conversion to CaO under the selected conditions. Calcination was performed in a muffle furnace at 1000 °C, and the resulting lime structure was confirmed via XRD analysis. Cooled samples were stored in sealed glass vials for subsequent use [
33].
2.5. Pretreatment of OMW
In this study, the pretreatment of OMW was experimentally conducted using a jar test apparatus to simulate coagulation, flocculation, and sedimentation under controlled laboratory conditions. The process was performed in six 1 L beakers, with continuous mixing and settling as described below. Total phenolic content (TPC), chemical oxygen demand (COD), and total suspended solids (TSS) were measured before and after treatment.
2.6. Chemical Pretreatment
Following [
18], coagulation was carried out with a 7%
w/
v ferric chloride solution added to 500 mL of wastewater adjusted to pH 7. The mixture was stirred at 200 rpm for 5 min to aggregate and destabilize suspended particles and colloids. Flocculation was then performed with 5 mL of polymeric quaternary amine, stirred at 30 rpm for 20 min. Sedimentation followed, and the mixture was filtered after a 50 min interval once stirring ceased.
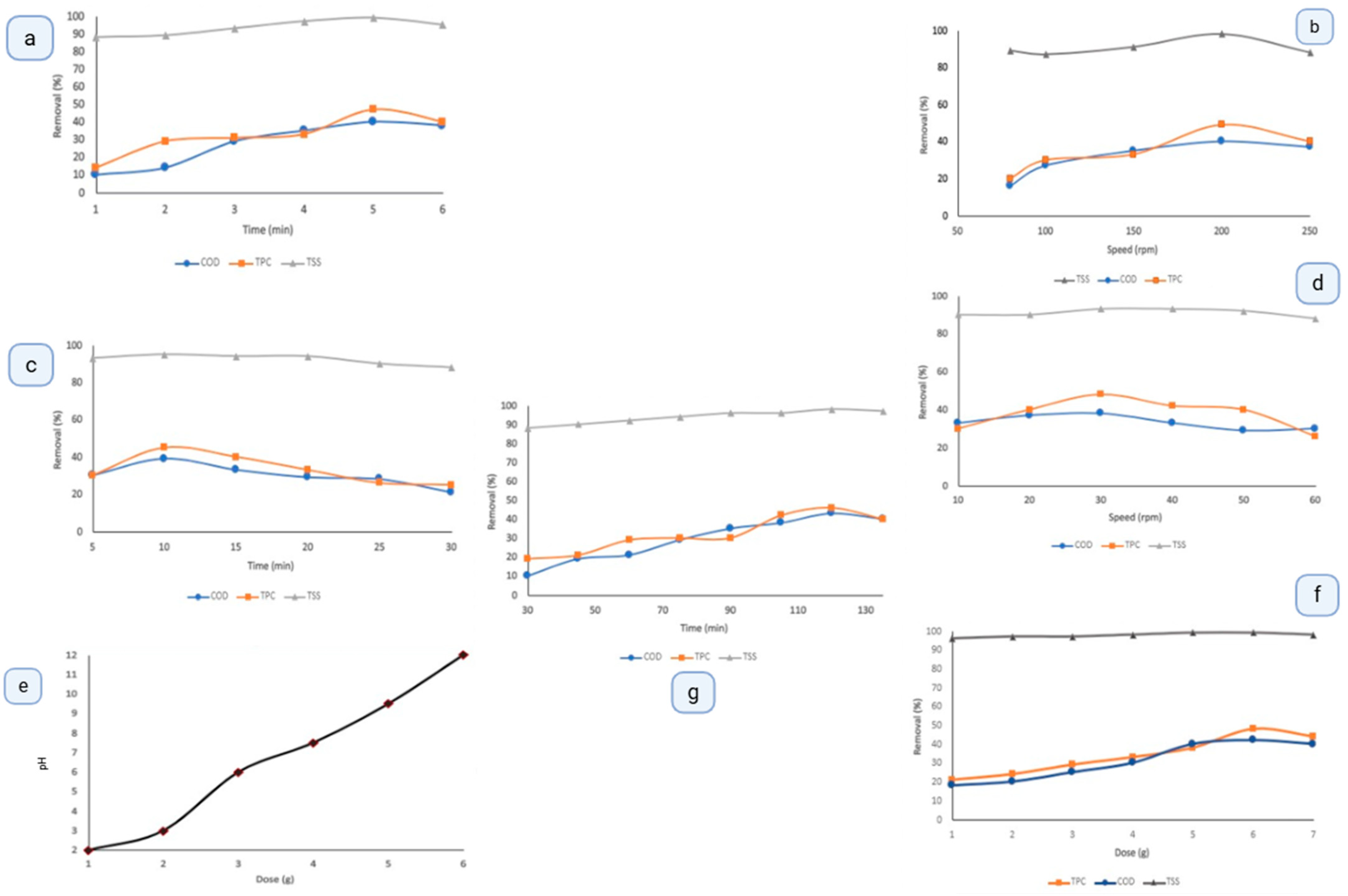
2.7. Pretreatment with Lime
OMW was treated using a lime-based coagulation process, with lime acting as the coagulant. Key parameters—including mixing speed and duration (rapid and slow), coagulant dosage, pH, and settling time—were systematically optimized. Experiments were conducted in triplicate using ~200 mL of OMW per set. During optimization, one parameter was varied while others were held constant. Lime doses of 1–6% (v/v) were tested, and optimal pH levels were determined. Rapid mixing speeds of 80–250 rpm and durations of 1–6 min, as well as slow mixing speeds of 10–60 rpm and durations of 5–45 min, were investigated. Settling times ranged from 10–60 min to determine the optimal conditions.
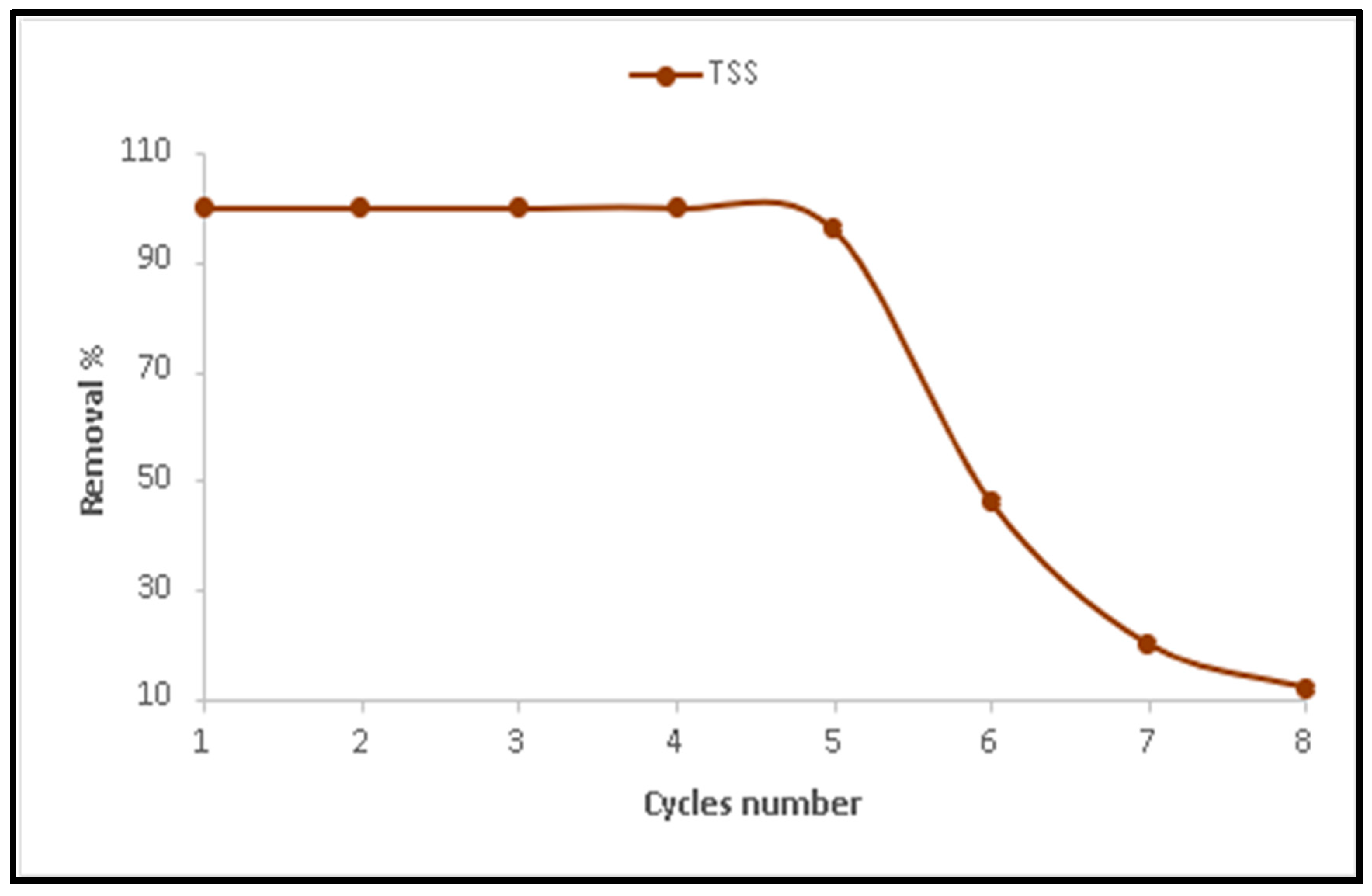
2.8. Reusability of Lime
Lime reusability was evaluated over eight consecutive cycles. In each cycle, 200 mL of OMW was treated with the solid lime residue recovered from the previous cycle. The previously optimized conditions for rapid mixing, slow mixing, and settling time were applied in all cycles to assess consistency and efficiency. The ability to reuse lime for several treatment cycles with only physical washing and drying demonstrates the robustness of CaO as a coagulant. This feature provides a significant economic advantage over commercial reagents such as alum or ferric salts, which require replenishment after each use and generate secondary sludge requiring neutralization.
