Three-Dimensional Distribution of Titanium Hydrides After Degradation of Magnesium/Titanium Hybrid Implant Material—A Study by X-Ray Diffraction Contrast Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterisation
2.3. Absorption Microtomography
2.4. X-Ray Diffraction Tomography
3. Results
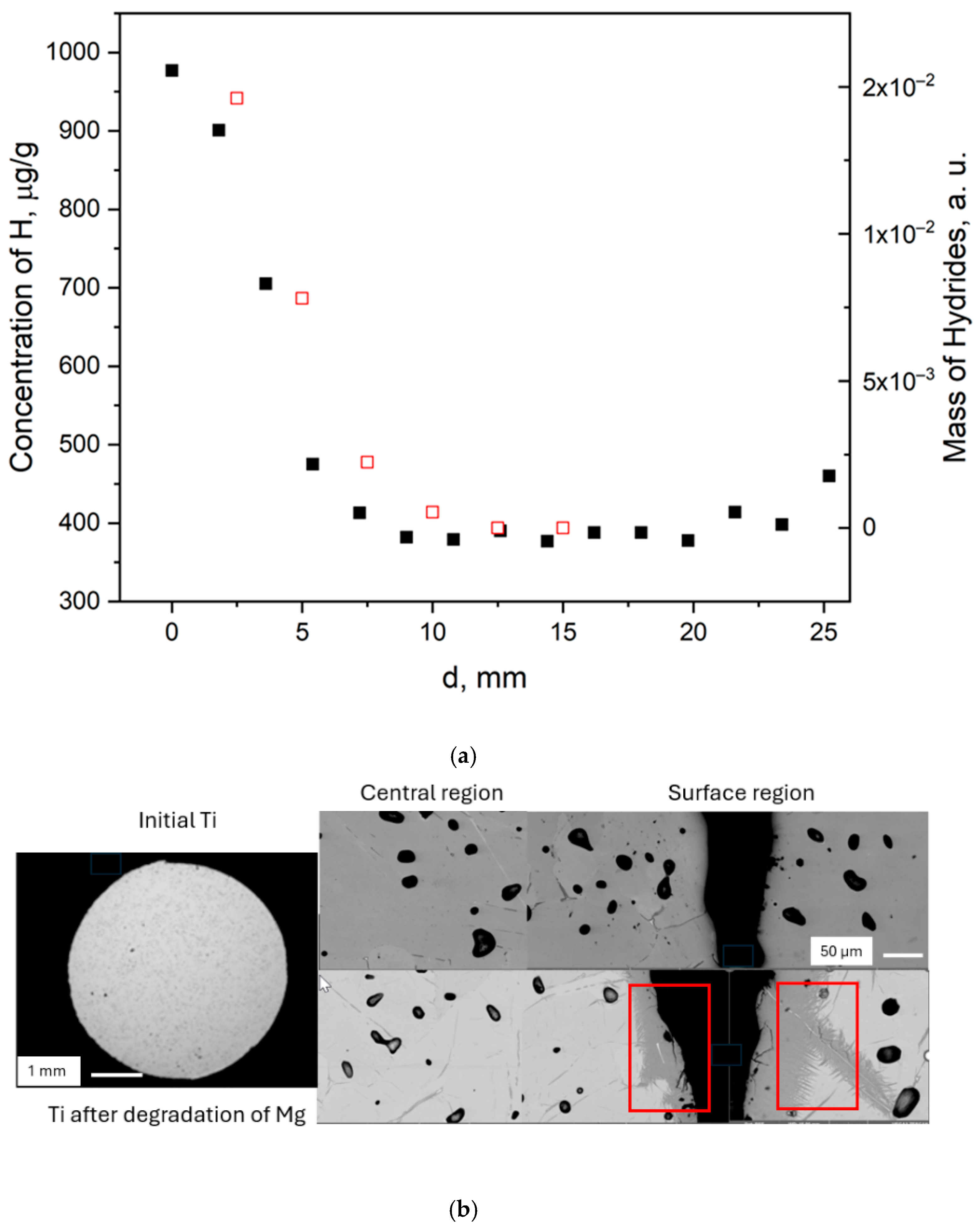
3.1. Macroscopic Sample Characterisation
3.2. Hydrogen Distribution by Chemical Composition and SEM
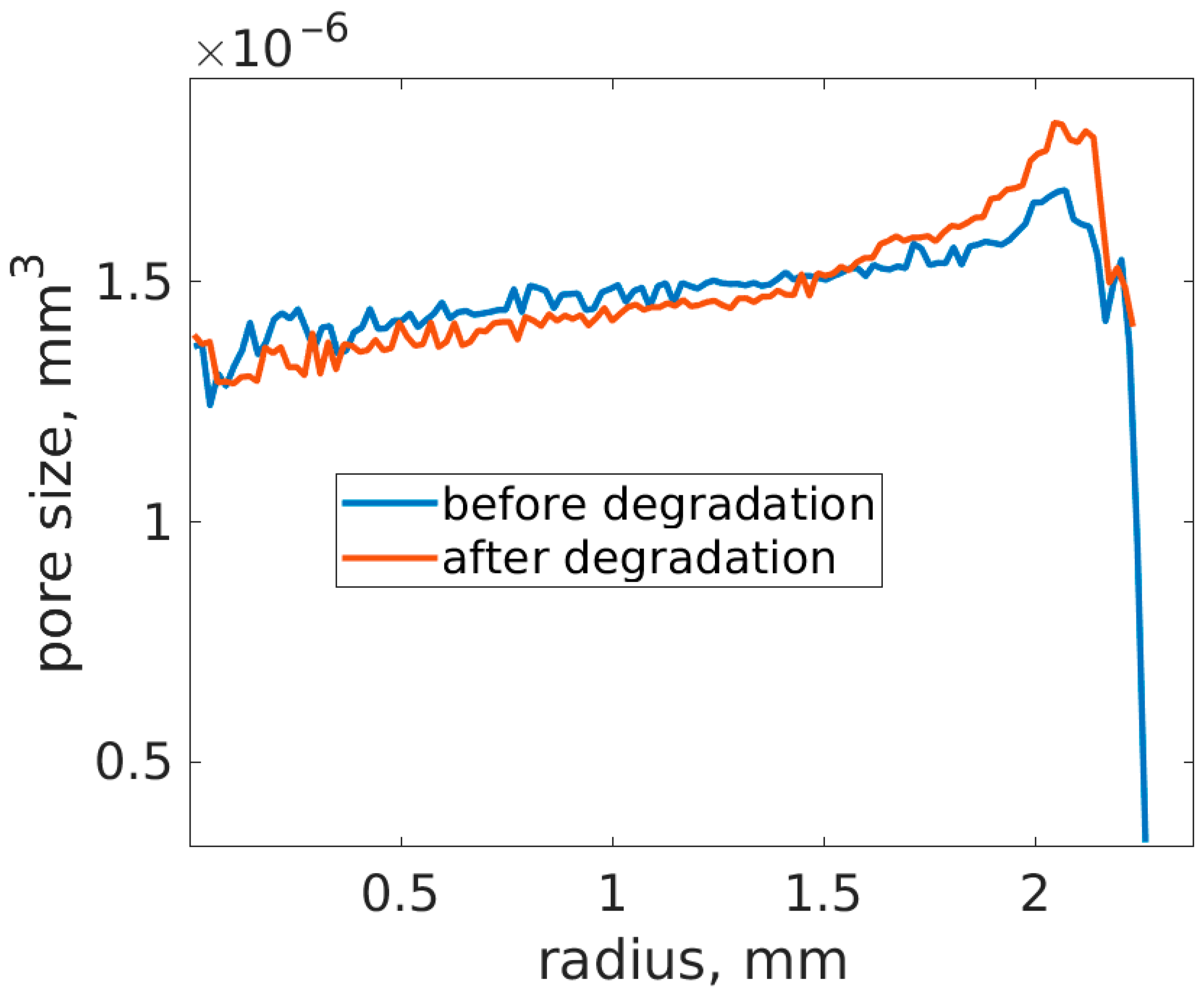
3.3. Distribution of Pores Within the Ti Part Before and After Degradation of Mg0.6Ca by Absorption Synchrotron Tomography
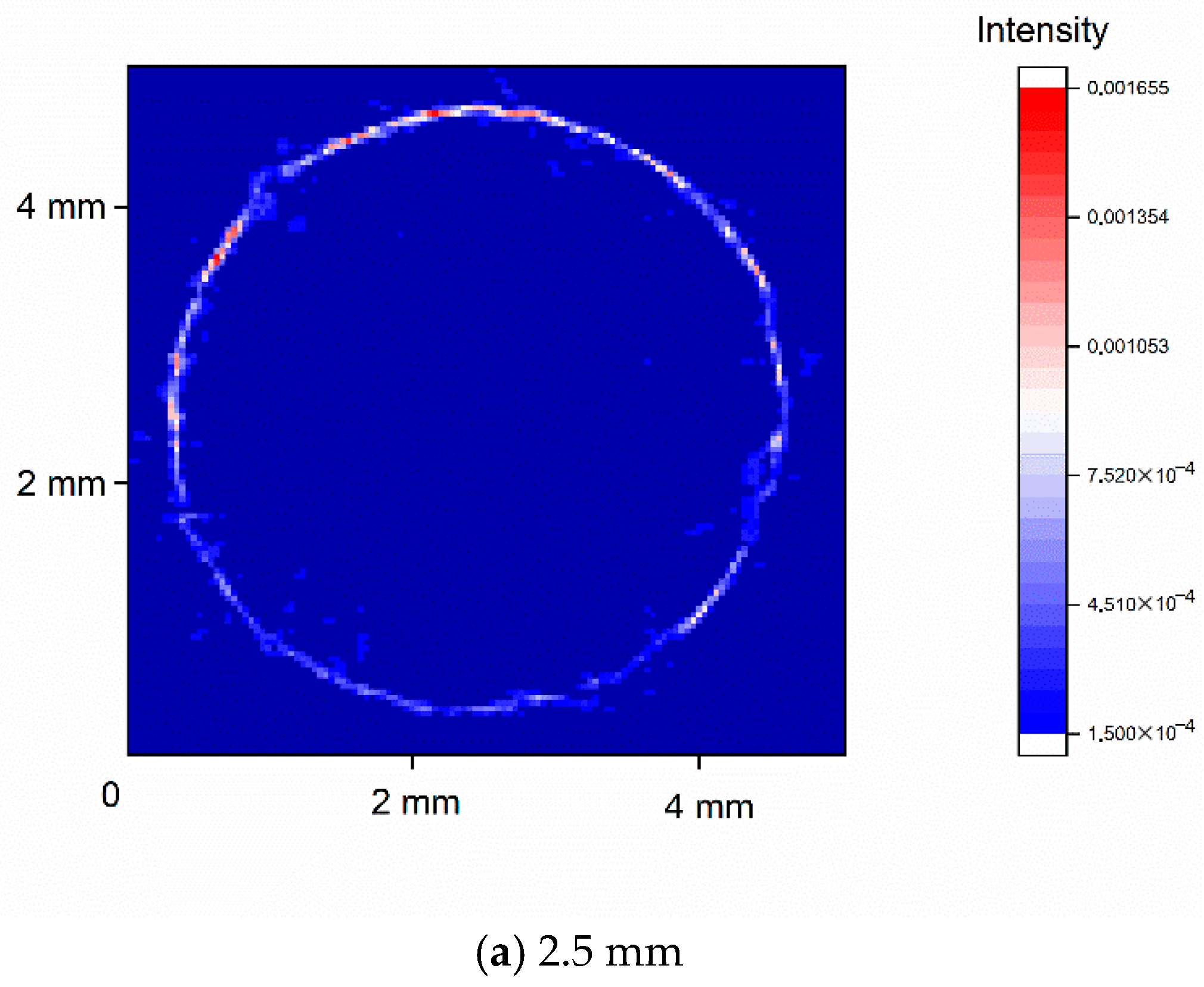
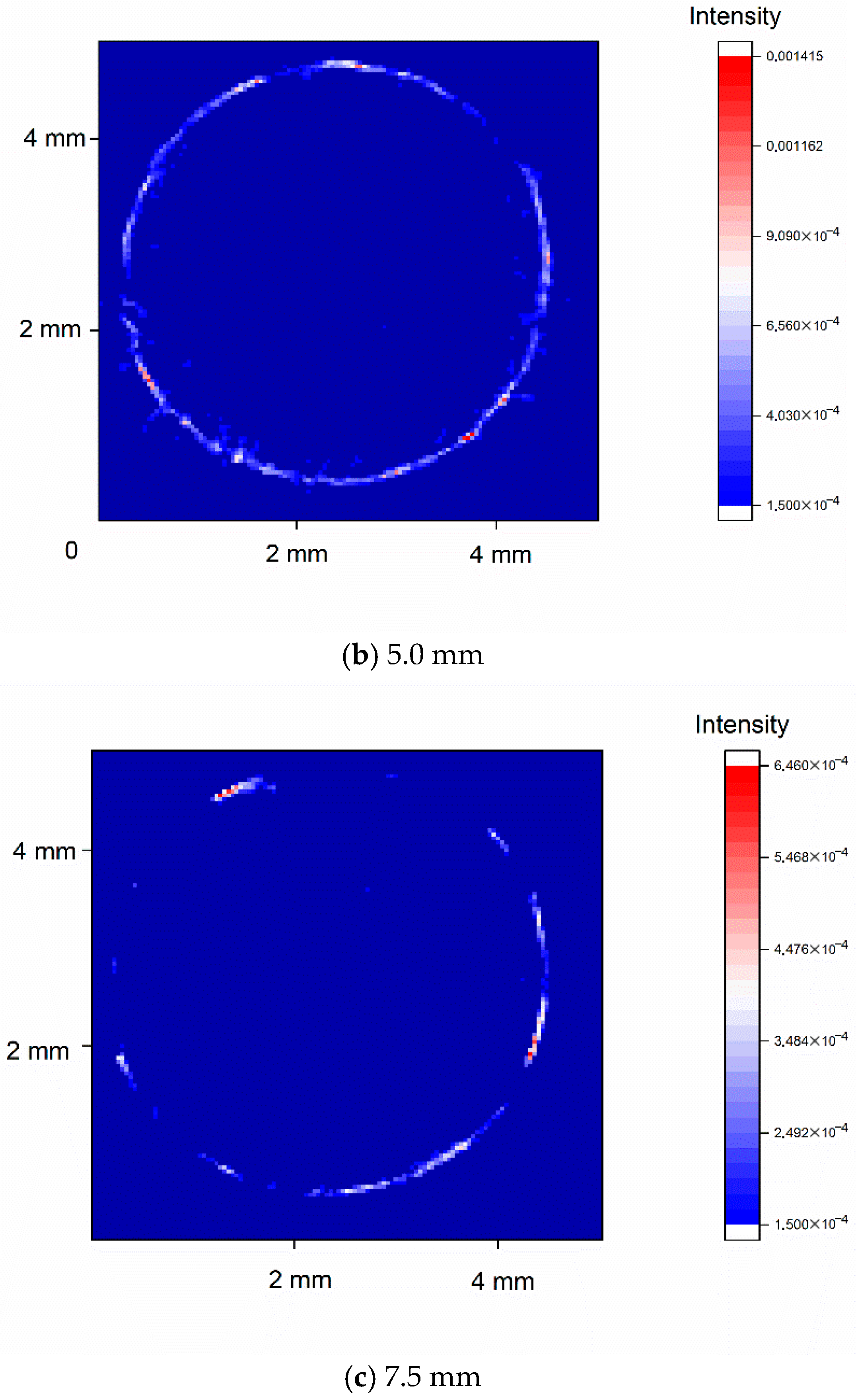
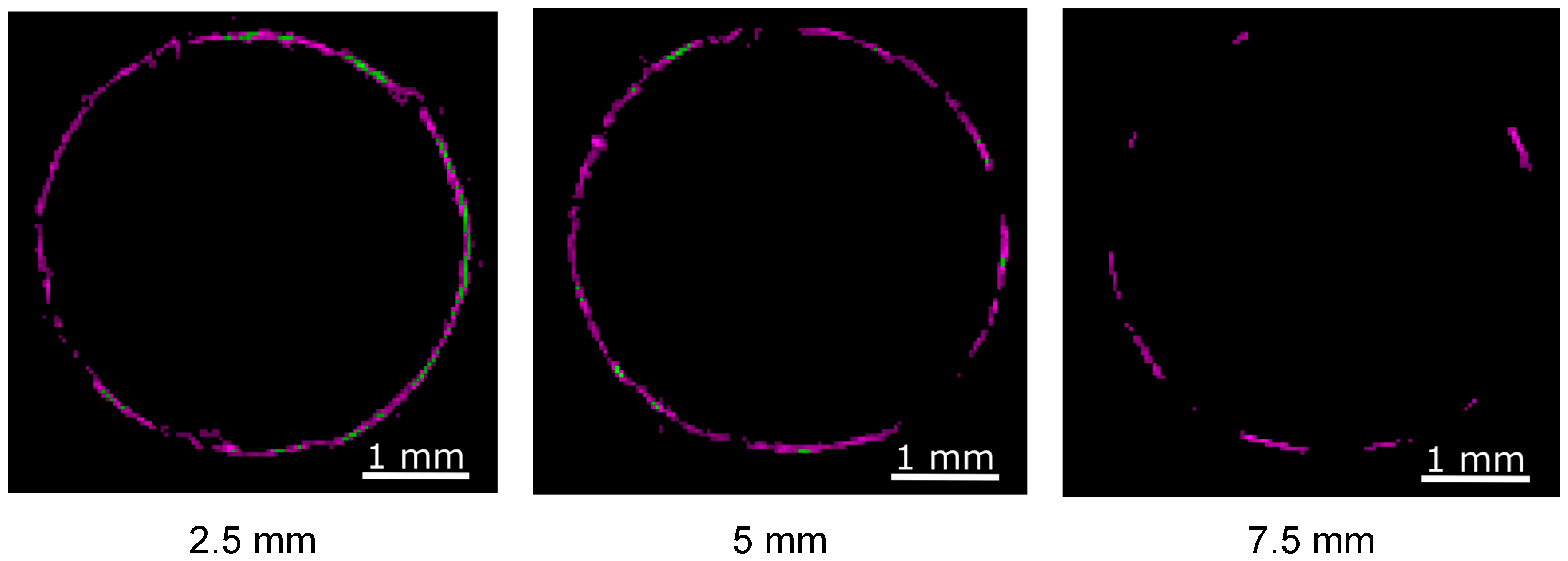
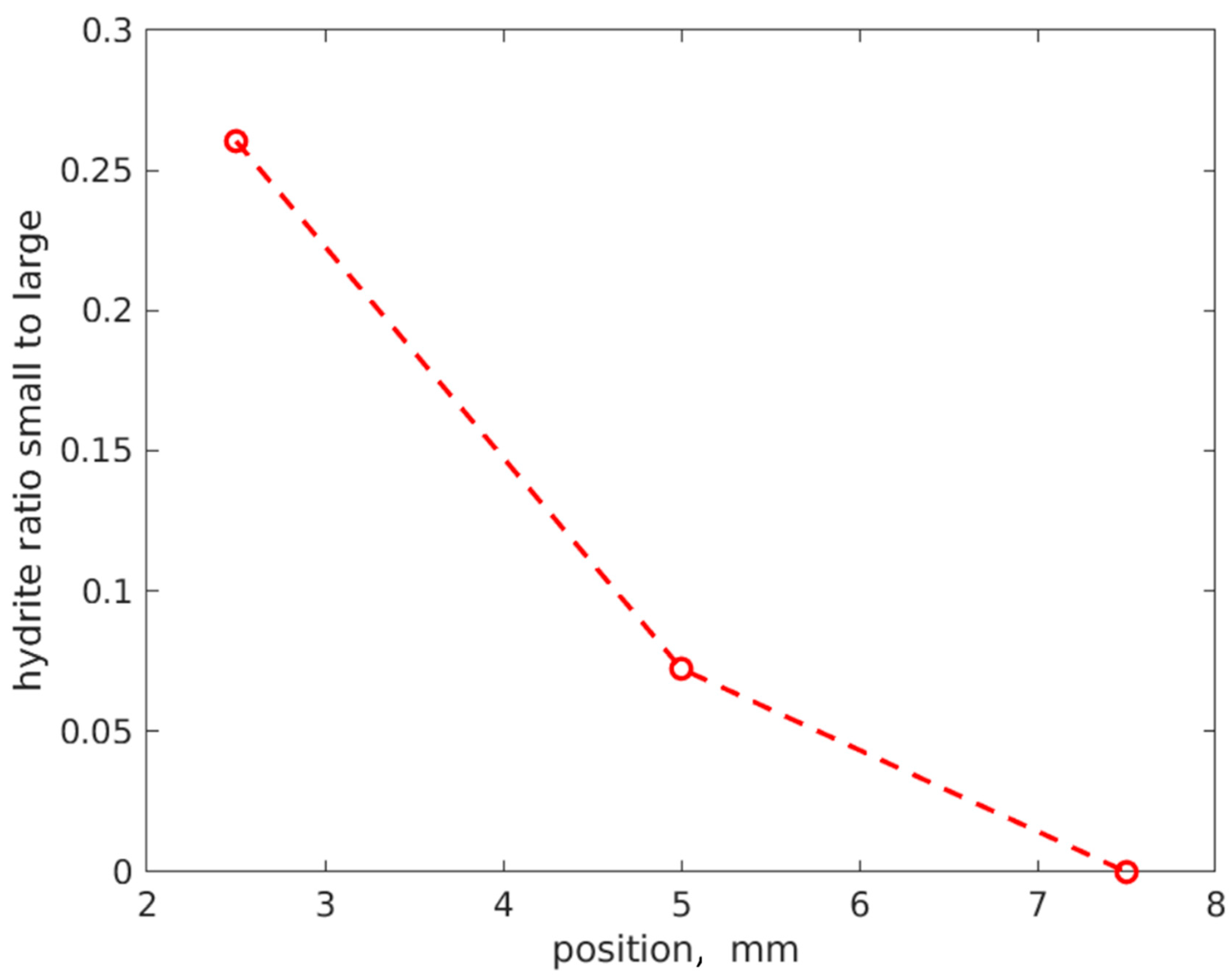
3.4. Three-Dimensional Distribution of TiH2 via XRD Tomography
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Translat. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Tian, L.; Sheng, Y.; Huang, L.; Chow, D.H.; Chau, W.H.; Tang, N.; Ngai, T.; Wu, C.; Lu, J.; Qin, L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials 2018, 180, 173–183. [Google Scholar] [CrossRef]
