Study on the Regulation Mechanism of Silane Coupling Agents’ Molecular Structure on the Rheological Properties of Fe3O4/CNT Silicone Oil-Based Magnetic Liquids
Abstract
1. Introduction
2. Experimental Materials and Instruments
2.1. Experimental Materials
2.2. Instruments
2.3. Material Preparation
3. Results and Discussion
3.1. Analysis of Material Preparation Results
3.1.1. TEM Characterization
3.1.2. XRD Characterization
3.1.3. IR Characterization
3.2. Rheological Property Analysis
3.2.1. Visco-Thermal Properties
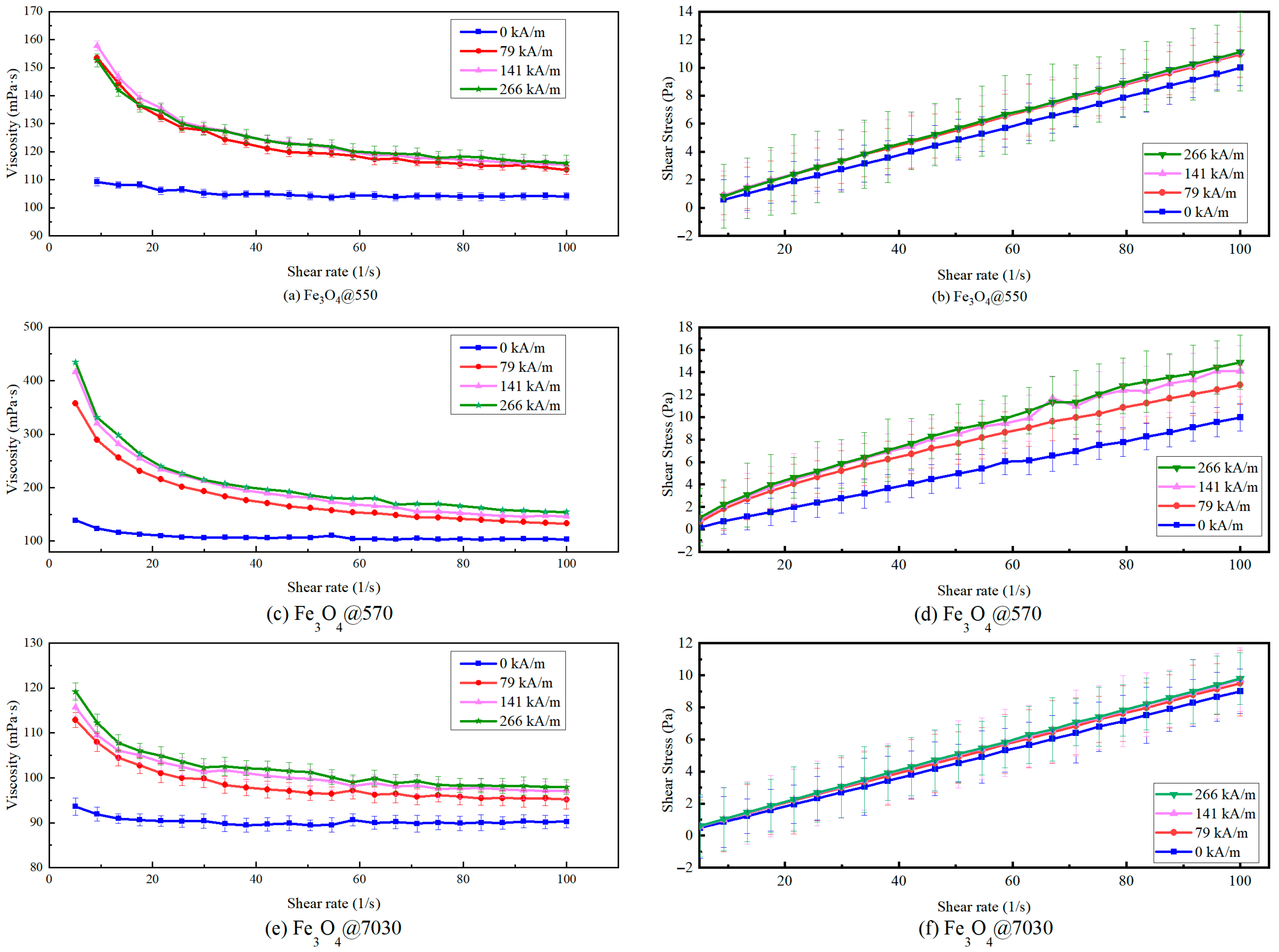
3.2.2. Magneto-Viscous Effect
3.2.3. Influence of Silane Coupling Agents on Shear-Thinning Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of Gadolinium on the Structure and Magnetic Properties of Nanocrystalline Powders of Iron Oxides Produced by the Extraction-Pyrolytic Method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef]
- Khonina, T.G.; Demin, A.M.; Tishin, D.S.; Germov, A.Y.; Uimin, M.A.; Mekhaev, A.V.; Minin, A.S.; Karabanalov, M.S.; Mysik, A.A.; Bogdanova, E.A.; et al. Magnetic Nanocomposite Materials Based on Fe3O4 Nanoparticles with Iron and Silica Glycerolates Shell: Synthesis and Characterization. Int. J. Mol. Sci. 2023, 24, 12178. [Google Scholar] [CrossRef] [PubMed]