2.9. Batch Adsorption Experiments for TPC Removal from Pretreated OMW
Pretreated OMW obtained after lime coagulation–flocculation was subjected to batch adsorption experiments using both micro- and nano-sized limestone. Maximum adsorption capacity was assessed by varying equilibrium time (2–48 h), adsorbent dose (0.5–32 g/L), and pH (2–12 ± 0.1). The removal efficiency was calculated using Equation (2):
where C
i and C
f refer to the TPC concentration before and after treatment, respectively. After treatment, limestone was removed, and measurements were conducted. After treatment, the adsorbent was separated by filtration, and the supernatant was analyzed for residual TPC concentration using the Folin–Ciocalteu method. These batch experiments provided the basis for determining the optimal operating conditions for phenolic removal and for subsequent column-scale validation. In this study, the adsorption behavior of limestone was evaluated with respect to both total phenolic content (TPC) and chemical oxygen demand (COD), as both parameters reflect the organic pollution load of OMW. The adsorption process targets phenolic compounds as the major contributors to toxicity and color, while COD represents the overall oxidizable organic fraction.
2.10. Column Experiments
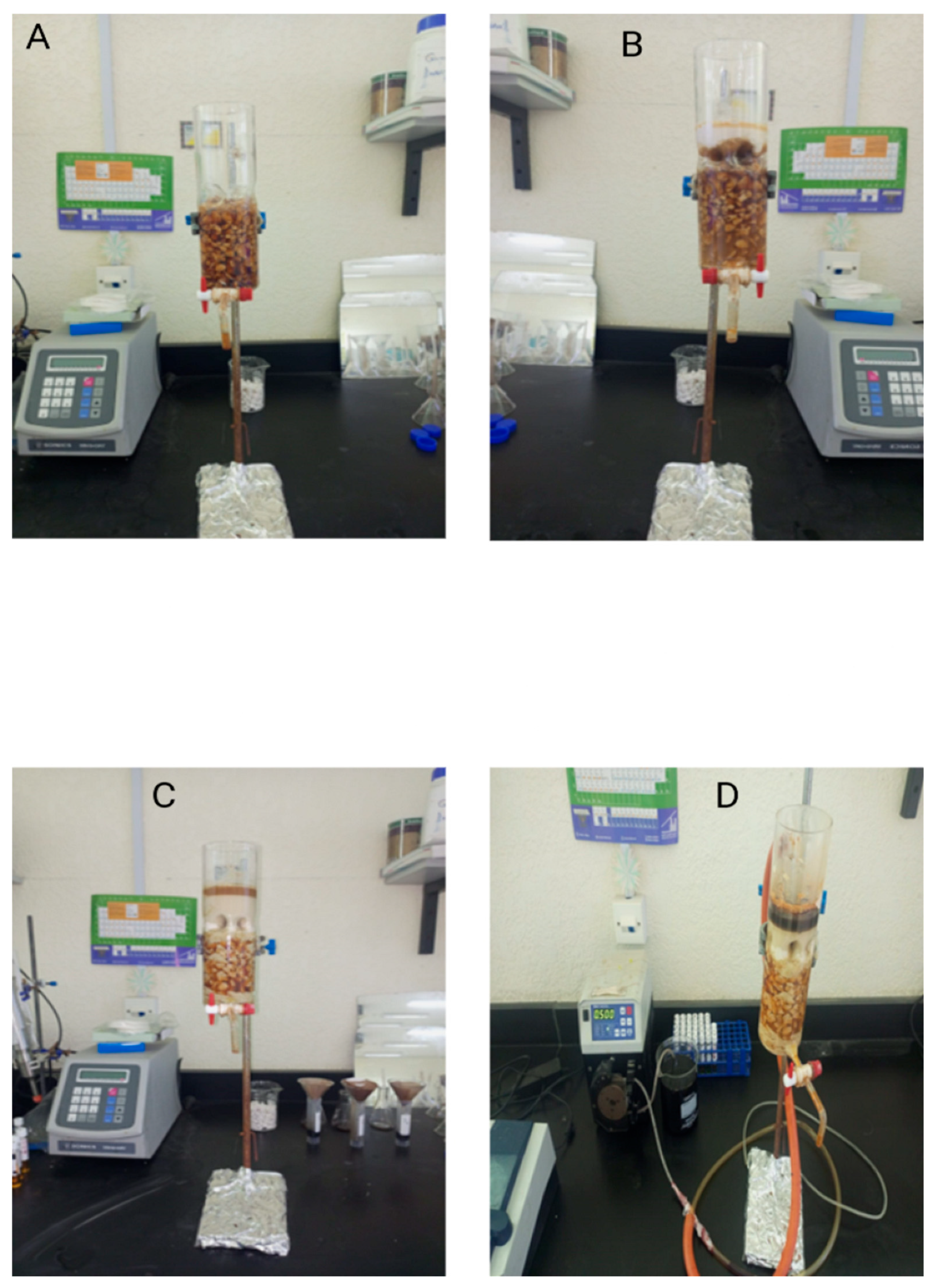
The column experiments assessed the efficiency of limestone and lime in removing phenol and COD from OMW. The experimental setup, illustrated in
Figure 2, utilized a column measuring 22 cm in length and 6.5 cm in diameter, operated under the following conditions:
C1: 350 g of coarse limestone (2–4 mm) with 2 L of pretreated OMW passed progressively; samples collected at variable contact times.
C2: 30 g of nanosized limestone (400–500 nm) particles with 2 L of pretreated OMW; samples collected at variable contact times.
C3: 380 g of limestone with mixed particle sizes and 4 L of pretreated OMW; sample collection varied.
C4: 380 g of mixed-size limestone combined with 12 g of lime; 2 L of untreated OMW was passed through, and flow rate (0.5 mL/min) was monitored via a pump. A breakthrough curve was constructed to determine the column’s adsorption kinetics, illustrating the rate at which substances pass through the column.
In these column configurations, lime and limestone acted jointly as a functional composite system for pollutant removal. Although no chemical bonding occurred between the two materials, their physical combination enhanced treatment performance by coupling lime’s strong coagulation and pH-adjusting ability with limestone’s surface adsorption capacity. This cooperative behavior justifies describing the setup as a “lime–limestone composite system” in a practical sense, reflecting its integrated action in reducing COD and phenolic compounds from OMW.
In the fourth column configuration (C4), a total of 380 g of mixed-size limestone was employed, consisting of approximately 70% coarse particles (2–4 mm) and 30% fine particles (400–500 nm) layered sequentially to improve permeability and surface contact. The limestone was combined with 12 g of lime, forming a composite filtration bed. For example, in a column filled to 22 cm height, the lower 10 cm consisted of coarse limestone to support flow and minimize clogging, while the upper 8 cm contained finer particles to maximize surface adsorption of dissolved organic compounds. The remaining upper layer (≈4 cm) comprised lime, enhancing coagulation and pH adjustment at the column inlet.
2.11. Analytical Methods
Chemical oxygen demand (COD) was determined using the dichromate method (HACH). Total phenolic content (TPC) was quantified via the Folin–Ciocalteu assay with gallic acid as the standard, while total suspended solids (TSS) were measured gravimetrically following APHA guidelines (2017). pH was monitored using a calibrated multiparameter probe. All experiments were conducted in triplicate, and results are presented as mean ± standard deviation. All experiments were conducted in triplicate, and results are presented as mean ± standard deviation.
