Crystallization Studies of Poly(Trimethylene Terephthalate) Nanocomposites—A Review
Abstract
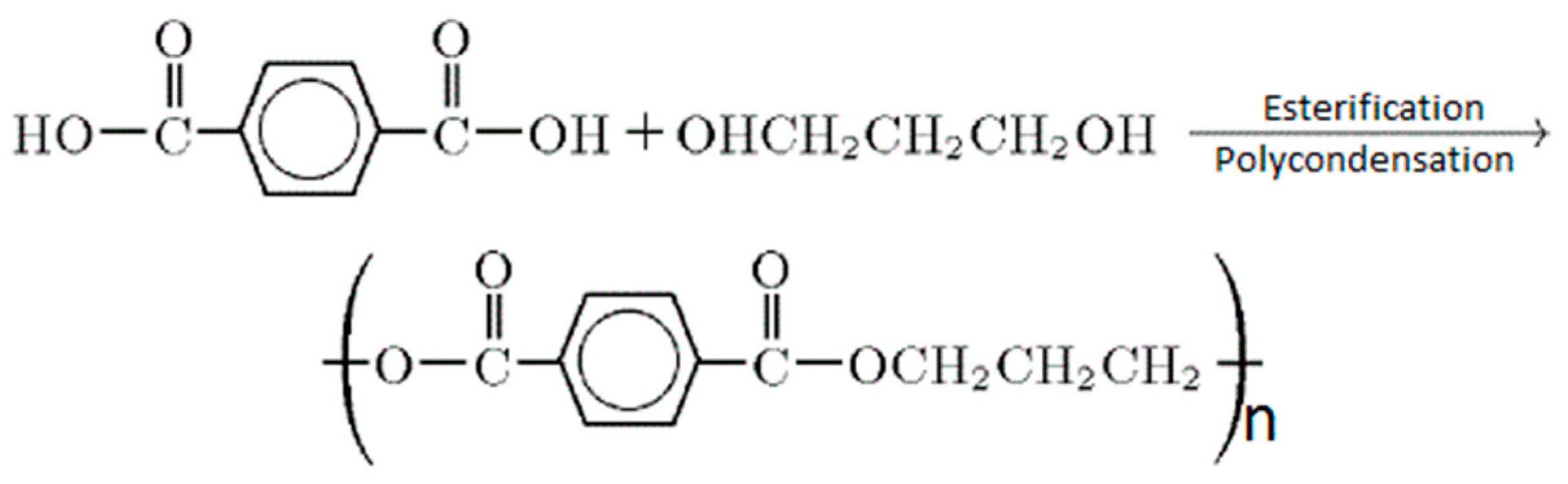
1. Introduction
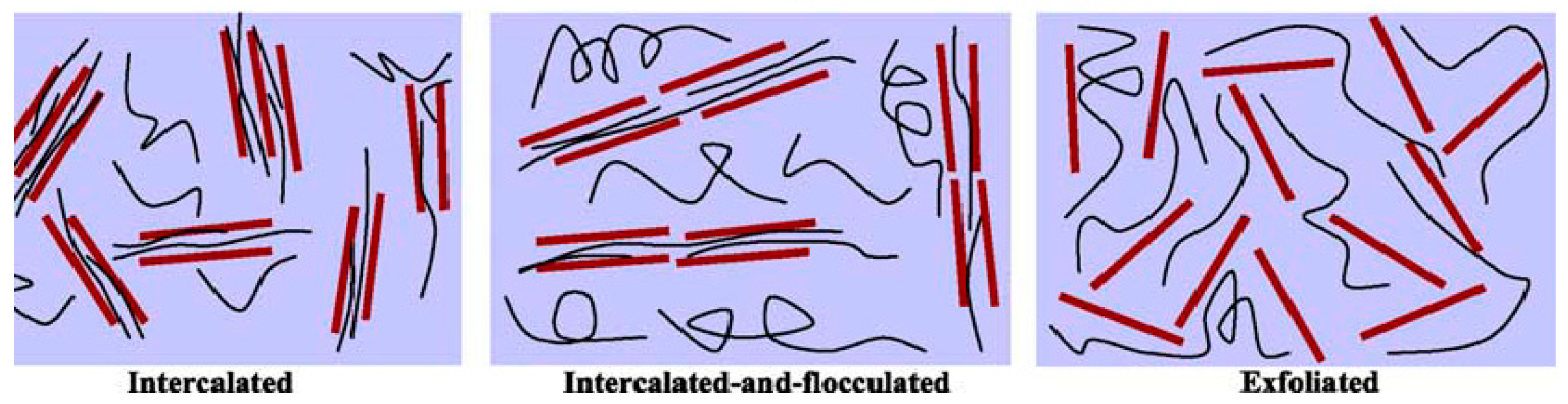
2. Nanocomposite
3. PTT Nanocomposites
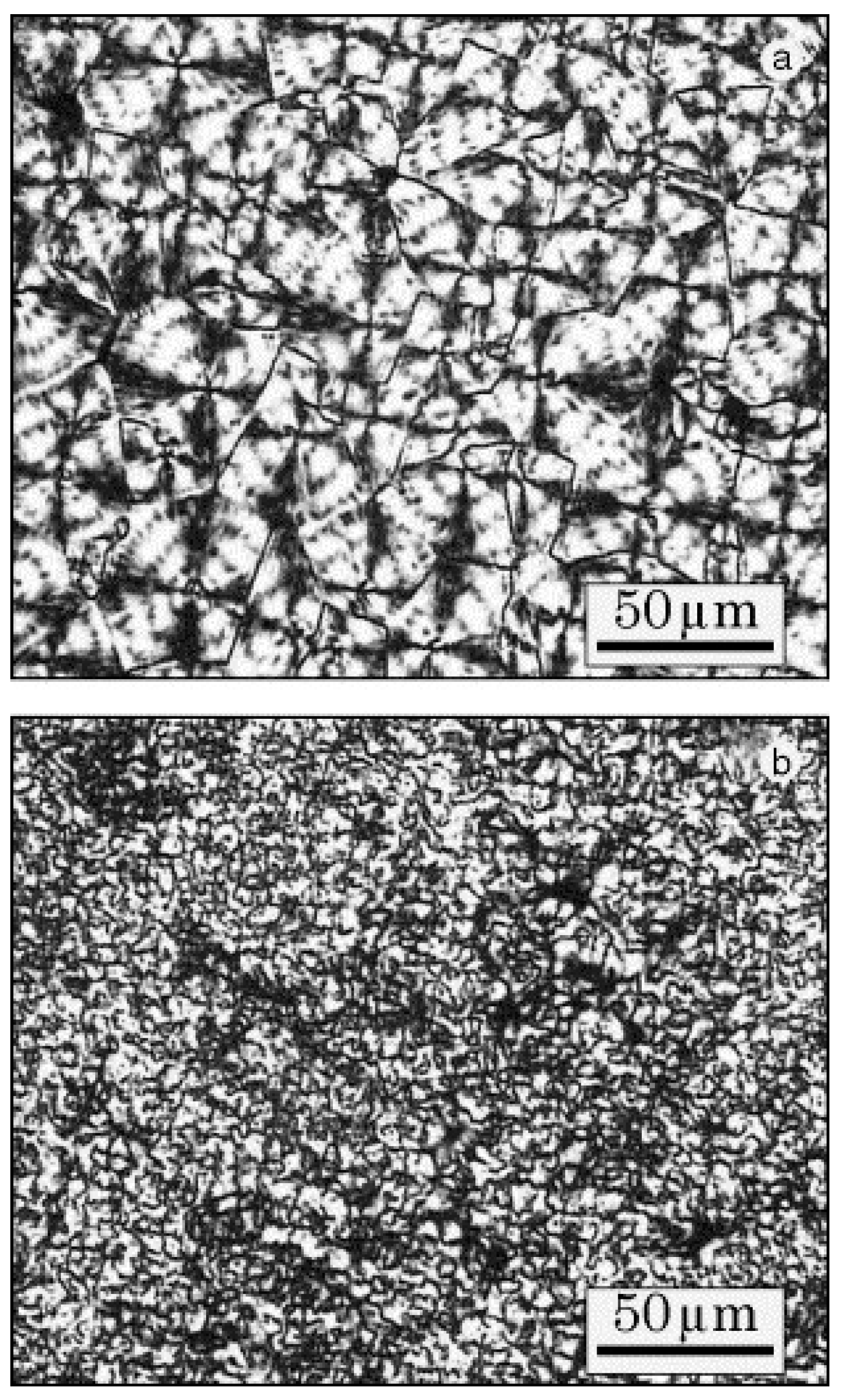
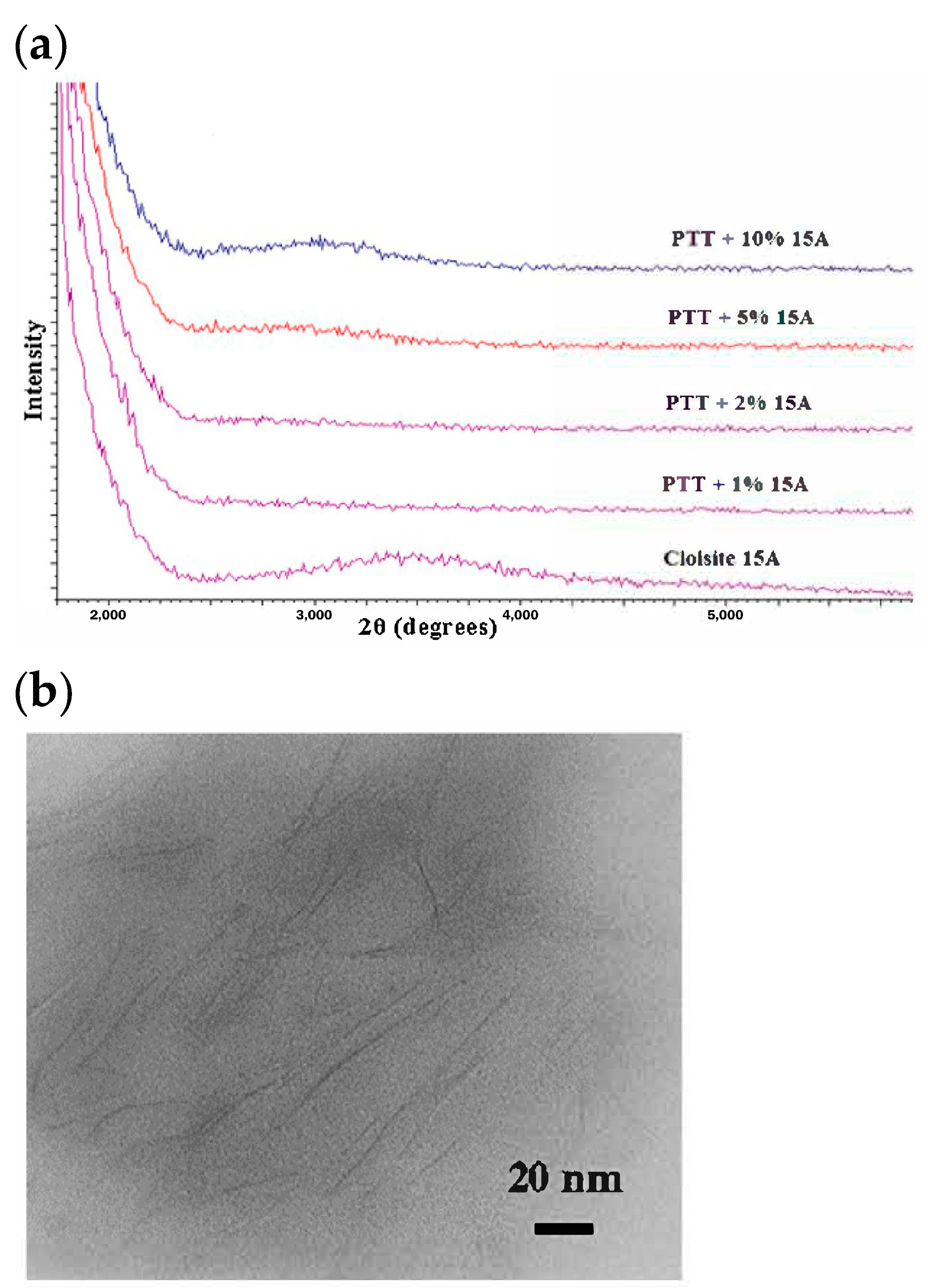
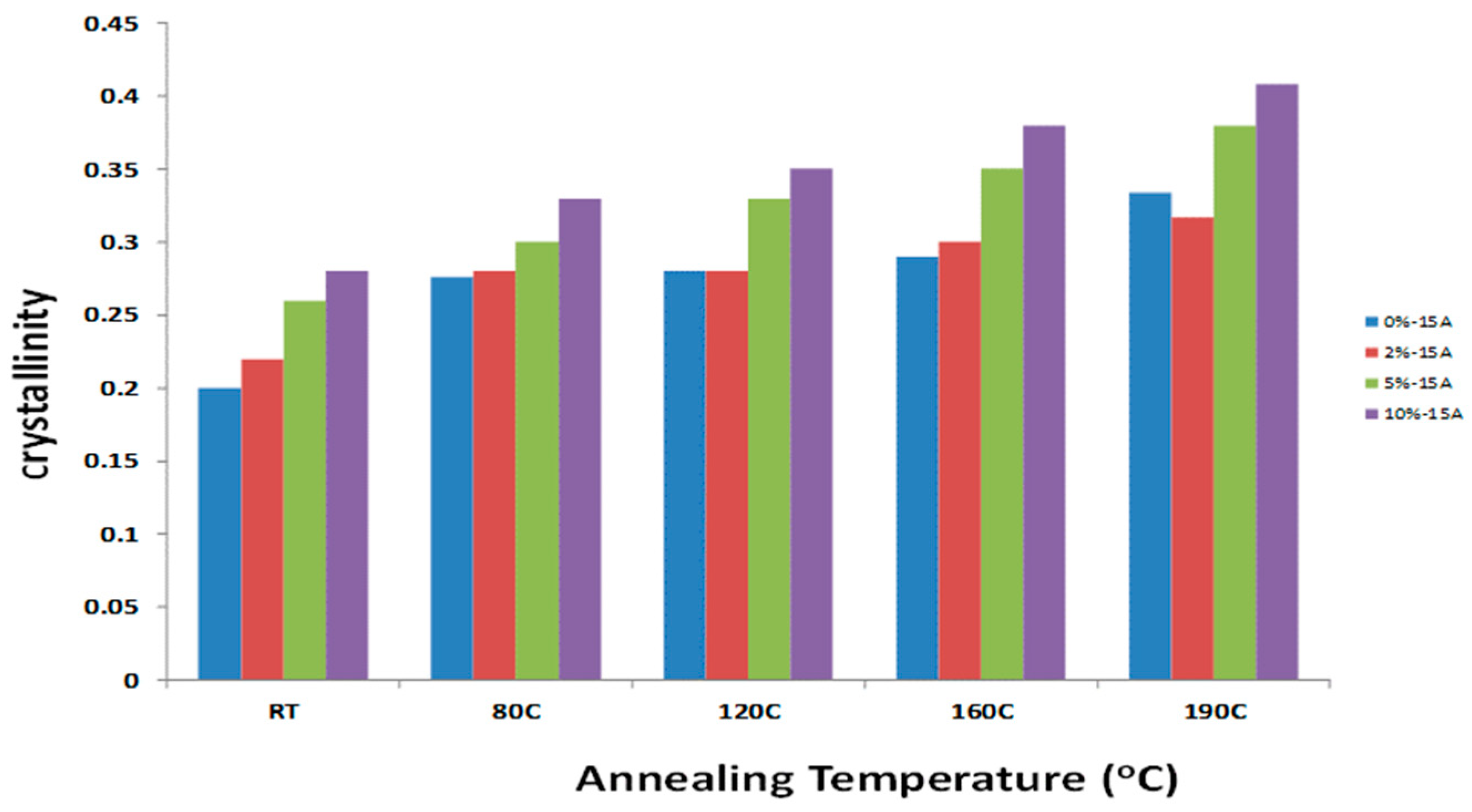
3.1. PTT/Orgnoclay Nanocomposites
3.2. PTT/ Mesoporous Silica Nanocomposite
3.3. PTT/Inorganic Nanocomposite
4. Conclusions
Funding
Conflicts of Interest
References
- Chuah, H.H. Crystallization kinetics of poly(trimethylene terephthalate). Polym. Eng. Sci. 2001, 41, 308–313. [Google Scholar] [CrossRef]
- Ward, I.M.; Wilding, M.A.; Brody, H.J. The mechanical properties and structure of poly(m-methylene terephthalate) fibers. J. Polym. Sci. Polym. Phys. 1976, 14, 263–274. [Google Scholar] [CrossRef]
- Whinfield, J.R.; Dikson, J.T. Improvements Relating to the Manufacture of Highly Polymeric Substances. British Patent 578079, 14 June 1946. [Google Scholar]
- Wang, B.W.; Li, C.Y.; Hanzlicek, J.; Cheng, S.Z.D.; Geil, P.H.; Grebowicz, J.; Ho, R.M. Poly(trimethylene teraphthalate) Crystal structure and Morphology in Different Length Scales. Polymer 2001, 42, 7171–7178. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.C.; Kim, Y.H. Structural Changes of Poly(trimethylene terephthalate) Film upon Uniaxial and Biaxial Drawing. Macromolecules 2000, 33, 7994–8001. [Google Scholar] [CrossRef]
- Kim, K.J.; Bae, J.H.; Kim, Y.H. Infrared Spectroscopic Analysis of Poly(trimethylene terephthalate). Polymer 2001, 42, 1023–1033. [Google Scholar] [CrossRef]
- Chuah, H.H. Orientation and Structure Development in Poly(trimethylene terephthalate) Tensile Drawing. Macromolecules 2001, 34, 6985–6993. [Google Scholar] [CrossRef]