- Hou, P.; Han, P.; Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Liu, J.; Zhang, Y.; Xu, H.; Cheng, P.; et al. Accelerating Corrosion of Pure Magnesium Co-implanted with Titanium in Vivo. Sci. Rep. 2017, 7, 41924. [Google Scholar] [CrossRef]
- Wang, J.; Bao, Z.; Wu, C.; Zhang, S.; Wang, N.; Wang, Q.; Yi, Z. Progress in partially degradable titanium-magnesium composites used as biomedical implants. Front. Bioeng. Biotechnol. 2022, 10, 996195. [Google Scholar] [CrossRef]
- Yang, X.; Huang, W.; Zhan, D.; Ren, D.; Ji, H.; Liu, Z.; Wang, Q.; Zhang, N.; Zhang, Z. Biodegradability and Cytocompatibility of 3D-Printed Mg-Ti Interpenetrating Phase Composites. Front. Bioeng. Biotechnol. 2022, 10, 891632. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, K.; Pan, M.; Chang, Y.; Gang, S.; Zhao, D. Ultrasonic spot welding of magnesium alloy to titanium alloy. Scr. Mater. 2017, 126, 58–62. [Google Scholar] [CrossRef]
- Murray, J.L. (Ed.) Mg-Ti (Magnesium-Titanium), 2nd ed.; ASM International: Novelty, OH, USA, 1990; Volume 3, pp. 2559–2560. [Google Scholar]
- Haramus, V.; Ebel, T.; Ramakrishnegowda, N.; Bußacker, S.; Schaper, J. Method for Producing a Metallic Implant. EP18156257.0, 12 December 2018. [Google Scholar]
- Garamus, V.M.; Limberg, W.; Serdechnova, M.; Mei, D.; Lamaka, S.V.; Ebel, T.; Willumeit-Römer, R. Degradation of Titanium Sintered with Magnesium: Effect of Hydrogen Uptake. Metals 2021, 11, 527. [Google Scholar] [CrossRef]