3. Results and Discussion
3.1. Characterization of Limestone and Olive Mill Wastewater (OMW) Samples
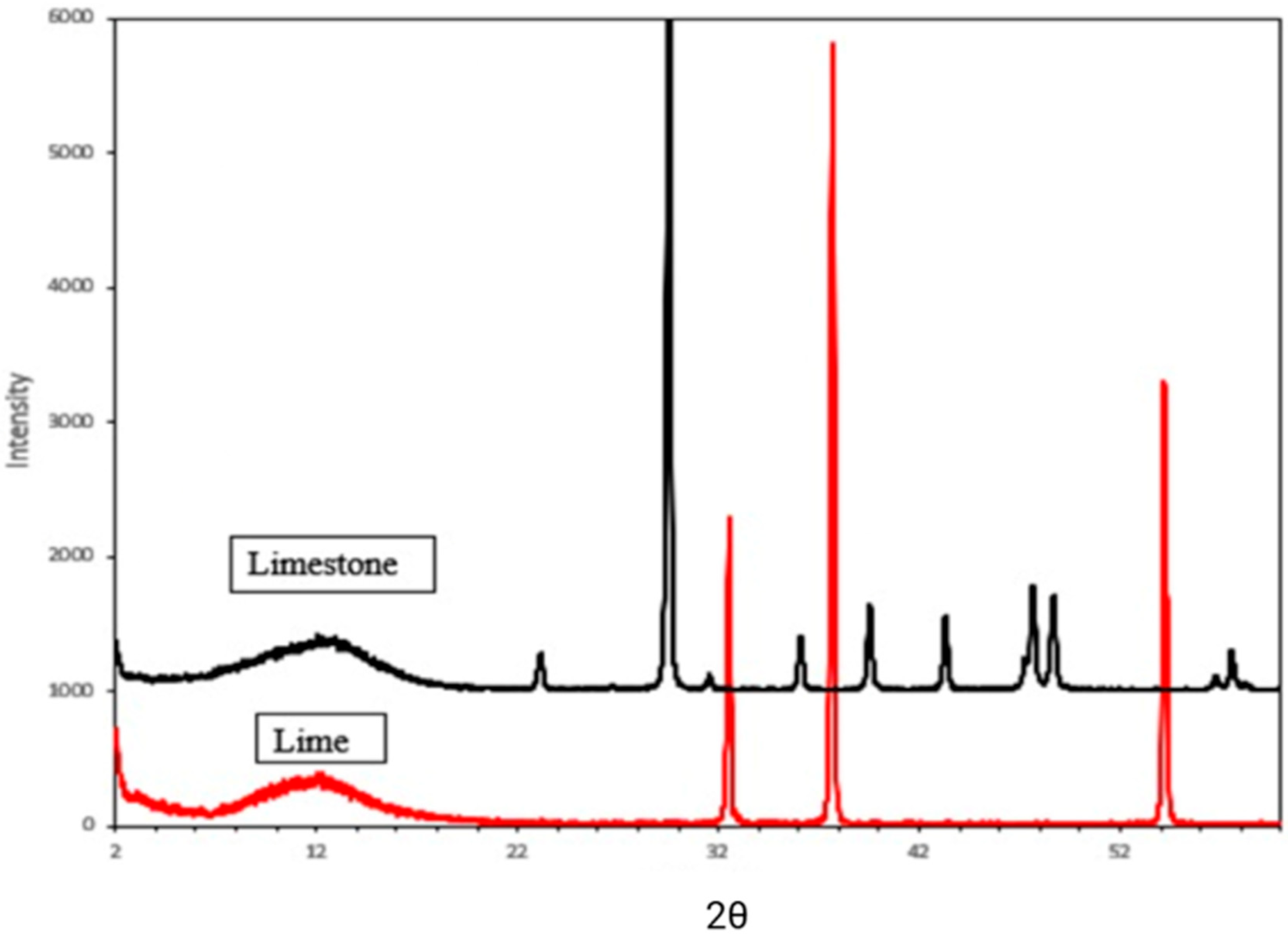
The X-ray diffraction (XRD) patterns of representative limestone samples display distinct peaks characteristic of calcite, shown in black in
Figure 3. These reflections at 3.85–3.86 Å (102), 3.03 Å (100), 2.84 Å (006), 2.49 Å (110), 2.28 Å (113), 2.09 Å (202), 1.97 Å (108), 1.87 Å (116), and 1.60 Å (212) confirm the dominance of calcite in the sample. Additionally, the presence of quartz is indicated by a peak at 3.33–3.34 Å (101) [
34]. The red XRD pattern in
Figure 3 demonstrates the conversion of approximately 85% of limestone to lime, with calcium oxide identified as the primary constituent. The XRD results (
Figure 3) confirm that the majority of CaCO
3 was decomposed into CaO during calcination. Semi-quantitative estimation based on the relative peak intensities suggests that approximately 85% of the limestone was converted to lime [
35].
The SEM images of the limestone samples (
Figure 4) show that the particles vary in both size and shape. At lower magnification, the surface appears to consist of irregular clusters of large and small particles, reflecting the heterogeneous nature of the sample. At higher magnification, the particle size is seen to range from the microscale (around 3.9 µm) down to the nanoscale (about 400–500 nm). Many of the smaller particles are attached to the larger ones, which increases the overall surface roughness and surface area of the sample. The irregular and angular shapes suggest that the particles were formed mainly through mechanical fracture rather than smooth natural weathering. This wide size distribution and rough morphology are important because they can affect the reactivity and adsorption properties of the limestone.
The surface area of the limestone samples, shifting from the micro- to nanoscale, was evaluated using the BET nitrogen adsorption method. The measured surface area ranged from 1.8 to 5.74 m
2/g, which is in good agreement with values reported in the literature [
36]. To further assess the adsorption characteristics of limestone as an adsorbent and its potential electrostatic interactions with dissolved TPC, the point of zero charge (pHpzc) was determined (
Figure 5). The pHpzc was found to be 8.3, consistent with previous studies [
37]. This indicates that the limestone surface is positively charged at pH values below 8.3 and negatively charged at pH values above this threshold.
The pHpzc of the raw limestone was determined to be 8.3, representing the surface behavior of the primary adsorbent. Lime (CaO) was not analyzed for pHpzc since it functioned as a coagulant, rapidly reacting with water to form Ca(OH)2 and altering surface chemistry dynamically. Moreover, the composite column bed (lime–limestone system) was a physical combination rather than a single chemically integrated phase, making direct pHpzc measurement unreliable. Future studies will include stabilized composite materials for accurate surface charge characterization.
3.2. Lime Coagulation Pretreatment: Evaluation and Optimization
The pretreatment process described in this section is fundamentally a coagulation–flocculation–sedimentation sequence using lime (CaO) as the primary coagulant. The term “pretreatment” in earlier drafts was intended to represent this integrated process. However, as coagulation is the main mechanism responsible for destabilizing suspended particles and initiating pollutant removal, the section has been revised to explicitly emphasize lime’s role as a coagulant. This adjustment ensures alignment between the terminology in the methodology and results sections. The parameters tested for the lime pretreatment process and their results are shown in
Figure 6, while
Table 2 summarizes the optimized conditions that achieved the best performance with acceptable efficiency. The results demonstrate that lime is an effective coagulant for removing TPC and COD from OMW, owing to its versatile chemical properties. Lime adjusts the wastewater pH, creating favorable conditions for coagulation and flocculation, and its highly reactive, soluble nature enhances its potential as a coagulant. The versatility of lime’s chemical behavior arises mainly from its strong alkalinity and high reactivity in aqueous media. Upon hydration, CaO rapidly converts to Ca(OH)
2, producing hydroxide ions (OH
−) that increase the pH and neutralize acidic compounds. This reaction promotes the precipitation of organic acids, phenolic compounds, and metal ions, while the released Ca
2+ ions enhance coagulation by neutralizing negatively charged colloids. For example, phenolic compounds in OMW can react with Ca
2+ to form insoluble calcium–phenolate complexes, facilitating their removal through sedimentation. These combined chemical effects explain lime’s multifunctional role as both a coagulant and a partial adsorbent in wastewater pretreatment.
pH adjustment not only promotes the coagulation of suspended particles but also converts phenolic compounds into forms that are more adsorbable and less soluble [
28]. Moreover, lime can adsorb phenolic compounds and organic matter through electrostatic interactions, forming complexes with calcium ions on its surface. Consequently, lime emerges as a highly effective, cost-efficient, and high-performance material for OMW pretreatment, particularly given its role in odor reduction and low cost [
38]. Under optimized conditions, the removal efficiencies for TSS, COD, and TPC were 99%, 43%, and 48%, respectively.
The raw olive mill wastewater (OMW) initially exhibited a pH of ≈5.0, confirming its acidic character. In contrast, a lime–water suspension (prepared by dispersing CaO in deionized water) displayed a highly alkaline pH of ≈12.4, resulting from CaO hydration to Ca(OH)2. When lime was added to OMW, the reaction between hydroxide ions (OH−) and acidic components raised the mixture’s pH from ≈5.0 to 12.0, establishing conditions favorable for coagulation and precipitation. If the lime mass were fixed but the pH of the OMW suspension was externally altered, the removal efficiency would likely decrease because effective coagulation depends not only on pH but also on the stoichiometric release of Ca2+ and OH− ions from lime dissolution. These ions are essential for charge neutralization and the formation of insoluble calcium–organic complexes responsible for pollutant removal.
Figure 7 shows the color changes in OMW after treatment with lime and a chemical coagulant compared to raw OMW. The raw OMW exhibits a dark brown to black color, reflecting its high content of phenolic compounds and organic matter. After lime pretreatment, the color noticeably lightens to a yellowish hue, indicating effective coagulation and partial removal of colored organic compounds. Treatment with the chemical coagulant also reduces the color, producing a reddish-brown solution, which suggests that it is somewhat effective but less efficient than lime in decolorization. These observations support the conclusion that lime is highly effective in removing chromophoric substances from OMW, likely due to pH adjustment, coagulation, and adsorption mechanisms.
Although UV–Vis spectroscopy was not included among the main analyses in this study, preliminary measurements were carried out to qualitatively examine the color change in OMW following treatment. The raw OMW exhibited strong absorbance peaks at ≈280 nm and weak band at ≈320–400 nm, corresponding to aromatic and polyphenolic compounds, respectively. After lime coagulation, a significant decline in absorbance intensity was observed across these regions, confirming the partial removal of colored organic constituents responsible for the characteristic dark brown coloration. These observations complement the COD and TPC reductions reported and visually demonstrated in
Figure 7.
3.3. The Efficiency of Lime Reusability
This part of the study aimed to evaluate the reusability of lime (CaO) in treating olive mill wastewater (OMW) and to monitor any changes in its effectiveness over multiple cycles. The results showed that lime performance remained relatively stable through the first five cycles, as illustrated in
Figure 8. During these cycles, lime reacted with water in the wastewater, converting into calcium hydroxide (Ca (OH)
2), also known as slaked lime:
The slaked lime continued to effectively treat OMW, producing similar results to the initial treatment. However, after the fifth cycle, a noticeable decline in treatment efficiency was observed, likely due to saturation of the active sites or accumulation of residual contaminants on the lime surface. An important point in adsorption treatment is how to handle the used adsorbent. It should be noted that adsorption does not break down pollutants but generally immobilizes pollutants rather than decomposing them. In this study, the dominant mechanism is physisorption, with possible minor contributions from surface complexation or chemisorption involving calcium ions. These interactions do not lead to chemical breakdown of the contaminants but instead trap them on the adsorbent surface, necessitating further handling or regeneration of the spent material. If not managed properly, the polluted limestone or lime could create new environmental problems. Techniques such as heating, chemical washing, or solvent treatment have been applied in other systems to clean adsorbents, but these methods usually involve extra costs, chemical inputs, and a gradual decrease in performance after several cycles. This drawback should be acknowledged, and future research should focus on regeneration options or alternative uses of the spent limestone, such as in construction products or soil improvement, to make the process more sustainable.
