Investigation of Phosphorus Dendrons and Their Properties for the Functionalization of Materials
Abstract
1. Introduction
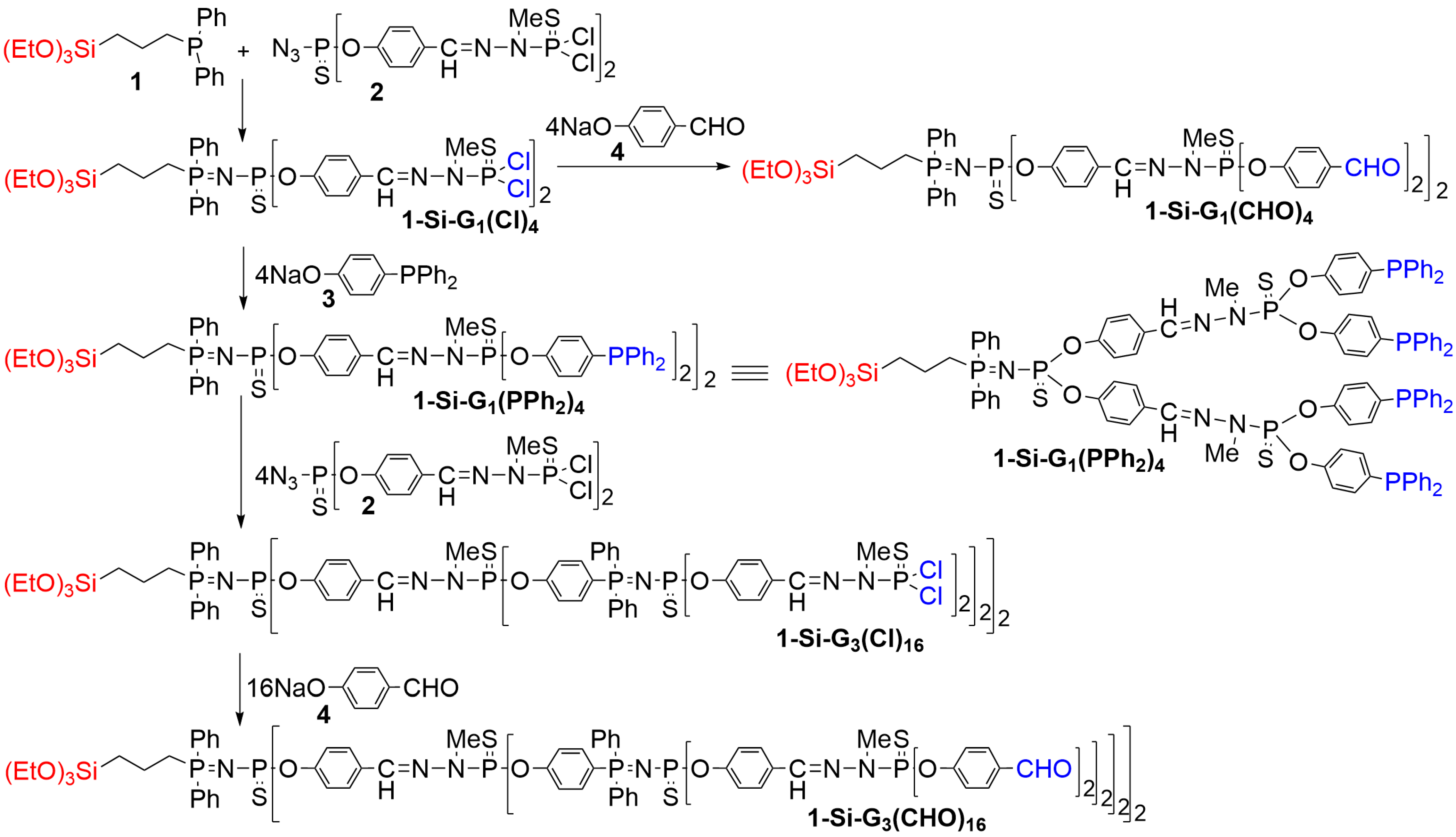
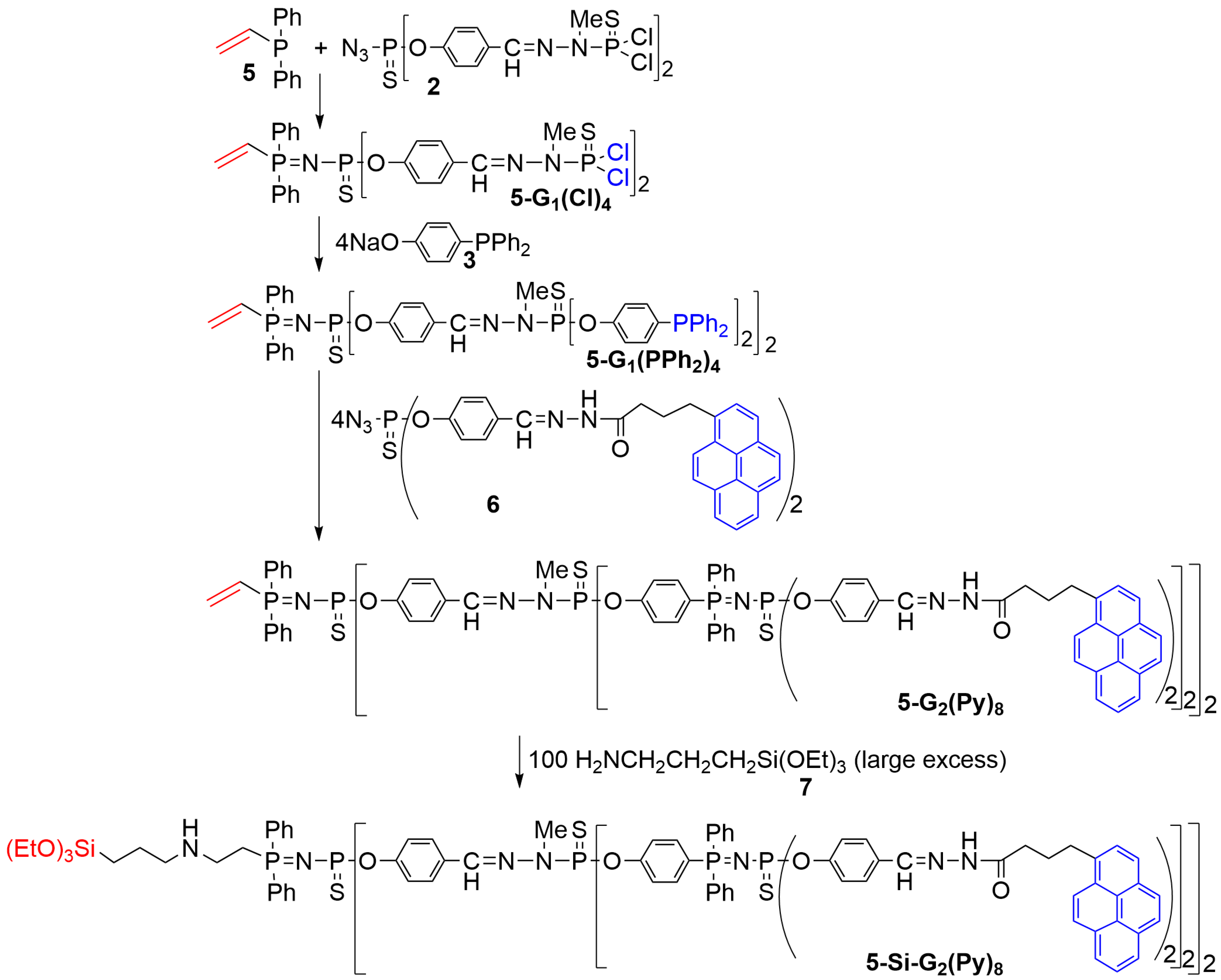
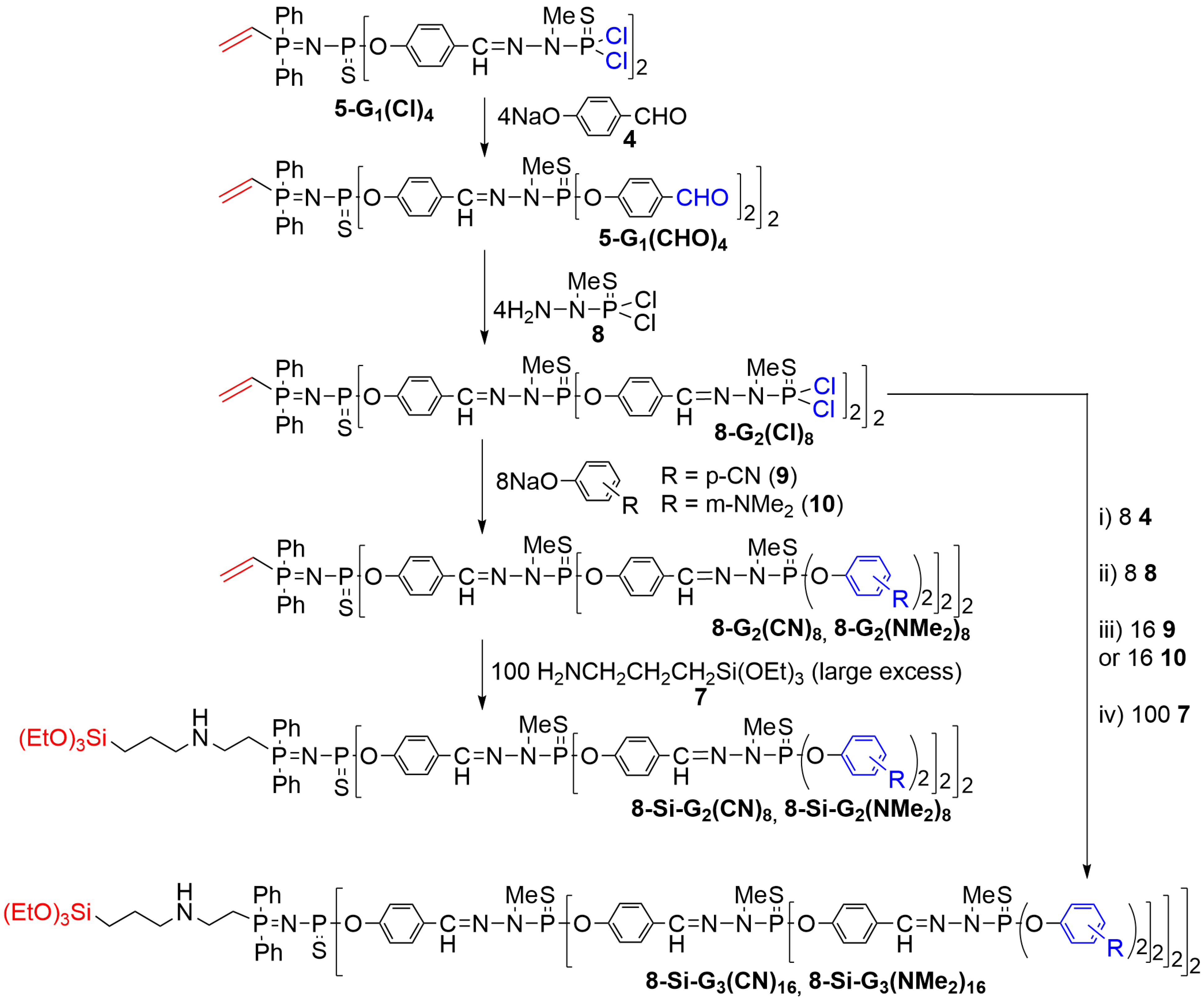
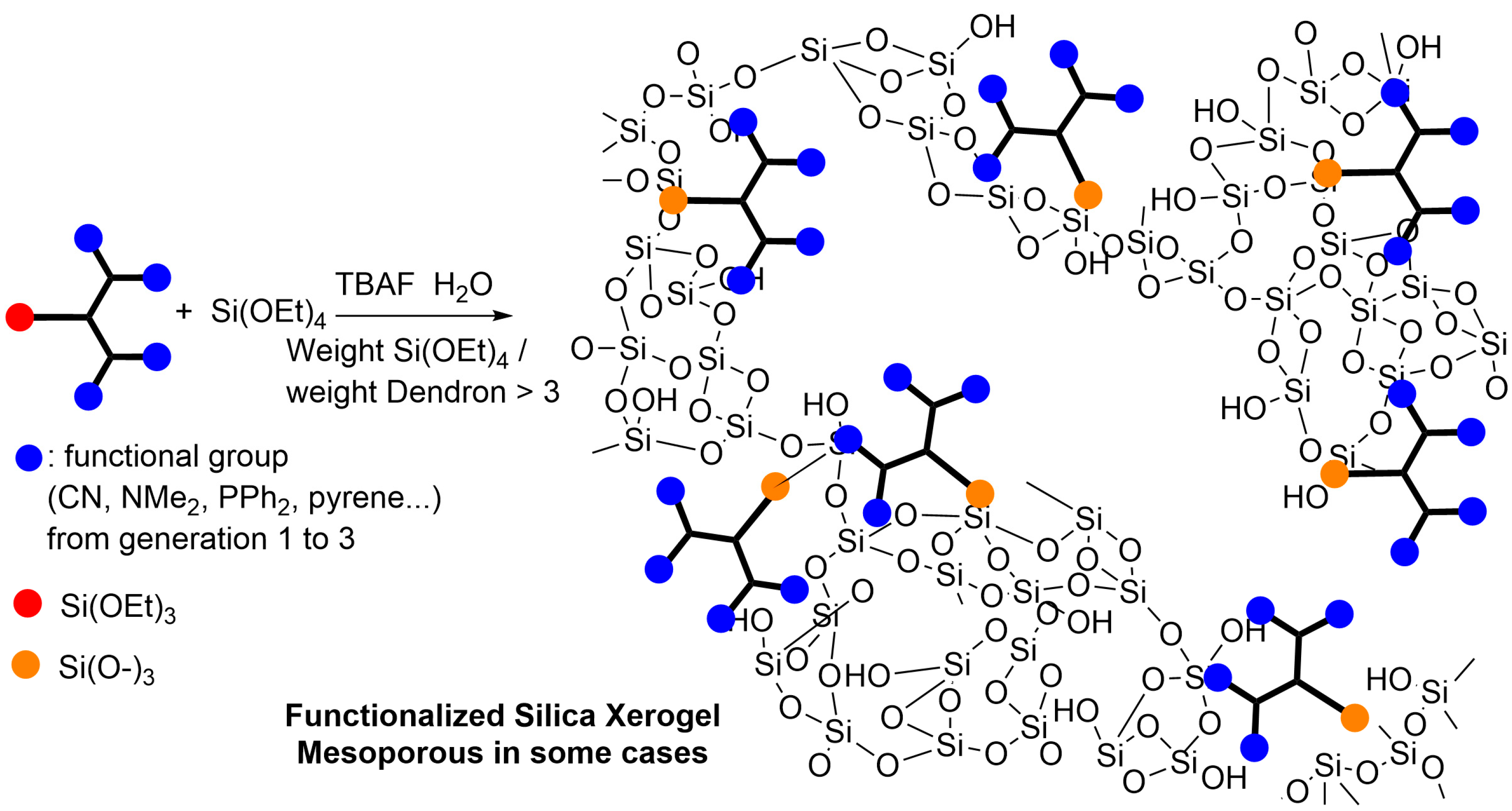
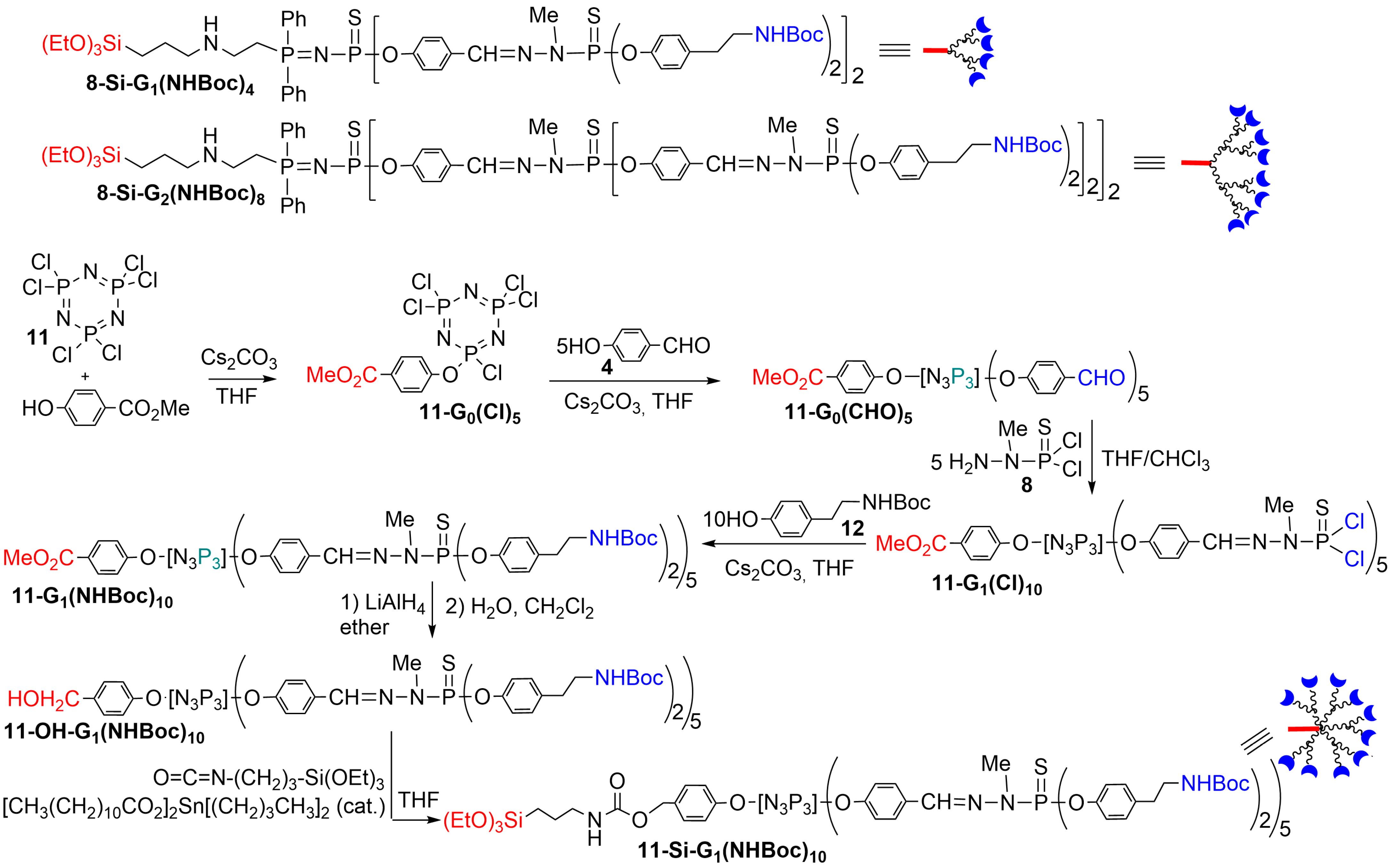
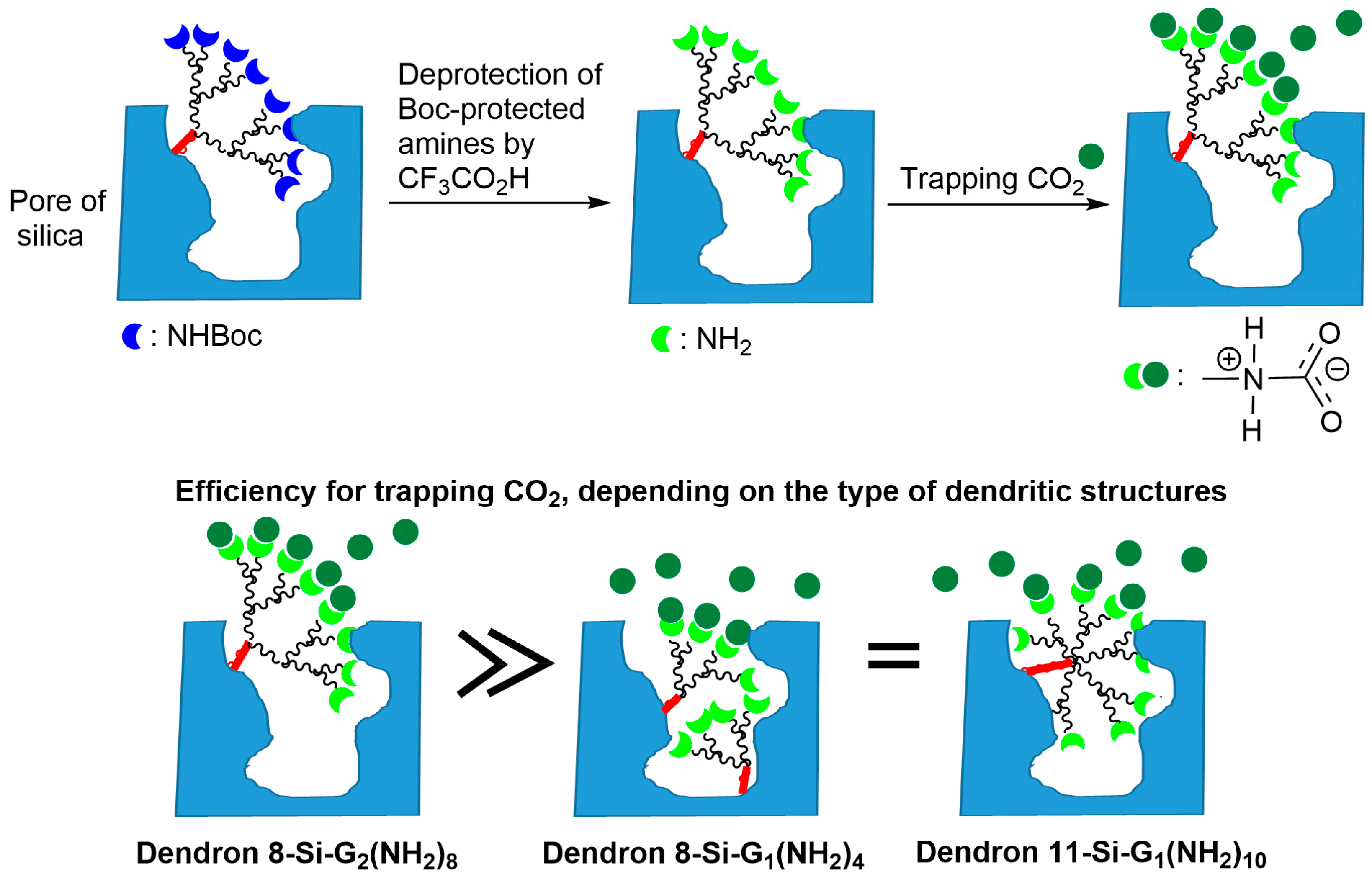
2. Phosphorus Dendrons Inside Materials and Their Properties
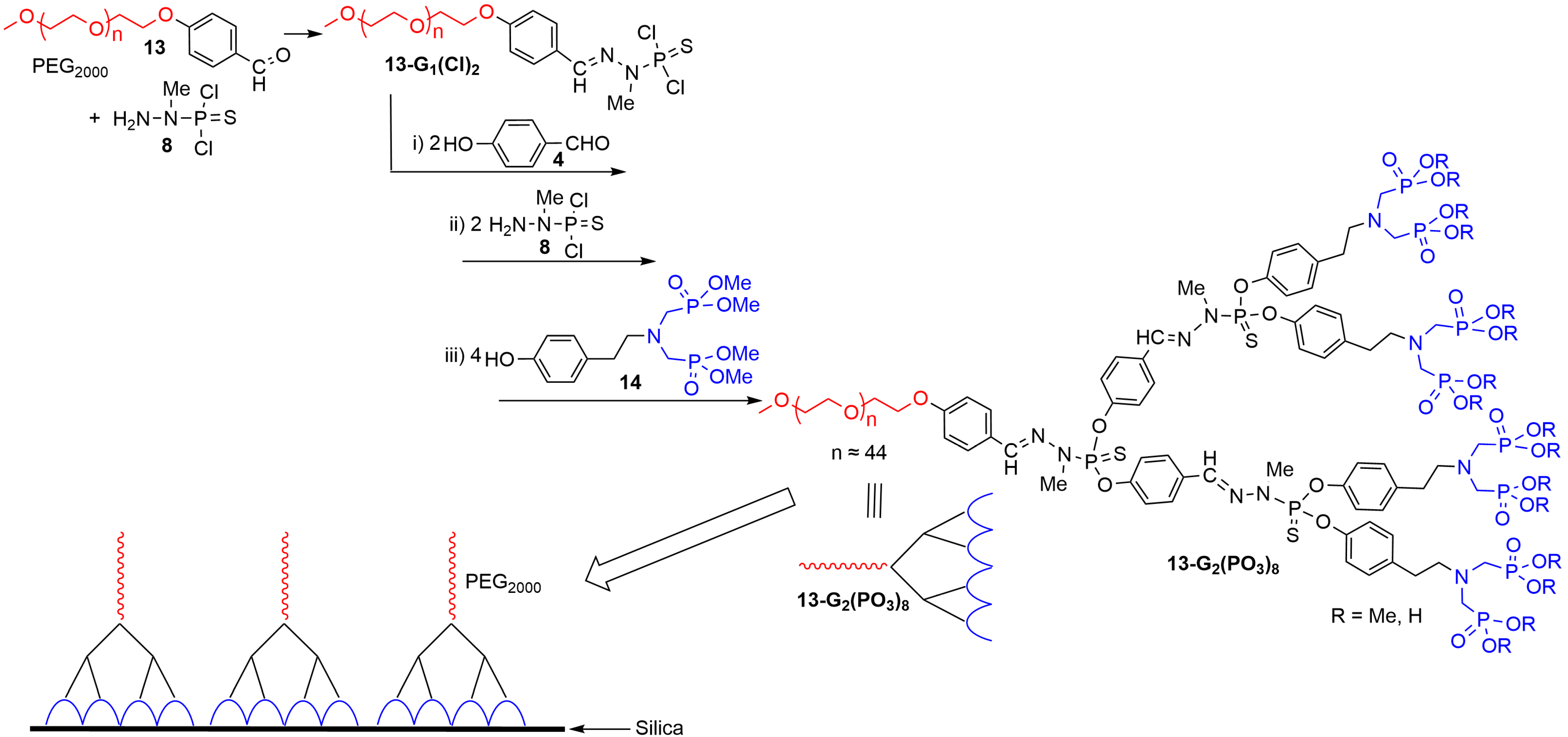
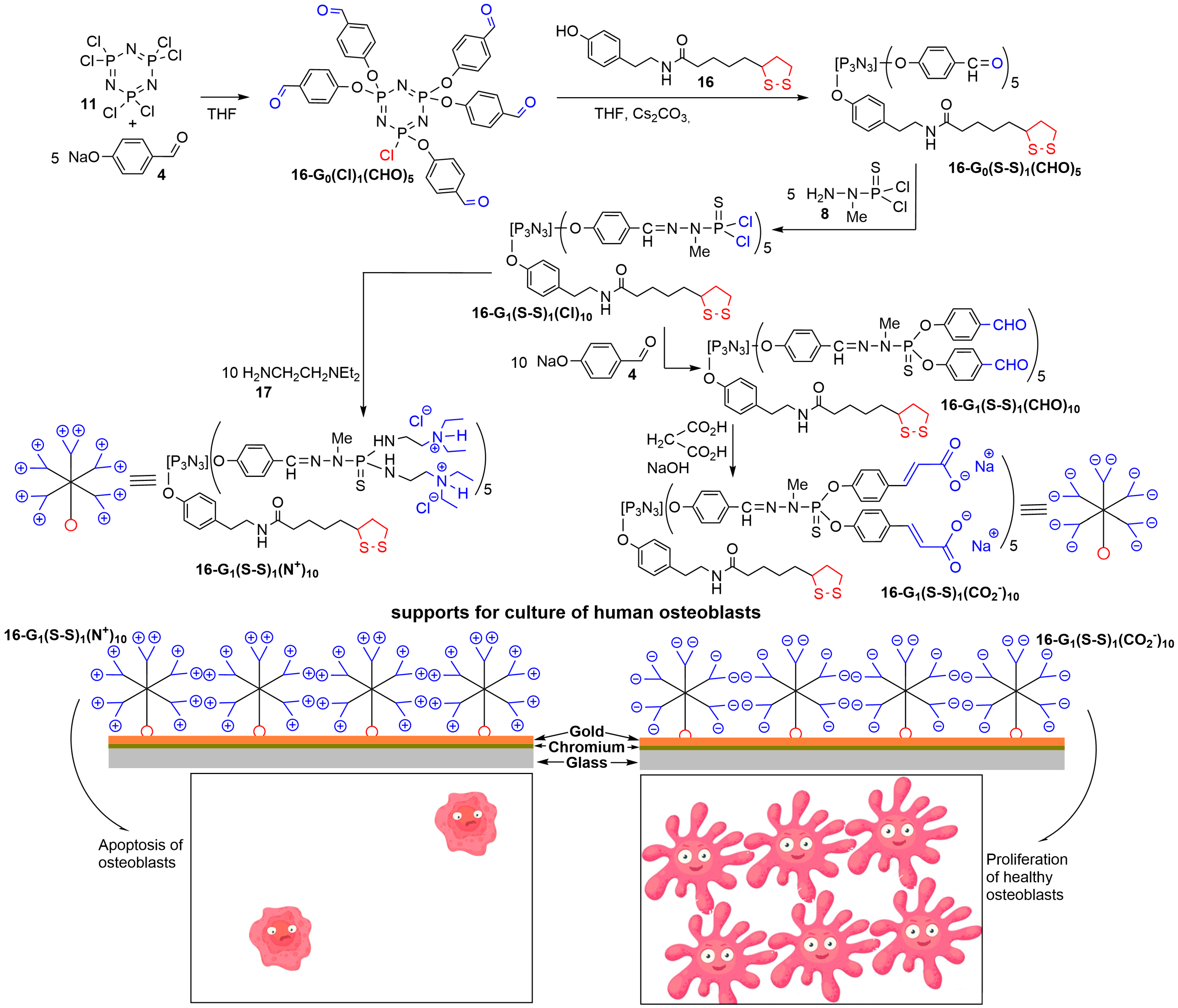
3. Phosphorus Dendrons on the Surface of Materials and Their Properties
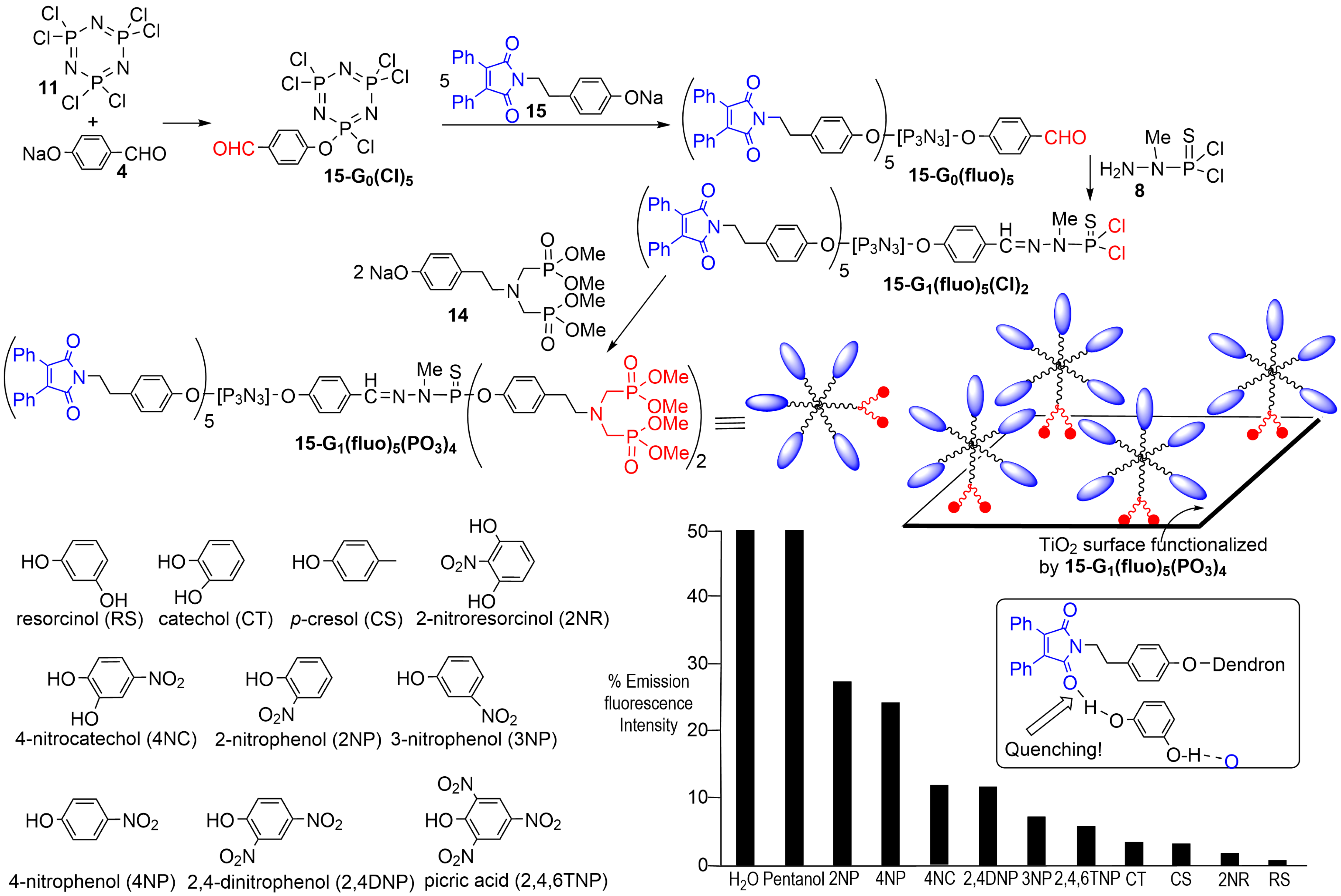
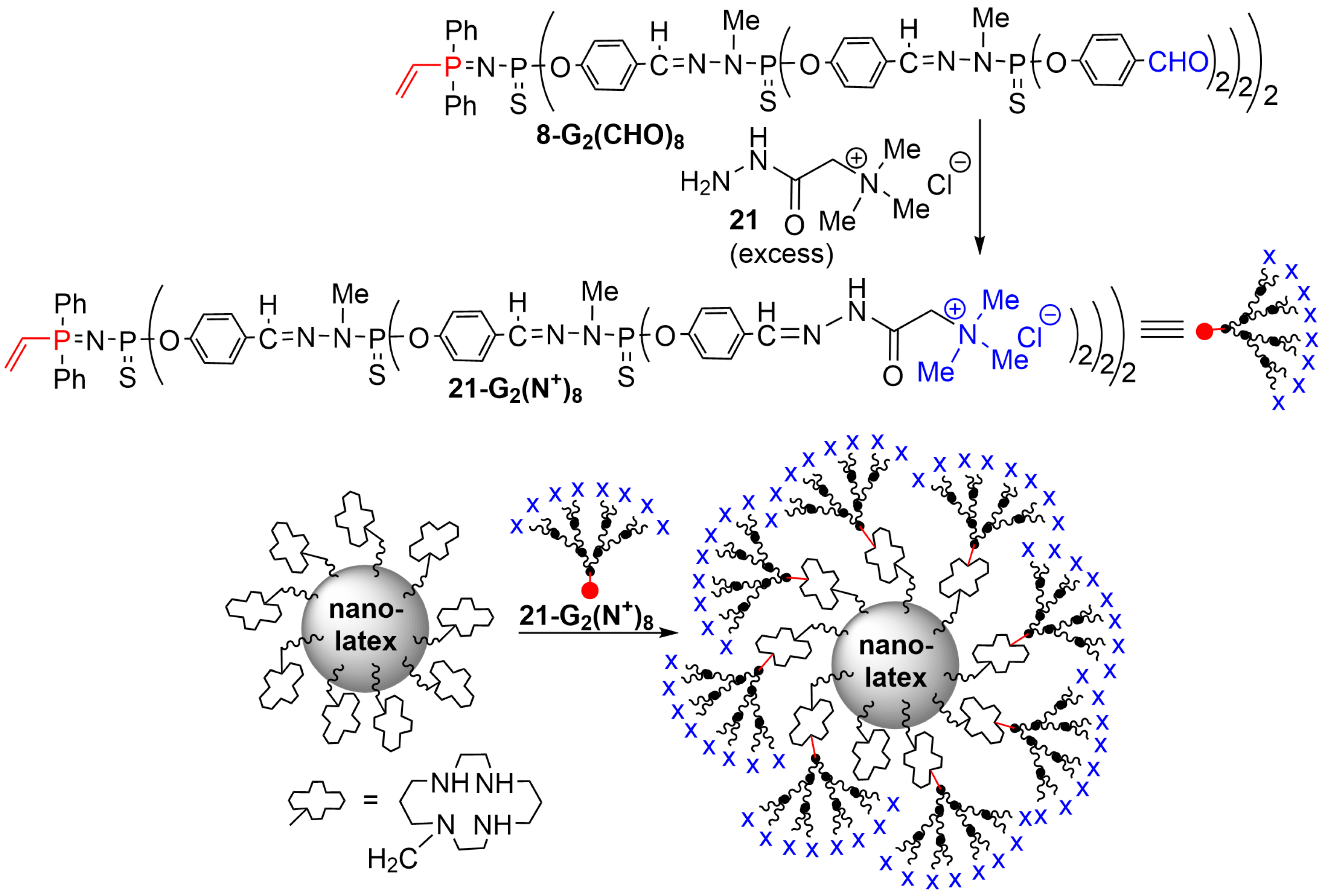
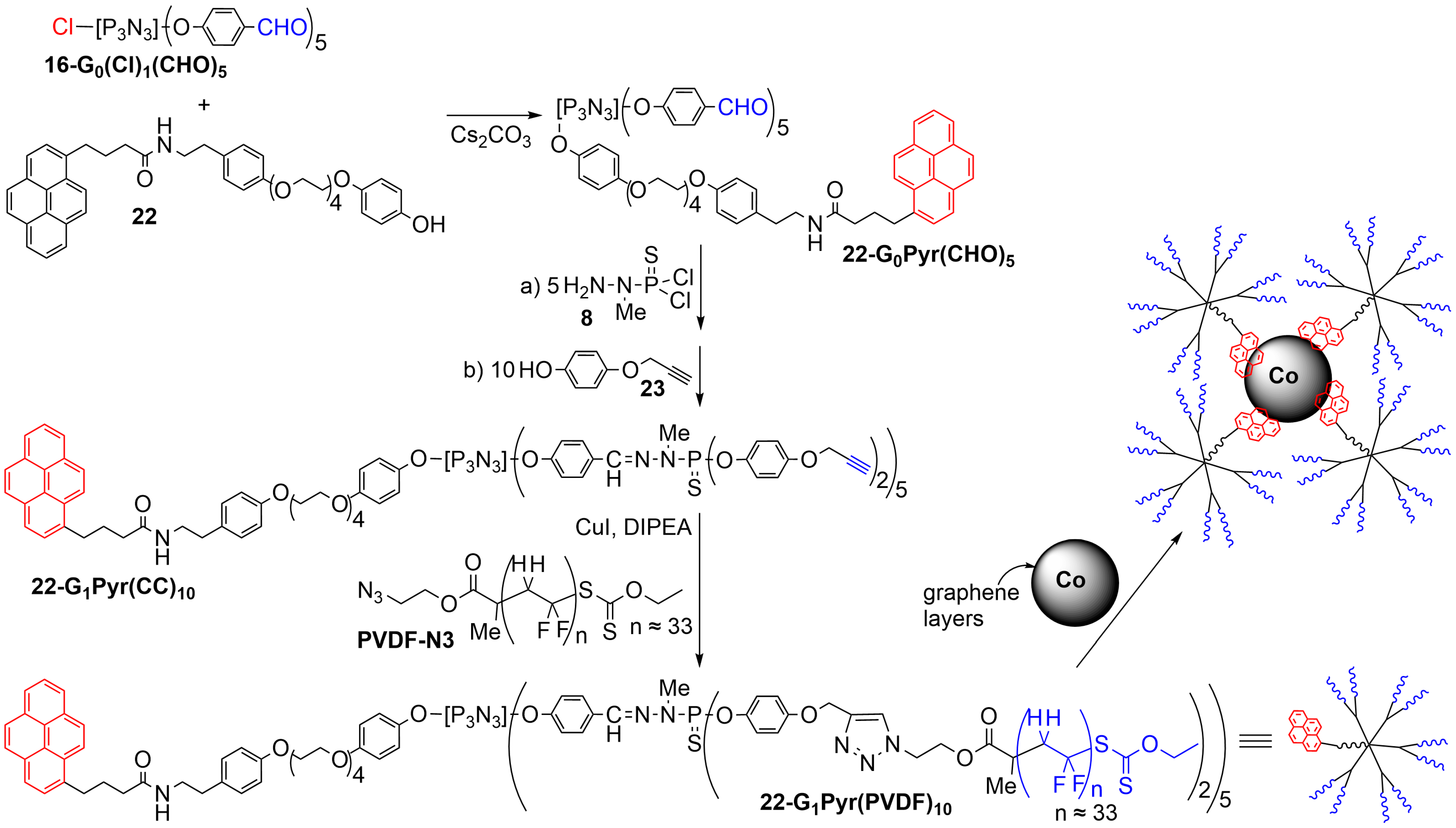
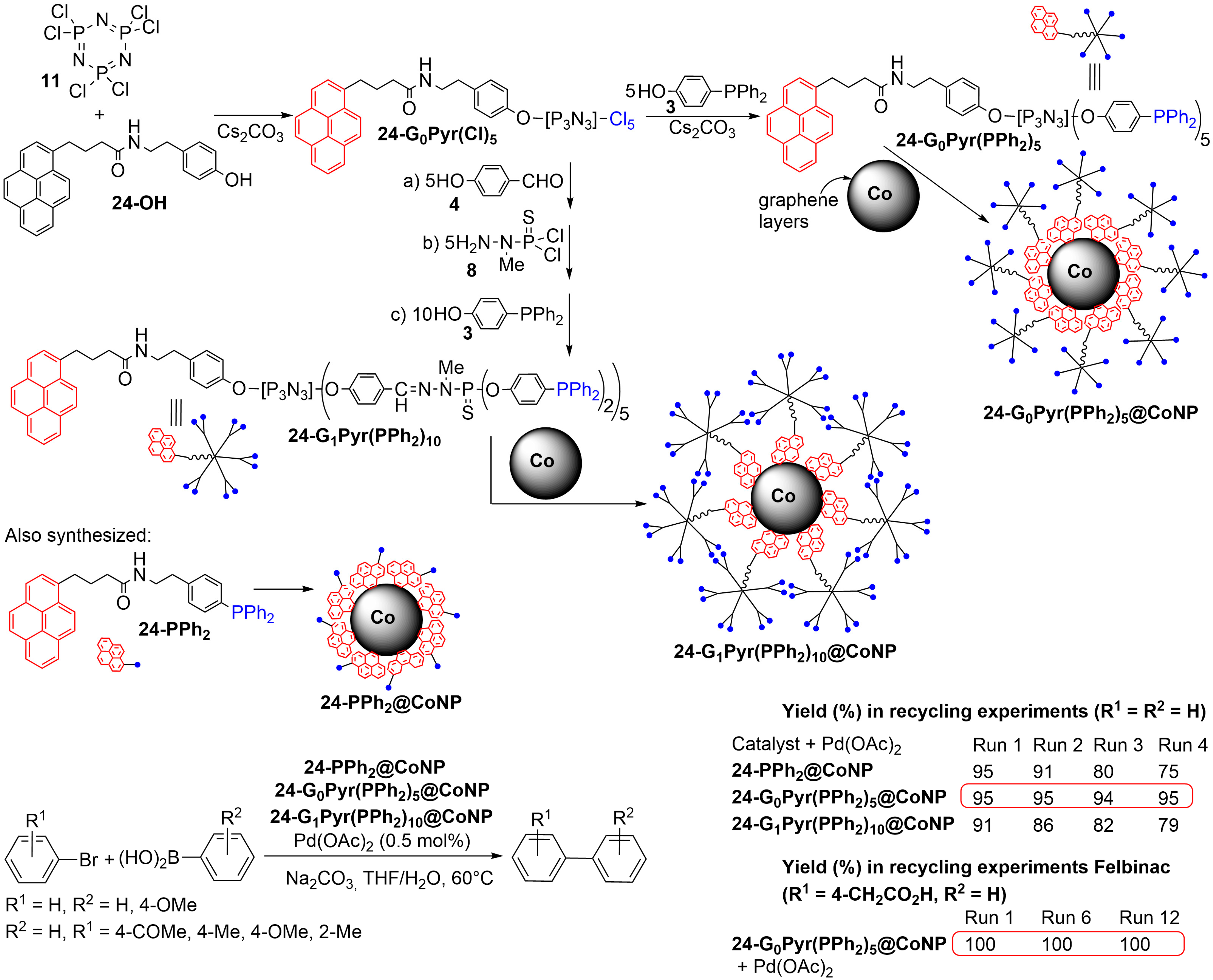
4. Phosphorus Dendrons on the Surface of Particles and Their Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawker, C.J.; Frechet, J.M.J. Control of Surface Functionality in the Synthesis of Dendritic Macromolecules using the Convergent- Growth Approach. Macromolecules 1990, 23, 4726–4729. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1→2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc.-Chem. Commun. 1990, 1990, 1010–1013. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168–2203. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Denkewalter, R.G.; Kolc, J.F.; Lukasavage, W.J. Macromolecular Highly Branched Homogeneous Compound Based on Lysine Units. U.S. Patent US4289872A, 15 September 1981. [Google Scholar]
- Worner, C.; Mulhaupt, R. Polynitrile-Functional and Polyamine-Functional Poly(Trimethylene Imine) Dendrimers. Angew. Chem.-Int. Edit. Engl. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- de Brabander van den Berg, E.M.M.; Meijer, E.W. Poly(Propylene Imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem.-Int. Edit. Engl. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem.-Int. Edit. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Turrin, C.O.; Rebout, C.; Delavaux-Nicot, B.; Ouali, A.; Zablocka, M.; Majoral, J.P. Phosphorus dendrimers as viewed by P-31 NMR spectroscopy; synthesis and characterization. C. R. Chim. 2010, 13, 1006–1027. [Google Scholar] [CrossRef]
- Galliot, C.; Larre, C.; Caminade, A.M.; Majoral, J.P. Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 1997, 277, 1981–1984. [Google Scholar] [CrossRef]