- Froes, F.H.; Senkov, O.N.; Qazi, J.I. Hydrogen as a temporary alloying element in titanium alloys: Thermohydrogen processing. Int. Mater. Rev. 2013, 49, 227–245. [Google Scholar] [CrossRef]
- Thiry, M.; Moosmann, J.; Hammel, J.; Wilde, F.; Bidola, P.; Beckmann, F. Brilliant Light for Materials Science: Industrial Applications of Synchrotron Radiation Based Microtomography at the Tomography Instruments by GEMS at PETRA III; SPIE: Bellingham, WA, USA, 2021; Volume 11840. [Google Scholar]
- Schell, N.; King, A.; Beckmann, F.; Fischer, T.; Müller, M.; Schreyer, A. The High Energy Materials Science Beamline (HEMS) at PETRA III. Mater. Sci. Forum 2013, 772, 57–61. [Google Scholar] [CrossRef]
- Moosmann, J.; Ershov, A.; Weinhardt, V.; Baumbach, T.; Prasad, M.S.; LaBonne, C.; Xiao, X.H.; Kashef, J.; Hofmann, R. Time-lapse X-ray phase-contrast microtomography for in vivo imaging and analysis of morphogenesis. Nat. Protoc. 2014, 9, 294–304. [Google Scholar] [CrossRef]
- King, A.; Schell, N.; Martins, R.V.; Beckmann, F.; Ruhnau, H.U.; Kiehn, R.; Marrow, T.J.; Ludwig, W.; Schreyer, A. Grain Tracking at the High Energy Materials Science Beamline of the Petra III Synchrotron Radiation Source. Mater. Sci. Forum 2010, 652, 70–73. [Google Scholar] [CrossRef]
