The Effect of Doping with Aluminum on the Optical, Structural, and Morphological Properties of Thin Films of SnO2 Semiconductors
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Scannig Electron Microscopy
3.2. X-Ray Diffraction
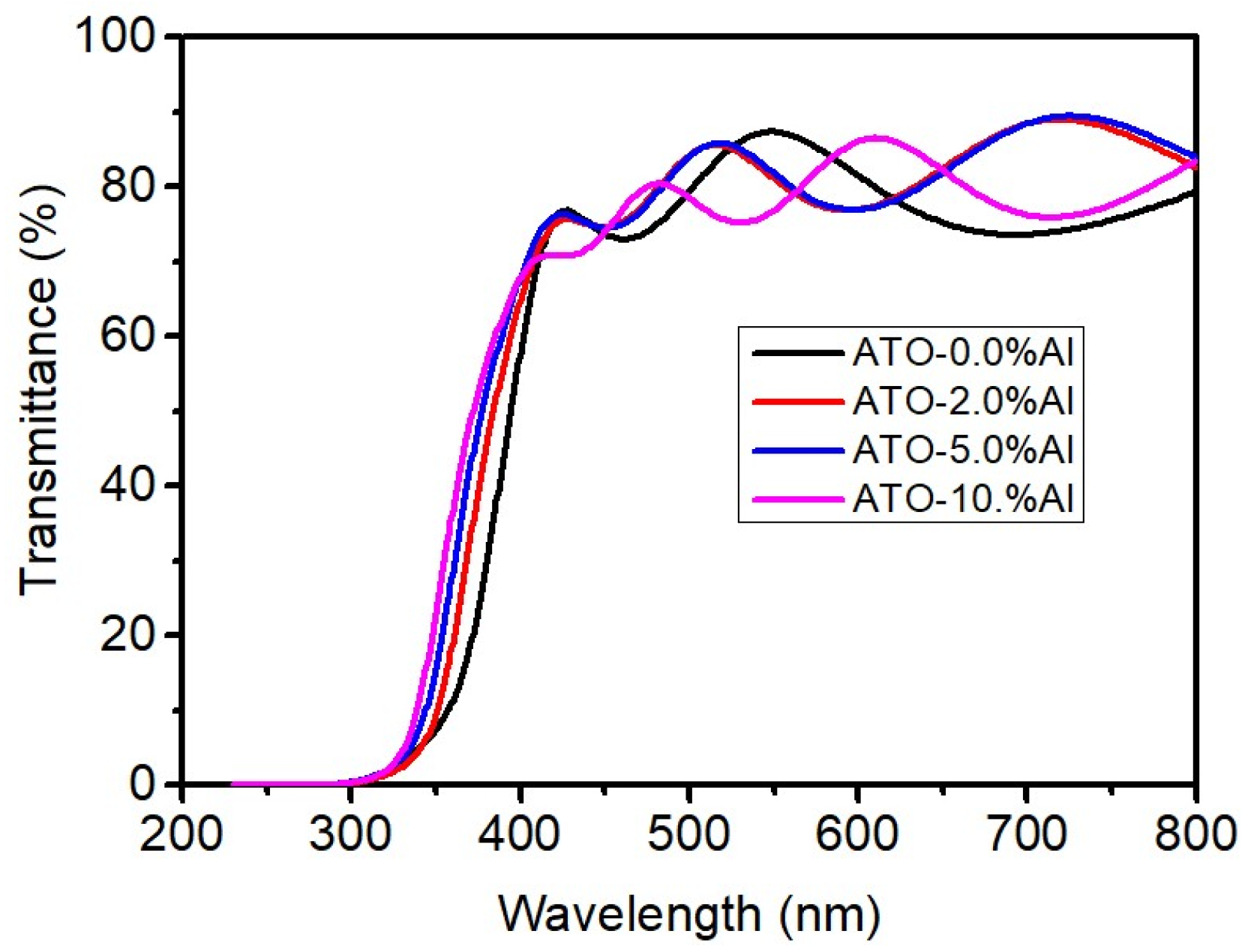
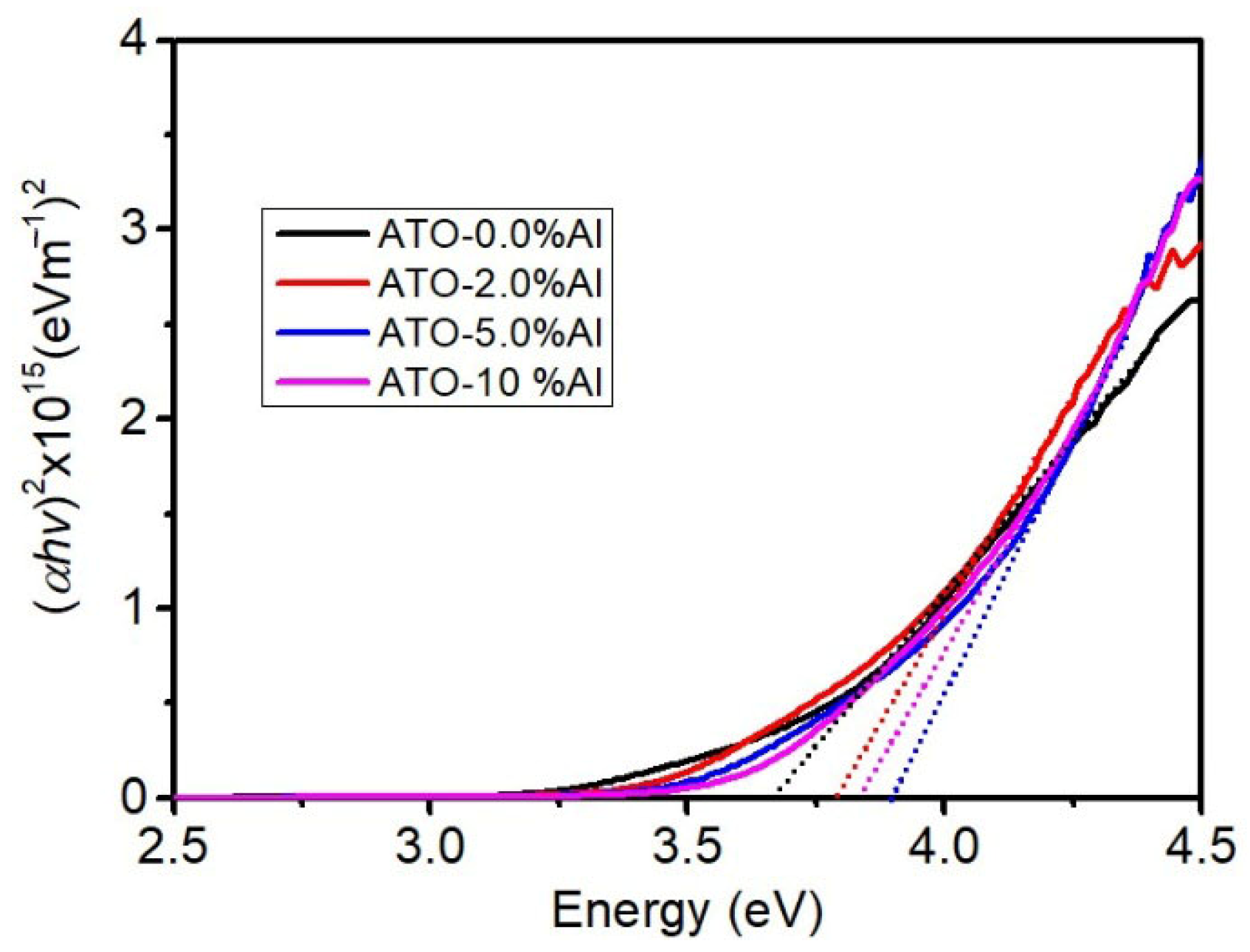
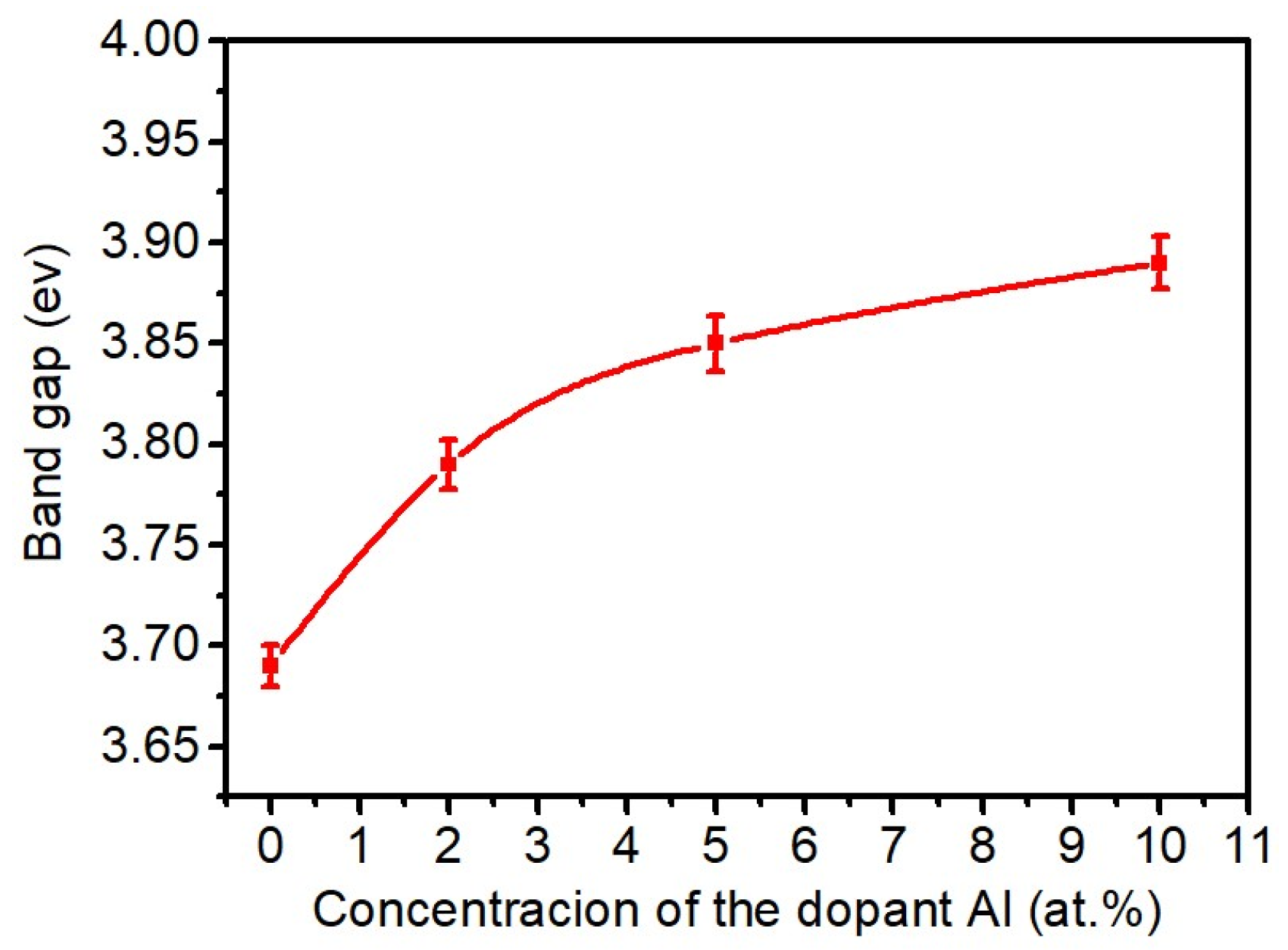
3.3. Transmittance and Bad Gap
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozati, S.M.; Ziabari, S.A.M. A review of various single layer, bilayer, and multilayer TCO materials and their applications. Mater. Chem. Phys. 2022, 292, 1–24. [Google Scholar] [CrossRef]
- Jarzebski, Z.M.; Marton, J.P. Physical properties of SnO2 materials: II. Electrical properties. J. Electrochem. Soc. 1976, 123, 299C–310C. [Google Scholar] [CrossRef]
- Amutha, T.; Rameshbabu, M.; Sasi Florence, S.; Senthilkumar, N.; Vetha Potheher, I.; Prabha, K. Studies on structural and optical properties of pure and transition metals (Ni, Fe and co-doped Ni–Fe) doped tin oxide (SnO2) nanoparticles for anti-microbial activity. Res. Chem. Intermed. 2019, 45, 1929–1941. [Google Scholar] [CrossRef]
- Tazikeh, S.; Akbari, A.; Talebi, A.; Talebi, E. Synthesis and characterization of tin oxide nanoparticles via the Co-precipitation method. Mat. Sci.-Pol. 2014, 32, 98–101. [Google Scholar] [CrossRef]
- Calnan, S.; Tiwari, A.N. High mobility transparent conducting oxides for thin film solar cells. Thin Solid Film. 2010, 518, 1839–1849. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Sol. Energy Mater. Sol. Cells 2010, 91, 1529–1598. [Google Scholar]
