Microwave-Assisted Hydrothermal Synthesis of Cu/Sr-Doped Hydroxyapatite with Prospective Applications for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CaO Preparation
2.3. Undoped HA and Cu/Sr-Doped Hydroxyapatite Synthesis Method from Biogenic Sources
2.4. Characterization Methods
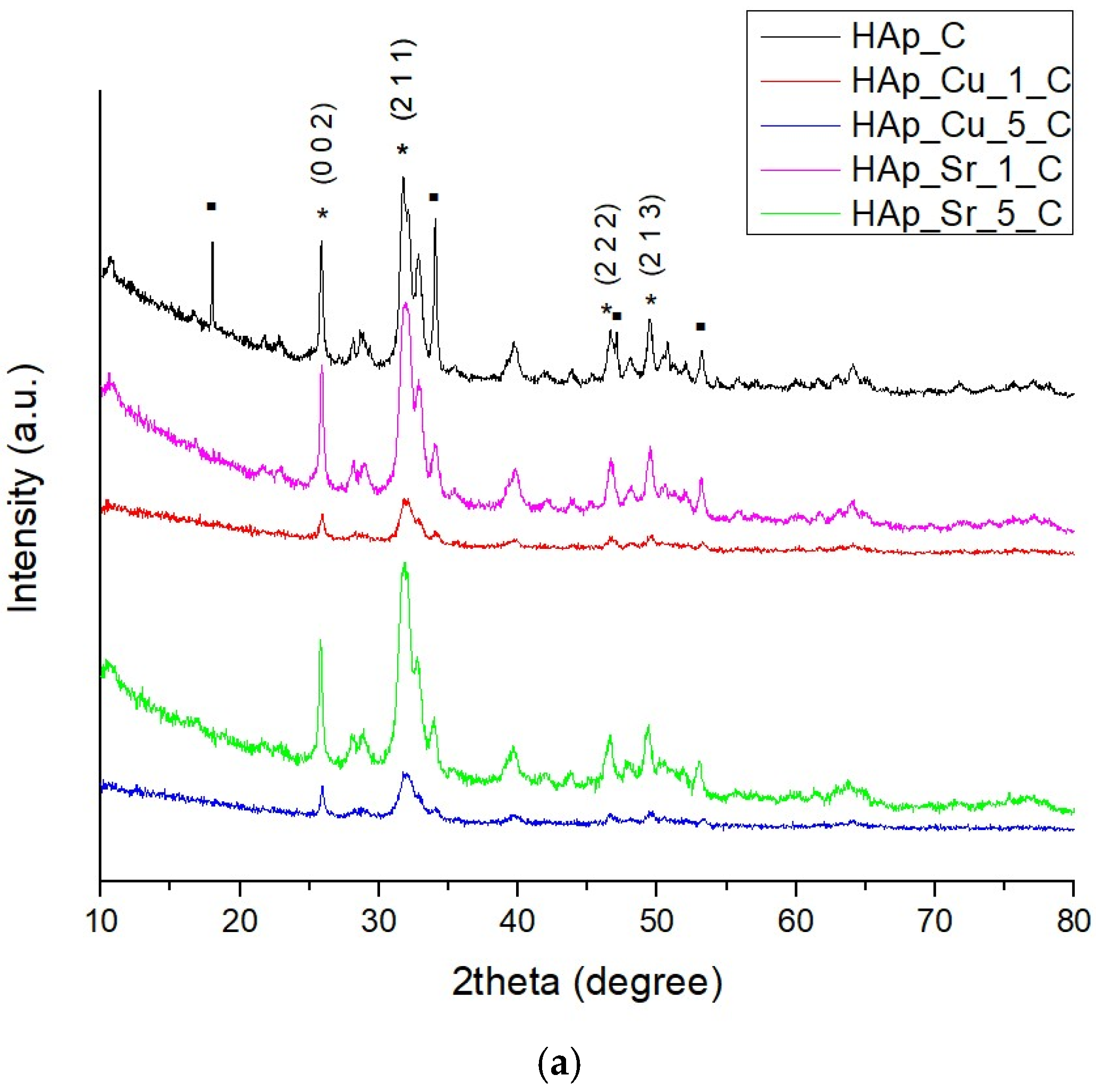
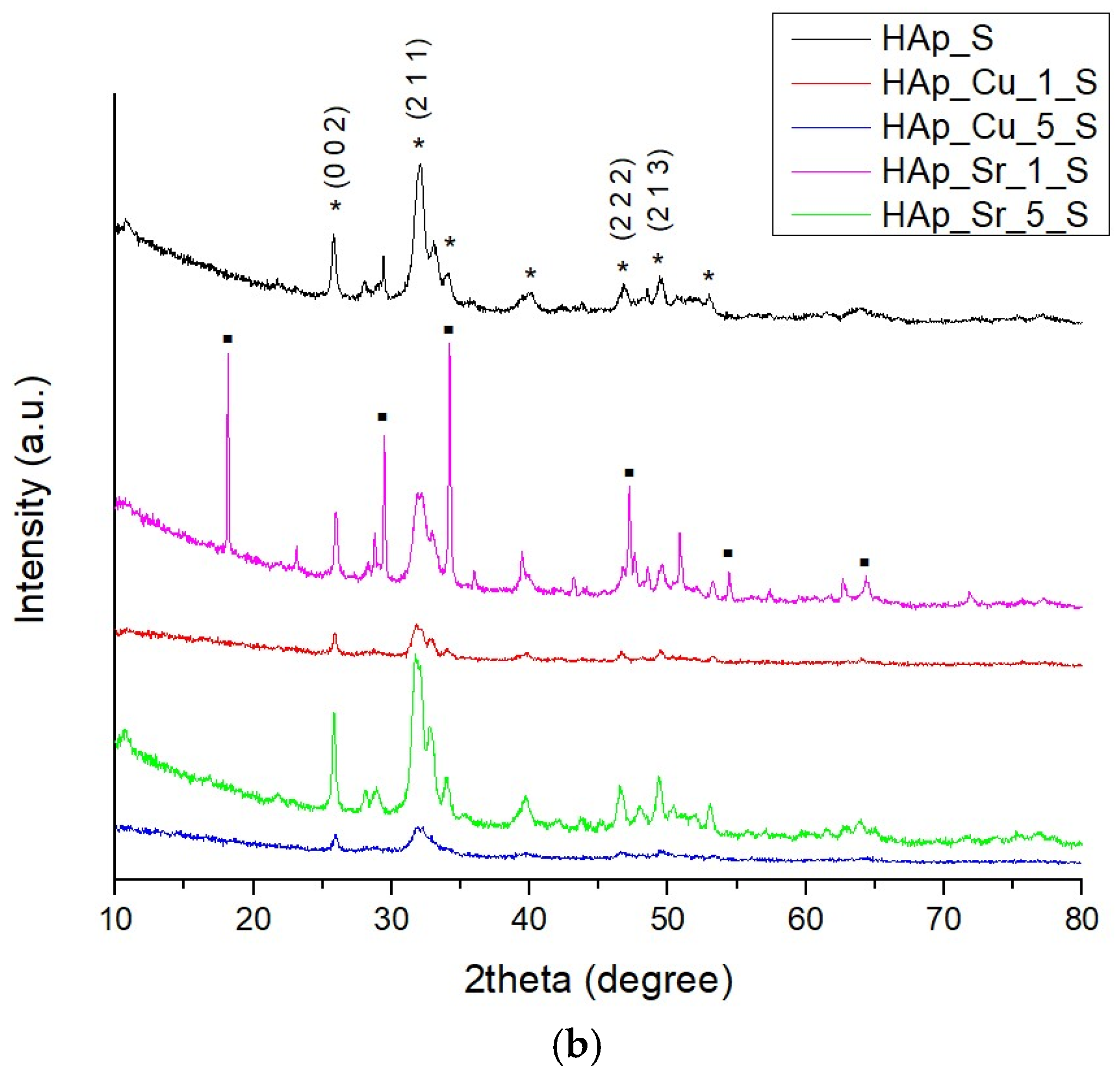
2.4.1. X-Ray Diffraction (XRD)
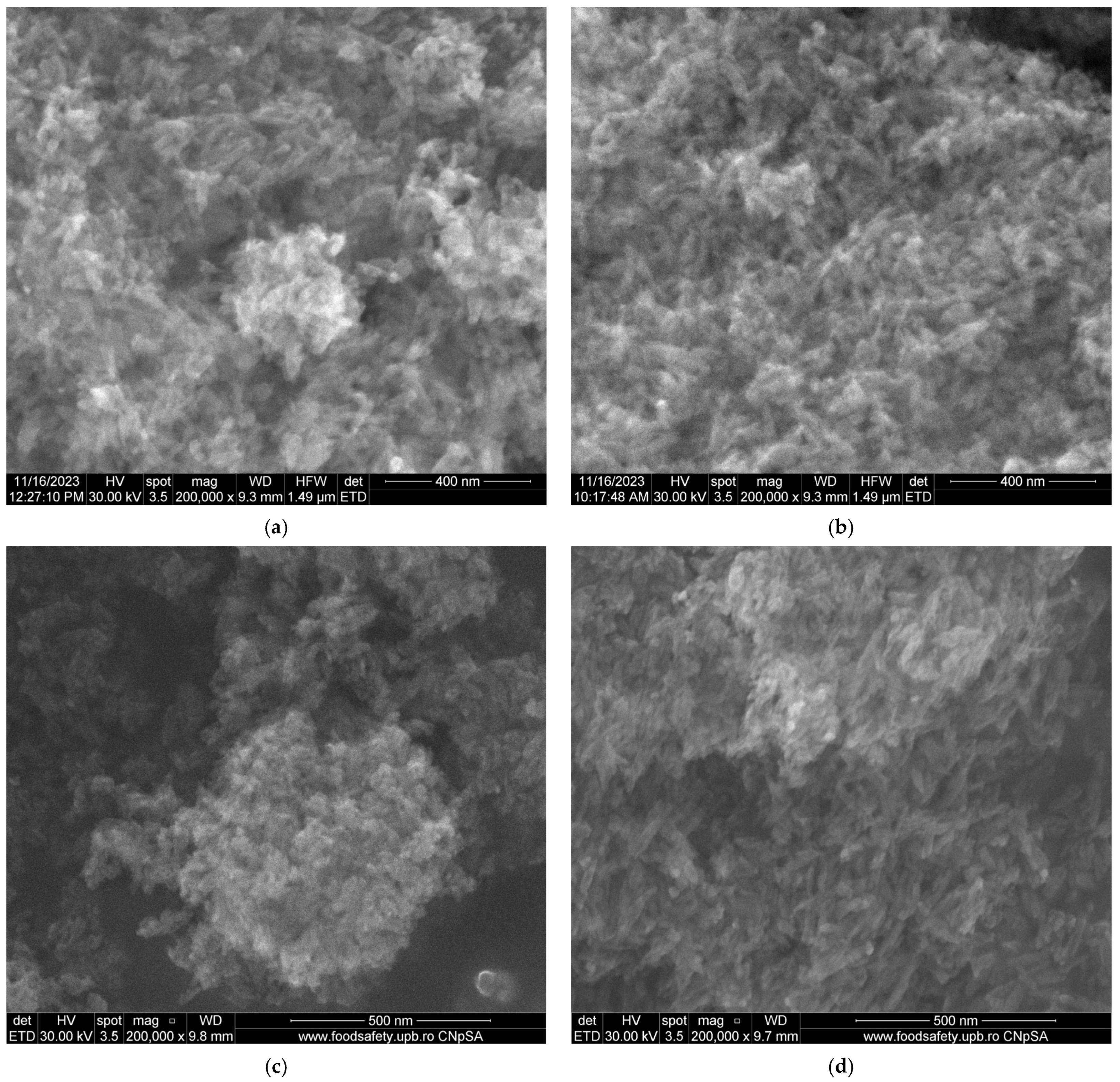
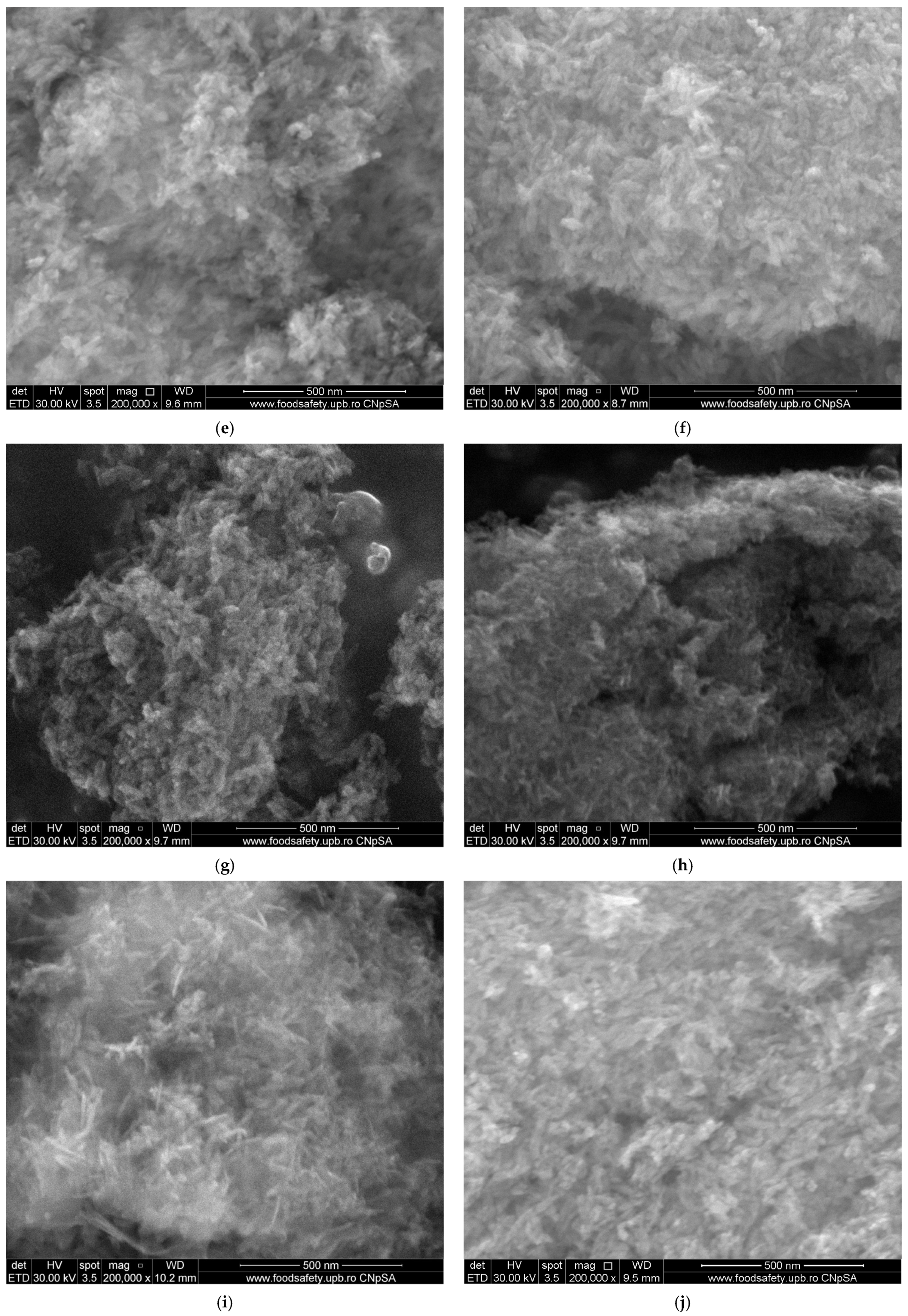
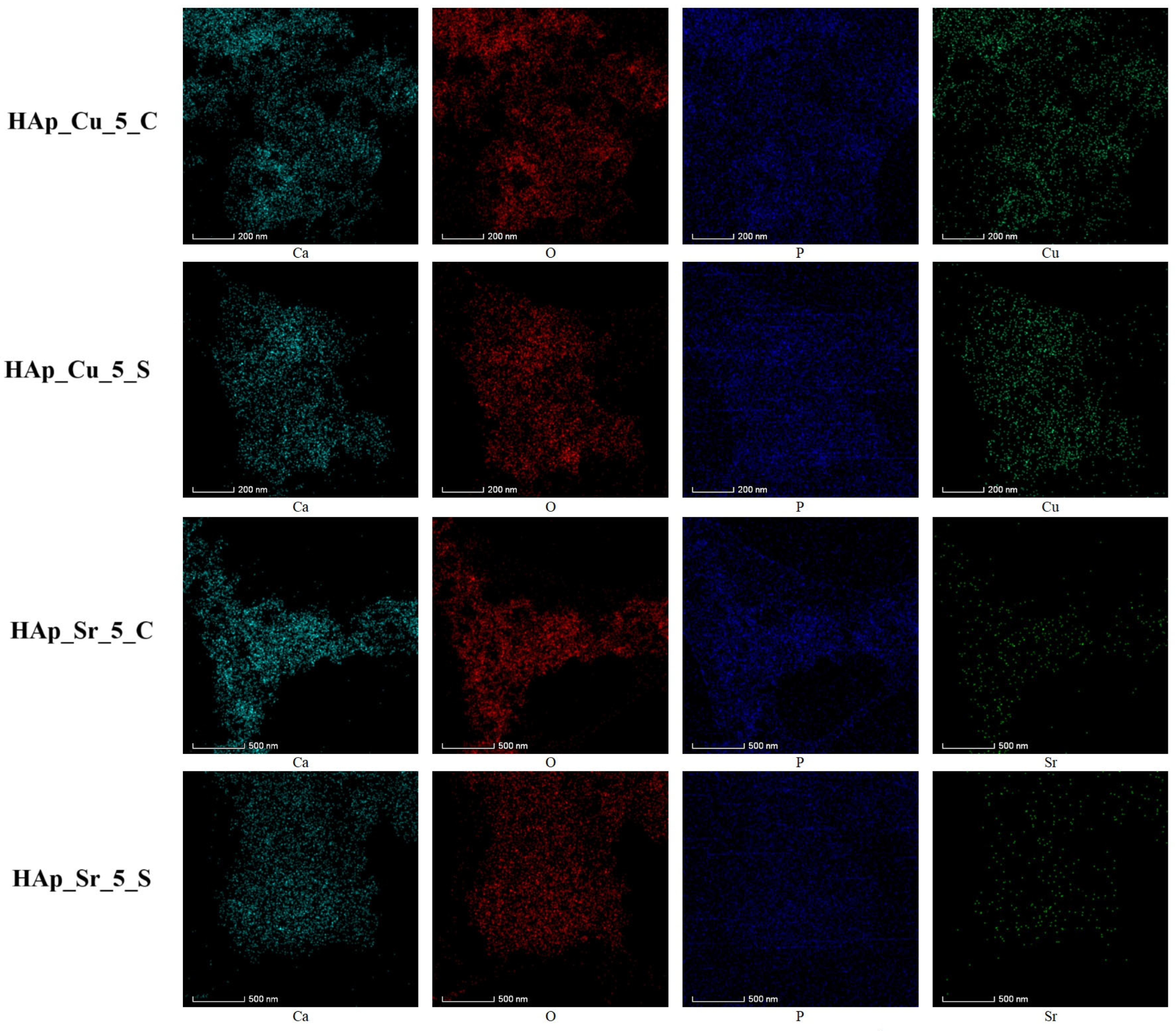
2.4.2. Scanning Electron Microscopy (SEM)
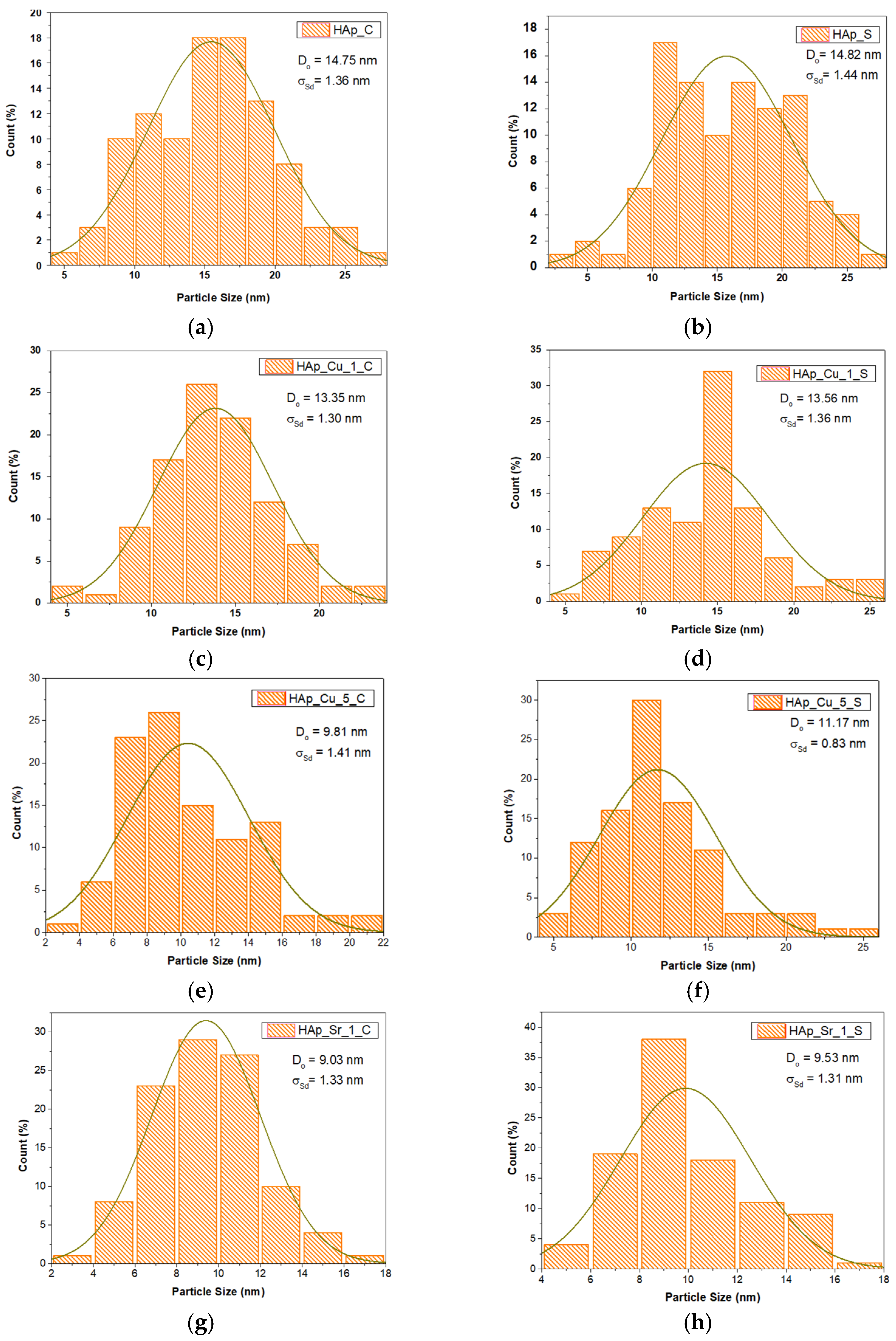
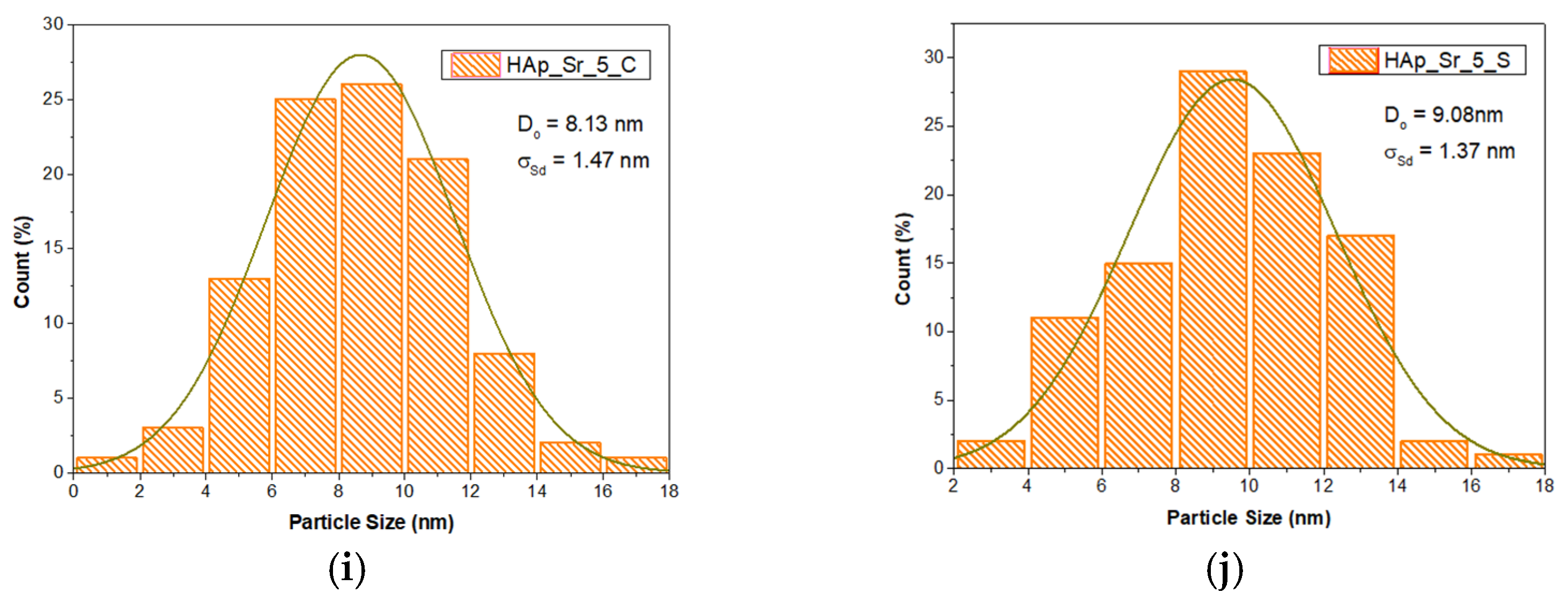
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.4.5. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
2.4.6. Biological Evaluation
Antimicrobial Assays
Biocompatibility Assay
2.5. Statistical Analysis
3. Results
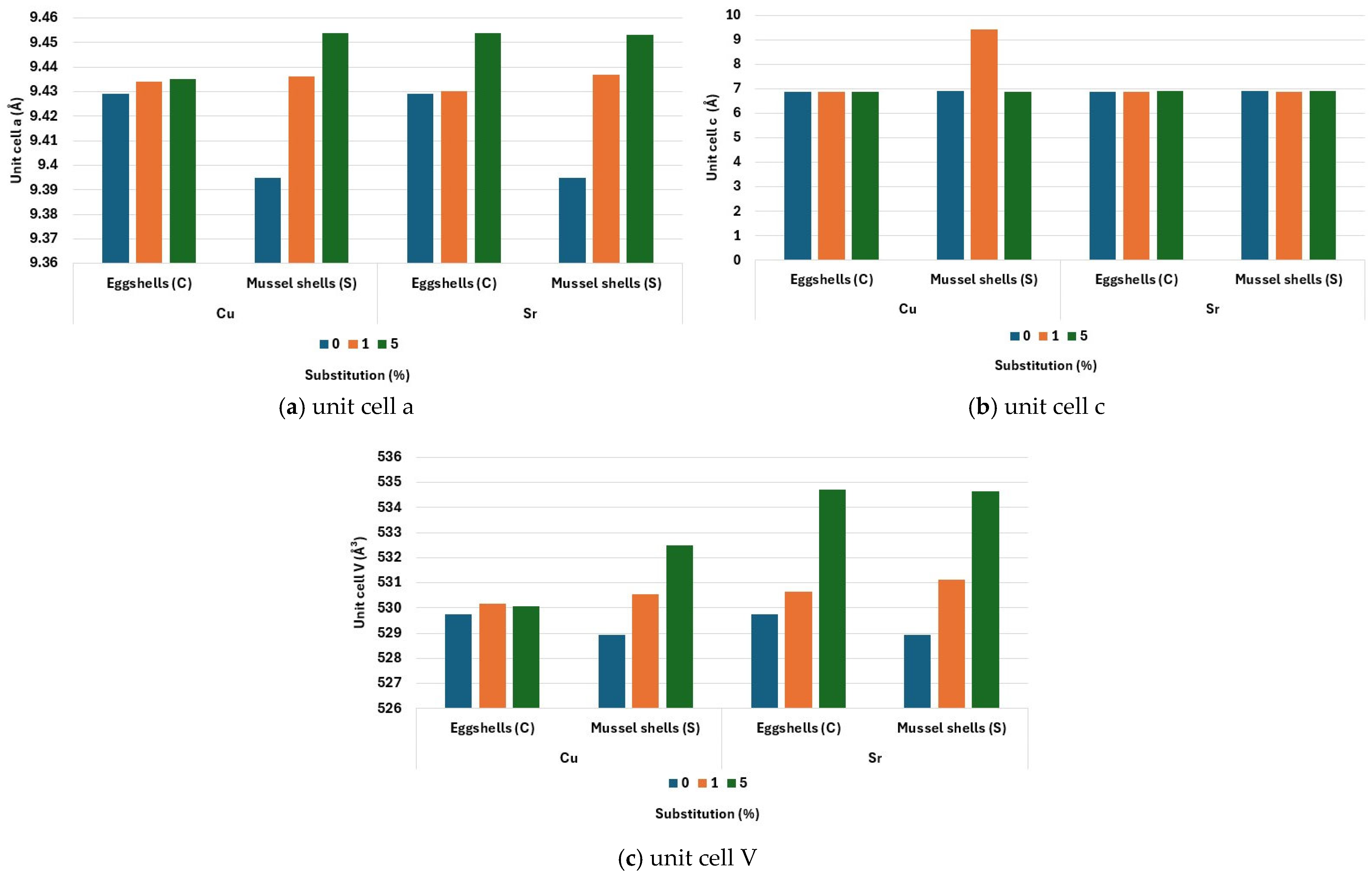
3.1. X-Ray Diffraction (XRD)
3.2. Scanning Electron Microscopy (SEM)
3.3. Transmission Electron Microscopy (TEM)
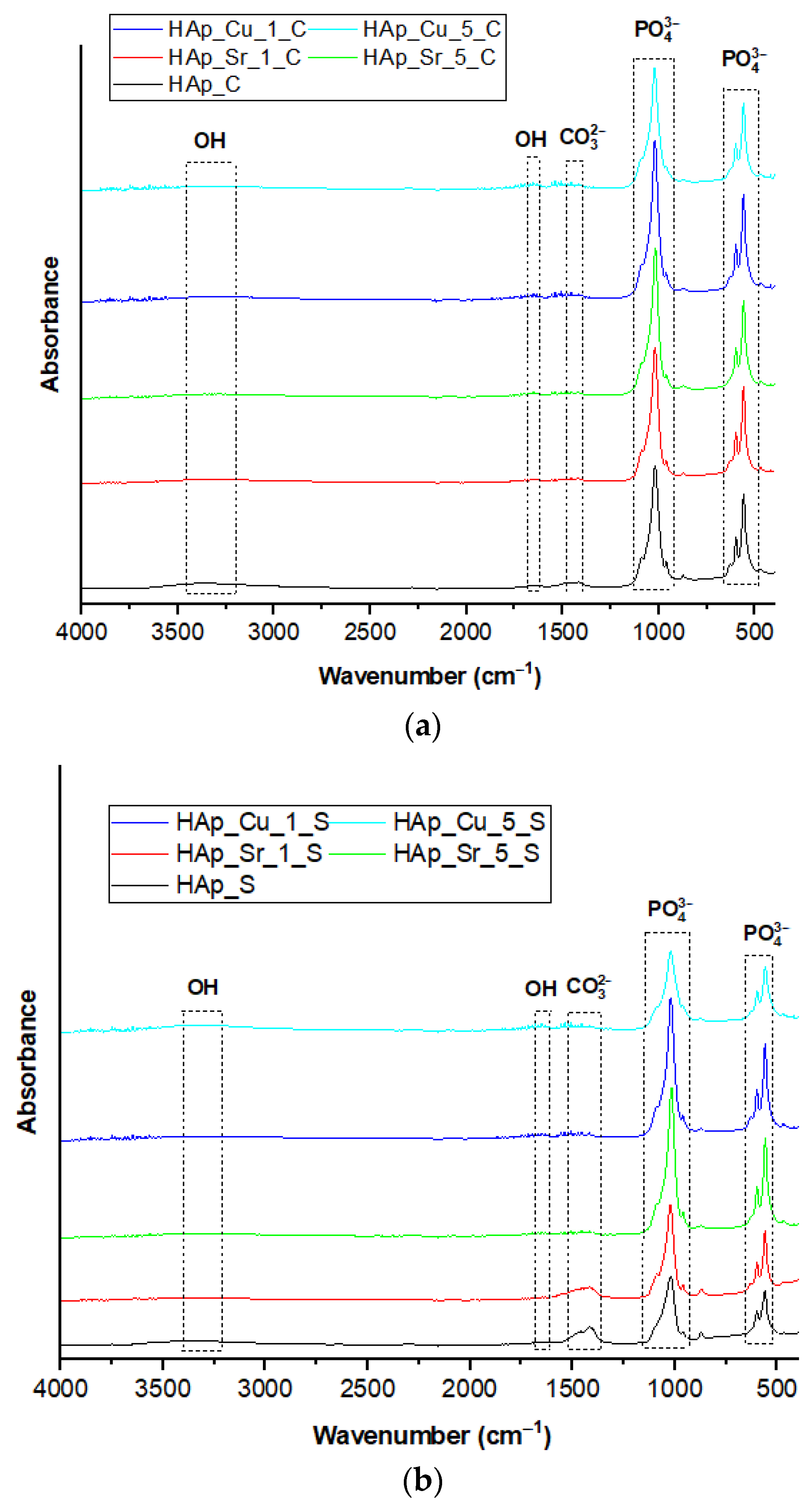
3.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.5. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
3.6. Biological Evaluation
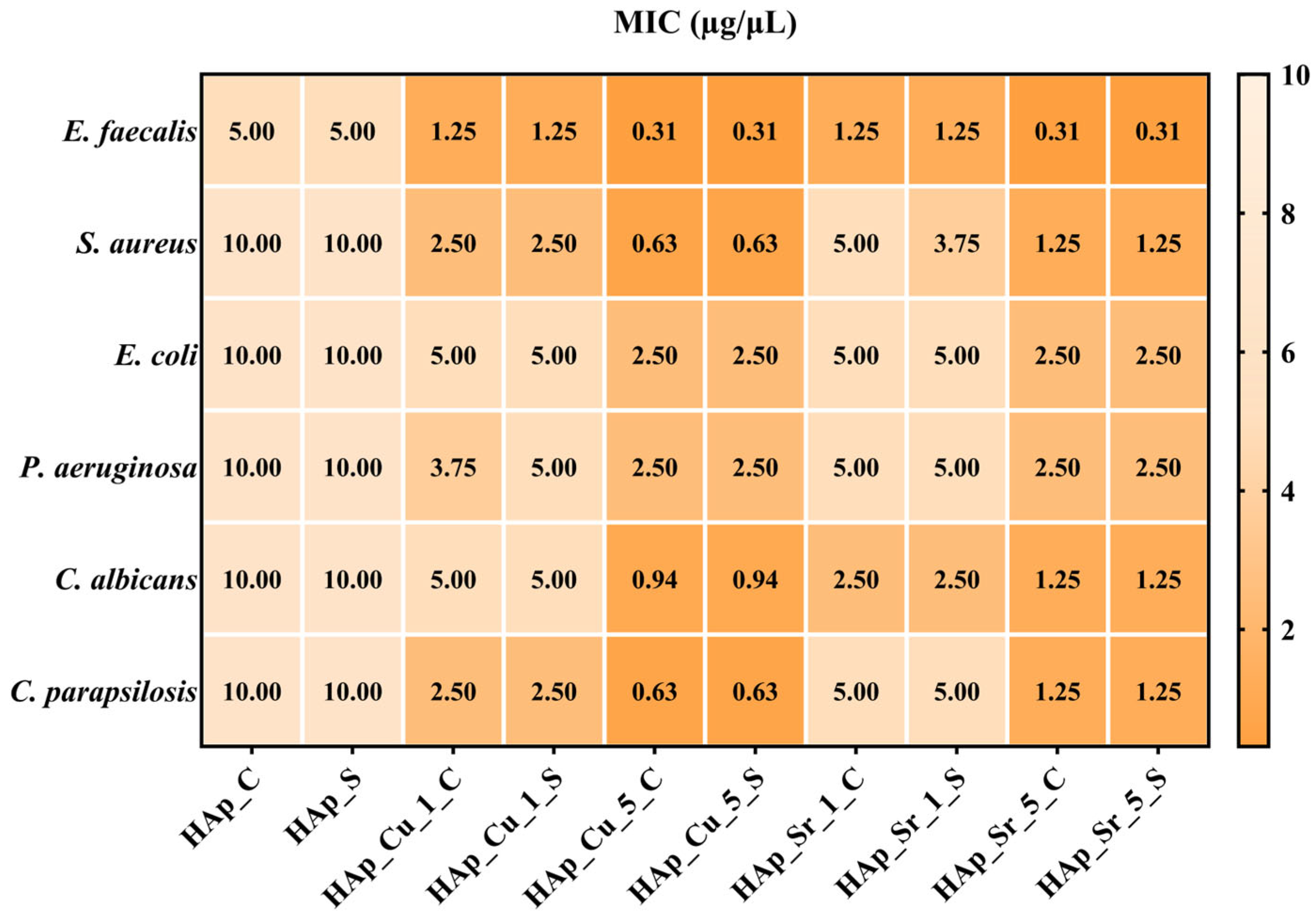
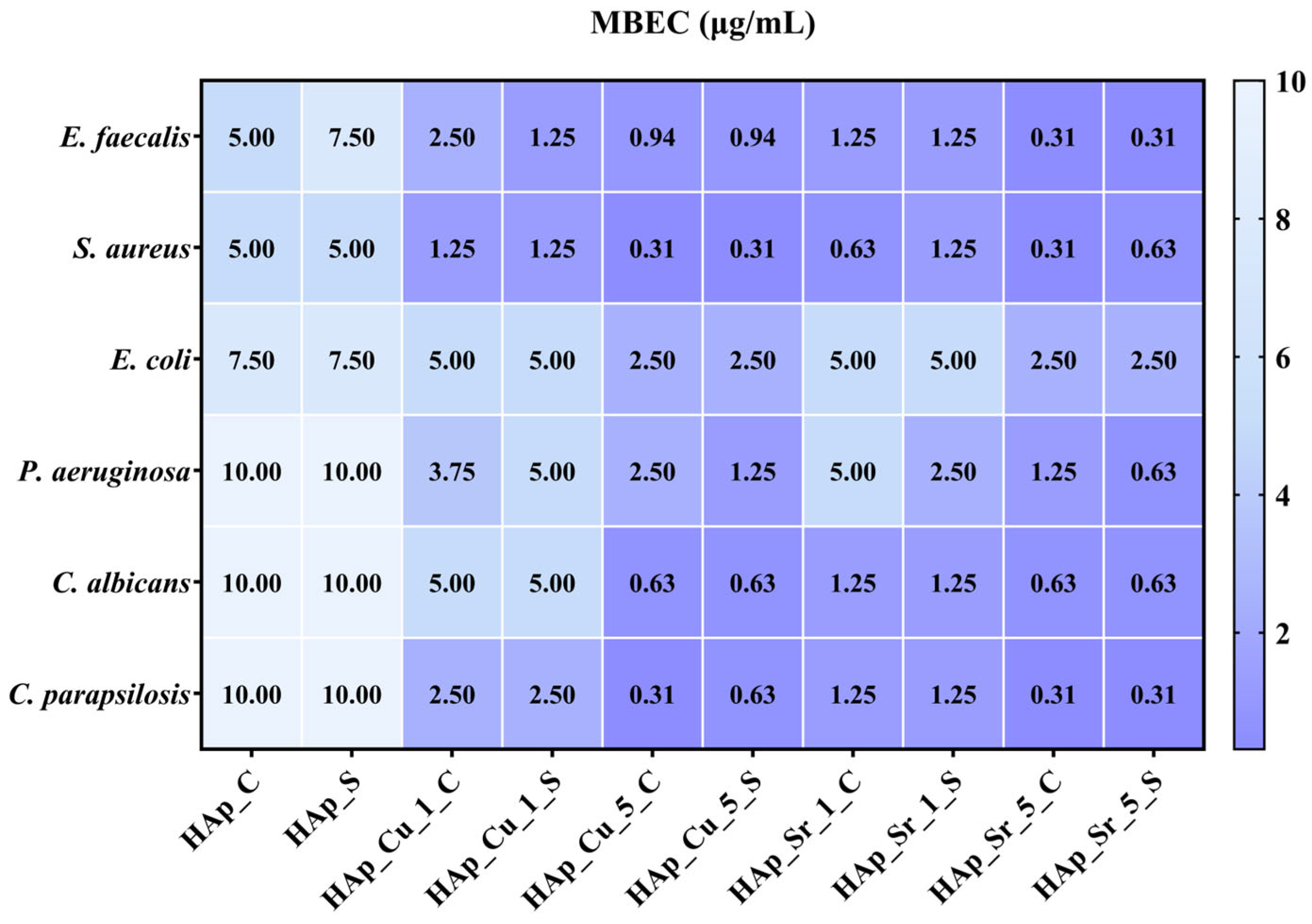
Antimicrobial Analysis
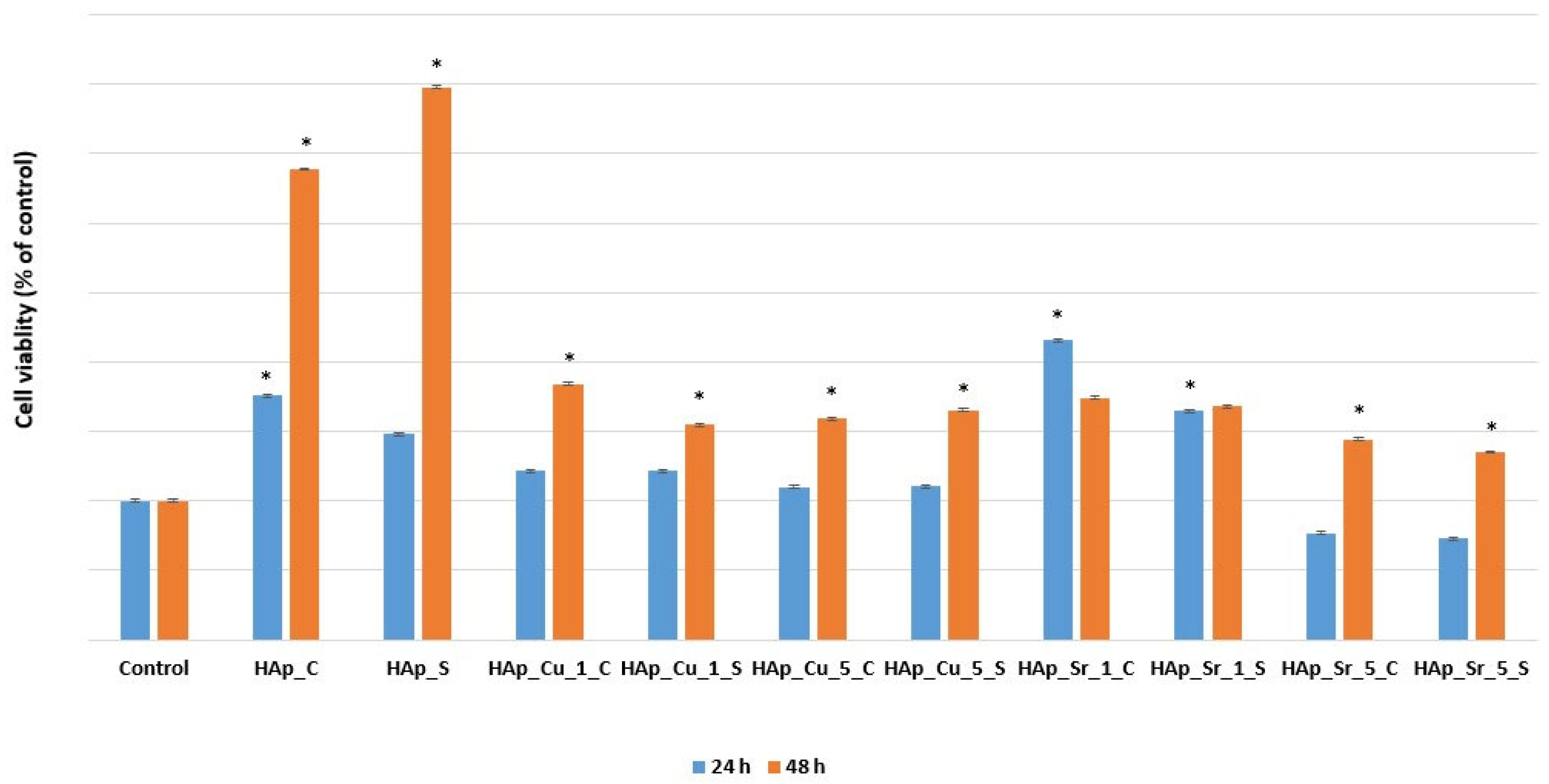
Biocompatibility Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Zarrabi, A.; Afghah, F.; Koc, B.; Abdolahinia, E.D.; Omidi, Y.; Barar, J.; Akbari-Fakhrabadi, A.; et al. Effect of Zinc-Doped Hydroxyapatite/Graphene Nanocomposite on the Physicochemical Properties and Osteogenesis Differentiation of 3d-Printed Polycaprolactone Scaffolds for Bone Tissue Engineering. Chem. Eng. J. 2021, 426, 131321. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Etinosa, P.O.; Obada, D.O. A Comparative Study of the Mechanical Properties of Sol-Gel Derived Hydroxyapatite Produced from a Novel Mixture of Two Natural Biowastes for Biomedical Applications. Mater. Chem. Phys. 2023, 297, 127434. [Google Scholar] [CrossRef]
