Improving Durability and Mechanical Properties of Silty Sand Stabilized with Geopolymer and Nanosilica Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
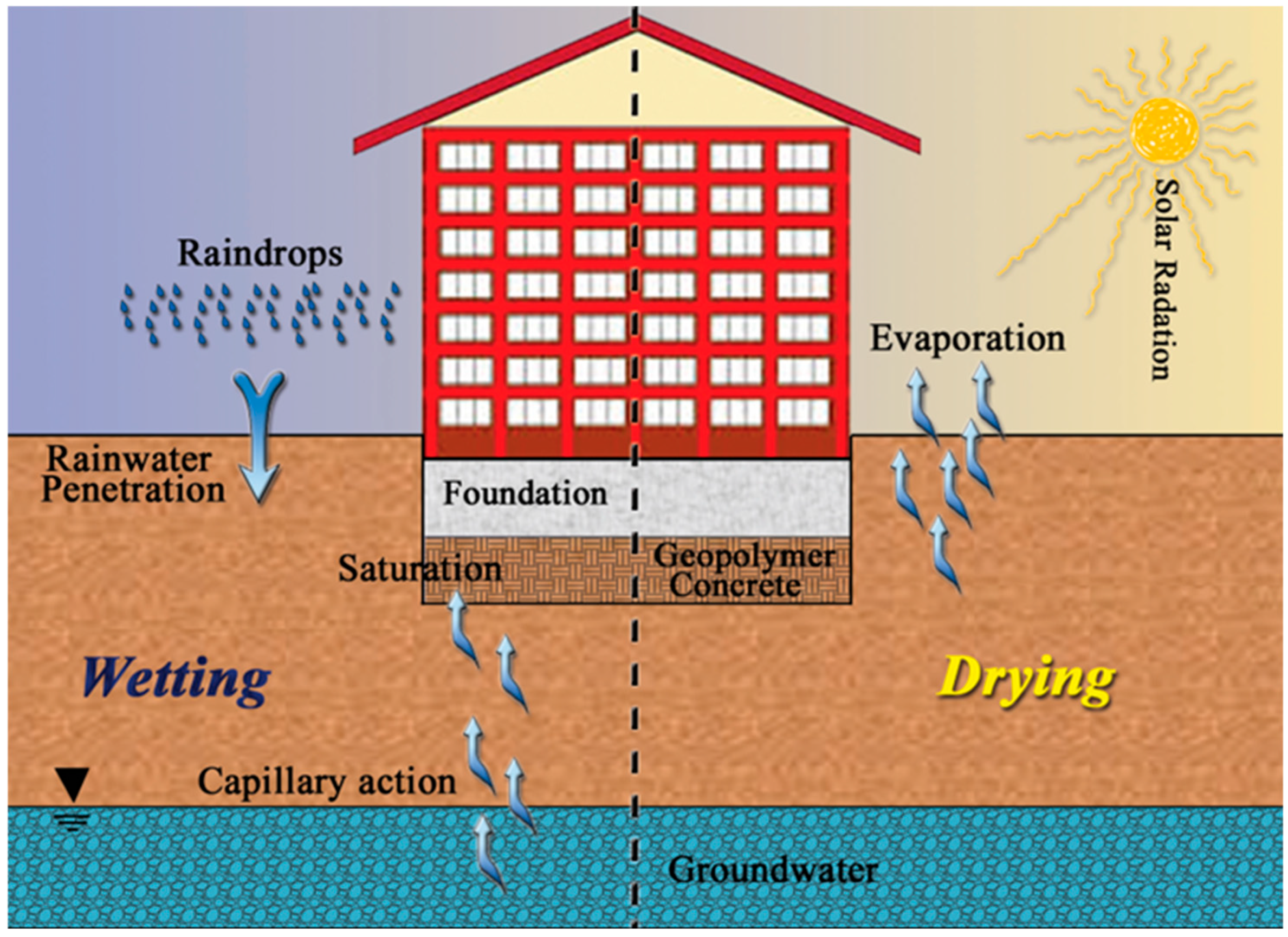
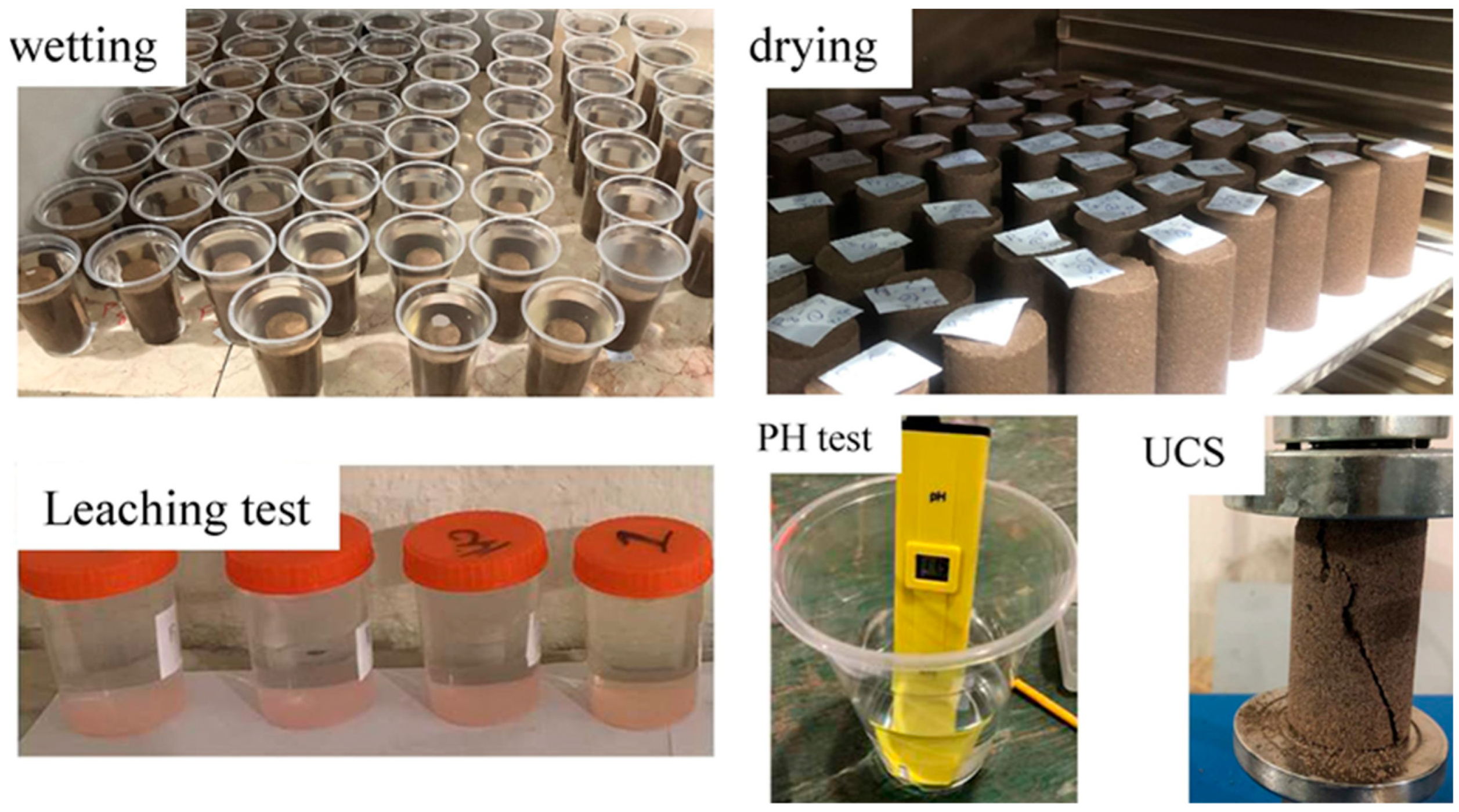
2.2. Wetting–Drying Cycle Test
2.3. Leaching Test
2.4. FE-SEM and EDX Tests
3. Results and Discussion
3.1. Investigation of the Saturation Conditions
3.2. Effect of Curing Time
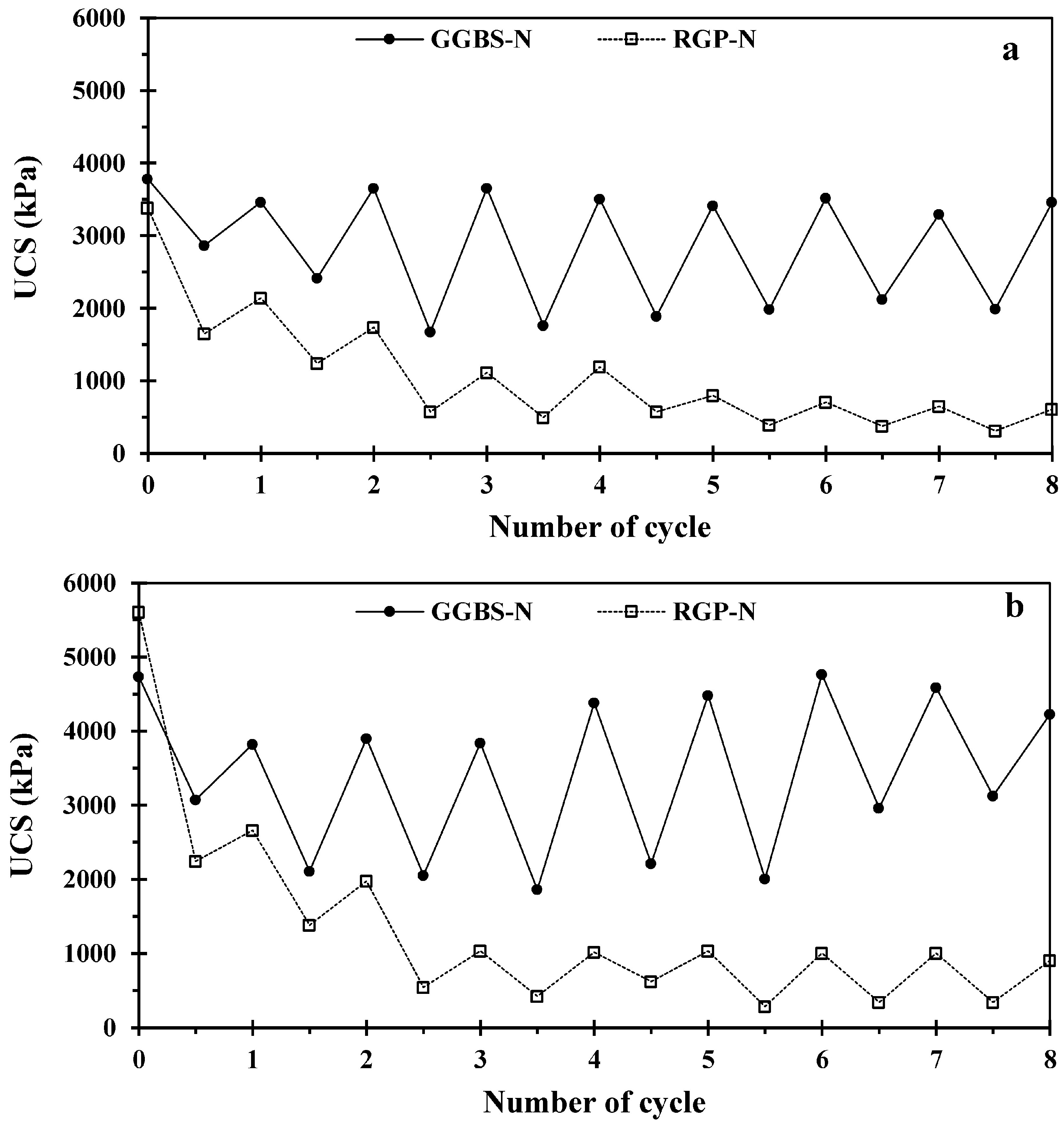
3.3. Wetting–Drying Cycle Test (W–D)
3.3.1. Mass Loss
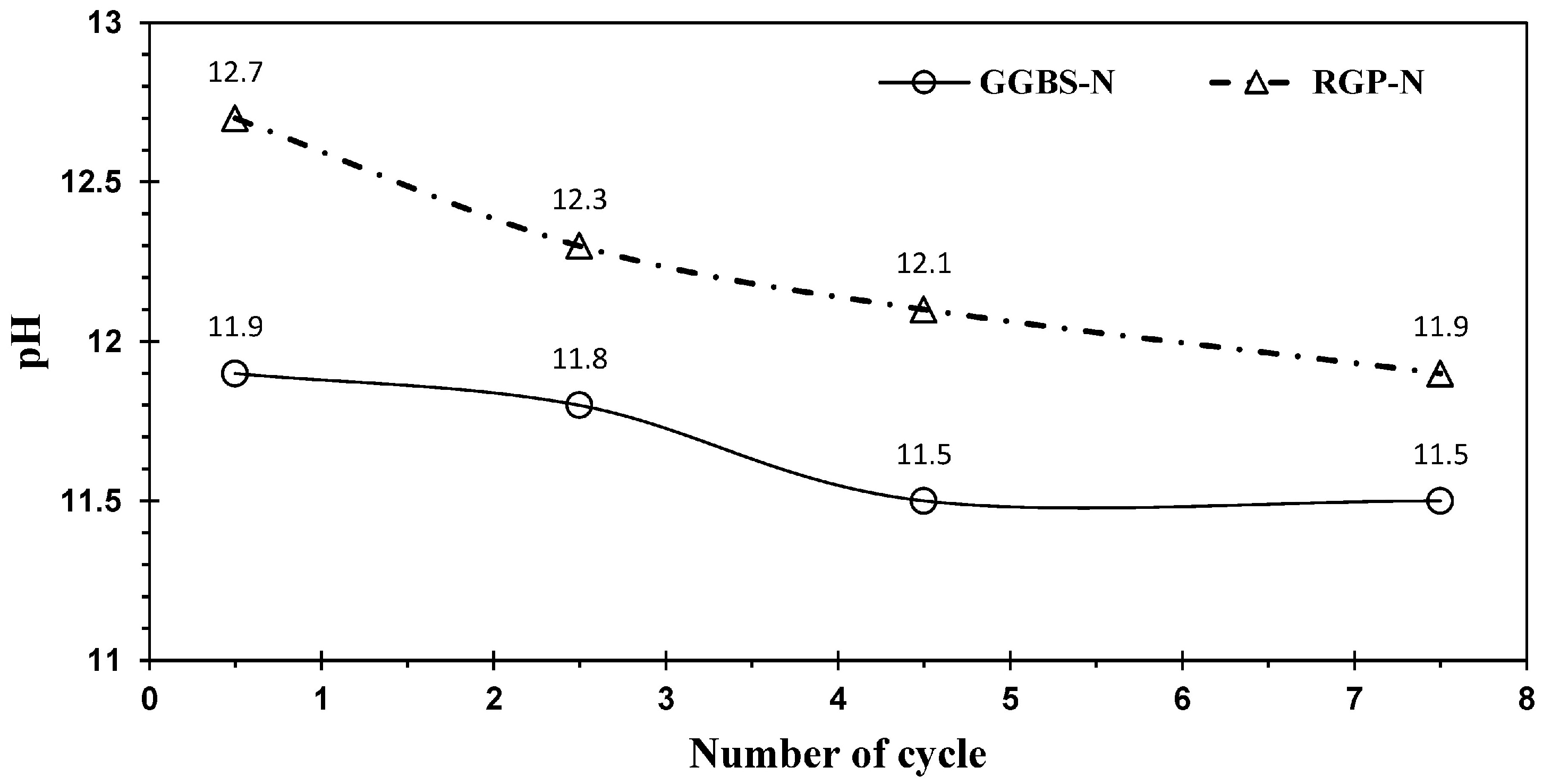
3.3.2. Leaching and pH Test Results
3.3.3. The Effects of W–D Cycles on Stress–Strain Behavior
4. FE-SEM and EDX Analysis
5. Conclusions
- Following saturation for 48 h, nearly all analyzed specimens showed a decline in strength as a result of saturation. However, they exhibited satisfactory stability and strength in contrast to unstabilized soil, which broke down after 5 h of saturation. Regarding the compressive strength of sample GGBS-N, it was observed that after 48 h of saturation, the UCS increased by 20%. This resulted from the ongoing chemical reactions following the seven-day curing, which resulted in the increased strength.
- The UCS of 14- and 45-day cured samples during W–D cycles illustrated the considerable drop after cycle one for RGP-based samples and remained constant after cycle three to the end (eighth cycle). However, the GGBS-based samples retained their initial strength even after 8 days of W–D cycles. Furthermore, the absorbed energy and elongation at break are inversely and directly related to the increase in cycle number in both group samples.
- The results of the durability test were indicated by the mass loss of the samples. For GGBS-N and RGP-N samples, a significant reduction in mass loss of 7% and 10%, respectively, was measured after cycle two, which was related to the leaching of the geopolymerization products after the W–D cycles.
- Leaching and pH tests indicated that the toxicity of all leached solutions was below the permissible limit. However, the high pH of the leached solution can pose a problem for the environment.