- Park, S.C.; Liang, Y.; Lee, H.S. Quantitative Analysis Method for Three-dimensional Orientation of PTT by Polarized FTIR-ATR Spectroscopy. Macromolecules 2004, 37, 5607–5614. [Google Scholar] [CrossRef]
- Chuah, H.H. Synthesis, properties and applications of poly(trimethylene terephthalate). In Modern Polyester: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; Wiley: Chichester, UK, 2004; pp. 361–397. [Google Scholar]
- Eberl, A.; Heumann, S.; Kotek, R.; Kaufmann, F.; Mitsche, S.; Cavaco-Paulo, A.; Gübitz, G.M. Enzymatic hydrolysis of PTT polymers and oligomers. J. Biotechnol. 2008, 135, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jakeways, R.; Ward, I.M.; Wilding, M.A.; Desborough, I.J.; Pass, M.G. Crystal Deformation in Aromatic Polyesters. J. Polym. Sci. Polym. Phys. 1975, 13, 799–813. [Google Scholar] [CrossRef]
- Luo, W.; Liao, Z.; Yan, J.; Li, Y.; Chen, X.; Mai, K.; Zhang, M. Photoinduced Energy Transfer in Poly(trimethylene terephthalate). Macromolecules 2008, 41, 3912–3918. [Google Scholar] [CrossRef]
- Chuah, H.H. Effect of process variables on bulk development of air-textured poly(trimethylene terephthalate) bulk continuous filament. J. Appl. Polym. Sci. 2004, 92, 1011–1017. [Google Scholar] [CrossRef]
- Wu, J.; Schultz, J.M.; Samon, J.M.; Pangelinan, A.B.; Chuah, H.H. In situ study of structure development in poly(trimethylene terephthalate) fibers during stretching by simultaneous synchrotron small- and wide-angle X-ray scattering. Polymer 2001, 42, 7141–7451. [Google Scholar] [CrossRef]
- Pyda, M.; Boller, A.; Grebowicz, J.; Chuah, H.; Lebedev, B.V.; Wunderlich, B. Heat Capacity of Poly(trimethylene terephthalate). J. Polym. Sci. Polym. Phys. 1998, 36, 2499–2511. [Google Scholar] [CrossRef]
- Gonzalez, C.C.; Perena, J.M.; Bello, A. Dynamic Mechanical Relaxations of Polyterephthalates Based on Trimethylene Glycol. J. Polym. Sci. Polym. Phys. 1988, 26, 1397–1408. [Google Scholar] [CrossRef]