- Surya, P.; Nithin, A.; Sundaramanickam, A.; Sathish, M. Synthesis and Characterization of Nano-Hydroxyapatite from Sardinella Longiceps Fish Bone and Its Effects on Human Osteoblast Bone Cells. J. Mech. Behav. Biomed. Mater. 2021, 119, 104501. [Google Scholar] [CrossRef] [PubMed]
- Firdaus Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Abdul Wahap, M.A. Extraction of Natural Hydroxyapatite for Biomedical Applications—A Review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef] [PubMed]
- Cursaru, L.M.; Iota, M.; Piticescu, R.M.; Tarnita, D.; Savu, S.V.; Savu, I.D.; Dumitrescu, G.; Popescu, D.; Hertzog, R.-G.; Calin, M. Hydroxyapatite from Natural Sources for Medical Applications. Materials 2022, 15, 5091. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Hsu, H.-C.; Wu, W.-H.; Ho, W.-F. Enhancing Bioactivity and Mechanical Properties of Nano-Hydroxyapatite Derived from Oyster Shells through Hydrothermal Synthesis. Nanomaterials 2024, 14, 1281. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from Fish Scale for Potential Use as Bone Scaffold or Regenerative Material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Nam, P.V.; Hoa, N.V.; Trung, T.S. Properties of Hydroxyapatites Prepared from Different Fish Bones: A Comparative Study. Ceram. Int. 2019, 45, 20141–20147. [Google Scholar] [CrossRef]
- Zubieta-Otero, L.F.; Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Rodriguez-Garcia, M.E. Bio-Inspired Synthesis of Bio-Hydroxyapatite/Synthetic Hydroxyapatite Hybrid Nanosystems. MedComm—Biomater. Appl. 2023, 2, e64. [Google Scholar] [CrossRef]
- Alturki, A.M.; Abu-Rayyan, A.; Abualnaja, K.M.; Alhashmialameer, D.; El-Saeed, R.A.; El-Shabasy, R.M. Physicomechanical and Morphological Properties of Hydroxyapatite Nanocrystals Substituted with Copper–Zirconium. J. Mater. Res. Technol. 2021, 14, 2312–2321. [Google Scholar] [CrossRef]
- Noviyanti, A.R.; Rahayu, I.; Fauzia, R.P.; Risdiana. The Effect of Mg Concentration to Mechanical Strength of Hydroxyapatite Derived from Eggshell. Arab. J. Chem. 2021, 14, 103032. [Google Scholar] [CrossRef]