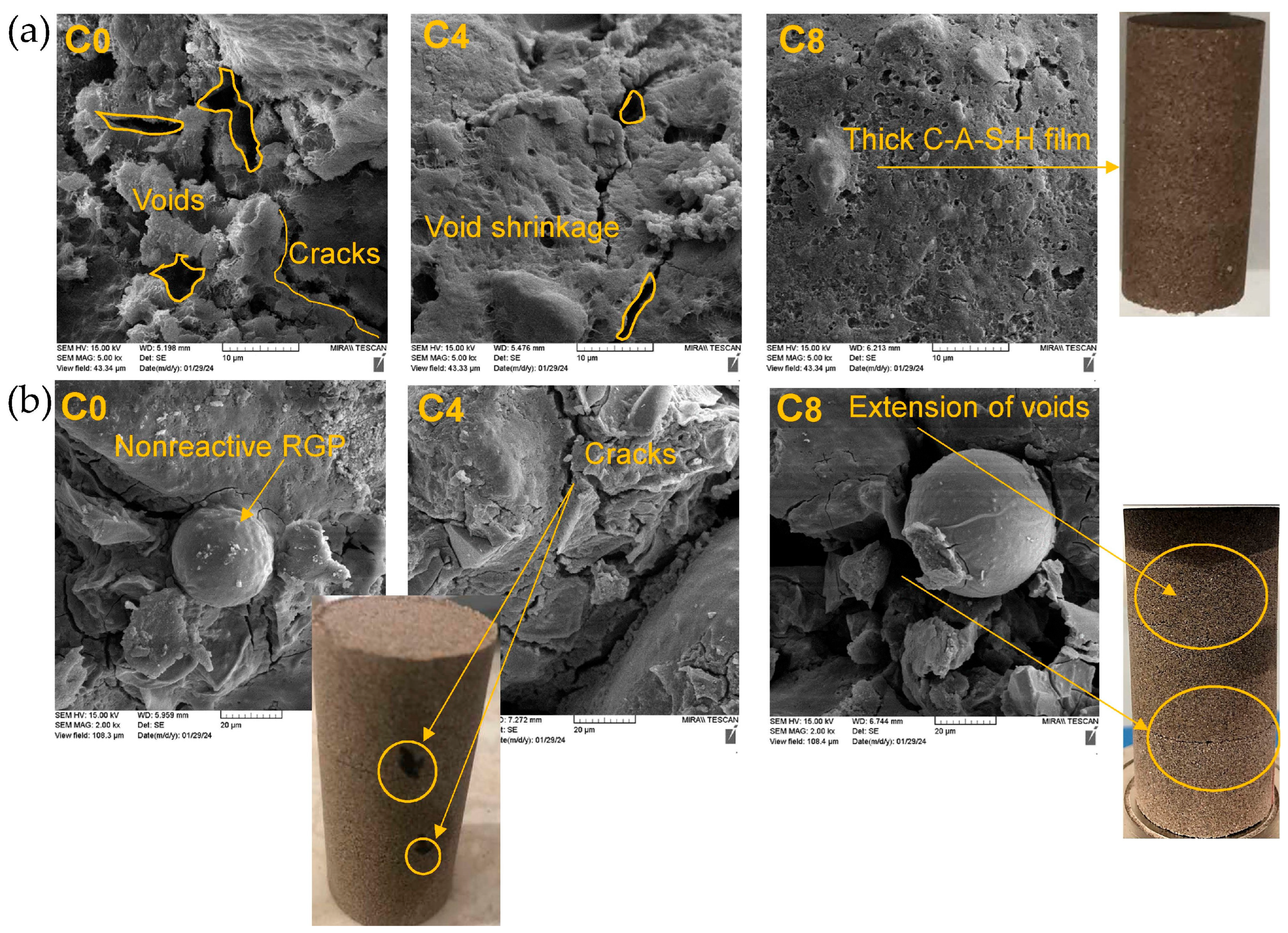
- SEM and EDS analysis showed that as the number of W–D cycles increased, the number of cracks and voids increased in RGP-N samples, which was reversed in GGBS-N samples.
- From the UCS and mass loss results, “GGBS-N” can be selected as a suitable blend for durability to W–D cycles. Ultimately, stabilizing silty sand with GGBS-based geopolymer is a suitable and durable method for geotechnical applications in harsh climates.
- The improvement in the mechanical performance of the stabilized soil, particularly UCS, can be attributed to the formation of geopolymer gels resulting from the alkali activation of aluminosilicate precursors. This process involves the dissolution of reactive silica and alumina species, followed by polycondensation reactions that generate a binding matrix, predominantly in the form of C(N)-A-S-H gels in sodium-activated, calcium-rich (GGBS) systems. These gels create a dense microstructure that enhances particle bonding, reduces porosity, and increases load-bearing capacity. In addition to strength gains, the formation of geopolymer gels may also contribute to improved soil durability, moisture resistance, and overall stability, highlighting their potential for sustainable soil improvement applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Namjoo, A.M.; Toufigh, M.M.; Toufigh, V. Experimental Investigation of Interface Behaviour between Different Types of Sand and Carbon Fibre Polymer. Eur. J. Environ. Civ. Eng. 2021, 25, 2317–2336. [Google Scholar] [CrossRef]
- Al-Taie, A.; Yaghoubi, E.; Wasantha, P.L.P.; Van Staden, R.; Guerrieri, M.; Fragomeni, S. Mechanical and Physical Properties and Cyclic Swell-Shrink Behaviour of Expansive Clay Improved by Recycled Glass. Int. J. Pavement Eng. 2023, 24, 2204436. [Google Scholar] [CrossRef]
- Yaghoubi, E.; Azadegan, O.; Li, J. Effect of Surface Layer Thickness on the Performance of Lime and Cement Treated Aggregate Surfaced Roads. Electron. J. Geotech. Eng. 2013, 18 F, 1081–1094. [Google Scholar]
- Abushama, W.J.; Tamimi, A.K.; Tabsh, S.W.; El-Emam, M.M.; Ibrahim, A.; Mohammed Ali, T.K. Influence of Optimum Particle Packing on the Macro and Micro Properties of Sustainable Concrete. Sustainability 2023, 15, 14331. [Google Scholar] [CrossRef]
- Pourabbas Bilondi, M.; Toufigh, M.M.; Toufigh, V. Experimental Investigation of Using a Recycled Glass Powder-Based Geopolymer to Improve the Mechanical Behavior of Clay Soils. Constr. Build. Mater. 2018, 170, 302–313. [Google Scholar] [CrossRef]
- Hossain, S.S.; Akhtar, F. Recent Progress of Geopolymers for Carbon Dioxide Capture, Storage and Conversion. J. CO2 Util. 2023, 78, 102631. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Nabizadeh Mashizi, M.; Bagheripour, M.H.; Jafari, M.M.; Yaghoubi, E. Mechanical and Microstructural Properties of a Stabilized Sand Using Geopolymer Made of Wastes and a Natural Pozzolan. Sustainability 2023, 15, 2966. [Google Scholar] [CrossRef]
- Bhojaraju, C.; Mousavi, S.S.; Brial, V.; DiMare, M.; Ouellet-Plamondon, C.M. Fresh and Hardened Properties of GGBS-Contained Cementitious Composites Using Graphene and Graphene Oxide. Constr. Build. Mater. 2021, 300, 123902. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Mousavi Ajarostaghi, S.S.; Bhojaraju, C. A Critical Review of the Effect of Concrete Composition on Rebar–Concrete Interface (RCI) Bond Strength: A Case Study of Nanoparticles. SN Appl. Sci. 2020, 2, 893. [Google Scholar] [CrossRef]
- Díaz-López, J.L.; Cabrera, M.; Agrela, F.; Rosales, J. Geotechnical and Engineering Properties of Expansive Clayey Soil Stabilized with Biomass Ash and Nanomaterials for Its Application in Structural Road Layers. Geomech. Energy Environ. 2023, 36, 100496. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.; Yu, Q. Investigation of the Effects of Graphene and Graphene Oxide Nanoplatelets on the Micro- and Macro-Properties of Cementitious Materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Ghazizadeh, S.; Duffour, P.; Skipper, N.T.; Bai, Y. Understanding the Behaviour of Graphene Oxide in Portland Cement Paste. Cem. Concr. Res. 2018, 111, 169–182. [Google Scholar] [CrossRef]