It is noteworthy that under certain conditions, it is possible that CaO (lime) is not fully transformed to Ca(OH)2 (slaked lime). Limited water contact, surface passivation by an early-formed hydroxide layer, short reaction time, or reduced reactivity of over-calcined particles can prevent full hydration of lime. Consequently, a fraction of anhydrate CaO may remain, potentially influencing the coagulation efficiency and pH stabilization during wastewater treatment.
Subsequent XRD analysis of the recovered solid showed distinct reflections at 2θ = 34.1°, 37.4°, and 53.9°, which correspond to crystalline Ca(OH)
2, verifying that hydration occurred during the coagulation stage. This transformation is consistent with earlier findings by [
27,
35] confirming that lime acted as slaked lime during treatment.
3.4. Adsorption Evolutions
3.4.1. Assessment of Adsorption Through Batch Experiments
The adsorption potential of limestone as an adsorbent was evaluated through batch experiments to determine its effectiveness in reducing chemical oxygen demand (COD) and total phenolic compounds (TPC) in olive mill wastewater (OMW). Initially, a chemical coagulant was applied to pre-treat the OMW, aiming to destabilize suspended particles and reduce the bulk load of contaminants. This pretreatment step was critical to enhance subsequent adsorption efficiency by facilitating better interaction between the adsorbent surface and dissolved pollutants.
Following pretreatment, both micro-sized and nano-sized limestone were employed as adsorbents in batch adsorption experiments. The use of different particle sizes allowed the assessment of surface area effects, as smaller particles generally offer a higher surface area-to-volume ratio, potentially increasing adsorption capacity. The concentration of TPCs in the treated wastewater was measured in parts per million (ppm) to quantify the removal efficiency, alongside monitoring COD levels to assess overall organic load reduction.
To further investigate the influence of pretreatment on adsorption, the experiments were repeated using lime (calcium oxide, CaO) as the chemical pretreatment agent. The subsequent adsorption step, again using micro- and nano-sized limestone, allowed the evaluation of how lime pretreatment affected the efficiency of TPC and COD removal. This approach provided insight into the synergistic effects of chemical coagulation and adsorption in reducing pollutant levels and highlighted the potential benefits of integrating pretreatment with adsorption for OMW remediation.
3.4.2. Influence of Limestone Particle Size and Operating Parameters on COD and TPC Removal
To assess the effect of limestone particle size and operating parameters on pollutant removal, adsorption studies were conducted using both chemically pretreated and lime-pretreated olive mill wastewater (OMW). The results, summarized in
Table 3, indicate that pollutant removal improved consistently when nano-sized limestone (Nano-LS) was used instead of micro-sized limestone (Micro-LS), due to its larger surface area and greater density of active sites.
Following chemical pretreatment, COD and TPC removal efficiencies were 54% and 67% for Micro-LS, and 58% and 69% for Nano-LS, respectively. When lime pretreatment was applied prior to adsorption, these efficiencies increased markedly reaching 68% and 87% for Micro-LS and 72% and 89% for Nano-LS. The improvement after lime pretreatment reflects the combined benefits of coagulation and adsorption: lime effectively removes suspended solids and partially oxidized organics, reducing turbidity and enabling better contact between dissolved pollutants and limestone surfaces.
Effect of Adsorbent Dose
Limestone dosages between 0.5 and 32 g/L were evaluated to determine the optimal amount for pollutant removal (
Figure 11 and
Figure 12). The highest removal was achieved at 2 g/L; beyond this level, efficiency declined due to particle aggregation [
36], which reduces the effective surface area available for adsorption. Aggregation reduces the number of accessible adsorption sites by increasing adsorption density, thereby limiting the effectiveness of the adsorbent. This trend was more pronounced for Nano-LS, where finer particles tend to agglomerate more readily at high dosages.
The highest removal was achieved at 2 g/L; beyond this level, efficiency declined due to particle aggregation, which reduces the effective surface area available for adsorption.
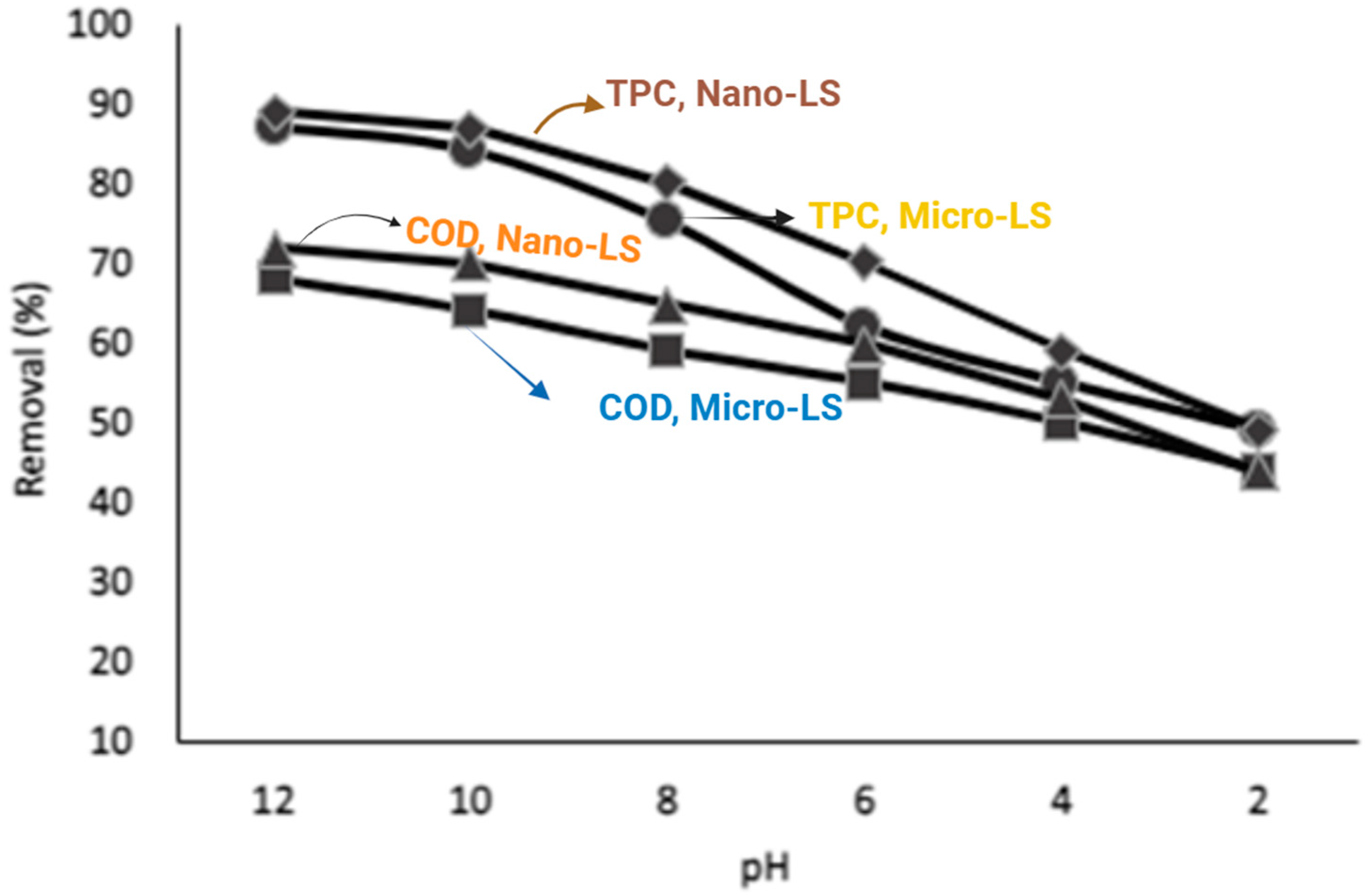
Effect of pH
The solution pH strongly affected adsorption efficiency (
Figure 13 and
Figure 14). Maximum COD and TPC removal occurred near pH 8, where partial deprotonation of phenolic compounds enhances electrostatic attraction with the positively charged limestone surface (pHpzc ≈ 8.3). Under lime pretreatment, OMW initially exhibited higher pH values (~12), which were gradually neutralized during treatment using HCL.
As shown in
Figure 14, adsorption efficiency was highest at elevated pH and gradually decreased at lower pH values. Higher acidity enhances limestone reactivity through the reaction (Equation (4)):
This reaction increases limestone’s ability to bind phenolic compounds. In acidic conditions, phenols form calcium phenolates on the limestone surface. The positively charged limestone surface further promotes electrostatic attraction, enhancing phenol removal [
12].
The initial pH of the raw olive mill wastewater (OMW) was approximately 5.0, reflecting its acidic composition dominated by organic acids and polyphenols. When lime (CaO) was introduced, it reacted with water to form calcium hydroxide (Ca(OH)2) according to the reaction (Equation (3)). The resulting hydroxide ions (OH−) neutralized the acidic species in the wastewater, leading to a marked pH increase. For example, when 2 g/L lime was added, the pH of the OMW rose from ≈5.0 to 12.0, which represents the optimal alkaline condition for efficient coagulation and removal of suspended solids and phenolic compounds.
The observed optimal adsorption performance at pH ≈ 8 aligns closely with the limestone’s point of zero charge (pHpzc = 8.3) (
Figure 5). At this pH, the surface charge of limestone approaches neutrality, facilitating both electrostatic attraction and complexation interactions with ionized phenolic species in OMW. Below the pHpzc, the positively charged surface enhances adsorption of negatively charged phenolic anions, whereas above this point, repulsion effects may occur. This correlation confirms that surface charge characteristics strongly influence adsorption efficiency.