- Bootchanont, A.; Wechprasit, T.; Areesamarn, N.; Pholprom, R.; Hwangphon, T.; Temprom, L.; Amonpattaratkit, P.; Klysubun, W.; Yimnirun, R. Comparison of Local Structure between Mg/Mn-Doped Natural and Synthetic Hydroxyapatites by X-Ray Absorption Spectroscopy. Radiat. Phys. Chem. 2020, 177, 109075. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Yetisgin, A.A.; Sahin, S.B.; Demir, E.; Cetinel, S. Enhanced Properties of Nickel–Silver Codoped Hydroxyapatite for Bone Tissue Engineering: Synthesis, Characterization, and Biocompatibility Evaluation. Environ. Res. 2023, 238, 117131. [Google Scholar] [CrossRef]
- Radulescu, D.-E.; Vasile, O.R.; Andronescu, E.; Ficai, A. Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 13157. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-Doped Hydroxyapatite: An Impasse or the Road to Follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Murugesan, V.; Vaiyapuri, M.; Murugeasan, A. Fabrication and Characterization of Strontium Substituted Chitosan Modify Hydroxyapatite for Biomedical Applications. Inorg. Chem. Commun. 2022, 142, 109653. [Google Scholar] [CrossRef]
- Nenen, A.; Maureira, M.; Neira, M.; Orellana, S.L.; Covarrubias, C.; Moreno-Villoslada, I. Synthesis of Antibacterial Silver and Zinc Doped Nano-Hydroxyapatite with Potential in Bone Tissue Engineering Applications. Ceram. Int. 2022, 48, 34750–34759. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Wang, C.; Sun, R.; Tang, Y. Effects of Physico-Chemical Properties of Ions-Doped Hydroxyapatite on Adsorption and Release Performance of Doxorubicin as a Model Anticancer Drug. Mater. Chem. Phys. 2022, 276, 125440. [Google Scholar] [CrossRef]
- Matić, T.; Zebić, M.L.; Miletić, V.; Cvijović-Alagić, I.; Petrović, R.; Janaćković, D.; Veljović, D. Sr,Mg Co-Doping of Calcium Hydroxyapatite: Hydrothermal Synthesis, Processing, Characterization and Possible Application as Dentin Substitutes. Ceram. Int. 2022, 48, 11155–11165. [Google Scholar] [CrossRef]
