Recent Advances in the Development and Industrial Applications of Wax Inhibitors: A Comprehensive Review of Nano, Green, and Classic Materials Approaches
Abstract
1. Introduction
2. Importance of Wax Inhibitors
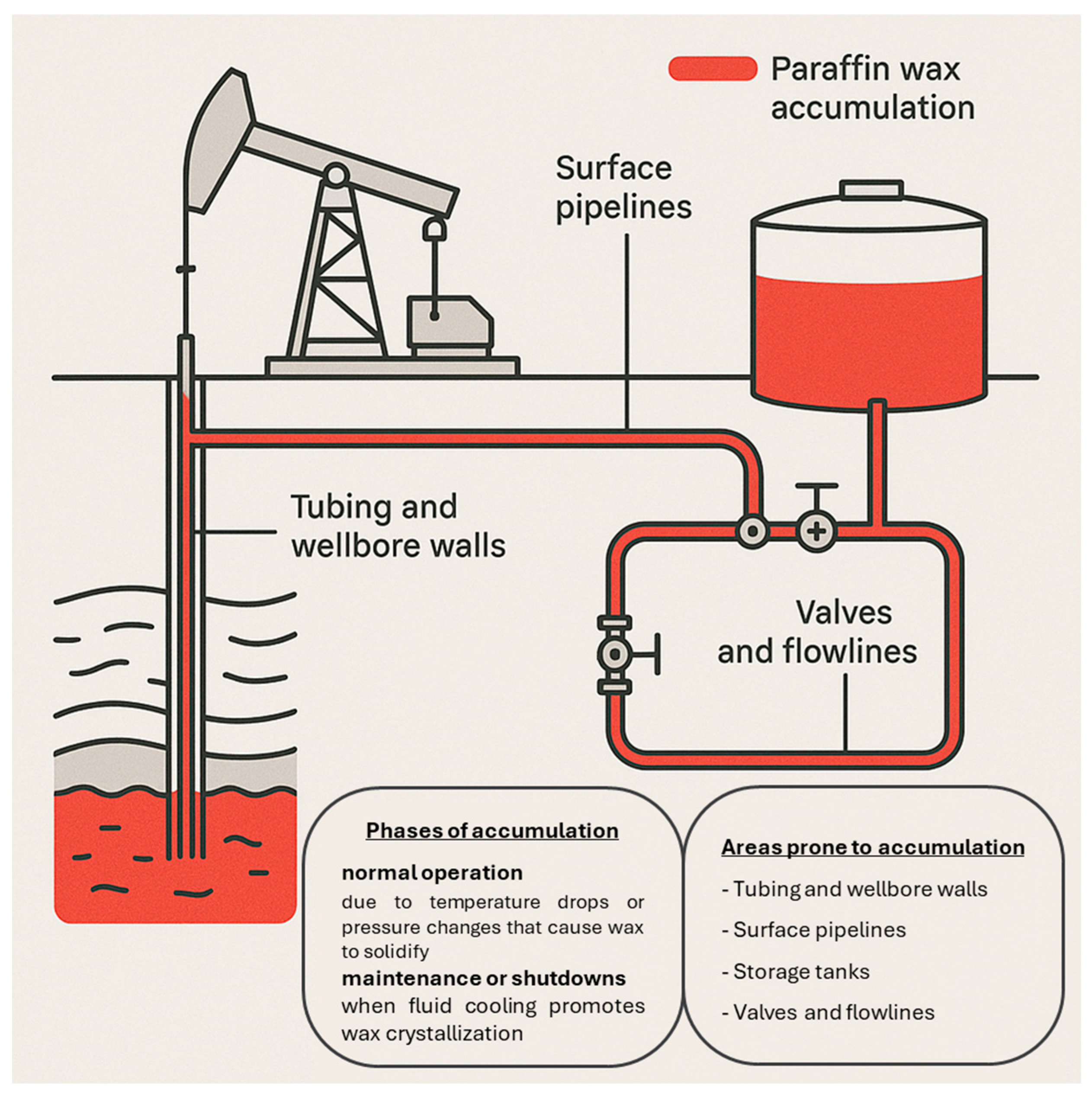
2.1. Wax Deposition Challenges Across Industrial Systems
2.2. Broader Impact on Industrial Operations
2.3. Applications of Wax Inhibitors in Industrial Setups
2.4. Economic Impact of Wax Inhibitors
3. Classic Wax Inhibitors
3.1. Types of Classic Wax Inhibitors Based on Source
3.2. Advantages and Disadvantages of Synthetic and Natural Inhibitors
3.3. Mechanisms and Interactions of Classic Wax Inhibitors
3.4. Integration, Outlook, and Safety Perspectives
4. Nano-Based Wax Inhibitors
4.1. Types of Nano-Based Wax Inhibitors
4.2. Advantages and Disadvantages of Nano-Based Wax Inhibitors
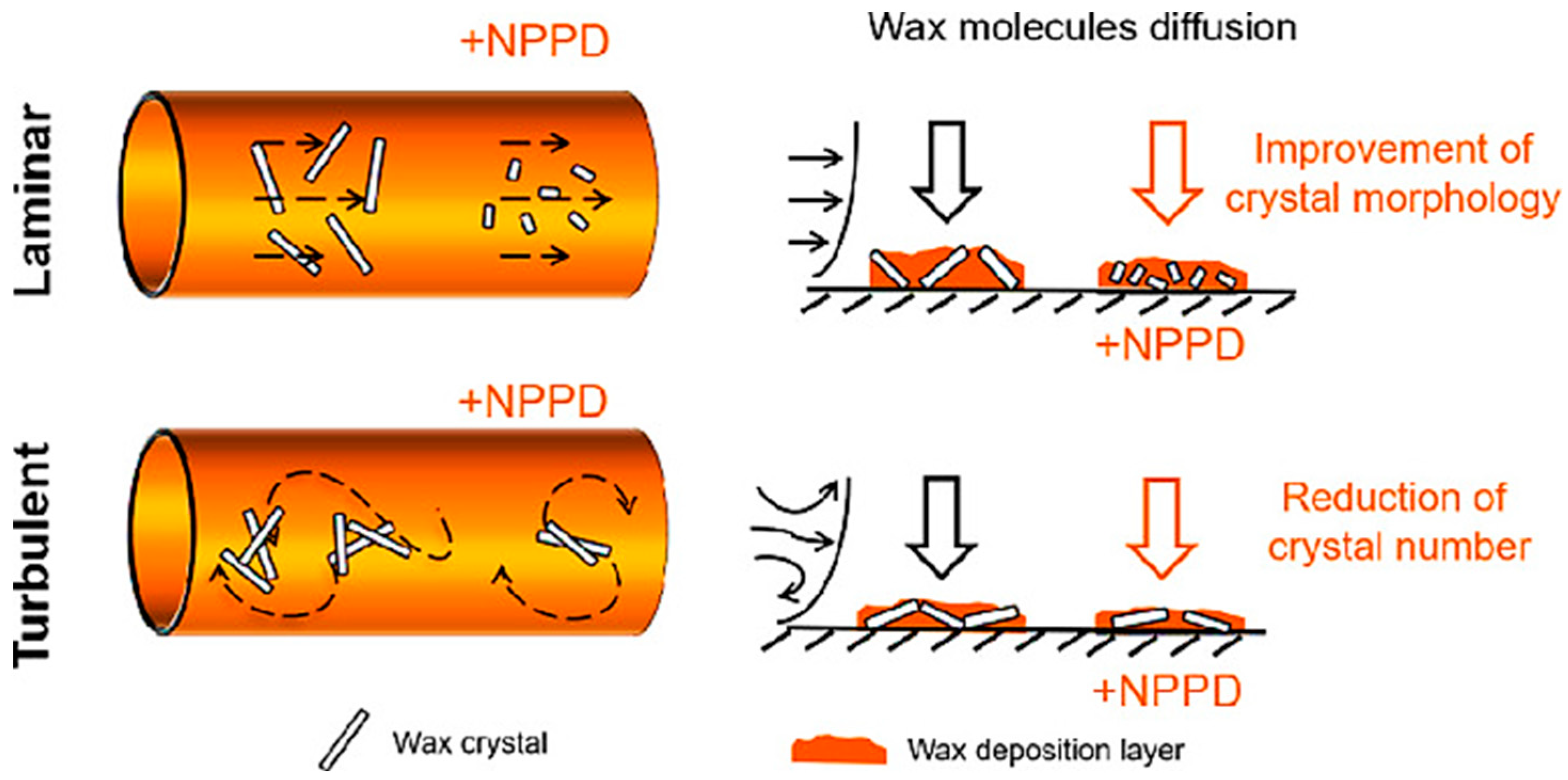
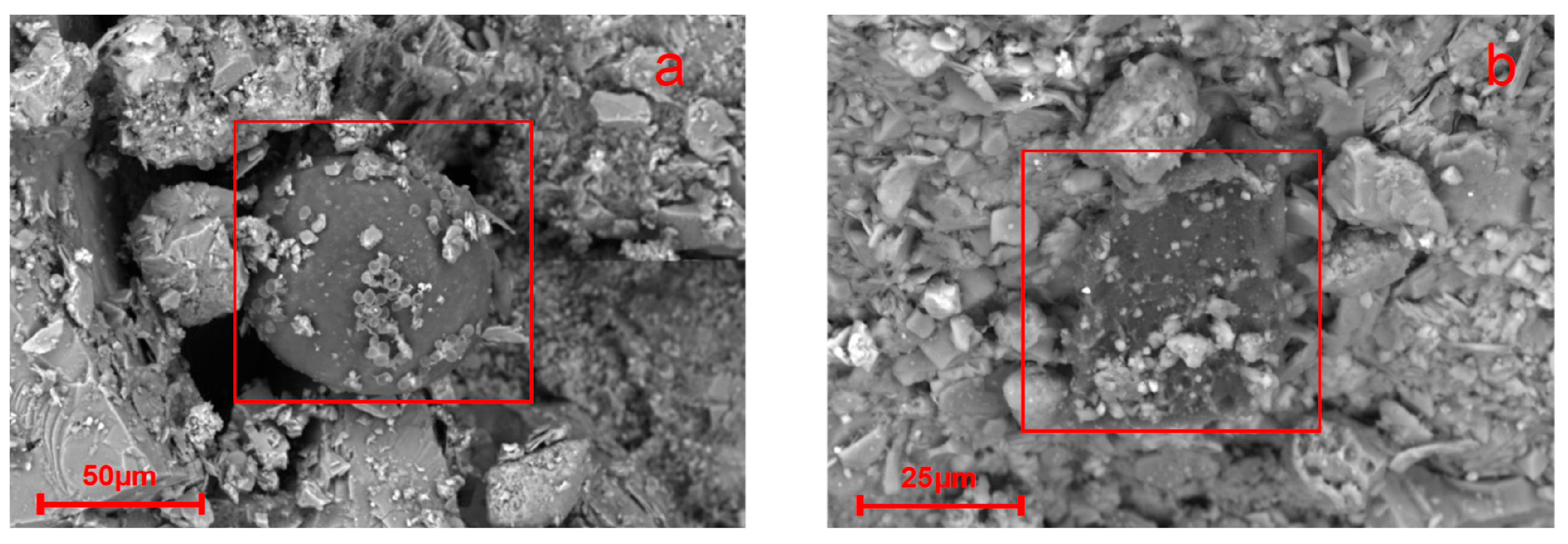
4.3. Mechanisms and Interactions of Nano-Based Wax Inhibitors
4.4. Market and Production Trends
5. Comparison and Future Perspectives
5.1. Nano-Based vs. Classic Wax Inhibitors: A Comparative Analysis
5.2. Research Trends and Gaps
5.3. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Shboul, T.; Sagala, F.; Nassar, N.N. Role of Surfactants, Polymers, Nanoparticles, and Its Combination in Inhibition of Wax Deposition and Precipitation: A Review. Adv. Colloid. Interface Sci. 2023, 315, 102904. [Google Scholar] [CrossRef]
- Ragunathan, T.; Husin, H.; Wood, C.D. Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition. Appl. Sci. 2020, 10, 479. [Google Scholar] [CrossRef]
- Aiyejina, A.; Chakrabarti, D.P.; Pilgrim, A.; Sastry, M.K.S. Wax Formation in Oil Pipelines: A Critical Review. Int. J. Multiph. Flow 2011, 37, 671–694. [Google Scholar] [CrossRef]
- Ala, B.S.K.; Daraboina, N. Chemical Management for Wax Deposition: Recent Developments and Future Prospects. Energy Fuels 2024, 38, 11437–11454. [Google Scholar] [CrossRef]
- Li, B.; Guo, Z.; Zheng, L.; Shi, E.; Qi, B. A Comprehensive Review of Wax Deposition in Crude Oil Systems: Mechanisms, Influencing Factors, Prediction and Inhibition Techniques. Fuel 2024, 357, 129676. [Google Scholar] [CrossRef]