- Caminade, A.M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Maraval, A.; Majoral, J.P. Phosphorus-containing dendrons: Synthesis, reactivity, properties, and use as building blocks for various dendritic architectures. Eur. J. Inorg. Chem. 2006, 2006, 887–901. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Zibarov, A.; Oukhrib, A.; Aujard Catot, J.; Turrin, C.-O.; Caminade, A.-M. AB5 Derivatives of Cyclotriphosphazene for the Synthesis of Dendrons and Their Applications. Molecules 2021, 26, 4017. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Portnoy, M. Dendrons and dendritic catalysts immobilized on solid support: Synthesis and dendritic effects in catalysis. J. Polym. Sci. Part. A-Polym. Chem. 2005, 43, 235–262. [Google Scholar] [CrossRef]
- Kehat, T.; Goren, K.; Portnoy, M. Dendrons on insoluble supports: Synthesis and applications. New J. Chem. 2007, 31, 1218–1242. [Google Scholar] [CrossRef]
- Walter, A.; Garofalo, A.; Parat, A.; Martinez, H.; Felder-Flesch, D.; Begin-Colin, S. Functionalization strategies and dendronization of iron oxide nanoparticles. Nanotechnol. Rev. 2015, 4, 581–593. [Google Scholar] [CrossRef]
- Paez, J.I.; Martinelli, M.; Brunetti, V.; Strumia, M.C. Dendronization: A Useful Synthetic Strategy to Prepare Multifunctional Materials. Polymers 2012, 4, 355–395. [Google Scholar] [CrossRef]
- Turrin, C.O.; Maraval, V.; Caminade, A.M.; Majoral, J.P.; Mehdi, A.; Reye, C. Organic-inorganic hybrid materials incorporating phosphorus-containing dendrimers. Chem. Mater. 2000, 12, 3848–3856. [Google Scholar] [CrossRef]
- Boury, B.; Corriu, R.J.P.; Nunez, R. Hybrid xerogels from dendrimers and arborols. Chem. Mater. 1998, 10, 1795–1804. [Google Scholar] [CrossRef]
- Kriesel, J.W.; Tilley, T.D. Synthesis and chemical functionalization of high surface area dendrimer-based xerogels and their use as new catalyst supports. Chem. Mater. 2000, 12, 1171–1179. [Google Scholar] [CrossRef]
- Staudinger, H.; Meyer, J. Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine. J. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar] [CrossRef]
- Pietrusiewicz, K.M.; Zablocka, M. Optically-active Phosphine Oxides.5. P-Chiral 2-Aminoethyl Phosphine Oxides. Tetrahedron Lett. 1988, 29, 1991–1992. [Google Scholar] [CrossRef]