- Dorcioman, G.; Grumezescu, V.; Stan, G.E.; Chifiriuc, M.C.; Gradisteanu, G.P.; Miculescu, F.; Matei, E.; Popescu-Pelin, G.; Zgura, I.; Craciun, V.; et al. Hydroxyapatite Thin Films of Marine Origin as Sustainable Candidates for Dental Implants. Pharmaceutics 2023, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Singh, J.P.; Pal, A.; Kaur, T. Encapsulation of Vancomycin in Copper Doped Hydroxyapatite Mesoporous Nanoparticles of Different Morphologies. J. Drug Deliv. Sci. Technol. 2020, 55, 101441. [Google Scholar] [CrossRef]
- Megha, M.; Joy, A.; Unnikrishnan, G.; Haris, M.; Thomas, J.; Deepti, A.; Chakrapani, P.S.B.; Kolanthai, E.; Muthuswamy, S. Structural and Biological Properties of Novel Vanadium and Strontium Co-Doped Hap for Tissue Engineering Applications. Ceram. Int. 2023, 49, 30156–30169. [Google Scholar] [CrossRef]
- Abdalla, M.M.; Sayed, O.; Lung, C.Y.K.; Rajasekar, V.; Yiu, C.K.Y. Applications of Bioactive Strontium Compounds in Dentistry. J. Funct. Biomater. 2024, 15, 216. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Z.; Yang, Y.; Jian, Y.; Li, R.; Dai, X.; Wu, W.; Zhong, J.; Chen, C. Synthesis, Characterization and Biological Performance Study of Sr-Doped Hydroxyapatite/Chitosan Composite Coatings. Mater. Chem. Phys. 2021, 270, 124752. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, D.; Sun, L.; Li, H.; Hanaor, D.A.; Schmidt, F.; Xu, K. Nanostructural Insights into the Dissolution Behavior of Sr-Doped Hydroxyapatite. J. Eur. Ceram. Soc. 2018, 38, 5554–5562. [Google Scholar] [CrossRef]
- Veljovic, D.; Matic, T.; Stamenic, T.; Kojic, V.; Dimitrijevic-Brankovic, S.; Lukic, M.J.; Jevtic, S.; Radovanovic, Z.; Petrovic, R.; Janackovic, D. Mg/Cu Co-Substituted Hydroxyapatite—Biocompatibility, Mechanical Properties and Antimicrobial Activity. Ceram. Int. 2019, 45, 22029–22039. [Google Scholar] [CrossRef]
- Jacobs, A.; Renaudin, G.; Charbonnel, N.; Nedelec, J.-M.; Forestier, C.; Descamps, S. Copper-Doped Biphasic Calcium Phosphate Powders: Dopant Release, Cytotoxicity and Antibacterial Properties. Materials 2021, 14, 2393. [Google Scholar] [CrossRef]