- Yadav, L.; Sihmar, A.; Kumar, S.; Dhaiya, H.; Vishwakarma, R. Review of Nano-Based Smart Coatings for Corrosion Mitigation: Mechanisms, Performance, and Future Prospects. Environ. Sci. Pollut. Res. 2024, 1–27. [Google Scholar] [CrossRef]
- Ahranjani, P.J.; Scampicchio, M.; Ferrentino, G. Advancing the Assessment of Oxidative Stability in Co-Stabilized Zein Nanoparticles and Xanthan Gum Pickering Emulsions Using Isothermal Calorimetry. Food Res. Int. 2025, 209, 116296. [Google Scholar] [CrossRef]
- Faraji Sarabmirza, R.; Joolaei Ahranjani, P.; Rashidi, L.; Mousavi, M.; Khodaiyan, F.; Rashidi Nodeh, H. An Investigation on Conjugated Linoleic Acid Content, Fatty Acid Composition, and Physicochemical Characteristics of Iranian Kurdish Butter Oil. Food Sci. Nutr. 2023, 11, 1051–1058. [Google Scholar] [CrossRef]
- Chen, G.; Tang, Y.; Zhang, J. Synthesis and Application of Polyaminoamide as New Paraffin Inhibitor from Vegetable Oil. Chem. Cent. J. 2011, 5, 82. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Y.; Zheng, Y.; Xing, C.; Hu, Y. Enhance Flows of Waxy Crude Oil in Offshore Petroleum Pipeline: A Review. J. Pet. Sci. Eng. 2022, 208, 109530. [Google Scholar] [CrossRef]
- Alnaimat, F.; Ziauddin, M. Wax Deposition and Prediction in Petroleum Pipelines. J. Pet. Sci. Eng. 2020, 184, 106385. [Google Scholar] [CrossRef]
- Cao, H.; Cao, X.; Zhao, X.; Guo, D.; Liu, Y.; Bian, J. Molecular Dynamics Simulation of Wax Molecules Aggregational Crystallization Behavior during Cooling of Crude Oil Mixture. Case Stud. Therm. Eng. 2022, 37, 102298. [Google Scholar] [CrossRef]
- Elkatory, M.R.; Soliman, E.A.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; El-Nemr, M.A.; Pantaleo, A. Mitigation and Remediation Technologies of Waxy Crude Oils’ Deposition within Transportation Pipelines: A Review. Polymers 2022, 14, 3231. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Yang, F.; Sun, G.; Xia, X.; Ashmawy, A.M.; Zeng, H. Advances in and Perspectives on Strategies for Improving the Flowability of Waxy Oils. Energy Fuels 2022, 36, 7987–8025. [Google Scholar] [CrossRef]
- Sharma, R.; Deka, B.; Mahto, V.; Vuthaluru, H.; Li, C.-Z. Investigation into the Flow Assurance of Waxy Crude Oil by Application of Graphene-Based Novel Nanocomposite Pour Point Depressants. Energy Fuels 2019, 33, 12330–12345. [Google Scholar] [CrossRef]