- Molinar-Díaz, J.; Woodliffe, J.L.; Steer, E.; Morley, N.A.; Brown, P.D.; Ahmed, I. Optimisation of the Flame Spheroidisation Process for the Rapid Manufacture of Fe3O4-Based Porous and Dense Microspheres. Molecules 2023, 28, 2523. [Google Scholar] [CrossRef] [PubMed]
- Stephen, P.S. Low Viscosity Magnetic Fluid Obtained by the Colloidal Suspension of Magnetic Particles. U.S. Patent US3215572 A, 2 November 1965. [Google Scholar]
- Xiaolong, Y.; Decai, L.I.; Wenming, Y.; Xing, F.; Cui, H. Magnetic field finite element analysis and experimental verification of magnetic fluid seal with large gap. J. Beijing Jiaotong Univ. 2013, 37, 181–184. [Google Scholar]
- Li, L.; Li, D.; Wang, L.; Liang, Z.; Zhang, Z. Stable silicone oil based ferrofluids with well low-temperature fluidity applied to magnetic fluid seal. J. Magn. Magn. Mater. 2023, 584, 171077. [Google Scholar] [CrossRef]
- Sharyna, S.; Krakov, M. Interplay between magnetophoresis and diffusion in magnetic fluid seals for vacuum devices and their lifespan. Vacuum 2025, 234, 114122. [Google Scholar] [CrossRef]