Visual Confirmation
Color changes observed after adsorption (
Figure 15 and
Figure 16) corroborate the analytical results. The dark brown color of untreated OMW progressively faded after both chemical and lime pretreatments, with the most pronounced decolorization observed in samples treated with Nano-LS. The color shift from dark to light yellow indicates the successful removal of chromophores phenolics and organic matter, consistent with measured decreases in TPC and COD.
Figure 15 illustrates the visible color changes in olive mill wastewater (OMW) after treatment following chemical pretreatment. The untreated OMW (tube A) displays a dark reddish-brown color, characteristic of high concentrations of phenolic compounds, tannins, and other organic pollutants. After treatment, a noticeable reduction in color intensity is observed, particularly in the sample treated with adsorption agents (tubes B and C), indicating the removal of a significant portion of colored organic matter. The gradual lightening of color corresponds with the reduction in total phenolic content (TPC) and COD levels, reflecting the effectiveness of the combined pretreatment and adsorption processes. The slightly different shades among treated samples may suggest variations in the extent of adsorption, potentially influenced by factors such as particle size of the adsorbent, contact time, and pH. Specifically, samples treated with nano-sized adsorbents (tube C) tend to show more pronounced decolorization due to their higher surface area and enhanced interaction with dissolved organic compounds. Overall, the visual color change provides qualitative confirmation of the chemical analyses, supporting the conclusion that chemical pretreatment followed by adsorption using limestone is effective in reducing both the organic load and coloration of OMW.
Figure 16 illustrates the color changes observed in olive mill wastewater (OMW) after lime pretreatment. The figure clearly shows a noticeable shift in coloration (from dark/black color to colorless solution), indicating a reduction in the intensity of the dark brown/black color typically associated with untreated OMW. This color change suggests effective removal or destabilization of suspended solids, polyphenols, and other chromophores during the lime treatment. The lighter color reflects the coagulant and adsorptive action of lime, which promotes precipitation of organic matter and enhances the clarity of wastewater. These visual changes corroborate the quantitative improvements observed in parameters such as total phenolic content (TPC) and chemical oxygen demand (COD), supporting the efficacy of lime as a pretreatment method.
Overall, these findings confirm that lime pretreatment enhances subsequent limestone adsorption, while nano-sized limestone provides a measurable improvement in pollutant removal. Consolidating the adsorption parameters under both pretreatment conditions offers a clearer understanding of the coupled mechanisms—coagulation, ion exchange, and surface complexation—that govern OMW purification.
The adsorption mechanism underlying the removal of phenolic compounds and organic matter by lime- and limestone-based systems can be explained by a combination of electrostatic attraction, surface complexation, and ion exchange phenomena. The measured pHPZC of the limestone (8.3) indicates that under the slightly to strongly alkaline conditions applied during treatment (pH 8–12), the adsorbent surface acquires a positive charge, enhancing the electrostatic attraction between the surface Ca2+/Ca–OH sites and the negatively charged phenolate ions present in the olive mill wastewater (OMW). Following lime pretreatment, surface hydroxylation and partial hydration of CaO to Ca(OH)2 introduce additional reactive –OH groups that promote surface complex formation with phenolic oxygen atoms, leading to the generation of calcium–phenolate complexes. Simultaneously, carbonation reactions may occur at the surface, forming CaCO3–Ca(OH)2 heterostructures that contribute to the immobilization of organics through surface precipitation and ion bridging. These synergistic interactions explain the enhanced adsorption capacity observed for nano-limestone due to its higher surface area and abundance of active binding sites.
Although this study did not include FTIR or XPS analysis of the spent adsorbents, future work will incorporate these spectroscopic techniques to provide molecular-level confirmation of the proposed mechanisms. Specifically, FTIR spectra are expected to show characteristic shifts in the –OH and aromatic C–O stretching bands, while XPS spectra should reveal variations in the O1s and Ca2p binding energies, confirming electrostatic and coordination interactions between the adsorbent surface and phenolic functional groups. This mechanistic framework, supported by pHPZC and morphological observations (SEM, XRD, and BET results), substantiates the strong affinity of lime-activated limestone surfaces for phenolic and organic contaminants in OMW.
Lime pretreatment enhanced pollutant removal by promoting coagulation and partial oxidation, improving subsequent adsorption performance. Nano-limestone consistently achieved higher efficiencies due to increased surface area and active site density.
3.4.3. Assessment of Adsorption Through Column Experiment
First Column Scenario (C1)
A column experiment was conducted to evaluate the optimal removal of TPC and COD from OMW. Four experimental scenarios were tested, with 2 L of lime pretreated OMW passed through each column, and samples collected at intervals ranging from 0.5 to 96 h. In the first column scenario (C1), 350 g of larger limestone particles were used. Sampling began at 0.5 h, with the final sample collected at 96 h (
Figure 17). The removal efficiency of both TPC and COD increased steadily over time, reaching a maximum at 48 h. After this point, efficiency gradually declined. Concurrently, the pH decreased, suggesting a possible correlation between pH reduction and decreased removal efficiency. This trend is consistent with [
39], who reported that higher pH values enhance removal efficiency. The post 48 h decline may be due to factors such as particle settling or saturation of adsorption sites. Under these conditions, the maximum TPC and COD removal efficiencies were 59% and 52%, respectively (
Figure 17).
Second Column Scenario (C2)
In the second column scenario (C2), 30 g of nano-sized limestone particles were used, and 2 L of lime-pretreated OMW was passed through the column. Samples were collected at intervals from 0.5 to 96 h (
Figure 17). The removal efficiency of TPC and COD increased steadily over time, reaching a maximum at 48 h. After this point, efficiency gradually declined, following a trend like that observed in C1, but with a higher overall adsorption capacity. This improvement is attributed to the smaller particle size of the nanoparticles, which provides a larger surface area and enhanced adsorption potential [
40]. Under these conditions, the total TPC and COD removal efficiencies reached 63% and 59%, respectively (
Figure 17).
Third Column Scenario (C3)
In the third column scenario (C3), a combination of large and nano-sized limestone particles (from C1 and C2) was used, with a total mass of 380 g. The larger particles were positioned at the bottom of the column, and the nanoparticles layered above them. Two liters of lime pretreated OMW were passed through the column, with samples collected at intervals from 0.5 to 96 h (
Figure 17). The removal efficiency of TPC and COD increased steadily over time, reaching a maximum at 48 h. Beyond this point, efficiency gradually declined, following the trend observed in C1 and C2, but with a higher overall adsorption capacity. This enhancement is attributed to the synergistic effect of combining large and nano-sized particles, optimizing both flow dynamics and available surface area for adsorption. The total TPC and COD removal efficiencies were 76% and 65%, respectively (
Figure 17).
Fourth Column Scenario (C4)
In the fourth column scenario (C4), a combination of lime and limestone particles (large and nano-sized) was used, consisting of 12 g of lime and 380 g of limestone. The larger limestone particles were positioned at the bottom, followed by a layer of nanoparticles, with an additional lime layer on top. Two liters of OMW with a pH of 2, without prior pretreatment, were passed through the column, and samples were collected at intervals from 0.5 to 96 h (
Figure 17). The removal efficiency followed a trend like that observed in the previous columns. This setup aimed to evaluate the effectiveness of adding lime directly in the column as an alternative to pretreatment. The results indicated slightly lower removal efficiencies than C3, with total TPC and COD removal of 72% and 62%, respectively (
Figure 17). Nevertheless, the close performance demonstrates that in-column lime addition can serve as a practical alternative to pre-treating OMW.
Figure 18 illustrates the color changes in OMW following treatment in the column experiments. A clear reduction in the dark brown coloration of the wastewater is observed across all scenarios, indicating effective removal of suspended solids, polyphenols, and other chromophoric compounds. The most pronounced lightning occurs in columns using nano-sized limestone or combined particle layers (C3 and C4), reflecting their higher adsorption capacity. In C4, where lime was added directly to the column without prior pretreatment, the color change is slightly less intense but still significant, demonstrating that in-column lime addition effectively reduces the visual intensity of OMW contaminants. Overall, the observed color changes corroborate the quantitative removal efficiencies of TPC, and COD reported for each column scenario, supporting the effectiveness of both particle size optimization and lime addition strategies.