- Lin, Q.; Qin, Y.; Sun, H.; Wang, X.; Yang, M.; Zhang, X.; Zhou, L.; Luo, F. SPE–UPLC–MS/MS for Determination of 36 Monomers of Alkylphenol Ethoxylates in Tea. Molecules 2023, 28, 3216. [Google Scholar] [CrossRef]
- Aji, A.; Mohyaldinn, M.E.; Mahmud, H. Ben. The Role of Polymer Nanocomposites in Sustainable Wax Deposition Control in Crude Oil Systems-Systematic Review. AIMS Environ. Sci. 2025, 12, 16–52. [Google Scholar] [CrossRef]
- Ruffolo, F.; Dinhof, T.; Murray, L.; Zangelmi, E.; Chin, J.P.; Pallitsch, K.; Peracchi, A. The Microbial Degradation of Natural and Anthropogenic Phosphonates. Molecules 2023, 28, 6863. [Google Scholar] [CrossRef]
- Norrman, J.; Solberg, A.; Sjoblom, J.; Paso, K. Nanoparticles for Waxy Crudes: Effect of Polymer Coverage and the Effect on Wax Crystallization. Energy Fuels 2016, 30, 5108–5114. [Google Scholar] [CrossRef]
- Ali, S.I.; Lalji, S.M.; Haneef, J.; Khan, M.A.; Yousufi, M.; Yousaf, N.; Saboor, A. Phenomena, Factors of Wax Deposition and Its Management Strategies. Arab. J. Geosci. 2022, 15, 133. [Google Scholar] [CrossRef]
- Hao, L.Z.; Al-Salim, H.S.; Ridzuan, N. A Review of the Mechanism and Role of Wax Inhibitors in the Wax Deposition and Precipitation. Pertanika J. Sci. Technol. 2019, 27, 499–526. [Google Scholar]
- Theyab, M.A. A Review of Wax Mitigation Methods through Hydrocarbon Production. J. Pet. Environ. Biotechnol. 2020, 9, 412. [Google Scholar]
- Ansari, F.; Shinde, S.B.; Paso, K.G.; Sjöblom, J.; Kumar, L. Chemical Additives as Flow Improvers for Waxy Crude Oil and Model Oil: A Critical Review Analyzing Structure–Efficacy Relationships. Energy Fuels 2022, 36, 3372–3393. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Da, H.; Hu, K.; Zhang, Y.; Teng, L. Influence of Pour Point Depressants (PPDs) on Wax Deposition: A Study on Wax Deposit Characteristics and Pipeline Pigging. Fuel Process. Technol. 2021, 217, 106817. [Google Scholar] [CrossRef]
- Elarbe, B.; Elganidi, I.; Ridzuan, N.; Yusoh, K.; Abdullah, N.; Vijayakumar, S. Synthesis, Characterization and Evaluation of Stearyl Acrylate-Co-Behenyl Acrylate Copolymer as a Pour Point Depressant of Waxy Crude Oil. J. Pet. Explor. Prod. Technol. 2022, 12, 1811–1828. [Google Scholar] [CrossRef]