- Maraval, V.; Laurent, R.; Donnadieu, B.; Mauzac, M.; Caminade, A.M.; Majoral, J.P. Rapid synthesis of phosphorus-containing dendrimers with controlled molecular architectures: First example of surface-block, layer-block, and segment-block dendrimers issued from the same dendron. J. Am. Chem. Soc. 2000, 122, 2499–2511. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements-BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Riegert, D.; Pla-Quintana, A.; Fuchs, S.; Laurent, R.; Turrin, C.O.; Duhayon, C.; Majoral, J.P.; Chaumonnot, A.; Caminade, A.M. Diversified Strategies for the Synthesis of Bifunctional Dendrimeric Structures. Eur. J. Org. Chem. 2013, 2013, 5414–5422. [Google Scholar] [CrossRef]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Q.; Wang, R. A critical review on new and efficient adsorbents for CO2 capture. Chem. Eng. J. 2024, 485, 141506. [Google Scholar] [CrossRef]
- Bilgili, F.; Kocak, E.; Bulut, U. The dynamic impact of renewable energy consumption on CO2 emissions: A revisited Environmental Kuznets Curve approach. Renew. Sustain. Energy Rev. 2016, 54, 838–845. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Stowe, H.M.; Hwang, G.S. Fundamental Understanding of CO2 Capture and Regeneration in Aqueous Amines from First-Principles Studies: Recent Progress and Remaining Challenges. Ind. Eng. Chem. Res. 2017, 56, 6887–6899. [Google Scholar] [CrossRef]
- Riegert, D.; Bareille, L.; Laurent, R.; Majoral, J.P.; Caminade, A.M.; Chaumonnot, A. Silica Functionalized by Bifunctional Dendrimers: Hybrid Nanomaterials for Trapping CO2. Eur. J. Inorg. Chem. 2016, 2016, 3103–3110. [Google Scholar] [CrossRef]
- Barriere, C.; Latour, V.; Fau, P.; Caminade, A.M.; Turrin, C.O. Low generation PEGylated phosphorus-containing dendrons with phosphonate anchors. Tetrahedron Lett. 2012, 53, 1908–1911. [Google Scholar] [CrossRef]
- Etienne, M.; Grosso, D.; Boissière, C.; Sanchez, C.; Walcarius, A. Electrochemical evidences of morphological transformation in ordered mesoporous titanium oxide thin films. Chem. Commun. 2005, 36, 4566–4568. [Google Scholar] [CrossRef] [PubMed]
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2017, 97, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Cata, A.; Ienascu, I.M.C.; Stefanut, M.N.; Rosu, D.; Pop, O.-R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics 2023, 15, 589. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Cejas-Sanchez, J.; Kajetanowicz, A.; Grela, K.; Caminade, A.