- Devi, S.C.; Khan, R.A. Effect of Graphene Oxide on Mechanical and Durability Performance of Concrete. J. Build. Eng. 2020, 27, 101007. [Google Scholar] [CrossRef]
- Gholampour, A.; Valizadeh Kiamahalleh, M.; Tran, D.N.H.; Ozbakkaloglu, T.; Losic, D. From Graphene Oxide to Reduced Graphene Oxide: Impact on the Physiochemical and Mechanical Properties of Graphene-Cement Composites. ACS Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef]
- Indumathi, P.; Shabhudeen, S.P.; Saraswathy, C.P. Synthesis and Characterization of Nano Silica from the Pods of Delonix Regia Ash. Int. J. Adv. Eng. Technol. 2011, 264–265, 1370–1375. [Google Scholar]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of Nano-SiO2 Addition on Properties of Hardened Cement Paste as Compared with Silica Fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. Improvement Compressive Strength of Concrete in Different Curing Media by Al2O3 Nanoparticles. Mater. Sci. Eng. A 2011, 528, 1183–1191. [Google Scholar] [CrossRef]
- Hosseini, P.; Hosseinpourpia, R.; Pajum, A.; Khodavirdi, M.M.; Izadi, H.; Vaezi, A. Effect of Nano-Particles and Aminosilane Interaction on the Performances of Cement-Based Composites: An Experimental Study. Constr. Build. Mater. 2014, 66, 113–124. [Google Scholar] [CrossRef]
- Wongkornchaowalit, N.; Lertchirakarn, V. Setting Time and Flowability of Accelerated Portland Cement Mixed with Polycarboxylate Superplasticizer. J. Endod. 2011, 37, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Hou, S. Study on the Rheological Properties of Portland Cement Pastes with Polycarboxylate Superplasticizers. Rheol. Acta 2013, 52, 707–718. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of Nano-TiO 2 on the Mechanical Properties of Cement Mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Aqel, M. Hybrid Effect of Carbon Nanotube and Nano-Clay on Physico-Mechanical Properties of Cement Mortar. Constr. Build. Mater. 2011, 25, 145–149. [Google Scholar] [CrossRef]
- Al-Taie, A.; Yaghoubi, E.; Tahmoorian, F.; Li, J.; Ahmed, B.A. Pavement Performance over Deep Trenches Backfilled with Recycled Aggregates under Different Compaction Efforts. Can. Geotech. J. 2024, 62, 1–26. [Google Scholar] [CrossRef]
- Perera, S.T.A.M.; Saberian, M.; Zhu, J.; Roychand, R.; Li, J. Effect of Crushed Glass on the Mechanical and Microstructural Behavior of Highly Expansive Clay Subgrade. Case Stud. Constr. Mater. 2022, 17, e01244. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of Glass Wastes for the Development of Geopolymer Composites–Durability, Thermal and Microstructural Properties: A Review. Constr. Build. Mater. 2019, 222, 673–687. [Google Scholar] [CrossRef]
- Manikandan, P.; Vasugi, V. Potential Utilization of Waste Glass Powder as a Precursor Material in Synthesizing Ecofriendly Ternary Blended Geopolymer Matrix. J. Clean. Prod. 2022, 355, 131860. [Google Scholar] [CrossRef]
- Pascual, A.B.; Tognonvi, M.T.; Tagnit-Hamou, A. Waste Glass Powder-Based Alkali-Activated Mortar. Int. J. Res. Eng. Technol 2014, 3, 32–36. [Google Scholar]
- Çelik, A.İ.; Tunç, U.; Bahrami, A.; Karalar, M.; Mydin, M.A.O.; Alomayri, T.; Özkılıç, Y.O. Use of Waste Glass Powder toward More Sustainable Geopolymer Concrete. J. Mater. Res. Technol. 2023, 24, 8533–8546. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. High Strength Geopolymer Binder Based on Waste-Glass Powder. Adv. Powder Technol. 2017, 28, 215–222. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of Inorganic Polymer (Geopolymer) Mortars Made of Glass Cullet. J. Mater. Sci. 2012, 47, 2782–2797. [Google Scholar] [CrossRef]
- Noolu, V.; Mallikarjuna Rao, G.; Sudheer Kumar Reddy, B.; Chavali, R.V.P. Strength and Durability Characteristics of GGBS Geopolymer Stabilized Black Cotton Soil. Mater. Today Proc. 2020, 43, 2373–2376. [Google Scholar] [CrossRef]
- AASHTO-T307; Standard Method of Test for Determining the Resilient Modulus of Soils and Aggregate Materials. American Association of State Highway and Transportation Officials: Washington, DC, USA, 2007.