- Sun, M.; Naderi, K.; Firoozabadi, A. Effect of Crystal Modifiers and Dispersants on Paraffin-Wax Particles in Petroleum Fluids. SPE J. 2019, 24, 32–43. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Shi, H.; Ma, K.; Ma, Q.; Gong, J. Role of a Nanocomposite Pour Point Depressant on Wax Deposition in Different Flow Patterns from the Perspective of Crystallization Kinetics. ACS Omega 2022, 7, 11200–11207. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Fei, R.; Wang, J.; Liu, S.; Li, M.; Han, S.; Zhou, F.; Yuan, S. Cooling Damage Characterization and Chemical-Enhanced Oil Recovery in Low-Permeable and High-Waxy Oil Reservoirs. Processes 2024, 12, 421. [Google Scholar] [CrossRef]
- Alpandi, A.H.; Husin, H.; Jeffri, S.I.; Sidek, A.; Mingyuan, L. Investigation on Wax Deposition Reduction Using Natural Plant-Based Additives for Sustainable Energy Production from Penara Oilfield Malaysia Basin. ACS Omega 2022, 7, 30730–30745. [Google Scholar] [CrossRef]
- Makwashi, N.; Zhao, D.; Abdulkadir, M.; Ahmed, T.; Muhammad, I. Study on Waxy Crudes Characterisation and Chemical Inhibitor Assessment. J. Pet. Sci. Eng. 2021, 204, 108734. [Google Scholar] [CrossRef]
- López, D.; Ríos, A.A.; Marín, J.D.; Zabala, R.D.; Rincon, J.A.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. SiO2-Based Nanofluids for the Inhibition of Wax Precipitation in Production Pipelines. ACS Omega 2023, 8, 33289–33298. [Google Scholar] [CrossRef]
- Modi, P.; Nagar, A. A Theoretical Approach of Distinctive Additives to Overcome the Challenges in the Field of Petroleum Industry. Rasayan J. Chem. 2024, 17, 1154–1162. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, Characteristics and Oil Industry Applications of Nanoemulsions: A Review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Ahamdi, N.; Ahranjani, P.J.; Rashidi, L.; Rezaei, K. Fortification of Sunflower Oil by Nanoemulsions Containing Vitamin-D3: Formation, Stability, and Release. Food Sci. Nutr. 2025, 13, e4677. [Google Scholar] [CrossRef]
- Peng, L.; Bahadoran, A.; Sheidaei, S.; Ahranjani, P.J.; Kamyab, H.; Oryani, B.; Arain, S.S.; Rezania, S. Magnetic Graphene Oxide Supported Tin Oxide (SnO) Nanocomposite as a Heterogeneous Catalyst for Biodiesel Production from Soybean Oil. Renew. Energy 2024, 224, 120050. [Google Scholar] [CrossRef]