-M.; Sebastian, R.M. Strategies for the Preparation of Phosphorus Janus Dendrimers and Their Properties. Molecules 2023, 28, 5570. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Shimizu, K.; Suzuki, N.; Yagi, S.; Sueyoshi, K.; Endo, T.; Hisamoto, H. Water detection in organic solvents using a copolymer membrane immobilised with a fluorescent intramolecular charge transfer-type dye: Effects of intramolecular hydrogen bonds. Analyst 2024, 149, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferrero, E.; Franc, G.; Mazeres, S.; Turrin, C.O.; Boissiere, U.; Caminade, A.M.; Majoral, J.P.; Sanchez, C. Optical properties of hybrid dendritic-mesoporous titania nanocomposite films. Chem.-Eur. J. 2008, 14, 7658–7669. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, S.; Mansell, J.P. The role of lysophosphatidic acid on human osteoblast formation, maturation and the implications for bone health and disease. Clin. Lipidol. 2013, 8, 123–135. [Google Scholar] [CrossRef]
- de Jong, E.R.; Deloch, N.; Knoll, W.; Turrin, C.O.; Majoral, J.P.; Caminade, A.M.; Koper, I. Synthesis and characterization of bifunctional dendrimers: Preliminary use for the coating of gold surfaces and the proliferation of human osteoblasts (HOB). New J. Chem. 2015, 39, 7194–7205. [Google Scholar] [CrossRef]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.D.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, B.J. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Lopez, J.L.; Khor, H.L.; Caminade, A.M.; Majoral, J.P.; Mittler, S.; Knoll, W.; Kim, D.H. Bioactive multilayer thin films of charged N,N-disubstituted hydrazine phosphorus dendrimers fabricated by layer-by-layer self-assembly. Thin Solid Film. 2008, 516, 1256–1264. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Namgung, R.; Singha, K.; Oh, I.K.; Kim, W.J. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug. Chem. 2011, 22, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Alami, O.; Laurent, R.; Tasse, M.; Coppel, Y.; Colliere, V.; Bignon, J.; Majoral, J.-P.; El Kazzouli, S.; El Brahmi, N.; Caminade, A.-M. Functionalization of graphene oxide surfaces with phosphorus dendrimer and dendron. Flatchem 2023, 42, 100564. [Google Scholar] [CrossRef]
- Eigler, S.; Hu, Y.; Ishii, Y.; Hirsch, A. Controlled functionalization of graphene oxide with sodium azide. Nanoscale 2013, 5, 12136–12139. [Google Scholar] [CrossRef] [PubMed]