- Bulina, N.V.; Eremina, N.V.; Vinokurova, O.B.; Ishchenko, A.V.; Chaikina, M.V. Diffusion of Copper Ions in the Lattice of Substituted Hydroxyapatite During Heat Treatment. Materials 2022, 15, 5759. [Google Scholar] [CrossRef]
- Burdusel, A.-C.; Neacsu, I.A.; Birca, A.C.; Chircov, C.; Grumezescu, A.-M.; Holban, A.M.; Curutiu, C.; Ditu, L.M.; Stan, M. Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration. J. Funct. Biomater. 2023, 14, 378. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, C.R.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Iordache, F.; Trusca, R.; Ciocan, L.T.; Ficai, A.; Andronescu, E. Nano-Hydroxyapatite Vs. Xenografts: Synthesis, Characterization, and in Vitro Behavio. Nanomaterials 2021, 11, 2289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Deng, H.; Yao, S.; Wang, Y. Regulatory Synthesis and Characterization of Hydroxyapatite Nanocrystals by a Microwave-Assisted Hydrothermal Method. Ceram. Int. 2020, 46, 2185–2193. [Google Scholar] [CrossRef]
- Karunakaran, G.; Kumar, G.S.; Cho, E.-B.; Sunwoo, Y.; Kolesnikov, E.; Kuznetsov, D. Microwave-Assisted Hydrothermal Synthesis of Mesoporous Carbonated Hydroxyapatite with Tunable Nanoscale Characteristics for Biomedical Applications. Ceram. Int. 2019, 45, 970–977. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Velázquez-Castillo, R.; Rivera-Muñoz, E.M.; Bucio-Galindo, L.; Mondragón-Galicia, G.; Manzano-Ramírez, A.; Ocampo, M.Á.; Apátiga-Castro, L.M. Crystal Growth and Structural Analysis of Hydroxyapatite Nanofibers Synthesized by the Hydrothermal Microwave-Assisted Method. Ceram. Int. 2017, 43, 451–457. [Google Scholar] [CrossRef]
- Patil, H.G.; Rajendran, A.; Lenka, N.; Kumar, B.S.; Murugesan, S.; Anandhan, S. Probing the Influence of Strontium Doping and Annealing Temperature on the Structure and Biocompatibility of Hydroxyapatite Nanorods. Dalton Trans. 2024, 53, 7812–7827. [Google Scholar] [CrossRef]