- Joolaei Ahranjani, P.; Polo, A.; Di Cagno, R.; Ferrentino, G. Comparative Evaluation of Stability and Interfacial Characteristics of Pickering Emulsions Co-Stabilized by Zein and Hydrocolloids: Role of Charge Profile, Emulsifying Ability, and Rheological Properties. Food Res. Int. 2025, 218, 116948. [Google Scholar] [CrossRef]
- Golmohammadi, G.; Joolaei Ahranjani, P.; Sereshti, H.; Dehghan, K.; Sadatfaraji, H.; Karami, S.; Rashidi Nodeh, H. Trace Isolation and Photometric Determination of Cadmium Ions in Water Using Thermally Reduced Graphene-Modified Hybrid Sol-Gel Composite. Sep. Sci. Technol. 2025, 60, 1421–1435. [Google Scholar] [CrossRef]
- Quadri, T.W.; Elugoke, S.E.; Verma, C.; Ebenso, E.E. Nanomaterials as Scale Inhibitors. Ind. Scale Inhib. Princ. Des. Appl. 2024, 304–326. [Google Scholar]
- Fakoya, M.F.; Shah, S.N. Emergence of Nanotechnology in the Oil and Gas Industry: Emphasis on the Application of Silica Nanoparticles. Petroleum 2017, 3, 391–405. [Google Scholar] [CrossRef]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A Comprehensive Review on Various Techniques Used for Synthesizing Nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Hasan, M.; Sun, B.; Bahaeddine, M.; Liang, Y.; Damulira, M.; Chen, L. Omniphobic/Superhydrophobic Surface Effect on Oil and Gas Flow: A Critical Review. Can. J. Chem. Eng. 2024, 102, 4241–4266. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid. Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Alkalbani, A.M.; Chala, G.T. A Comprehensive Review of Nanotechnology Applications in Oil and Gas Well Drilling Operations. Energies 2024, 17, 798. [Google Scholar] [CrossRef]
- Sfameni, S.; Rando, G.; Plutino, M.R. Sustainable Secondary-Raw Materials, Natural Substances and Eco-Friendly Nanomaterial-Based Approaches for Improved Surface Performances: An Overview of What They Are and How They Work. Int. J. Mol. Sci. 2023, 24, 5472. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Zeeshan, S.; Khubaib, M.; Ikram, A.; Hussain, F.; Yassin, H.; Qazi, A. A Review of Major Trends, Opportunities, and Technical Challenges in Biodiesel Production from Waste Sources. Energy Convers. Manag. X 2024, 23, 100675. [Google Scholar] [CrossRef]