- Lottermoser, L.; Beckmann, F.; Moosmann, J.; Garamus, V.; Iskhakova, K.; Zeller-Plumhoff, B.; Wieland, F. Parallel Usage of X-Ray Diffraction Contrast Tomography and Micro-Computed Tomography for Multi-Modal Characterization at the HEMS P07 Beamline; SPIE: Bellingham, WA, USA, 2024; Volume 13152. [Google Scholar]
- Kieffer, J.; Valls, V.; Blanc, N.; Hennig, C. New tools for calibrating diffraction setups. J. Synchrotron Radiat. 2020, 27, 558–566. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Zhu, T.; Li, M. Lattice variations of Ti-6Al-4V alloy with hydrogen content. Mater. Charact. 2011, 62, 724–729. [Google Scholar] [CrossRef]
- Schmidt, P. Optimierung Der Mechanischen Eigenschaften von Beta-Titanlegierungen Durch Die Verwendung von Wasserstoff Als Temporäres Legierungselement; Lehrstuhl für Materialkunde und Werkstoffprüfung, Universität Siegen: Siegen, Germany, 2020. [Google Scholar]
- Nelson, H.G.; Williams, D.P.; Stein, J.E. Environmental hydrogen embrittlement of an α-β titanium alloy: Effect of microstructure. Metall. Trans. 1972, 3, 473–479. [Google Scholar] [CrossRef]