- Aina, V.; Bergandi, L.; Lusvardi, G.; Malavasi, G.; Imrie, F.E.; Gibson, I.R.; Cerrato, G.; Ghigo, D. Sr-Containing Hydroxyapatite: Morphologies of Ha Crystals and Bioactivity on Osteoblast Cells. Mater. Sci. Eng. C 2013, 33, 1132–1142. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their in Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Baldassarre, F.; Altomare, A.; Mesto, E.; Lacalamita, M.; Dida, B.; Mele, A.; Bauer, E.M.; Puzone, M.; Tempesta, E.; Capelli, D.; et al. Structural Characterization of Low-Sr-Doped Hydroxyapatite Obtained by Solid-State Synthesis. Crystals 2023, 13, 117. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Ghegoiu, L.; Buton, N.; Motelica-Heino, M. Copper Doped Hydroxyapatite Nanocomposite Thin Films: Synthesis, Physico–Chemical and Biological Evaluation. BioMetals 2024, 37, 1487–1500. [Google Scholar] [CrossRef]
- Tuntun, S.M.; Sahadat Hossain, M.; Uddin, M.N.; Shaikh, M.A.A.; Bahadur, N.M.; Ahmed, S. Crystallographic Characterization and Application of Copper Doped Hydroxyapatite as a Biomaterial. New J. Chem. 2023, 47, 2874–2885. [Google Scholar] [CrossRef]
- Anand, A.; Sengupta, S.; Galusek, D.; Beltrán, A.M.; Galusková, D.; Boccaccini, A.R. A New Approach to Overcome Cytotoxic Effects of Cu by Delivering Dual Therapeutic Ions (Sr, Cu). J. Trace Elem. Med. Biol. 2025, 87, 127565. [Google Scholar] [CrossRef]
- Al-Hammadi, A.H.; Al-Adhreai, A.a.A.; Abdulwahab, A.M.; Al-Adhreai, A.; Salem, A.; Alaizeri, Z.M.; Alsaeedy, M.; Katib Alanazi, F. An Investigation on the Structural, Morphological, Optical, and Antibacterial Activity of Sr:Cus Nanostructures. Sci. Rep. 2024, 14, 25169. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Spoiala, A.; Chircov, C.; Dolete, G.; Oprea, O.-C.; Vasile, B.-S.; Crainiceanu, S.A.; Nicoara, A.-I.; Marinas, I.C.; Stan, M.S.; et al. Antioxidant, Antitumoral, Antimicrobial, and Prebiotic Activity of Magnetite Nanoparticles Loaded with Bee Pollen/Bee Bread Extracts and 5-Fluorouracil. Antioxidants 2024, 13, 895. [Google Scholar] [CrossRef]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing—35th Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025.