- Jing, J.; Cao, L.; Shan, Y.; Sun, J.; Tan, J. Optimizing the Low-temperature Performance of High-wax-content Crude Oil with Emulsion-based Wax Inhibitors: Mechanisms and Efficacy. Ann. N. Y. Acad. Sci. 2024, 1541, 255–265. [Google Scholar] [CrossRef]
- Mosleh, N.; Ahranjani, P.J.; Parandi, E.; Nodeh, H.R.; Nawrot, N.; Rezania, S.; Sathishkumar, P. Titanium Lanthanum Three Oxides Decorated Magnetic Graphene Oxide for Adsorption of Lead Ions from Aqueous Media. Environ. Res. 2022, 214, 113831. [Google Scholar] [CrossRef]
- Sher, F.; Ziani, I.; Hameed, M.; Ali, S.; Sulejmanović, J. Advanced Nanomaterials Design and Synthesis for Accelerating Sustainable Biofuels Production–A Review. Curr. Opin. Green. Sustain. Chem. 2024, 47, 100925. [Google Scholar] [CrossRef]
- Basik, M.; Mobin, M. Environmentally Sustainable Corrosion Inhibitors in the Oil and Gas Industry. In Environmentally Sustainable Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 405–421. [Google Scholar]
- Verma, C.; Chauhan, D.S.; Aslam, R.; Banerjee, P.; Aslam, J.; Quadri, T.W.; Zehra, S.; Verma, D.K.; Quraishi, M.A.; Dubey, S. Principles and Theories of Green Chemistry for Corrosion Science and Engineering: Design and Application. Green Chem. 2024, 26, 4270–4357. [Google Scholar] [CrossRef]

| Inhibitor Type | Environmental/Safety Notes | Status/Trends |
|---|---|---|
| Polymeric PPDs (traditional) | Many are relatively inert high molecular weight polymers. Not acutely toxic, but not readily biodegradable—can accumulate as microplastics if released. Often solvent-carried (solvent can be hazardous) [15]. | New formulations aim for biodegradable polymers (e.g., polyesters, modified natural polymers) to replace legacy polymers. Generally low environmental risk in contained systems, but disposal of pipeline scrapper wax containing polymer needs consideration. |
| Alkylphenol Surfactants (APEs) | Persistent and toxic to aquatic life. APEs degrade to alkylphenols, which bioaccumulate and disrupt endocrine systems in fish and wildlife [16]. Banned or restricted in many regions. | Largely being phased out in oilfield chemicals. Replaced by more benign surfactants (e.g., alcohol ethoxylates, glyceride-based surfactants). Regulatory pressure has prompted greener dispersants. |