- Vasanthan, N.; Yamen, M. Crystallization Studies of Poly(trimethylene terephthalate) Using Thermal Analysis and Far Infrared spectroscopy. J. Polym. Sci. Polym. Phys. 2007, 45, 349–355. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Wu, Q.; Song, L.; Wu, G. Thermal Analysis of the Melting Process of Poly(trimethylene terephthalate) using FTIR micro-spectroscopy. Eur. Polym. J. 2005, 41, 2216–2223. [Google Scholar] [CrossRef]
- Hong, P.D.; Chuang, W.T.; Hsu, C.F. Crystallization kinetics and morphology of poly(trimethylene terephthalate). Polymer 2002, 43, 3335–3343. [Google Scholar] [CrossRef]
- Chuang, W.T.; Hong, P.D.; Chuah, H.H. Effects of crystallization behavior on morphological change in poly(trimethylene terephthalate) spherulites. Polymer 2004, 45, 2413–2425. [Google Scholar] [CrossRef]
- He, X.J.; Chuan, H.H.; Ellison, M.S. Raman spectroscopy study of poly(trimethylene terephthalate) crystallization. Polym. Bull. 2004, 51, 285–291. [Google Scholar] [CrossRef]
- Chuang, W.T.; Yeh, W.J.; Hong, P.D. Melting behavior of poly(trimethylene terephthalate). J. Appl. Polym. Sci. 2002, 83, 2426–2433. [Google Scholar] [CrossRef]
- Srimoaon, P.; Dangseeyun, N.; Supaphol, P. Multiple melting behavior in isothermally crystallized poly(trimethylene terephthalate). Eur. Polym. J. 2004, 40, 599–608. [Google Scholar] [CrossRef]
- Huang, J.M.; Chang, F.C. Crystallization kinetics of poly(trimethylene terephthalate). J. Polym. Sci. Polym. Phys. 2000, 38, 934–941. [Google Scholar] [CrossRef]
- Xue, M.L.; Sheng, J.; Yu, Y.L.; Chuah, H.H. Nonisothermal crystallization kinetics and spherulite morphology of poly(tremethylene terephthalate). Eur. Polym. J. 2004, 40, 811–818. [Google Scholar] [CrossRef]
- Desborough, I.J.; Hall, I.H.; Neisser, J.Z. The structure of poly(trimethylene terephthalate). Polymer 1979, 20, 545–552. [Google Scholar] [CrossRef]