- Wan-Li, S.; Seung-Bok, C.; Myeong-Woo, C. Lubrication property of magnetorheological fluid with nano-silica additives under magnetic field. J. Iron Steel Res. Engl. Ed. 2012, 5, 519–523. [Google Scholar]
- Rui, H.; Hansheng, C.; Haoran, Z.; Humao, W.; Junpeng, G.; Songshan, Y.; Hougen, L.; Yi, G.; Chunxia, X.; Jianwu, Y.; et al. Research on Magnetic Fluid Precise Localized Lubrication Thrust Cylindrical Roller Bearing with Low-Friction Micro-Vibration and High Load-Bearing Capacity; IOP Publishing Ltd.: Bristol, UK, 2024; Volume 2853, p. 012016. [Google Scholar]
- Laru, R.; Dragoi, D.D. Inductive tilt sensor with magnets and magnetic fluid. Sens. Actuators A Phys. 2005, 120, 424–428. [Google Scholar]
- Hang, M.; Yang, W. Research on the sensing properties and vibration reduction performance of self-sensing self-tuning magnetic fluid damper. Meas. Sci. Technol. 2025, 36, 015121. [Google Scholar]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Yudhisthira, S.; Hillel, P.; Tcipi, F.; Dina, G.L.; BursteinChaim, N.; SukenikGil, M. Alkyl phosphonate/phosphate coating on magnetite nano-particles: A comparison with fatty acids. Langmuir 2001, 17, 7907–7911. [Google Scholar]