- Horpibulsuk, S.; Suksiripattanapong, C.; Samingthong, W.; Rachan, R.; Arulrajah, A. Durability against Wetting–Drying Cycles of Water Treatment Sludge–Fly Ash Geopolymer and Water Treatment Sludge–Cement and Silty Clay–Cement Systems. J. Mater. Civ. Eng. 2016, 28, 4015078. [Google Scholar] [CrossRef]
- Bin-Shafique, S.; Rahman, K.; Azfar, I. The Effect of Freezing-Thawing Cycles on Performance of Fly Ash Stabilized Expansive Soil Subbases. In Proceedings of the Geo-Frontiers 2011: Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; pp. 697–706, ISBN 9780784411650. [Google Scholar]
- ASTM D422; Standard Test Method for Particle-Size Analysis of Soils. ASTM: West Conshohocken, PA, USA, 2007; pp. 1–8.
- ASTM D698-07; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2007; Volume 12, pp. 1–13.
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental Feasibility Study of Geopolymer as the Next-Generation Soil Stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- ASTM D 854 D854; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2010; Volume 2458000, pp. 1–7.
- Burciaga-Díaz, O.; Durón-Sifuentes, M.; Díaz-Guillén, J.A.; Escalante-García, J.I. Effect of Waste Glass Incorporation on the Properties of Geopolymers Formulated with Low Purity Metakaolin. Cem. Concr. Compos. 2020, 107, 103492. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Das, S.K.; Mustakim, S.M.; Kandi, S.K.; Rajput, P. Integrated Management of Ferrochrome Slag: Metal Recovery, Cr (VI) Stabilization, and Sustainable Reuse in Construction Materials. J. Environ. Manag. 2025, 390, 126268. [Google Scholar] [CrossRef] [PubMed]
- Kampala, A.; Horpibulsuk, S.; Prongmanee, N.; Chinkulkijniwat, A. Influence of Wet-Dry Cycles on Compressive Strength of Calcium Carbide Residue–Fly Ash Stabilized Clay. J. Mater. Civ. Eng. 2014, 26, 633–643. [Google Scholar] [CrossRef]
- Jafari Kermanipour, M.; Bagheripour, M.H.; Yaghoubi, E. Mechanical and Microstructural Characterization of a Nano-Stabilized Sandy Soil. Geotech. Geol. Eng. 2024, 42, 6131–6146. [Google Scholar] [CrossRef]
- Rios, S.; Cristelo, N.; Viana da Fonseca, A.; Ferreira, C. Structural Performance of Alkali-Activated Soil Ash versus Soil Cement. J. Mater. Civ. Eng. 2016, 28, 4015125. [Google Scholar] [CrossRef]
- Ladd, R. Preparing Test Specimens Using Undercompaction. Geotech. Test. J. 1978, 1, 16–23. [Google Scholar] [CrossRef]
- ASTM D 2166; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2013; pp. 1–7.
- Blight, G.E. Unsaturated Soil Mechanics in Geotechnical Practice; CRC Press: Boca Raton, FL, USA, 2013; ISBN 1315882930. [Google Scholar]
- Head, K.H.; Epps, R. Manual of Soil Laboratory Testing; Pentech Press: London, UK, 1980; Volume 1. [Google Scholar]
- ASTM D559/D559M-15; Standard Test Methods for Wetting and Drying Compacted Soil-Cement Mixtures. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–6.
- Mohammadzadeh, M.A.; Toufigh, M.M.; Toufigh, V. Durability and Strength of Geopolymer with Recycled Glass Powder Base for Clay Stabilization. KSCE J. Civ. Eng. 2023, 27, 156–168. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Effect of Hydrophobic Surface-Modified Fine Aggregates on Efflorescence Control in Geopolymer. Cem. Concr. Compos. 2022, 126, 104337. [Google Scholar] [CrossRef]
- Alyamani, A.; Lemine, O.M. FE-SEM Characterization of Some Nanomaterial. In Scanning Electron Microscopy; IntechOpen: London, UK, 2012; ISBN 9535100920. [Google Scholar]