- Wang, C.; Cui, X.; Liu, J.; Zhou, X.; Cheng, X.; Sun, P.; Lu, G. Design of superior ethanol gas sensor based on Al-doped NiO nanorod-flowers. ACS Sens. 2016, 1, 131–136. [Google Scholar] [CrossRef]
- Samson, S.; Fonstad, C.G. Defect structure and electronic donor levels in stannic oxide crystals. J. Appl. Phys. 1973, 44, 4618–4621. [Google Scholar] [CrossRef]
- Deyu, G.K.; Muñoz-Rojas, D.; Rapenne, L.; Deschanvres, J.-L.; Klein, A.; Jiménez, C.; Bellet, D. SnO2 films deposited by ultrasonic spray pyrolysis: Influence of Al incorporation on the properties. Molecules 2019, 24, 2797–2812. [Google Scholar] [CrossRef]
- Lei, M.; Hu, Q.R.; Wang, S.L.; Tang, W.H. Structural and optical properties of Al-doped SnO2 nanowires. Mat. Lett. 2010, 64, 19–21. [Google Scholar] [CrossRef]
- Weidner, M.; Jia, J.; Shigesato, Y.; Klein, A. Comparative study of sputter-deposited SnO2 films doped with antimony or tantalum: Comparative study of sputter-deposited SnO2:Sb and SnO2:Ta. Phys. Status Solidi. B Basic Res. 2016, 253, 923–928. [Google Scholar] [CrossRef]
- Weidner, M.; Fuchs, A.; Bayer, T.J.M.; Rachut, K.; Schnell, P.; Deyu, G.K.; Klein, A. Defect modulation doping. Adv. Funct. Mater. 2019, 29, 11467–11479. [Google Scholar] [CrossRef]