- Alami, O.; Laurent, R.; Tasse, M.; Coppel, Y.; Bignon, J.; El Kazzouli, S.; Majoral, J.-P.; El Brahmi, N.; Caminade, A.-M. “Click” Chemistry for the Functionalization of Graphene Oxide with Phosphorus Dendrons: Synthesis, Characterization and Preliminary Biological Properties. Chem.-Eur. J. 2023, 29, e202302198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yu, T.; Wang, R.; Wang, X.; Jing, Y. Synthesis and structure–activity relationship of ethacrynic acid analogues on glutathione-s-transferase P1-1 activity inhibition. Bioorg. Med. Chem. 2005, 13, 4056–4062. [Google Scholar] [CrossRef]
- Koechel, D.A. Ethacrynic-acid and related diuretics: Relationship of structure to beneficial and detrimental actions. Annu. Rev. Pharmacol. Toxicol. 1981, 21, 265–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, C.; Song, D.; Zhao, G.; Jing, Y. Ethacrynic Acid Butyl-Ester Induces Apoptosis in Leukemia Cells through a Hydrogen Peroxide–Mediated Pathway Independent of Glutathione S-Transferase P1-1 Inhibition. Cancer Res. 2007, 67, 7856–7864. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lee, H.; Rho, S.B.; Park, M.K.; Lee, C.H. Ethacrynic Acid: A Promising Candidate for Drug Repurposing as an Anticancer Agent. Int. J. Mol. Sci. 2023, 24, 6712. [Google Scholar] [CrossRef] [PubMed]
- El Brahmi, N.; Mignani, S.M.; Caron, J.; El Kazzouli, S.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. Investigations on dendrimer space reveal solid and liquid tumor growth-inhibition by original phosphorus-based dendrimers and the corresponding monomers and dendrons with ethacrynic acid motifs. Nanoscale 2015, 7, 3915–3922. [Google Scholar] [CrossRef] [PubMed]
- Franc, G.; Kakkar, A.K. “Click” methodologies: Efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev. 2010, 39, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, M.; Wafer, C.; Morin, J.F. Recent Advances in Click Chemistry Applied to Dendrimer Synthesis. Molecules 2015, 20, 9263–9294. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.; Aubert, G.; Laurent, R.; Caminade, A.M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.P. Original Multivalent Copper(II)-Conjugated Phosphorus Dendrimers and Corresponding Mononuclear Copper(II) Complexes with Antitumoral Activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.