- Poulin-Dandurand, S.; Perez, S.; Revol, J.F.; Brisse, F. The crystal structure of poly(trimethylene terephthalate) by X-ray and electron diffraction. Polymer 1979, 20, 419–426. [Google Scholar] [CrossRef]
- Yaman, M.; Ozkaya, S.; Vasanthan, N. Structural and conformational changes during thermally-induced crystallization of poly(trimethylene terephthalate) by infrared spectroscopy. J. Polym. Sci. Polym. Phys. 2008, 46, 1497–1504. [Google Scholar] [CrossRef]
- Vasanthan, N.; Ozkaya, S.; Yaman, M. Morphological and conformational changes of poly(trimethylene terephthalate) during isothermal melt Crystallization. J. Phys. Chem. B 2010, 114, 13069–13075. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, N. Crystallization and Solid-State Characterization of Poly(trimethylene terephthalate) and its Nanocomposites. In Poly Trimethylene Terephthalate: Based Blends, IPNs, Composites and Nanocomposites; Ajitha, A.R., Thomas, S., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer Nature: Singapore, 2023; pp. 129–147. [Google Scholar]
- Vasanthan, N.; Manne, N.J. Strain-induced crystallization and conformational transition of poly(trimethylene terephthalate) films during uniaxial deformation probed by polarized infrared spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 12596–12603. [Google Scholar] [CrossRef]
- Vasanthan, N.; Manne, N.J.; Krishnama, A. The effect of molecular orientation on cold crystallization of amorphous-crystallizable polymers: The case of poly(trimethylene terephthalate). Ind. Eng. Chem. Res. 2013, 52, 17920–17926. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/inorganic hybrid network materials by sol-gel Approach. Chem. Mater. 1996, 8, 1667–1669. [Google Scholar] [CrossRef]