- White, M.E.; Bierwagen, O.; Tsai, M.Y.; Speck, J.S. Electron transport properties of antimony doped SnO2 single crystalline thin films grown by plasma-assisted molecular beam epitaxy. J. Appl. Phys. 2009, 106, 093704–093710. [Google Scholar] [CrossRef]
- Dai, C.M.; Su, C.S.; Chuu, D.S. Growth of highly oriented tin oxide thin films by laser evaporation deposition. Appl. Phys. Lett. 1990, 57, 1879–1881. [Google Scholar] [CrossRef]
- Kane, J.; Schweizer, H.; Kern, W. Chemical Vapor Deposition of Antimony-Doped Tin Oxide Films Formed from Dibutyl Tin Diacetate. J. Electrochem. Soc. 1976, 123, 270–277. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, J.-M.; Lim, J.-H.; Park, J.; Park, J.-S. A study on the thermoelectric properties of ALD-grown aluminum-doped tin oxide with respect to nanostructure modulations. Ceram. Int. 2018, 44, 1978–1983. [Google Scholar] [CrossRef]
- Terán, U.G.; Díaz Cano, A.I.; Torchynska, T.; Rodriguez, I.C.B.; El Filali, B.; Cervantes, J.E. Impact of Cu doping on morphology, structural and optical characteristics of SnO2 thin films prepared by spray pyrolysis. MRS Adv. 2024, 9, 1849–1853. [Google Scholar] [CrossRef]
- Bilgin, V.; Akyuz, I.; Ketenci, E.; Kose, S.; Atay, F. Electrical, structural and surface properties of fluorine doped tin oxide films. Appl. Surf. Sci. 2010, 256, 6586–6591. [Google Scholar] [CrossRef]
- Saoula, I.; Siad, C.; Djedidi, K.; Allag, N.; Chala, A. Effect of (Al, Zn, Cu, and Sr) doping on structural, optical and electrical properties of sprayed SnO2 thin films. Acta Metall. Slovaka 2023, 29, 59–62. [Google Scholar] [CrossRef]