- Kim, J.; Plancher, E.; Tasan, C.C. Hydrogenation-induced lattice expansion and its effects on hydrogen diffusion and damage in Ti–6Al–4V. Acta Mater. 2020, 188, 686–696. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D. High fugacity hydrogen effects at room temperature in titanium based alloys. J. Alloys Compd. 2005, 404, 613–616. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, W.; Zhang, G.; Wang, B. Surface and phase transformation characteristics of titanium hydride film under irradiation of pulsed ion beam. Appl. Surf. Sci. 2013, 285, 557–563. [Google Scholar] [CrossRef]
- Ebel, T.; Ferri, O.M.; Limberg, W.; Oehring, M.; Pyczak, F.; Schimansky, F.P. Metal injection moulding of titanium and titanium-aluminides. In Proceedings of the 1st International Conference on Powder Processing, Consolidation and Metallurgy of Titanium, Brisbane, Australia, 4–7 December 2011; pp. 153–160. [Google Scholar]
- Wang, T.; Eichhorn, F.; Grambole, D.; Grötzschel, R.; Herrmann, F.; Kreissig, U.; Möller, W. A new Ti/H phase transformation in the H2+ titanium alloy studied by x-ray diffraction, nuclear reaction analysis, elastic recoil detection analysis and scanning electron microscopy. J. Phys. Condens. Matter. 2002, 14, 11605–11614. [Google Scholar] [CrossRef]