- Tzavalaz, S.; Drakonakis, V.; Mouzakis, D.E.; Fischer, D.; Gregoriou, V.G. Effect of carboxy-functionalized mutiwall carbon nanotubes on the crystallization and chain conformation of poly(ethylene terephthalate) PET in PET-MWNT nanocomposites. Macromolecules 2006, 39, 9150–9156. [Google Scholar] [CrossRef]
- Sun, T.; Garces, J.M. High-performance polypropylene-clay nanocomposites by in-situ polymerization with metallocene/clay catalysts. Adv. Mater. 2002, 14, 128–130. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurachi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid by montmorillonite intercalated with ε-caprolactam. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 983–986. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Ginnelis, E.P. Polymer-layered silicate nanocomposites: In-situ intercalative polymerization of ∈-caprolactone in layered silicates. Chem. Mater. 1993, 5, 1064–1066. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Feng, M.; Zhang, Y.; Yang, M.; Shen, D. Hydrogen bonding in polyamide 66/clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2313–2321. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, K.; Okamoto, M. Structure-property relationship in biodegradable poly(butylene succinate)/layered silicate nanocomposites. Macromolecules 2003, 36, 2355–2367. [Google Scholar]
- Vasanthan, N.; Ly, H.; Ghosh, S. Impact of nanoclay on isothermal cold crystallization kinetics and polymorphism of poly (L -lactic acid) nanocomposites. J. Phys. Chem. 2011, 115, 9556–9562. [Google Scholar] [CrossRef]
- Linkoln, D.M.; Vaia, R.A.; Krishnamoorti, R. Isothermal crystallization of nylon-6/montmorillonite nanocomposites. Macromolecules 2004, 37, 4554–4561. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Q. Non-isothermal crystallization behaviors of polyamide 6/clay nanocomposites. Euro Polym. J. 2002, 38, 1383–1389. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Crystallization behavior of nylon 6 nanocomposites. Polymer 2003, 44, 3945–3961. [Google Scholar] [CrossRef]