- Obada, D.O.; Salami, K.A.; Oyedeji, A.N.; Fasanya, O.O.; Suleiman, M.U.; Ibisola, B.A.; Atta, A.Y.; Dodoo-Arhin, D.; Kuburi, L.S.; Dauda, M.; et al. Solution Combustion Synthesis of Strontium-Doped Hydroxyapatite: Effect of Sintering and Low Compaction Pressure on the Mechanical Properties and Physiological Stability. Mater. Lett. 2021, 304, 130613. [Google Scholar] [CrossRef]
- Poovendran, K.; Wilson, K.S.J.; Revathy, M.S.; Ayeshamariam, A.; Kaviyarasu, K. Functionalization Effect of Hap with Copper (Cu) Having Excellent Dielectric Applications. Surf. Interfaces 2020, 19, 100474. [Google Scholar] [CrossRef]
- Chadha, R.K.; Singh, K.L.; Sharma, C.; Singh, A.P.; Naithani, V. Effect of Microwave and Conventional Processing Techniques on Mechanical Properties of Strontium Substituted Hydroxyapatite. Ceram. Int. 2020, 46, 1091–1098. [Google Scholar] [CrossRef]
- Unabia, R.B.; Bonebeau, S.; Candidato, R.T.; Jouin, J.; Noguera, O.; Pawłowski, L. Investigation on the Structural and Microstructural Properties of Copper-Doped Hydroxyapatite Coatings Deposited Using Solution Precursor Plasma Spraying. J. Eur. Ceram. Soc. 2019, 39, 4255–4263. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wang, S.; Jia, F.; Bian, A.; Wang, K.; Xie, L.; Yan, K.; Qiao, H.; Lin, H.; et al. Electrodeposited Dopamine/Strontium-Doped Hydroxyapatite Composite Coating on Pure Zinc for Anti-Corrosion, Antimicrobial and Osteogenesis. Mater. Sci. Eng. C 2021, 129, 112387. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Ai, F.; Yan, J.; Zhou, K. Fabrication and Characterization of Sr-Doped Hydroxyapatite Porous Scaffold. JOM 2021, 73, 1745–1753. [Google Scholar] [CrossRef]
- Shavandi, A.; Wilton, V.; Bekhit, A.E.-D.A. Synthesis of Macro and Micro Porous Hydroxyapatite (Ha) Structure from Waste Kina (Evechinus Chloroticus) Shells. J. Taiwan Inst. Chem. Eng. 2016, 65, 437–443. [Google Scholar] [CrossRef]
- Ma, Q.; Rubenis, K.; Sigurjónsson, Ó.E.; Hildebrand, T.; Standal, T.; Zemjane, S.; Locs, J.; Loca, D.; Haugen, H.J. Eggshell-Derived Amorphous Calcium Phosphate: Synthesis, Characterization and Bio-Functions as Bone Graft Materials in Novel 3d Osteoblastic Spheroids Model. Smart Mater. Med. 2023, 4, 522–537. [Google Scholar] [CrossRef]
- Sasireka, A.; Renji, R.; Mohan Raj, R.; Vignesh, S.; Raj, V.; Ashraf, I.M.; Shkir, M. Exploration on in Vitro Bioactivity, Antibacterial Activity and Corrosion Behavior of Strontium Doped Hydroxyapatite Reinforced Chitosan-Polypyrrole/Tnt for Bone Regeneration. Inorg. Chem. Commun. 2022, 142, 109621. [Google Scholar] [CrossRef]
- Chen, T.; Wu, X.; Zhang, P.; Wu, W.; Dai, H.; Chen, S. Strontium-Doped Hydroxyapatite Coating Improves Osteo/Angiogenesis for Ameliorative Graft-Bone Integration Via the Macrophage-Derived Cytokines-Mediated Integrin Signal Pathway. ACS Appl. Mater. Interfaces 2024, 16, 15687–15700. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.Q.; Gangadoo, S.; Lu, Z.; Berndt, C.C.; Newsom, E.T.; Zreiqat, H.; Truong, V.K.; Ang, A.S.M. Strontium-Doped Hardystonite Plasma Sprayed Coatings with Robust Antimicrobial Activity. Mater. Today Chem. 2022, 24, 100822. [Google Scholar] [CrossRef]
- Jamarun, N.; Afriska, L.N.; Wellia, D.V.; Prasejati, A.; Amirullah, T.Y.; Wulandari, W.; Trycahyani, N.A. Investigation of the Antibacterial Activity of Synthesized Hydroxyapatite Sr-Doped Nanocomposite; JAPS: Gwalior, India, 2024; Volume 14, pp. 264–269. [Google Scholar]
- Hassanain, M.; Abdel-Ghafar, H.M.; Hamouda, H.I.; El-Hosiny, F.I.; Ewais, E.M.M. Enhanced Antimicrobial Efficacy of Hydroxyapatite-Based Composites for Healthcare Applications. Sci. Rep. 2024, 14, 26426. [Google Scholar] [CrossRef]
- Noori, A.; Hoseinpour, M.; Kolivand, S.; Lotfibakhshaiesh, N.; Ebrahimi-Barough, S.; Ai, J.; Azami, M. Exploring the Various Effects of Cu Doping in Hydroxyapatite Nanoparticle. Sci. Rep. 2024, 14, 3421. [Google Scholar] [CrossRef]
- Prabha, R.D.; Ding, M.; Bollen, P.; Ditzel, N.; Varma, H.K.; Nair, P.D.; Kassem, M. Strontium Ion Reinforced Bioceramic Scaffold for Load Bearing Bone Regeneration. Mater. Sci. Eng. C 2020, 109, 110427. [Google Scholar] [CrossRef]
- Sheng, X.; Li, C.; Wang, Z.; Xu, Y.; Sun, Y.; Zhang, W.; Liu, H.; Wang, J. Advanced Applications of Strontium-Containing Biomaterials in Bone Tissue Engineering. Mater. Today Bio 2023, 20, 100636. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Li, M.; Wang, S.; Fu, J.; Yang, M.; Yan, C.; Liu, Y.; Zheng, Y. Dose-Dependent Enhancement of in Vitro Osteogenic Activity on Strontium-Decorated Polyetheretherketone. Sci. Rep. 2025, 15, 3063. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Boanini, E.; Gazzano, M.; Giardino, R.; Bigi, A. Interaction of Sr-Doped Hydroxyapatite Nanocrystals with Osteoclast and Osteoblast-Like Cells. J. Biomed. Mater. Res. Part A 2009, 89, 594–600. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhi, W.; Xiao, D.; Li, H.; Duan, K.; Qu, S.; Weng, J. The Synergistic Effect of Micro/Nano-Structured and Cu2+-Doped Hydroxyapatite Particles to Promote Osteoblast Viability and Antibacterial Activity. Biomed. Mater. 2017, 12, 035006. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Cheng, Y.; Ma, L.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Bao, H.; Li, X.; et al. Nanosized Strontium Substituted Hydroxyapatite Prepared from Egg Shell for Enhanced Biological Properties. J. Biomater. Appl. 2018, 32, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Kundu, B.; Chanda, A.; Nandi, S.K. Mg63 Osteoblast Cell Response on Zn Doped Hydroxyapatite (Hap) with Various Surface Features. Ceram. Int. 2017, 43, 3752–3760. [Google Scholar] [CrossRef]
- Huang, H.; Qiang, L.; Fan, M.; Liu, Y.; Yang, A.; Chang, D.; Li, J.; Sun, T.; Wang, Y.; Guo, R.; et al. 3d-Printed Tri-Element-Doped Hydroxyapatite/Polycaprolactone Composite Scaffolds with Antibacterial Potential for Osteosarcoma Therapy and Bone Regeneration. Bioact. Mater. 2024, 31, 18–37. [Google Scholar] [CrossRef]
- Terra, J.; Dourado, E.R.; Eon, J.-G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The Structure of Strontium-Doped Hydroxyapatite: An Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef]
- Kawabata, K.; Yamamoto, T. First-Principles Calculations of the Elastic Properties of Hydroxyapatite Doped with Divalent Ions. J. Ceram. Soc. Jpn. 2010, 118, 548–549. [Google Scholar] [CrossRef][Green Version]
- Klinkla, R.; Kaewmaraya, T.; Bootchanon, A.; Saisopa, T.; Fongkaew, I.; Yimnirun, R.; Khamkongkaeo, A.; Rattanachai, Y.; Sailuam, W. Effects of Sr and Mg Doping on Elastic, Mechanical, and Optical Properties of Hydroxyapatite: A First-Principles Study. Results Phys. 2024, 57, 107352. [Google Scholar] [CrossRef]