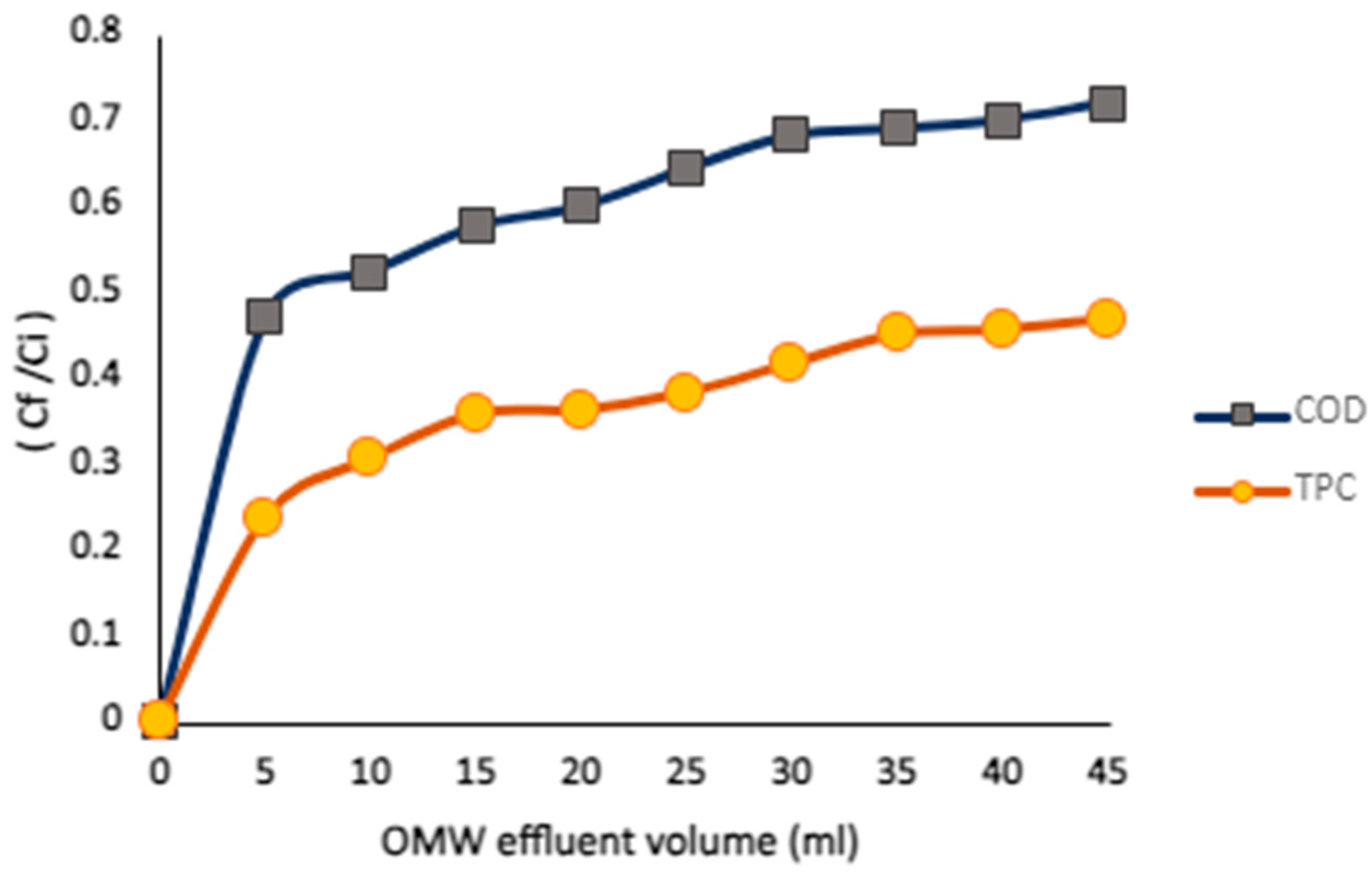
In the fourth column experiment (C4), a pump was used to circulate OMW through the column at a flow rate of 0.5 mL/min, without any pH adjustment.
Figure 19 presents the breakthrough curve obtained from this setup. The breakthrough ratio (C
F/C
I) was used to evaluate the effectiveness of the adsorption process at different OMW volumes. Starting with an OMW volume of 5 L, the ratios gradually increased, reaching a volume of 45 L. Higher breakthrough ratios indicate a greater rise in effluent concentration relative to the influent, reflecting the extent of adsorbent saturation or “leakage” in the system. This trend is consistent with some reported observations [
41]. The gradual increase highlights the progressive loading of the adsorbent and emphasizes the need for further optimization to maintain adsorption efficiency as the OMW volume increases.
Table 4 presents all column scenarios, detailing the types and sizes of limestone particles used, along with the corresponding maximum total removal of TPC and COD. It is noteworthy that all scenarios reached their peak removal efficiency at 48 h.
The results presented in this work demonstrate that combining lime pretreatment with nano-limestone adsorption provides synergistic removal effects not reported in earlier studies using conventional natural adsorbents. Lime pretreatment not only reduces suspended solids and partially neutralizes acidity but also modifies the surface chemistry of the wastewater, creating favorable conditions for subsequent adsorption. The enhanced performance observed with nano-limestone, particularly in dual-layer column configurations, reflects both its increased surface area and the improved accessibility of phenolic compounds following lime coagulation. Compared to previously reported systems using activated carbon, clay, or biochar, the lime–nano-limestone composite achieves comparable phenolic and COD removal efficiencies (up to 89% and 72%, respectively) under milder operational conditions and at a significantly lower cost. In addition, the demonstrated reusability of lime for five consecutive treatment cycles highlights its potential for long-term application with minimal secondary waste generation. This integrated and locally adaptable approach thus represents a significant advancement beyond earlier adsorption–coagulation combinations, aligning technical performance with sustainability and economic feasibility.
3.5. Comparative Insights from Other Regions
The Jordanian results show that lime pretreatment and local adsorbents can reduce OMW pollutants effectively. However, international experience suggests that combining pretreatment with a polishing step such as adsorption, and in some cases membranes or electrochemical methods, achieves more reliable outcomes. To place the Jordan findings in context, the results were compared using common indicators like COD and TPC removal, adsorbent capacity, regeneration performance, and sludge generation.
The use of Jordanian limestone offers several distinct advantages over similar materials from other regions. Geologically, Jordanian limestones are abundant and widely distributed across central and northern parts of the country, ensuring low extraction and transportation costs. Their high calcite purity and low levels of heavy metal impurities make them particularly suitable for wastewater treatment compared with limestones from industrialized or dolomitic formations elsewhere [
23,
27]. In addition, the heterogeneous particle morphology—comprising both micro- and nanoscale fragments formed by mechanical processes—enhances the material’s surface reactivity and adsorption capacity without requiring chemical activation or modification.
From an environmental and economic standpoint, utilizing locally sourced Jordanian limestone supports circular economy principles by valorizing an industrial byproduct that is often discarded for not meeting commercial whiteness standards. This reduces waste generation and promotes sustainable resource use while offering a cost-effective alternative to imported adsorbents such as activated carbon or synthetic nanomaterials. Consequently, the adoption of Jordanian limestone for olive mill wastewater treatment represents both a scientifically viable and regionally sustainable solution. Although calcination is an energy-consuming step, it was conducted only once to produce a reusable lime fraction, which maintained over 99% TSS removal efficiency for five consecutive treatment cycles (
Figure 8). When normalized per treatment cycle, the energy cost remains significantly lower than those associated with commercial coagulants (e.g., alum or ferric chloride) that require continuous chemical replenishment.
In Mediterranean and North African studies, lime coagulation is commonly used as a low-cost pre-treatment to reduce COD and phenolics, followed by adsorption (activated carbon, biochar, clays, or olive-stone chars) for phenolic removal, while membranes or electrochemical methods are applied when higher-quality effluent or water reuse is required [
28].
OMW treatment can be improved through a stepwise approach: initial lime coagulation to remove oils, solids, and part of the COD, followed by filtration and a polishing step such as activated carbon or membrane treatment [
42]. Activated carbon is particularly effective in reducing residual phenols and organics, with combined processes achieving up to 99.7% phenol removal and about 80% COD reduction. In addition, the dewatered cakes generated during treatment can be burned to provide thermal energy, potentially covering the heating needs of both the mill and the treatment system.
Table 5 presents a comparison of treatment performance across representative studies. The results of this study show that in scenario C3, the combination of large and nano-sized limestone particles enhanced TPC and COD removal to 76% and 65%, respectively. Although slightly lower than phenolic removal reported for commercial activated carbon or biochar in Mediterranean and North African studies (85–97%) (
Table 5), this approach offers advantages in cost, availability, and operational simplicity. The improvement over C1 and C2 suggests that layering different particle sizes optimizes flow and surface area, making locally sourced limestone a practical, low-cost option for OMW treatment where expensive adsorbents are not feasible.
4. Conclusions
This study demonstrates that lime pretreatment followed by limestone adsorption provides an efficient and sustainable strategy for olive mill wastewater (OMW) treatment. All pretreatment and adsorption experiments were performed experimentally under laboratory conditions, confirming the practical applicability of the proposed lime and nano-limestone treatment system. Lime coagulation effectively reduced total suspended solids (TSS) by 99% and significantly lowered chemical oxygen demand (COD) and total phenolic content (TPC), while its reusability over multiple cycles confirmed operational stability. Subsequent adsorption using limestone—particularly in its nanoscale form—further enhanced pollutant removal, achieving up to 72% COD and 89% TPC reduction.
The improved performance of nano-limestone is attributed to its higher surface area and greater density of active sites, which facilitate enhanced adsorption interactions. Although the BET surface area of nano-limestone (≈5 m2/g) is relatively modest compared with synthetic adsorbents such as activated carbon, it represents a significant increase over bulk limestone (≈1–2 m2/g). This enhancement confirms that mechanical size reduction to the nanoscale effectively increases the reactive surface area and improves adsorption potential for OMW contaminants. Integrating lime pretreatment with limestone adsorption therefore represents a co-friendly, locally applicable, and environmentally friendly approach suitable for regions with limited access to advanced treatment technologies.
While these findings highlight the promise of lime–limestone composites, future studies should investigate regeneration methods, long-term performance under continuous flow, and economic feasibility at pilot scale to validate the process for real-world application.



 (Yellow triangular) Micro-LS for % removal of TPC.
(Yellow triangular) Micro-LS for % removal of TPC.  (Brown circle) Nano-LS for % removal of TPC.
(Brown circle) Nano-LS for % removal of TPC.  (Blue diamond) Nano-LS for % removal of COD
(Blue diamond) Nano-LS for % removal of COD  (Gold square). Micro -LS for % removal of COD.
(Gold square). Micro -LS for % removal of COD.
 (Yellow triangular) Micro-LS for % removal of TPC.
(Yellow triangular) Micro-LS for % removal of TPC.  (Brown circle) Nano-LS for % removal of TPC.
(Brown circle) Nano-LS for % removal of TPC.  (Blue diamond) Nano-LS for % removal of COD
(Blue diamond) Nano-LS for % removal of COD  (Gold square). Micro -LS for % removal of COD.