- Chang, J.H.; Park, K.M. Polyimide nanocomposites: Comparison of their properties with precursor polymer nanocomposites. Polym. Eng. Sci. 2001, 41, 2226–2230. [Google Scholar] [CrossRef]
- Jain, S.; Goossens, H.; van Duin, M.; Lemstra, P. Effect of in-situ prepared silica nano-particles on non-isothermal crystallization of polypropylene. Polymer 2005, 46, 3343–3354. [Google Scholar] [CrossRef]
- Guo, R.; Ma, X.; Hu, C.; Jiang, Z. Novel PVA-silica nanocomposite membrane for pervaporative Dehydration of Ethylene Glycol Aqueous Solution. Polymer 2007, 48, 2939–2945. [Google Scholar] [CrossRef]
- Sengupta, R.; Bandyopadhyay, A.; Sabharwal, S.; Chaki, T.; Bhowmick, A. Polyamide-6, 6/in-situ silica hybrid nanocomposites by sol-gel technique: Synthesis, characterization and properties. Polymer 2005, 46, 3343–3354. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer-layered silicate nanocomposite: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Hu, X.; Lesser, A.J. Effect of a silicate filler on the crystal morphology of poly(trimethylene terephthalate)/clay nanocomposites. J. Polym. Sci. Polym. Phys. 2003, 41, 2275–2289. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Yan, D. Crystallization, morphology, and dynamic mechanical properties of poly(trimethylene terephthalate)/clay nanocomposites. Eur. Polym. J. 2003, 39, 2359–2366. [Google Scholar] [CrossRef]
- Chang, J.-H.; Kim, S.J.; Im, S. Poly(trimethylene terephthalate) nanocomposite fibers by in- situ intercalation polymerization: Thermo-mechanical properties and morphology. Polymer 2004, 45, 5171–5181. [Google Scholar] [CrossRef]
- Smith, L.; Vasanthan, N. Effect of clay on melt crystallization, crystallization kinetics and spherulitic morphology of poly(trimethylene terephthalate) nanocomposites. Thermochim. Acta 2015, 617, 152–162. [Google Scholar] [CrossRef]