- Garrido-García, L.F.; Pérez-Martínez, A.L.; Reyes-Gasga, J.; Aguilar-Del-Valle, M.d.P.; Wong, Y.H.; Rodríguez-Gómez, A. A Simple Methodology to Gain Insights into the Physical and Compositional Features of Ternary and Quaternary Compounds Based on the Weight Percentages of Their Constituent Elements: A Proof of Principle Using Conventional EDX Characterizations. Ceramics 2024, 7, 1275–1300. [Google Scholar] [CrossRef]
- Roshan, K.; Choobbasti, A.J.; Kutanaei, S.S. Evaluation of the Impact of Fiber Reinforcement on the Durability of Lignosulfonate Stabilized Clayey Sand under Wet-Dry Condition. Transp. Geotech. 2020, 23, 100359. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.A.B.; Ahmad, R.; Rahim, S.Z.A.; Rychta, M.; Wnuk, I.; Nabiałek, M.; Muskalski, K.; Tahir, M.F.M. Syafwandi Evaluation on the Mechanical Properties of Ground Granulated Blast Slag (GGBS) and Fly Ash Stabilized Soil via Geopolymer Process. Materials 2021, 14, 2833. [Google Scholar] [CrossRef]
- Moazami, D.; Bilondi, M.P.; Rahnama, A.; Zaresefat, M.; Moretti, L. Recycled Glass Powder and Calcium Carbide Residue Geopolymer to Stabilise Silty Sand Soil: Mechanical Performances and Statistical Analysis. Heliyon 2025, 11, e41738. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Vollpracht, A. Leaching of Monolithic Geopolymer Mortars. Cem. Concr. Res. 2020, 136, 106161. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of Blast Furnace Slag Grades on Fly Ash Based Geopolymer Waste Forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Tome, S.; Etoh, M.A.; Etame, J.; Sanjay, K. Characterization and Leachability Behaviour of Geopolymer Cement Synthesised from Municipal Solid Waste Incinerator Fly Ash and Volcanic Ash Blends. Recycling 2018, 3, 50. [Google Scholar] [CrossRef]
- Saride, S.; Puppala, A.J.; Chikyala, S.R. Swell-Shrink and Strength Behaviors of Lime and Cement Stabilized Expansive Organic Clays. Appl. Clay Sci. 2013, 85, 39–45. [Google Scholar] [CrossRef]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Impact of Wetting-Drying Cycles on the Microstructure and Mechanical Properties of Lime-Stabilized Gypseous Soils. Eng. Geol. 2014, 174, 11–21. [Google Scholar] [CrossRef]
- Das, B.M.; Sobhan, K. Principles of Geotechnical Engineering; CL Engineering: Don Hua Lo, Thailand, 1990. [Google Scholar]
- Fakhrabadi, A.; Ghadakpour, M.; Choobbasti, A.J.; Kutanaei, S.S. Evaluating the Durability, Microstructure and Mechanical Properties of a Clayey-Sandy Soil Stabilized with Copper Slag-Based Geopolymer against Wetting-Drying Cycles. Bull. Eng. Geol. Environ. 2021, 80, 5031–5051. [Google Scholar] [CrossRef]
- Das, S.K.; Nayak, M.K.; Patro, S.K.; Suda, Y. Durability Properties of Ambient Cured Geopolymer Mortar Made from Rice Husk Ash–Based Alkali Activator: A Comparative Study with Conventional Alkali Activator. Adv. Civ. Eng. Mater. 2023, 12, 314–328. [Google Scholar] [CrossRef]
- Nguyễn, H.H.; Nguyễn, P.H.; Lương, Q.-H.; Meng, W.; Lee, B.Y. Mechanical and Autogenous Healing Properties of High-Strength and Ultra-Ductility Engineered Geopolymer Composites Reinforced by PE-PVA Hybrid Fibers. Cem. Concr. Compos. 2023, 142, 105155. [Google Scholar] [CrossRef]
- Furtos, G.; Prodan, D.; Sarosi, C.; Moldovan, M.; Łach, M.; Melnychuk, M.; Korniejenko, K. Advanced Geopolymer-Based Composites for Antimicrobial Application. Materials 2023, 16, 7414. [Google Scholar] [CrossRef] [PubMed]