- Durdureanu-Angheluta, A.; Ardeleanu, R.; Pinteala, M.; Harabagiu, V.; Chiriacc, H.; Simionescu, B.C. Silane covered magnetite particles. preparation and characterisation. Dig. J. Nanomater. Biostruct. 2008, 3, 33–40. [Google Scholar]
- Durdureanu-Angheluta, A.; Pricop, L.; Stoica, I.; Peptu, C.-A.; Dascalu, A.; Marangoci, N.; Doroftei, F.; Chiriac, H.; Pinteala, M.; Simionescu, B.C. Synthesis and characterization of magnetite particles covered with α-trietoxysilil-polydimethylsiloxane. J. Magn. Magn. Mater. 2010, 322, 2956–2968. [Google Scholar] [CrossRef]
- Wilson, K.S.; Goff, J.D.; Riffle, J.S.; Harris, L.A.; Pierre, T.G.S. Polydimethylsiloxane-magnetite nanoparticle complexes and dispersions in polysiloxane carrier fluids. Polym. Adv. Technol. 2005, 16, 200–211. [Google Scholar] [CrossRef]
- Chen, H.J.; Wang, Y.M.; Qu, J.M.; Hong, R.Y.; Li, H.Z. Preparation andcharacterization of silicone oil based ferrofluid. Appl. Surf. Sci. 2011, 257, 10802–10807. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Fakharpour, M.; Salehi, S. Synthesis and study of electrochemical, optical, and magnetic properties of Fe3O4/MWCNT-COOH nanocomposite. Eur. Phys. J. Plus 2025, 140, 459. [Google Scholar] [CrossRef]