| Aromatic Solvents (Toluene/Xylene) | Volatile Organic Compounds (VOC)—flammable, toxic to handle. Cause air pollution (smog) and health hazards on exposure [17]. Spills can contaminate water/soil. | Usage is minimized; often recovered or incinerated after use. The industry is moving toward solvent-free inhibition methods due to safety and environmental regulations. |
| Phosphonate Inhibitors | Some older wax inhibitors contained phosphonates—these are non-biodegradable and can lead to nutrient pollution (algal blooms) in water if discharged [18] | Rare in modern wax formulations; if used, effluent treatment is required. Focus on reducing phosphorus-based additive use. |
| Natural Oil-Based Inhibitors | Derived from renewable sources (vegetable oils, fatty acids). Generally biodegradable and low toxicity., e.g., plant oils used as solvent or PPD base leave less persistent residues [17]. | Increasing interest as sustainable alternatives. Field trialed in some regions for environmental compliance. Need to ensure consistent quality and performance. |
| Nano-Based Inhibitors (e.g., nanoparticles, nanoemulsions, nanocomposites) | Depending on components—can be designed to be environmentally friendly (using green synthesis and biodegradable polymers) [19]. Nanoparticles like silica or clay are usually inert; concern is any toxicity of nano-size particles if released. | Research trend toward green nanocomposites. The goal is to be high performance with minimal eco-toxicity. Regulators may scrutinize nanoparticle discharges; however, encapsulating NPs in polymer mitigates free nanoparticle release. |
| Inhibitor Type | Mechanism of Action | Interaction Type | Functional Features | Ref. |
|---|---|---|---|---|
| Synthetic Polymer (Classic) | Co-crystallization with wax molecules; disrupts lattice growth | Hydrophobic insertion, steric hindrance | Temperature-stable, scalable, less biodegradable | [4] |
| Natural (Plant-Based) | Surface adsorption; modification of interfacial energy | Hydrogen bonding, amphiphilic interaction | Biodegradable, eco-friendly, limited efficacy in harsh conditions | [29] |