-M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original Multivalent Gold(III) and Dual Gold(III)-Copper(II) Conjugated Phosphorus Dendrimers as Potent Antitumoral and Antimicrobial Agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, Y.; Qiu, J.; Laurent, R.; Li, J.; Bignon, J.; Mignani, S.; Caminade, A.-M.; Shi, X.; Majoral, J.-P. Potent Anticancer Efficacy of First-In-Class Cu-II and Au-III Metaled Phosphorus Dendrons with Distinct Cell Death Pathways. Chem.-Eur. J. 2020, 26, 5903–5910. [Google Scholar] [CrossRef]
- Apartsin, E.K.; Knauer, N.; Kahlert, U.D.; Caminade, A.-M. Amphiphilic Triazine-Phosphorus Metallodendrons Possessing Anti-Cancer Stem Cell Activity. Pharmaceutics 2022, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Sandulesco, G. On a new series of reactants of the carbonyl group, their use for the extraction of ketonic substances and for the microchemical characterisation of aldehydes and ketones. Helv. Chim. Acta 1936, 19, 1096–1107. [Google Scholar]
- Larpent, C.; Amigoni-Gerbier, S.; De Sousa Delgado, A. Synthesis of metal-complexing nanoparticles by post-functionalisation of reactive nanolatexes produced by microemulsion polymerisation. C. R. Chim. 2003, 6, 1275–1283. [Google Scholar] [CrossRef]
- Larpent, C.; Genies, C.; Delgado, A.P.D.; Caminade, A.M.; Majoral, J.P.; Sassi, J.F.; Leising, F. Giant dendrimer-like particles from nanolatexes. Chem. Commun. 2004, 2004, 1816–1817. [Google Scholar] [CrossRef] [PubMed]
- Marmillon, C.; Gauffre, F.; Gulik-Krzywicki, T.; Loup, C.; Caminade, A.M.; Majoral, J.P.; Vors, J.P.; Rump, E. Organophosphorus dendrimers as new gelators for hydrogels. Angew. Chem. Int. Ed. 2001, 40, 2626–2629. [Google Scholar] [CrossRef]
- Apartsin, E.K.; Grigoryeva, A.E.; Malrin-Fournol, A.; Ryabchikova, E.I.; Venyaminova, A.G.; Mignani, S.; Caminade, A.M.; Majoral, J.P. Hydrogels of Polycationic Acetohydrazone-Modified Phosphorus Dendrimers for Biomedical Applications: Gelation Studies and Nucleic Acid Loading. Pharmaceutics 2018, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- El Ghzaoui, A.; Gauffre, F.; Caminade, A.M.; Majoral, J.P.; Lannibois-Drean, H. Self-assembly of water-soluble dendrimers into thermoreversible hydrogels and macroscopic fibers. Langmuir 2004, 20, 9348–9353. [Google Scholar] [CrossRef] [PubMed]
- Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Covalently functionalized cobalt nanoparticles as a platform for magnetic separations in organic synthesis. Angew. Chem. Int. Ed. 2007, 46, 4909–4912. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Bao, L.; Yu, Z.; Lu, X. Carbon encapsulated nanoparticles: Materials science and energy applications. Chem. Soc. Rev. 2024, 53, 11100–11164. [Google Scholar] [CrossRef] [PubMed]
- Kainz, Q.M.; Reiser, O. Polymer- and Dendrimer-Coated Magnetic Nanoparticles as Versatile Supports for Catalysts, Scavengers, and Reagents. Acc. Chem. Res. 2014, 47, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.; Muellen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Folgado, E.; Guerre, M.; Mimouni, N.; Colliere, V.; Bijani, C.; Ching, K.M.C.; Caminade, A.M.; Ladmiral, V.; Ameduri, B.; Ouali, A. pi-Stacking Interactions of Graphene-Coated Cobalt Magnetic Nanoparticles with Pyrene-Tagged Dendritic Poly(Vinylidene Fluoride). Chempluschem 2019, 84, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mery, D.; Astruc, D. Dendritic catalysis: Major concepts and recent progress. Coord. Chem. Rev. 2006, 250, 1965–1979. [Google Scholar] [CrossRef]
- Ming, C.; Kwong, F.Y. Palladium-catalyzed cross-coupling reactions of aryl mesylates. Chem. Soc. Rev. 2011, 40, 4963–4972. [Google Scholar] [CrossRef]
- Pandit, A.P.; Bhanushali, V.P.; Patel, S.A.; Kandekar, U.Y.; Kakad, V.D.; Deshpande, T.C. Topical felbinac loaded cubosomal hydrogel for promising treatment of inflammatory pain. J. Dispers. Sci. Technol. 2024, 1–11. [Google Scholar] [CrossRef]
- Keller, M.; Colliere, V.; Reiser, O.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Pyrene-Tagged Dendritic Catalysts Noncovalently Grafted onto Magnetic Co/C Nanoparticles: An Efficient and Recyclable System for Drug Synthesis. Angew. Chem. Int. Ed. 2013, 52, 3626–3629. [Google Scholar] [CrossRef] [PubMed]
- Barath, E. Hydrogen Transfer Reactions of Carbonyls, Alkynes, and Alkenes with Noble Metals in the Presence of Alcohols/Ethers and Amines as Hydrogen Donors. Catalysts 2018, 8, 671. [Google Scholar] [CrossRef]
- Asri, H.; Dautel, O.; Ouali, A. Terpyridine-Ru Complexes Noncovalently Supported on Cobalt Magnetic Nanoparticles for Nitroarene Transfer Hydrogenation. ACS Appl. Nano Mater. 2020, 3, 11811–11818. [Google Scholar] [CrossRef]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalton Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pena, N.G.; Redon, R.; Herrera-Gomez, A.; Leticia Fernandez-Osorio, A.; Bravo-Sanchez, M.; Gomez-Sosa, G. Solventless synthesis of ruthenium nanoparticles. Appl. Surf. Sci. 2015, 340, 25–34. [Google Scholar] [CrossRef]