- Barbarey, M.S.; Seleman, M.M.E.-S.; El Kheshen, A.A.; Zawrah, M. Processing and Characterization of Geopolymer Based on Ladle Furnace Slag and Fly Ash: The Impact of Chemical Composition on the Biodiesel Production. J. Pet. Min. Eng. 2024, 26, 54–63. [Google Scholar] [CrossRef]
- Lingyu, T.; Dongpo, H.; Jianing, Z.; Hongguang, W. Durability of Geopolymers and Geopolymer Concretes: A Review. Rev. Adv. Mater. Sci. 2021, 60, 1–14. [Google Scholar] [CrossRef]
- Dinh, H.L.; Liu, J.; Doh, J.H.; Ong, D.E.L. Influence of Si/Al Molar Ratio and ca Content on the Performance of Fly Ash-Based Geopolymer Incorporating Waste Glass and GGBFS. Constr. Build. Mater. 2024, 411, 134741. [Google Scholar] [CrossRef]
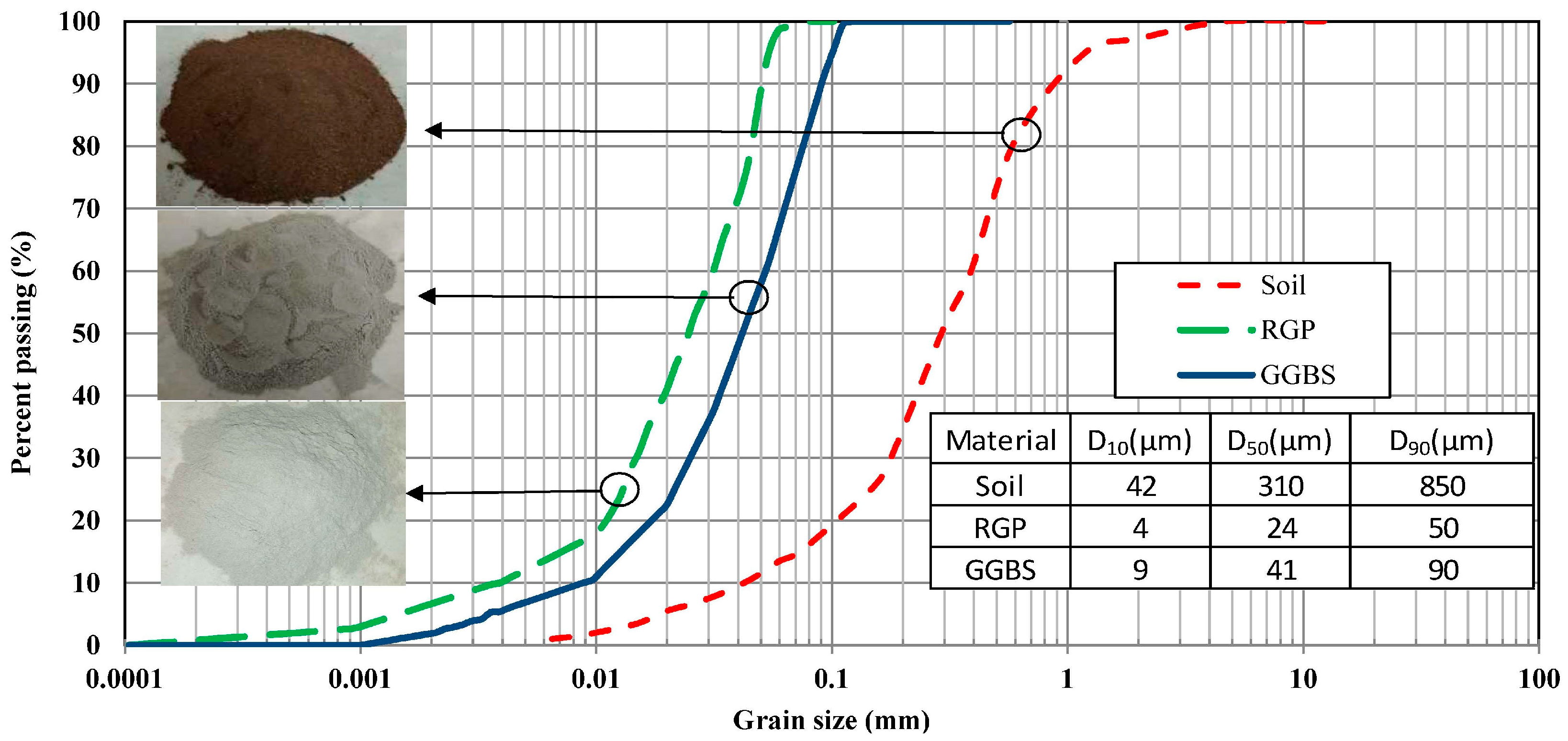





| Property | Result |
|---|---|
| Soil classification (USCS) | Silty Sand (SM) |
| Coefficient of uniformity (Cu) | 9.262 |
| Coefficient of curvature (Cc) | 1.939 |
| OMC [37] | 13% |
| MDD [37] | 1.57 gr/cm3 |
| Specific gravity (Gs) [39] | 2.64 |
| Component Oxides (%) | GGBS | RGP |
|---|---|---|
| SiO2 | 36.50 | 73.77 |
| Al2O3 | 13.04 | 1.91 |
| Fe2O3 | 0.36 | 0.03 |
| CaO | 38.94 | 8.87 |
| MgO | 8.72 | 2.14 |
| Na2O | 0.11 | 11.55 |
| K2O | 0.31 | 0.28 |
| P2O5 | 0.11 | 0 |
| SO3 | 1.76 | 0.78 |
| TiO2 | 0.01 | 0 |
| Mn2O3 | 0.32 | 0 |
| LoI | 1.03 | 0.22 |
| Sample Type | Water Content (%) | Sand (%) | GGBS (%) | RGP (%) | Nanomaterials (%) | Alkali Activator (NaOH) |
|---|---|---|---|---|---|---|
| NS | 13 | 100 | 0 | 0 | 0 | 13% (7MOL) |
| GGBS | 13 | 80 | 20 | 0 | 0 | |
| RGP | 13 | 80 | 0 | 20 | 0 | |
| GGBS-N | 13 | 80 | 18 | 0 | 2 | |
| RGP-N | 13 | 80 | 0 | 18 | 2 |
| Samples | K+ | Na+ | Ca2+ | Si4+ | Al3+ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C8 | C1 | C8 | C1 | C8 | C1 | C8 | C1 | C8 | |
| GGBS-N | 34.26 | 2.55 | 2880 | 175 | 0.47 | 0.15 | 52.7 | 13.6 | 10.31 | <0.1 |
| RGP-N | 26.14 | 4.31 | 3902 | 496 | 0.6 | 0.26 | 2266 | 48.5 | 7.44 | <0.1 |
| Metal | GGBS-N (mg/L) | RGP-N (mg/L) | USEPA Limit (mg/L) |
|---|---|---|---|
| As | 1.07 | 0.93 | 5 |
| Bi | <0.05 | <0.05 | N/A |
| Cd | <0.05 | <0.05 | 1 |
| Cr | <0.05 | <0.05 | 5 |
| Cu | 0.12 | <0.05 | N/A |
| Ni | <0.05 | <0.05 | N/A |
| Pb | <0.05 | <0.05 | 5 |
| Se | 0.05 | 0.07 | N/A |
| Zn | <0.05 | <0.05 | 100 |
| Mix ID | Si/Al | NA/Al | UCS (MPa) | |||
|---|---|---|---|---|---|---|
| C0 | C8 | C0 | C8 | C0 | C8 | |
| GGBS-N | 3.27 | 3.95 | 0.86 | 0.54 | 4.7 | 4.2 |
| RGP-N | 7.2 | 7.33 | 3.29 | 0 | 5.6 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari Kermanipour, M.; Bagheripour, M.H.; Yaghoubi, E. Improving Durability and Mechanical Properties of Silty Sand Stabilized with Geopolymer and Nanosilica Composites. J. Compos. Sci. 2025, 9, 397. https://doi.org/10.3390/jcs9080397
Jafari Kermanipour M, Bagheripour MH, Yaghoubi E. Improving Durability and Mechanical Properties of Silty Sand Stabilized with Geopolymer and Nanosilica Composites. Journal of Composites Science. 2025; 9(8):397. https://doi.org/10.3390/jcs9080397
Chicago/Turabian StyleJafari Kermanipour, Mojtaba, Mohammad Hossein Bagheripour, and Ehsan Yaghoubi. 2025. "Improving Durability and Mechanical Properties of Silty Sand Stabilized with Geopolymer and Nanosilica Composites" Journal of Composites Science 9, no. 8: 397. https://doi.org/10.3390/jcs9080397
APA StyleJafari Kermanipour, M., Bagheripour, M. H., & Yaghoubi, E. (2025). Improving Durability and Mechanical Properties of Silty Sand Stabilized with Geopolymer and Nanosilica Composites. Journal of Composites Science, 9(8), 397. https://doi.org/10.3390/jcs9080397