- Pal, A.; Nasker, P.; Paul, S.; Roy Chowdhury, A.; Sinha, A.; Das, M. Strontium Doped Hydroxyapatite from Mercenaria Clam Shells: Synthesis, Mechanical and Bioactivity Study. J. Mech. Behav. Biomed. Mater. 2019, 90, 328–336. [Google Scholar] [CrossRef]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Abdullah, H. Synthesis and Characterizations of Natural Limestone-Derived Nano-Hydroxyapatite (Hap): A Comparison Study of Different Metals Doped Haps on Antibacterial Activity. RSC Adv. 2021, 11, 15896–15904. [Google Scholar] [CrossRef]
- Oladipupo, O.F.; Adekola, A.H.; Ofudje, E.A.; Al-Ahmary, K.M.; Al-Mhyawi, S.R.; Alshdoukhi, I.F.; Alrahili, M.R.; Alsaiari, A.A. Eggshell Derived Scaffold of Hydroxyapatite-Ammonium Bicarbonate Nano-Composite: Bioactivity and Cytotoxicity Studies. Heliyon 2024, 10, e36493. [Google Scholar] [CrossRef] [PubMed]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V.M. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and in Vitro Bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Akobundu, U.U.; Ifijen, I.H.; Duru, P.; Igboanugo, J.C.; Ekanem, I.; Fagbolade, M.; Ajayi, A.S.; George, M.; Atoe, B.; Matthews, J.T. Exploring the Role of Strontium-Based Nanoparticles in Modulating Bone Regeneration and Antimicrobial Resistance: A Public Health Perspective. RSC Adv. 2025, 15, 10902–10957. [Google Scholar] [CrossRef] [PubMed]
- Ngece, K.; Khwaza, V.; Paca, A.M.; Aderibigbe, B.A. The Antimicrobial Efficacy of Copper Complexes: A Review. Antibiotics 2025, 14, 516. [Google Scholar] [CrossRef]
- Golovchak, R.; Mahlovanyi, B.; Shpotyuk, Y.; Kus-Liskiewicz, M.; Kozianska, J.; Zadrag-Tecza, R.; Zagula, G.; Trzyna-Sowa, M.; Kovalskiy, A.; Gala-Bladzinska, A.; et al. Copper Strontium Phosphate Glasses with High Antimicrobial Efficacy. Sci. Rep. 2025, 15, 4677. [Google Scholar] [CrossRef]
- Safarova, Y.; Nessipbekova, A.; Syzdykova, A.; Olzhayev, F.; Umbayev, B.; Kassenova, A.; Fadeeva, I.V.; Askarova, S.; Rau, J.V. Strontium- and Copper-Doped Ceramic Granules in Bone Regeneration-Associated Cellular Processes. J. Funct. Biomater. 2024, 15, 352. [Google Scholar]
- Wan, B.; Wang, R.; Sun, Y.; Cao, J.; Wang, H.; Guo, J.; Chen, D. Building Osteogenic Microenvironments with Strontium-Substituted Calcium Phosphate Ceramics. Front. Bioeng. Biotechnol. 2020, 8, 591467. [Google Scholar] [CrossRef]
- Alashi, S.; Alkhouri, I.; Alghoraibi, I.; Kochaji, N.; Houri, A.; Karkoutly, M. Evaluating Various Properties of Nanohydroxyapatite Synthesized from Eggshells and Dual-Doped with Si4+ and Zn2+: An in Vitro Study. Heliyon 2024, 10, e35907. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Liao, Z.; Wei, Y.; Hang, R.; Huang, D. Engineered Functional Doped Hydroxyapatite Coating on Titanium Implants for Osseointegration. J. Mater. Res. Technol. 2023, 27, 122–152. [Google Scholar] [CrossRef]

| Source | Code |
|---|---|
| Eggshell (C) | HAp_C |
| HAp_Cu_1_C | |
| HAp_Cu_5_C | |
| HAp_Sr_1_C | |
| HAp_Sr_5_C | |
| HAp_S | |
| Mussel shells (S) | HAp_Cu_1_S |
| HAp_Cu_5_S | |
| HAp_Sr_1_S | |
| HAp_Sr_5_S |
| Code | GOF | R Values | Lattice Parameters | ||||
|---|---|---|---|---|---|---|---|
| Rexp (%) | Rwp (%) | Rp (%) | Unit Cell a (Ȧ) | Unit Cell c (Ȧ) | Unit Cell Volume (Ȧ3) | ||
| HAp_C | 2.657 | 8.596 | 14.013 | 11.616 | 9.429 | 6.880 | 529.762 |
| HAp_Cu_1_C | 1.062 | 13.308 | 13.718 | 11.275 | 9.434 | 6.877 | 530.176 |
| HAp_Cu_5_C | 0.965 | 12.698 | 12.480 | 10.272 | 9.435 | 6.874 | 530.076 |
| HAp_Sr_1_C | 2.242 | 7.892 | 11.818 | 9.707 | 9.430 | 6.889 | 530.657 |
| HAp_Sr_5_C | 1.600 | 6.725 | 8.508 | 6.754 | 9.454 | 6.898 | 534.699 |
| HAp_S | 3.069 | 8.494 | 14.882 | 12.218 | 9.395 | 6.918 | 528.933 |
| HAp_Cu_1_S | 1.013 | 13.257 | 13.348 | 10.947 | 9.436 | 6.880 | 530.546 |
| HAp_Cu_5_S | 1.059 | 12.710 | 13.082 | 10.632 | 9.454 | 6.878 | 532.480 |
| HAp_Sr_1_S | 3.481 | 7.851 | 14.651 | 11.315 | 9.437 | 6.886 | 531.137 |
| HAp_Sr_5_S | 1.919 | 6.888 | 9.543 | 7.678 | 9.453 | 6.897 | 534.638 |
| Code | Phase Content (%) | Crystal System | Space Group | Crystallinity (%) | Average Crystallite Size (nm) | |
|---|---|---|---|---|---|---|
| HAp | Ca(OH)2 | |||||
| HAp_C | 92.2 | 7.8 | Hexagonal | P63/m | 50.6 | 34.4 |
| HAp_Cu_1_C | 100 | - | Hexagonal | P63/m | 36.22 | 23.3 |
| HAp_Cu_5_C | 100 | - | Hexagonal | P63/m | 41.92 | 36.6 |
| HAp_Sr_1_C | 100 | - | Hexagonal | P63/m | 52.08 | 21.9 |
| HAp_Sr_5_C | 100 | - | Hexagonal | P63/m | 54.53 | 24.2 |
| HAp_S | 100 | - | Hexagonal | P63/m | 49.92 | 35.4 |
| HAp_Cu_1_S | 100 | - | Hexagonal | P63/m | 34.4 | 28.1 |
| HAp_Cu_5_S | 100 | - | Hexagonal | P63/m | 30.4 | 35.5 |
| HAp_Sr_1_S | 80.9 | 19.1 | Hexagonal | P63/m | 51.53 | 57.3 |
| HAp_Sr_5_S | 100 | - | Hexagonal | P63/m | 52.68 | 25.6 |
| Sample | Cu | Sr |
|---|---|---|
| (wt. %) | (wt. %) | |
| HAp_C | n.d. | 0.03 |
| HAp_S | n.d. | 0.08 |
| HAp_Cu_1_C | 0.76 | 0.03 |
| HAp_Cu_1_S | 0.77 | 0.10 |
| HAp_Cu_5_C | 3.29 | 0.03 |
| HAp_Cu_5_S | 3.58 | 0.09 |
| HAp_Sr_1_C | n.d. | 1.04 |
| HAp_Sr_1_S | n.d. | 1.23 |
| HAp_Sr_5_C | n.d. | 6.34 |
| HAp_Sr_5_S | n.d. | 5.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, D.-E.; Vasile, B.S.; Vasile, O.R.; Neacsu, I.A.; Trusca, R.D.; Surdu, V.-A.; Birca, A.C.; Dolete, G.; Ilie, C.-I.; Andronescu, E. Microwave-Assisted Hydrothermal Synthesis of Cu/Sr-Doped Hydroxyapatite with Prospective Applications for Bone Tissue Engineering. J. Compos. Sci. 2025, 9, 427. https://doi.org/10.3390/jcs9080427
Radulescu D-E, Vasile BS, Vasile OR, Neacsu IA, Trusca RD, Surdu V-A, Birca AC, Dolete G, Ilie C-I, Andronescu E. Microwave-Assisted Hydrothermal Synthesis of Cu/Sr-Doped Hydroxyapatite with Prospective Applications for Bone Tissue Engineering. Journal of Composites Science. 2025; 9(8):427. https://doi.org/10.3390/jcs9080427
Chicago/Turabian StyleRadulescu, Diana-Elena, Bogdan Stefan Vasile, Otilia Ruxandra Vasile, Ionela Andreea Neacsu, Roxana Doina Trusca, Vasile-Adrian Surdu, Alexandra Catalina Birca, Georgiana Dolete, Cornelia-Ioana Ilie, and Ecaterina Andronescu. 2025. "Microwave-Assisted Hydrothermal Synthesis of Cu/Sr-Doped Hydroxyapatite with Prospective Applications for Bone Tissue Engineering" Journal of Composites Science 9, no. 8: 427. https://doi.org/10.3390/jcs9080427
APA StyleRadulescu, D.-E., Vasile, B. S., Vasile, O. R., Neacsu, I. A., Trusca, R. D., Surdu, V.-A., Birca, A. C., Dolete, G., Ilie, C.-I., & Andronescu, E. (2025). Microwave-Assisted Hydrothermal Synthesis of Cu/Sr-Doped Hydroxyapatite with Prospective Applications for Bone Tissue Engineering. Journal of Composites Science, 9(8), 427. https://doi.org/10.3390/jcs9080427