| Nanoemulsion | Interfacial tension reduction; steric stabilization of wax crystals | Surfactant-like interface disruption | Effective at low concentrations, enhanced dispersion, thermodynamically stable | [33] |
| Functionalized Nanoparticle | Adsorption to wax nuclei; lattice capping and morphology alteration | Electrostatic and van der Waals interactions | High specificity, enhanced control under extreme pressure/temperature | [31] |
| Polymer Nanocomposite | Synergistic co-crystallization and nanoparticle interference | Combined steric + polar interaction | High performance, tunable properties, emerging green synthesis methods | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joolaei Ahranjani, P.; Sadatfaraji, H.; Dehghan, K.; Edlabadkar, V.A.; Khadka, P.; Nwobodo, I.; Turaga, V.R.; Disney, J.; Rashidi Nodeh, H. Recent Advances in the Development and Industrial Applications of Wax Inhibitors: A Comprehensive Review of Nano, Green, and Classic Materials Approaches. J. Compos. Sci. 2025, 9, 395. https://doi.org/10.3390/jcs9080395
Joolaei Ahranjani P, Sadatfaraji H, Dehghan K, Edlabadkar VA, Khadka P, Nwobodo I, Turaga VR, Disney J, Rashidi Nodeh H. Recent Advances in the Development and Industrial Applications of Wax Inhibitors: A Comprehensive Review of Nano, Green, and Classic Materials Approaches. Journal of Composites Science. 2025; 9(8):395. https://doi.org/10.3390/jcs9080395
Chicago/Turabian StyleJoolaei Ahranjani, Parham, Hamed Sadatfaraji, Kamine Dehghan, Vaibhav A. Edlabadkar, Prasant Khadka, Ifeanyi Nwobodo, VN Ramachander Turaga, Justin Disney, and Hamid Rashidi Nodeh. 2025. "Recent Advances in the Development and Industrial Applications of Wax Inhibitors: A Comprehensive Review of Nano, Green, and Classic Materials Approaches" Journal of Composites Science 9, no. 8: 395. https://doi.org/10.3390/jcs9080395
APA StyleJoolaei Ahranjani, P., Sadatfaraji, H., Dehghan, K., Edlabadkar, V. A., Khadka, P., Nwobodo, I., Turaga, V. R., Disney, J., & Rashidi Nodeh, H. (2025). Recent Advances in the Development and Industrial Applications of Wax Inhibitors: A Comprehensive Review of Nano, Green, and Classic Materials Approaches. Journal of Composites Science, 9(8), 395. https://doi.org/10.3390/jcs9080395