- Krishnama, A.; Vasanthan, N. Effect of hydrophilicity of clay on cold crystallization of poly(trimethylene terephthalate) nanocomposites. Ind. Eng. Chem. Res. 2015, 54, 8183–8192. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(Aryl Ether Ether Ketone Ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Yin, L.; Wu, D.; Yao, Z.; Feng, S.; Zhang, M.; Gao, Y. Crystallization behavior of poly(trimethylene terephthalate)/mesoporous silica SBA-15 composite prepared by in- situ polymerization. Thermochem. Acta 2013, 565, 72–81. [Google Scholar] [CrossRef]
- Yao, C.; Yang, G. Poly(trimethylene terephthalate)/silica nanocomposites prepared by dual in-situ polymerization: Synthesis, morphology, crystallization behavior and mechanical properties. Polym. Int. 2010, 59, 492–500. [Google Scholar] [CrossRef]
- Li, P.; Yao, C.; Yang, G.; Li, P.; Yao, C.; Yang, G. Synthesis, thermal properties and crystalline morphology of poly (trimethylene terephthalate)/ZnO nanocomposites prepared by dual in-situ polymerization. Polym. Adv. Technol. 2016, 27, 1451–1457. [Google Scholar] [CrossRef]
- Bodempudi, A.; Vasanthan, N. Crystallization studies of poly(trimethylene terephthalate)/silica nanocomposites prepared by sol–gel technique. ACS Omega 2018, 3, 17797–17804. [Google Scholar] [CrossRef] [PubMed]





| Sample | PTT | PTT/MNa | PTT/M10A | PTT/M15A | PTT/M20A | PTT/M25A | PTT/M30B | PTT/M93A |
|---|---|---|---|---|---|---|---|---|
| Tg (°C) | 45.9 | 45 | 43.8 | 43 | 43.7 | 44 | 43.8 | 44.7 |
| Tcc onset (°C) | 66.9 | 65.5 | 64.8 | 65.9 | 64.4 | 65.6 | 64.1 | 67.2 |
| Tcc (°C) | 69.4 | 68.1 | 67.2 | 68.3 | 66.9 | 68 | 66.7 | 69.7 |
| ΔHcc (J/g) | 30.8 | 34.2 | 32.3 | 30.9 | 34.4 | 35.3 | 32.9 | 31 |
| Tm onset (°C) | 221.1 | 219.5 | 221.2 | 221 | 217 | 222.2 | 219 | 221.7 |
| Tm (°C) | 227.7 | 226 | 227 | 227.3 | 226.6 | 227.9 | 227 | 228.6 |