- Ali, A.M. Synthesis of magnetic MWCNTs nanohybrids and application in remediation of chromium ions in refinery wastewater. Eng. Technol. J. 2022, 35, 838–841. [Google Scholar] [CrossRef]
- Paidar, R.; Gholikandi, G.B.; Alighardashi, A.; Shahamat, Y.D.; Barzaki, H.R. Bio-ZnO/Fe3O4@MWCNTs magnetic nanocomposites promoted green synthesis of pyrrole derivatives and removal of cefixime and amoxiciline from aqueous solution. Polycycl. Aromat. Compd. 2024, 44, 1991–2007. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Chang, C. Effect of MWCNTs content on the magnetic and wave absorb-ing properties of ferrite-MWCNTs composites. Synth. Met. 2011, 161, 44–50. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Fu, Y.Q.; Zong, E.M.; Jiang, S.T.; Li, J.B.; Zhu, J.Q.; Zhu, Y.Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2021, 252, 117158. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Zhang, Z. Rheological and magnetic properties of stable poly alpha olefins based ferrofluids with high viscosity and magnetization. SSRN Electron. J. 2022, 564, 170096. [Google Scholar]
- Li, L.; Li, D.; Zhang, Z. Colloidal stability of magnetite nanoparticles coated by oleic acid and 3-(N,N-Dimethylmyristylammonio)propanesulfonate in solvents. Front. Mater. 2022, 9, 893072. [Google Scholar] [CrossRef]