- Garcia-Pena, N.G.; Caminade, A.M.; Ouali, A.; Redon, R.; Turrin, C.O. Solventless synthesis of Ru(0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis. RSC Adv. 2016, 6, 64557–64567. [Google Scholar] [CrossRef]
- Svenson, S. The dendrimer paradox—High medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Daniel Pedro-Hernandez, L.; Martinez-Garcia, M. Dendrimer Applications: A Brief Review. Curr. Org. Chem. 2021, 25, 1247–1269. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendrimers, Dendrons, and the Dendritic State: Reflection on the Last Decade with Expected New Roles in Pharma, Medicine, and the Life Sciences. Pharmaceutics 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]



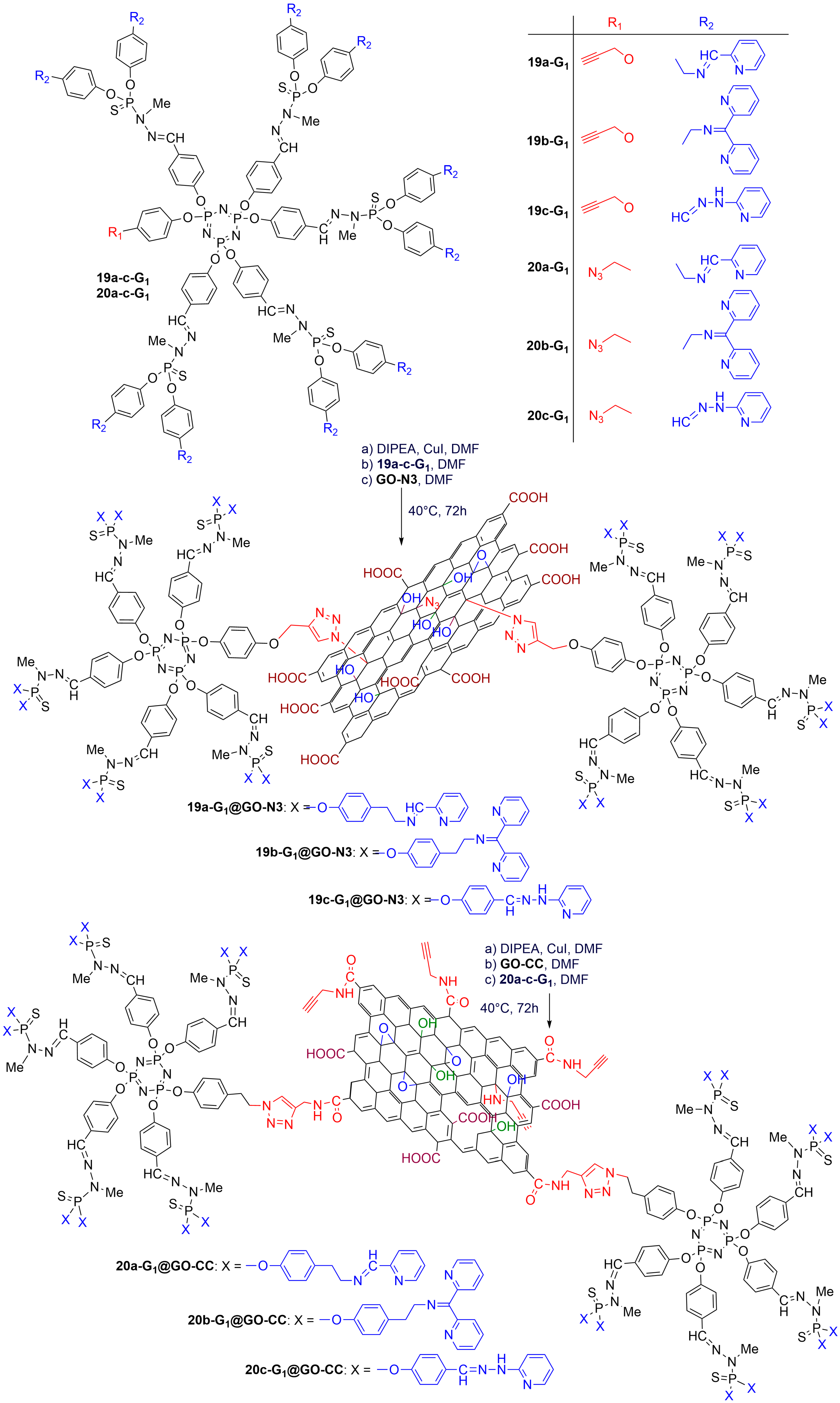


| Dendron 10−5 M | HCT116 % Viability | Dendron@GO 10−5 M | HCT116 % Viability |
|---|---|---|---|
| GO (alone) | 80.0 ± 3.5 | ||
| 19a-G1 | 0.6 ± 0.2 | 19a-G1@GO-N3 | 82.1 ± 2.9 |
| 19b-G1 | 1.8 ± 0.7 | 19b-G1@GO-N3 | 74.2 ± 1.5 |
| 19c-G1 | 99.8 ± 3.8 | 19c-G1@GO-N3 | 94.7 ± 2.4 |
| 20a-G1 | 4.2 ± 0.4 | 20a-G1@GO-CC | 60.5 ± 1.2 |
| 20b-G1 | 9.8 ± 0.2 | 20b-G1@GO-CC | 92.5 ± 1.4 |
| 20c-G1 | 95.5 ± 2.1 | 20c-G1@GO-CC | 98.8 ± 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrin, C.-O.; Maraval, V.; Caminade, A.-M. Investigation of Phosphorus Dendrons and Their Properties for the Functionalization of Materials. J. Compos. Sci. 2025, 9, 382. https://doi.org/10.3390/jcs9080382
Turrin C-O, Maraval V, Caminade A-M. Investigation of Phosphorus Dendrons and Their Properties for the Functionalization of Materials. Journal of Composites Science. 2025; 9(8):382. https://doi.org/10.3390/jcs9080382
Chicago/Turabian StyleTurrin, Cédric-Olivier, Valérie Maraval, and Anne-Marie Caminade. 2025. "Investigation of Phosphorus Dendrons and Their Properties for the Functionalization of Materials" Journal of Composites Science 9, no. 8: 382. https://doi.org/10.3390/jcs9080382
APA StyleTurrin, C.-O., Maraval, V., & Caminade, A.-M. (2025). Investigation of Phosphorus Dendrons and Their Properties for the Functionalization of Materials. Journal of Composites Science, 9(8), 382. https://doi.org/10.3390/jcs9080382