| ΔHm (J/g) | 60.3 | 66.2 | 63.1 | 63.5 | 66.9 | 65.9 | 65.2 | 67 |
| Tc onset (°C) | 186.8 | 188.2 | 195.7 | 194.3 | 195.3 | 195.9 | 194.7 | 190.8 |
| Tc (°C) | 175.6 | 179.2 | 190.4 | 188.7 | 189.5 | 191 | 189.2 | 183.6 |
| ΔHc (J/g) | 49.8 | 57.2 | 56.4 | 56.3 | 61.5 | 61.2 | 56.4 | 56.1 |
| T0 (°C) | Zt | n | t1/2 (s) | |
|---|---|---|---|---|
| PTT | 186 | 2.95 | 2.33 | 0.54 |
| 190 | 1.05 | 2.32 | 0.77 | |
| 194 | 0.38 | 2.28 | 1.32 | |
| 198 | 0.13 | 2.24 | 2.28 | |
| 202 | 0.07 | 2.32 | 3.92 | |
| PTT/DK2 | 196 | 3.63 | 2.52 | 0.53 |
| 200 | 0.84 | 2.59 | 0.92 | |
| 204 | 0.18 | 2.73 | 1.64 | |
| 208 | 0.04 | 2.53 | 3.06 | |
| 212 | 0.01 | 2.58 | 5.39 |
| Clay (%) | ΔH (J/g) | Crystallinity (%) | Tc Onset (°C) | Tc Maximum (°C) | t1/2 (s) | n | Zc (min−n) |
|---|---|---|---|---|---|---|---|
| 0 | −53 | 36 | 198 | 189 | 88.2 | 3.48 | 0.2021 |
| 1 | −56 | 39 | 198 | 188 | 75.1 | 3.58 | 0.2058 |
| 2 | −57 | 40 | 197 | 189 | 74 | 3.59 | 0.206 |
| 5 | −59 | 43 | 197 | 190 | 70.2 | 3.61 | 0.2082 |
| 10 | −61 | 46 | 199 | 195 | 43.8 | 3.72 | 0.2364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasanthan, N. Crystallization Studies of Poly(Trimethylene Terephthalate) Nanocomposites—A Review. J. Compos. Sci. 2025, 9, 417. https://doi.org/10.3390/jcs9080417
Vasanthan N. Crystallization Studies of Poly(Trimethylene Terephthalate) Nanocomposites—A Review. Journal of Composites Science. 2025; 9(8):417. https://doi.org/10.3390/jcs9080417
Chicago/Turabian StyleVasanthan, Nadarajah. 2025. "Crystallization Studies of Poly(Trimethylene Terephthalate) Nanocomposites—A Review" Journal of Composites Science 9, no. 8: 417. https://doi.org/10.3390/jcs9080417
APA StyleVasanthan, N. (2025). Crystallization Studies of Poly(Trimethylene Terephthalate) Nanocomposites—A Review. Journal of Composites Science, 9(8), 417. https://doi.org/10.3390/jcs9080417