- Shliomis, M.I. Effective viscosity of magnetic suspensions. J. Exp. Theor. Phys. 1972, 34, 1291–1294. [Google Scholar]

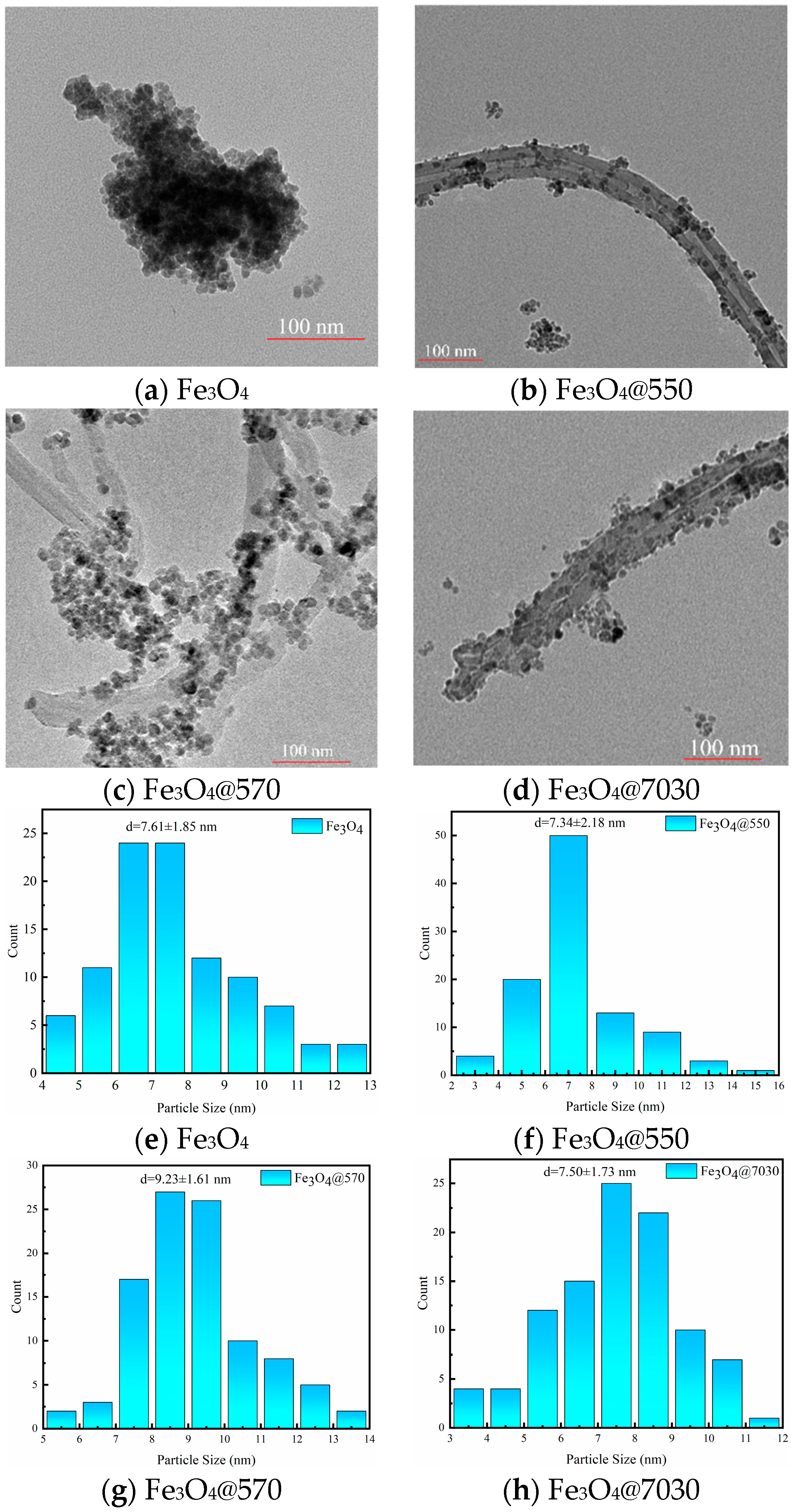

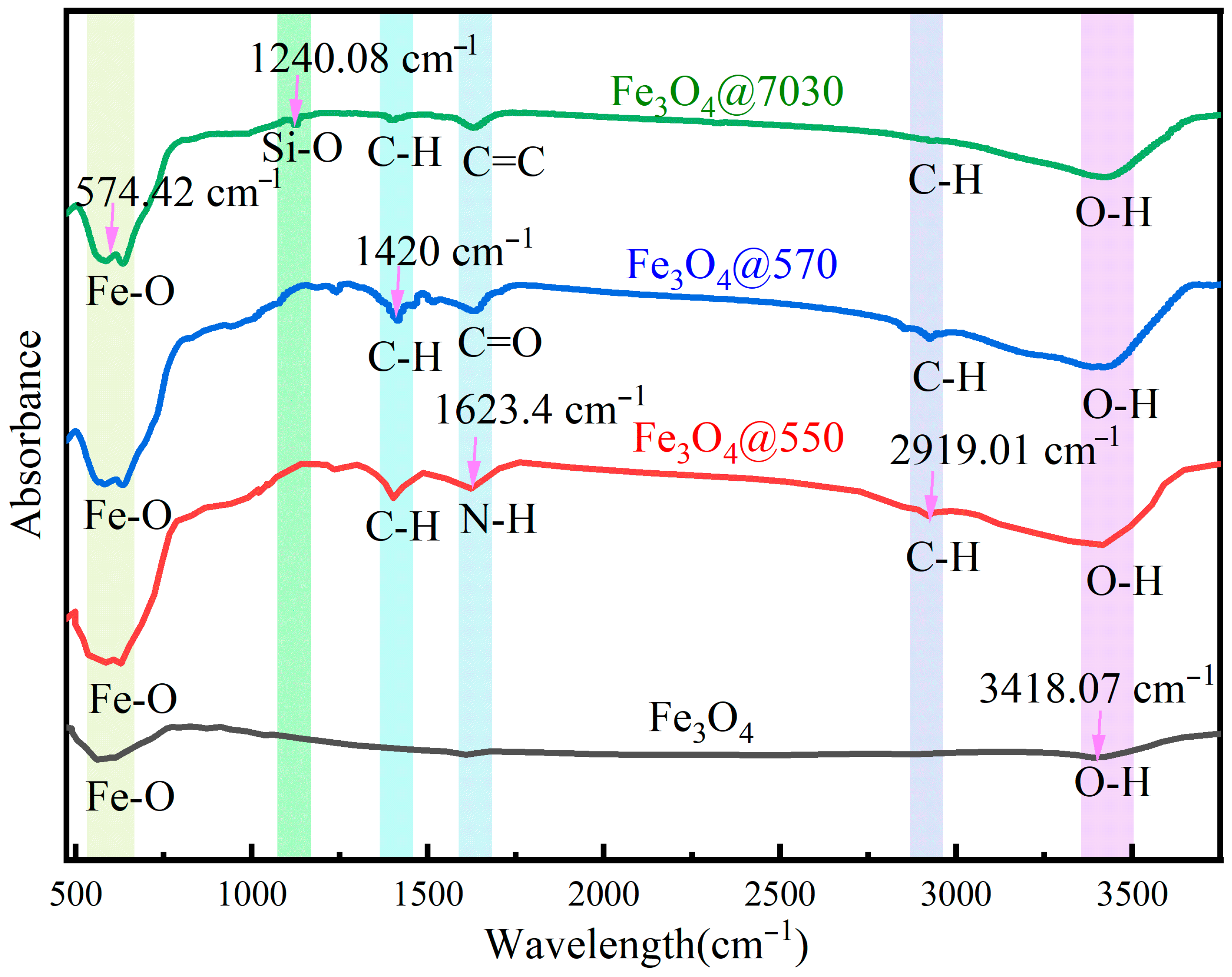
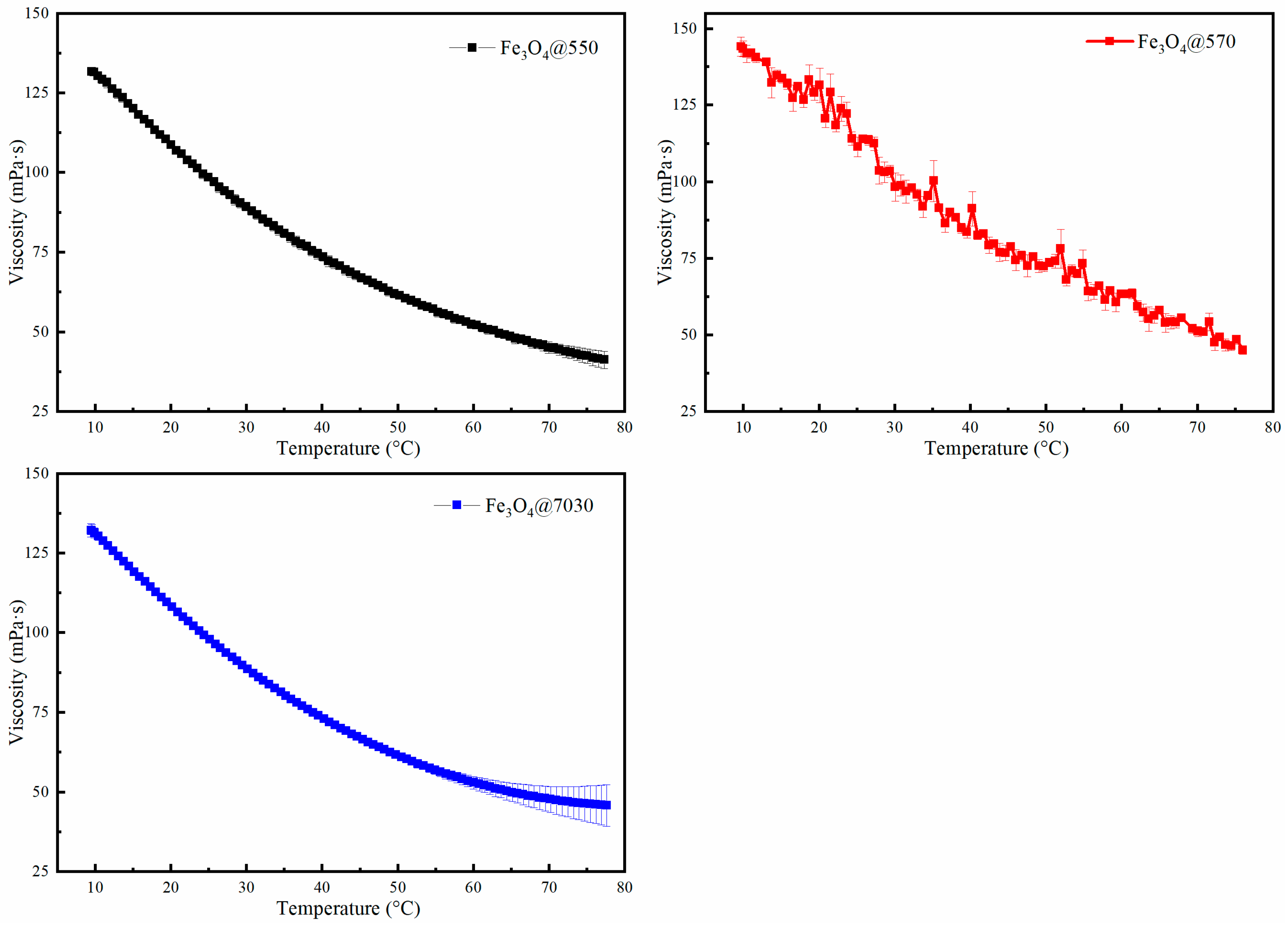
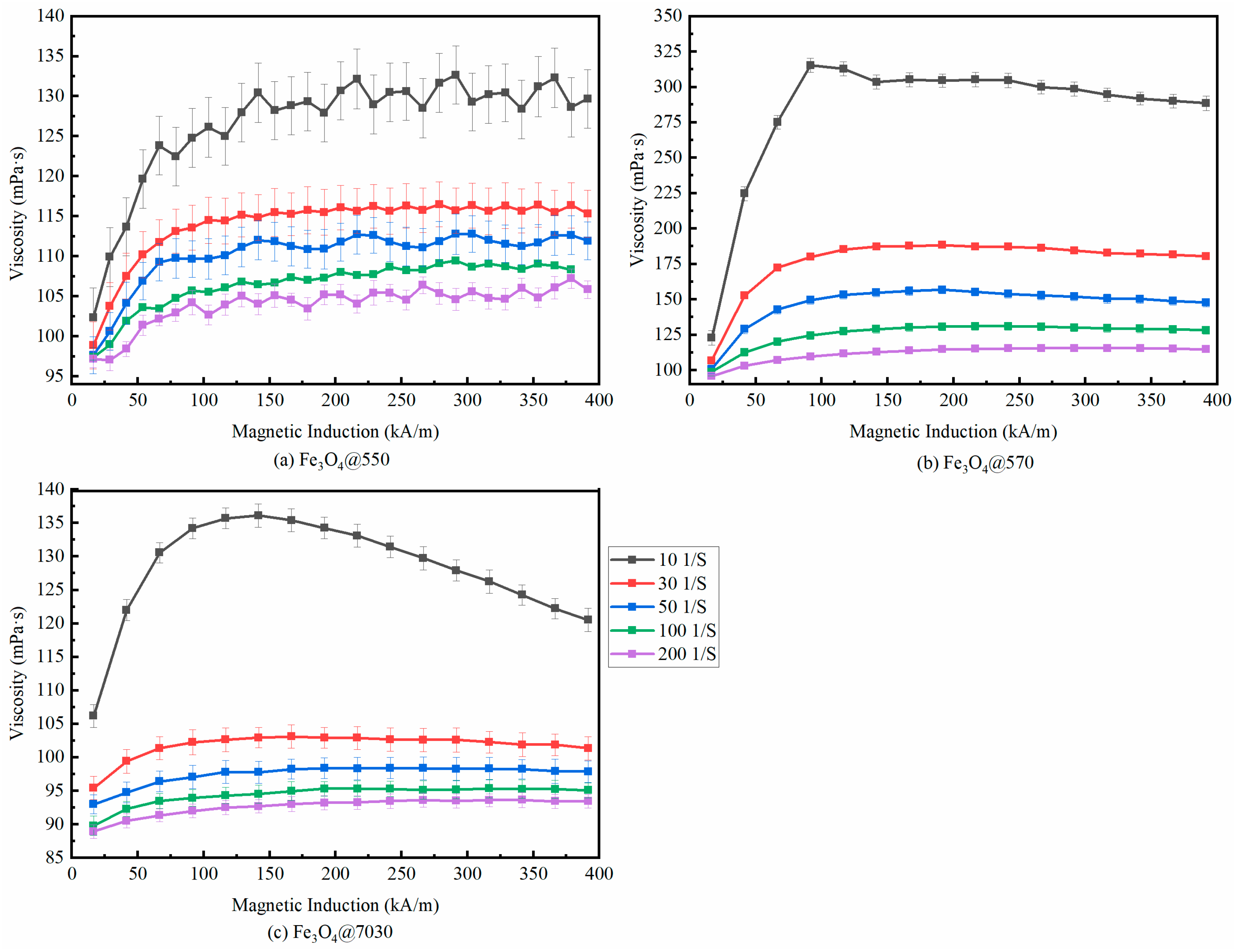
| Sample | (mPa·s) | (mPa·s) | (kJ/mol) |
|---|---|---|---|
| Fe3O4@550 | 119.9 | 43.75 | 14.26 |
| Fe3O4@570 | 133.96 | 50.10 | 13.63 |
| Fe3O4@7030 | 119.07 | 44.90 | 13.56 |
| Shear Rate (s−1) | Fe3O4@550 | Fe3O4@570 | Fe3O4@7030 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Max (mPa·s) | Min (mPa·s) | Change in Value | Max (mPa·s) | Min (mPa·s) | Change in Value | Max (mPa·s) | Min (mPa·s) | Change in Value | |
| 10 | 132.7 | 102.3 | 29.7% | 311.8 | 119.27 | 161.4% | 135.99 | 106.11 | 28.2% |
| 30 | 116.36 | 98.87 | 17.7% | 188.36 | 106.71 | 76.5% | 103.04 | 95.45 | 8.0% |
| 50 | 112.91 | 97.67 | 15.6% | 156.63 | 100.35 | 56.1% | 98.37 | 92.86 | 6.0% |
| 100 | 109.53 | 97.59 | 12.2% | 130.67 | 98.28 | 33.0% | 95.51 | 90 | 6.1% |