(Gold square). Micro -LS for % removal of COD.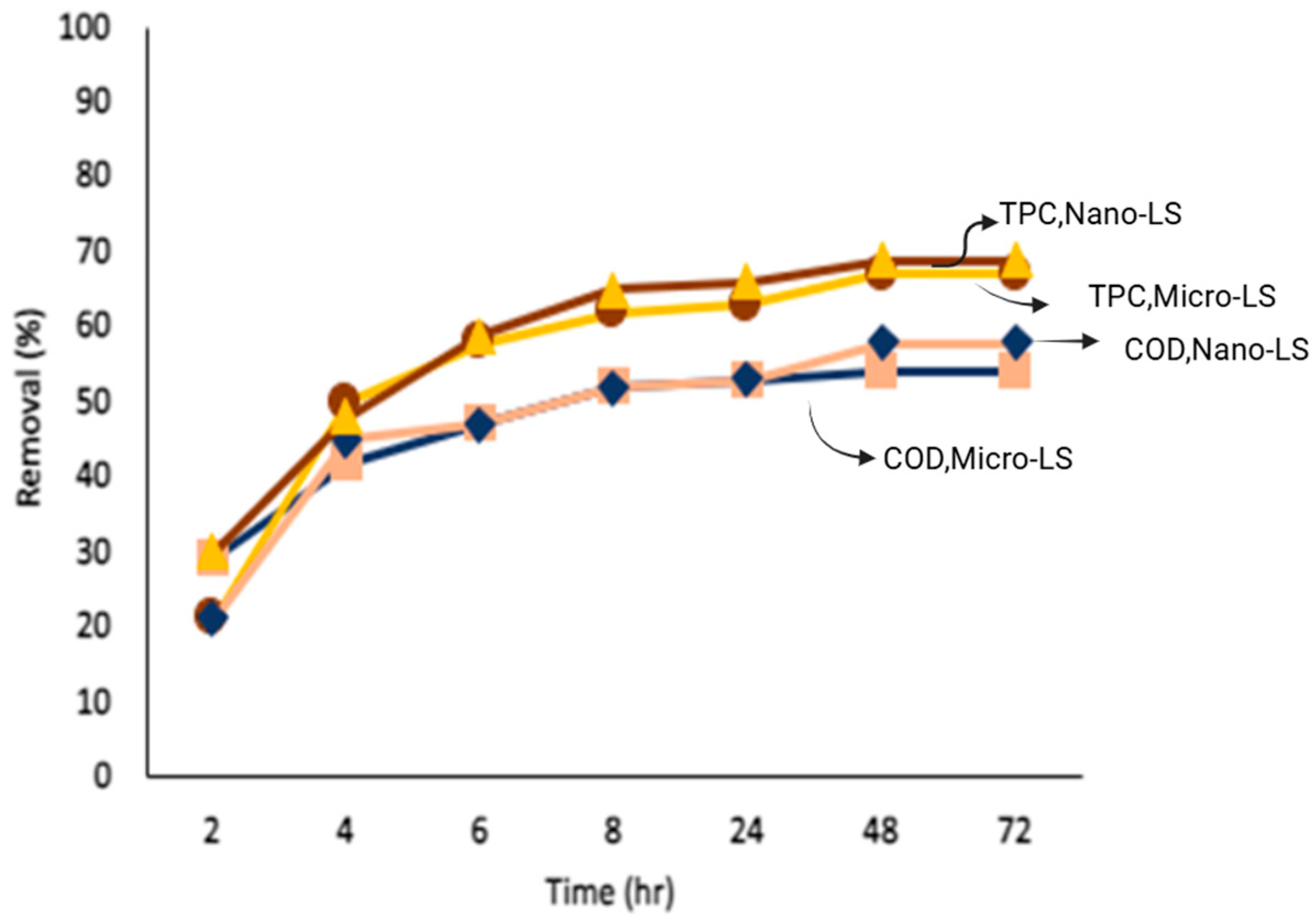
 (diamond) Nano-LS for % removal of TPC.
(diamond) Nano-LS for % removal of TPC.  (circle) Micro-LS for % removal of TPC.
(circle) Micro-LS for % removal of TPC.  (triangular) Nano-LS for % removal of COD.
(triangular) Nano-LS for % removal of COD.  (square) Micro -LS for % removal of COD.
(square) Micro -LS for % removal of COD.
 (diamond) Nano-LS for % removal of TPC.
(diamond) Nano-LS for % removal of TPC.  (circle) Micro-LS for % removal of TPC.
(circle) Micro-LS for % removal of TPC.  (triangular) Nano-LS for % removal of COD.
(triangular) Nano-LS for % removal of COD.  (square) Micro -LS for % removal of COD.
(square) Micro -LS for % removal of COD.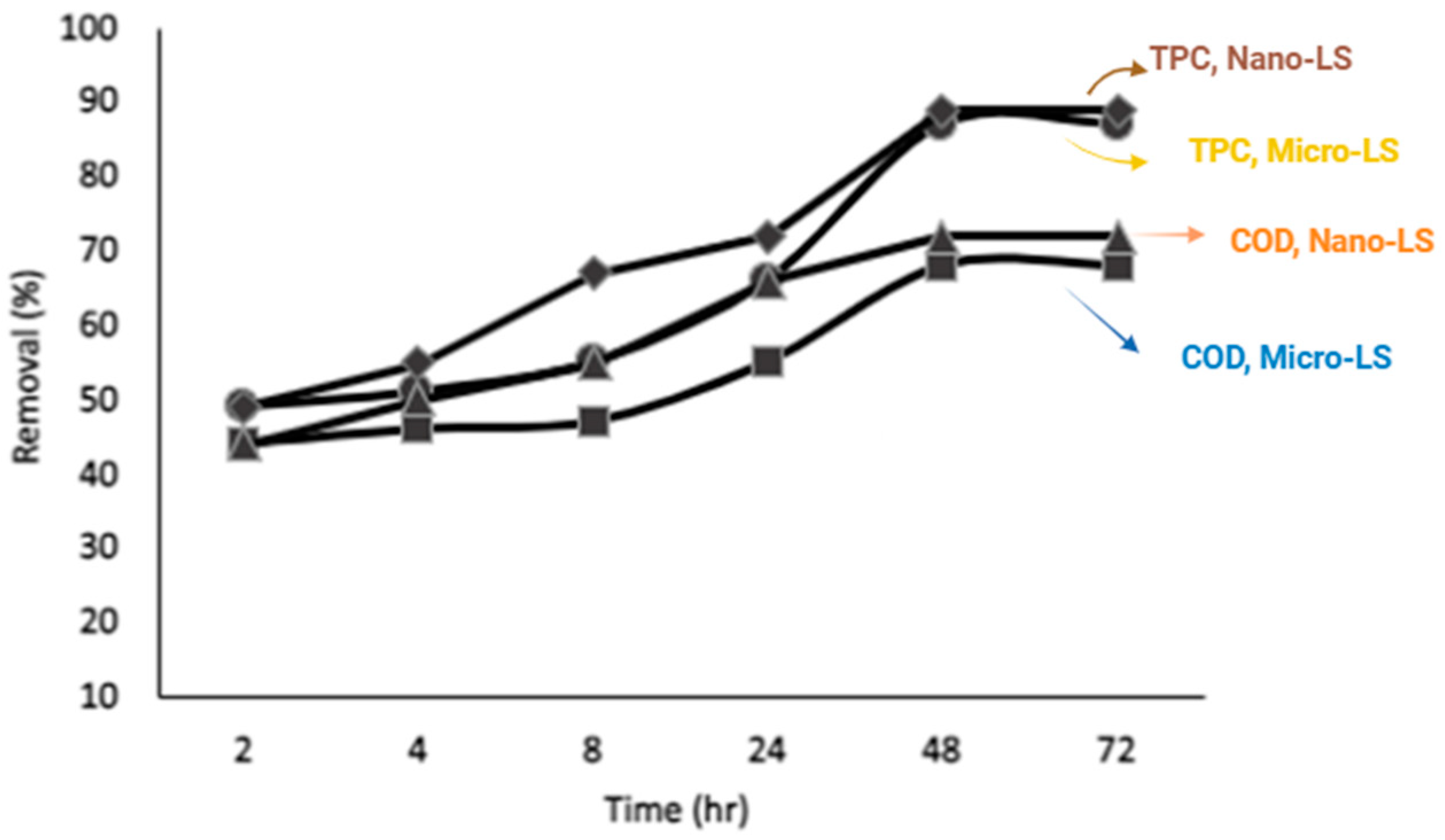
 (Blue triangular) Micro -LS for % removal of COD.
(Blue triangular) Micro -LS for % removal of COD.  (Yellow Gold square) Nano-LS for % removal of COD.
(Yellow Gold square) Nano-LS for % removal of COD.  (Red diamond) Micro-LS for % removal of TPC.
(Red diamond) Micro-LS for % removal of TPC.  (Green circle) Nano-LS for % removal of TPC.
(Green circle) Nano-LS for % removal of TPC.
 (Blue triangular) Micro -LS for % removal of COD.
(Blue triangular) Micro -LS for % removal of COD.  (Yellow Gold square) Nano-LS for % removal of COD.
(Yellow Gold square) Nano-LS for % removal of COD.  (Red diamond) Micro-LS for % removal of TPC.
(Red diamond) Micro-LS for % removal of TPC.  (Green circle) Nano-LS for % removal of TPC.