- Zhu, Y.; Wook Heo, T.; Rodriguez, J.N.; Weber, P.K.; Shi, R.; Baer, B.J.; Morgado, F.F.; Antonov, S.; Kweon, K.E.; Watkins, E.B.; et al. Hydriding of titanium: Recent trends and perspectives in advanced characterization and multiscale modeling. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101020. [Google Scholar] [CrossRef]
- Yan, S.; Song, G.-L.; Li, Z.; Wang, H.; Zheng, D.; Cao, F.; Horynova, M.; Dargusch, M.S.; Zhou, L. A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Tang, Y.; Ji, X.; Wang, H. Advanced Materials Prepared via Metallic Reduction Reactions for Electrochemical Energy Storage. Small Methods 2020, 4, 2000613. [Google Scholar] [CrossRef]
- Zu, D.; Xu, Z.; Zhang, A.; Wang, H.; Wei, H.; Ou, G.; Huang, K.; Zhang, R.; Li, L.; Hu, S.; et al. Room temperature Mg reduction of TiO2: Formation mechanism and application in photocatalysis. Chem. Commun. 2019, 55, 7675–7678. [Google Scholar] [CrossRef]
- Videm, K.; Lamolle, S.; Monjo, M.; Ellingsen, J.E.; Lyngstadaas, S.P.; Haugen, H.J. Hydride formation on titanium surfaces by cathodic polarization. Appl. Surf. Sci. 2008, 255, 3011–3015. [Google Scholar] [CrossRef]
- Ariyaratnam, S.V.; Surplice, N.A.; Adem, E.H. Solubility of hydrogen in titanium wires and films at 300-k. J. Mater. Sci. Lett. 1987, 6, 1349–1350. [Google Scholar] [CrossRef]