| 200 | 107.21 | 97.29 | 10.12% | 115.62 | 95.73 | 20.8% | 93.66 | 89.01 | 5.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zeng, X.; Yang, S.; Wang, B.; Tian, X.; Shen, W. Study on the Regulation Mechanism of Silane Coupling Agents’ Molecular Structure on the Rheological Properties of Fe3O4/CNT Silicone Oil-Based Magnetic Liquids. J. Compos. Sci. 2025, 9, 423. https://doi.org/10.3390/jcs9080423
Li W, Zeng X, Yang S, Wang B, Tian X, Shen W. Study on the Regulation Mechanism of Silane Coupling Agents’ Molecular Structure on the Rheological Properties of Fe3O4/CNT Silicone Oil-Based Magnetic Liquids. Journal of Composites Science. 2025; 9(8):423. https://doi.org/10.3390/jcs9080423
Chicago/Turabian StyleLi, Wenyi, Xiaotong Zeng, Shiyu Yang, Bingxue Wang, Xiangju Tian, and Weihao Shen. 2025. "Study on the Regulation Mechanism of Silane Coupling Agents’ Molecular Structure on the Rheological Properties of Fe3O4/CNT Silicone Oil-Based Magnetic Liquids" Journal of Composites Science 9, no. 8: 423. https://doi.org/10.3390/jcs9080423
APA StyleLi, W., Zeng, X., Yang, S., Wang, B., Tian, X., & Shen, W. (2025). Study on the Regulation Mechanism of Silane Coupling Agents’ Molecular Structure on the Rheological Properties of Fe3O4/CNT Silicone Oil-Based Magnetic Liquids. Journal of Composites Science, 9(8), 423. https://doi.org/10.3390/jcs9080423