(Green circle) Nano-LS for % removal of TPC.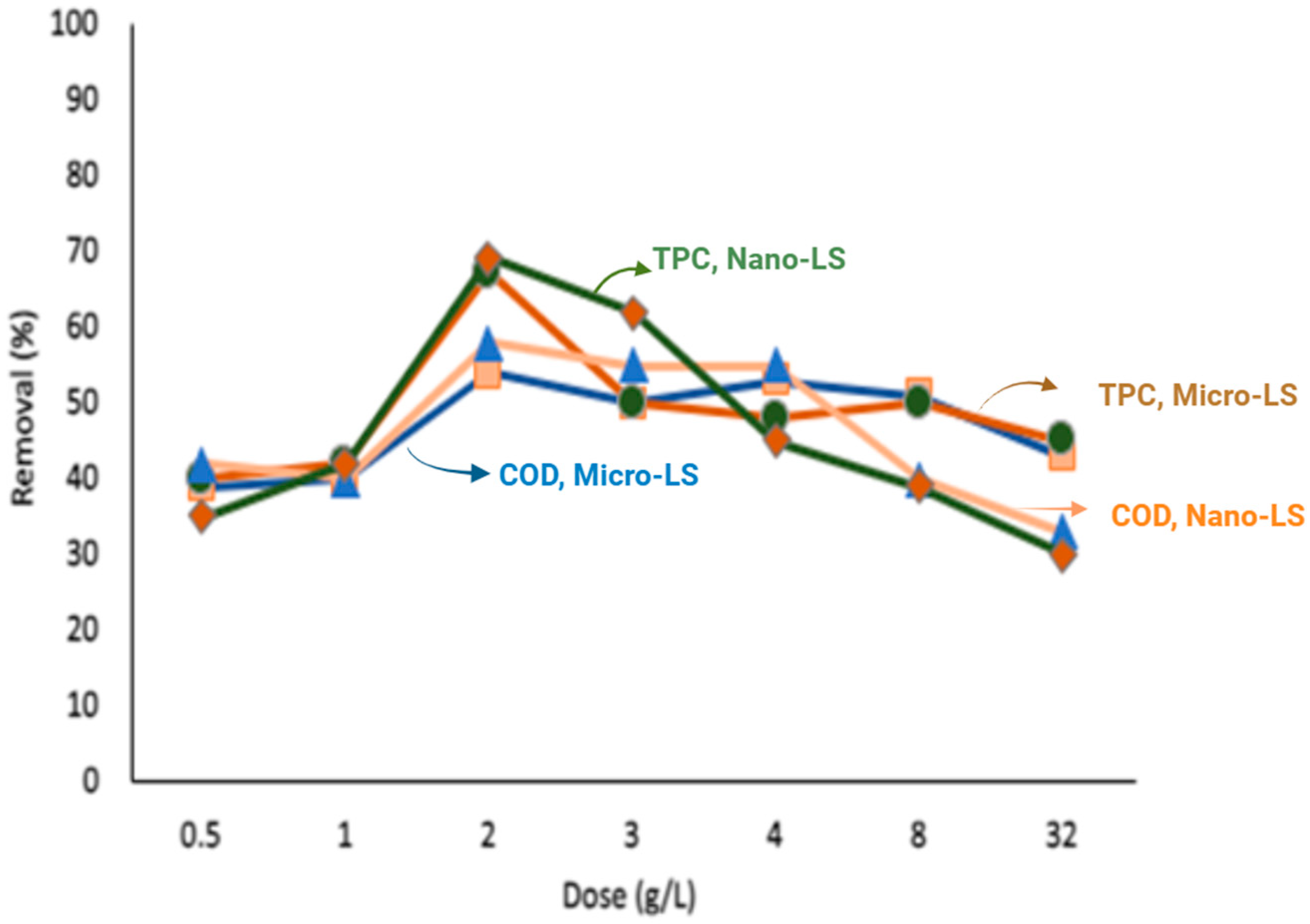
 (diamond) Nano-LS for % removal of TPC.
(diamond) Nano-LS for % removal of TPC.  (circle) Micro-LS for % removal of TPC.
(circle) Micro-LS for % removal of TPC.  (triangular) Nano-LS for % removal of COD.
(triangular) Nano-LS for % removal of COD.  (square) Micro -LS for % removal of COD.
(square) Micro -LS for % removal of COD.
 (diamond) Nano-LS for % removal of TPC.
(diamond) Nano-LS for % removal of TPC.  (circle) Micro-LS for % removal of TPC.
(circle) Micro-LS for % removal of TPC.  (triangular) Nano-LS for % removal of COD.
(triangular) Nano-LS for % removal of COD.  (square) Micro -LS for % removal of COD.
(square) Micro -LS for % removal of COD.
 (Blue triangular) Micro -LS for % removal of COD.
(Blue triangular) Micro -LS for % removal of COD.  (Gold square) Nano-LS for % removal of COD
(Gold square) Nano-LS for % removal of COD  (red circle) Micro-LS for % removal of TPC
(red circle) Micro-LS for % removal of TPC  (Yellow diamond) Nano-LS for % removal of TPC.
(Yellow diamond) Nano-LS for % removal of TPC.
 (Blue triangular) Micro -LS for % removal of COD.
(Blue triangular) Micro -LS for % removal of COD.  (Gold square) Nano-LS for % removal of COD
(Gold square) Nano-LS for % removal of COD  (red circle) Micro-LS for % removal of TPC
(red circle) Micro-LS for % removal of TPC  (Yellow diamond) Nano-LS for % removal of TPC.
(Yellow diamond) Nano-LS for % removal of TPC.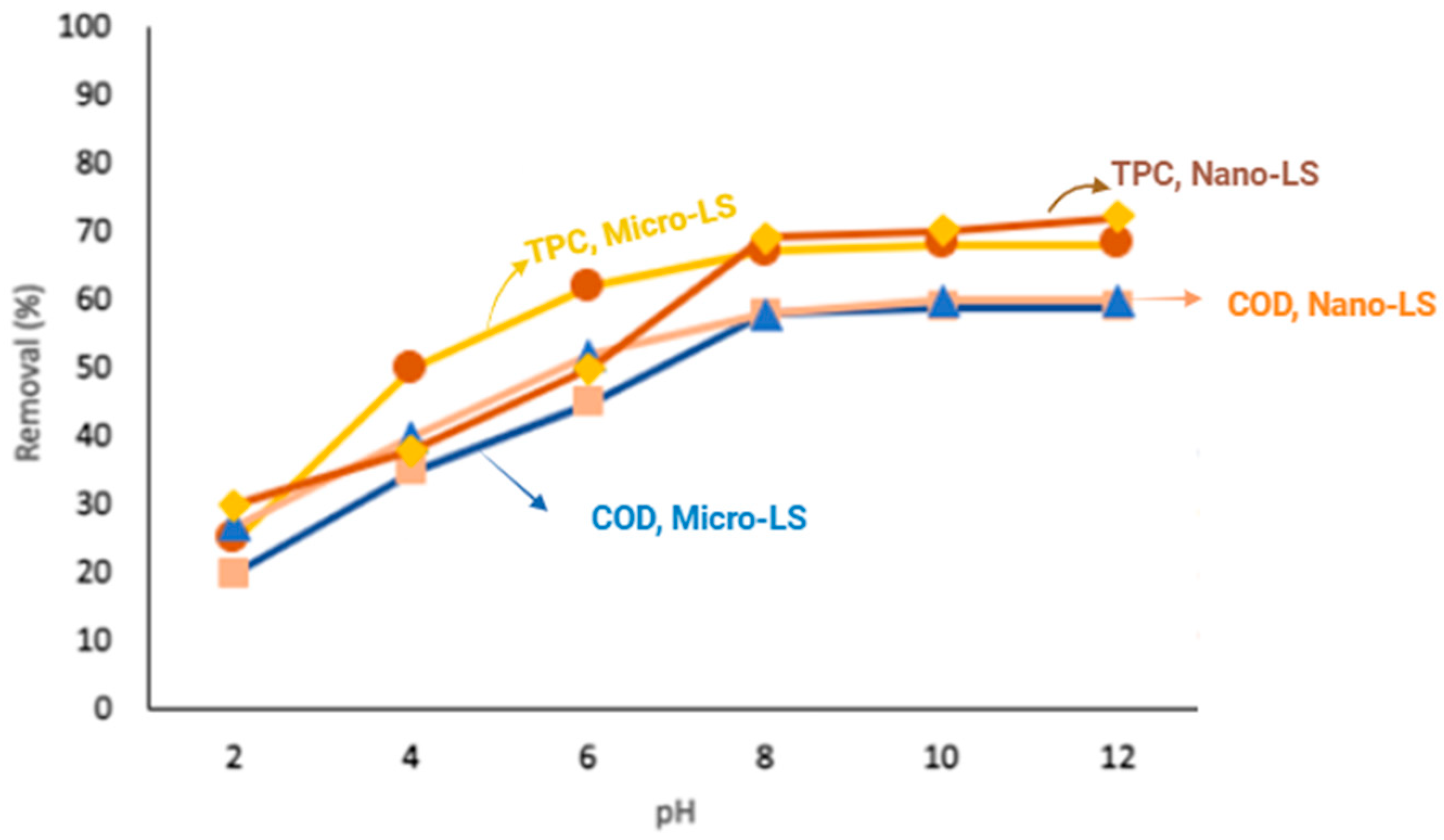
 (diamond) Nano-LS for % removal of TPC.
(diamond) Nano-LS for % removal of TPC.  (circle) Micro-LS for % removal of TPC.
(circle) Micro-LS for % removal of TPC.  (triangular) Nano-LS for % removal of COD.
(triangular) Nano-LS for % removal of COD.  (square) Micro -LS for % removal of COD.
(square) Micro -LS for % removal of COD.
 (diamond) Nano-LS for % removal of TPC.
(diamond) Nano-LS for % removal of TPC.  (circle) Micro-LS for % removal of TPC.
(circle) Micro-LS for % removal of TPC.  (triangular) Nano-LS for % removal of COD.
(triangular) Nano-LS for % removal of COD.  (square) Micro -LS for % removal of COD.
(square) Micro -LS for % removal of COD.