- Lewkowicz, I. Titanium-Hydrogen. Solid State Phenom. 1996, 49–50, 239–280. [Google Scholar] [CrossRef]
- Wen, J.; Allain, N.; Fleury, E. Hydrogen evolution and its effects on cold rolling behavior in commercial pure titanium. Mater. Charact. 2016, 121, 139–148. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Xia, J.; Chen, Y. Effect of Hydrogen Precharging on Mechanical and Electrochemical Properties of Pure Titanium. Adv. Eng. Mater. 2020, 22, 1901182. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.; Suckert, M.; Dahms, M.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Metal Injection Molding (MIM) of Magnesium and Its Alloys. Metals 2016, 6, 118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garamus, V.M.; Wieland, D.C.F.; Moosmann, J.P.; Beckmann, F.; Lottermoser, L.; Serdechnova, M.; Blawert, C.; Fazel, M.; Nidadavolu, E.P.S.; Limberg, W.; et al. Three-Dimensional Distribution of Titanium Hydrides After Degradation of Magnesium/Titanium Hybrid Implant Material—A Study by X-Ray Diffraction Contrast Tomography. J. Compos. Sci. 2025, 9, 396. https://doi.org/10.3390/jcs9080396
Garamus VM, Wieland DCF, Moosmann JP, Beckmann F, Lottermoser L, Serdechnova M, Blawert C, Fazel M, Nidadavolu EPS, Limberg W, et al. Three-Dimensional Distribution of Titanium Hydrides After Degradation of Magnesium/Titanium Hybrid Implant Material—A Study by X-Ray Diffraction Contrast Tomography. Journal of Composites Science. 2025; 9(8):396. https://doi.org/10.3390/jcs9080396
Chicago/Turabian StyleGaramus, Vasil M., D. C. Florian Wieland, Julian P. Moosmann, Felix Beckmann, Lars Lottermoser, Maria Serdechnova, Carsten Blawert, Mohammad Fazel, Eshwara P. S. Nidadavolu, Wolfgang Limberg, and et al. 2025. "Three-Dimensional Distribution of Titanium Hydrides After Degradation of Magnesium/Titanium Hybrid Implant Material—A Study by X-Ray Diffraction Contrast Tomography" Journal of Composites Science 9, no. 8: 396. https://doi.org/10.3390/jcs9080396
APA StyleGaramus, V. M., Wieland, D. C. F., Moosmann, J. P., Beckmann, F., Lottermoser, L., Serdechnova, M., Blawert, C., Fazel, M., Nidadavolu, E. P. S., Limberg, W., Ebel, T., Willumeit-Römer, R., & Zeller-Plumhoff, B. (2025). Three-Dimensional Distribution of Titanium Hydrides After Degradation of Magnesium/Titanium Hybrid Implant Material—A Study by X-Ray Diffraction Contrast Tomography. Journal of Composites Science, 9(8), 396. https://doi.org/10.3390/jcs9080396