- Rodriguez, I.C.B.; El Filali, B.; Torchynska, T.; Douda, J.; Ibarra, I.C.R. Optical and structural properties of Sn doped ZnO thin films synthesized by spray pyrolysis. MRS Adv. 2023, 8, 1434–1437. [Google Scholar] [CrossRef]
- Minami, T.; Miyata, T.; Ohtani, Y.; Kuboi, T. Effect of thickness on the stability of transparent conducting impurity-doped ZnO thin films in a high humidity environment. Phys. Status Solidi Rapid Res. Lett. 2007, 1, R31–R33. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, K.; Kim, K.; Yoon, J.S.; Choi, D.-S.; Yoo, Y.; Kim, C.; Kang, S.; Kim, J.H. Electrical, structural, optical, and adhesive characteristics of aluminum-doped tin oxide thin films for transparent flexible thin-film transistor applications. Materials 2019, 12, 137–142. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, X.; Miao, J.; Zhang, R.; Zhang, J.; Zhou, M.; Lu, W. Enhanced performance of an Al-doped SnO2 anode for the electrocatalytic oxidation of organic pollutants in water. Mater. Today Commun. 2020, 24, 101164–101172. [Google Scholar] [CrossRef]
- Salameh, B.; Alsmadi, A.M.; Hegazy, A.R. Optoelectronic properties and optical transitions in low concentrated aluminum-doped tin oxide thin films. J. Alloys Compd. 2023, 969, 172412–172424. [Google Scholar] [CrossRef]
- Doyan, A.; Susilawati, I.Y.D.; Gunawan, E.R.; Taufik, M. Characterization thin film nano particle of aluminum tin oxide (AlTO) as touch screen. J. Phys. Conf. Ser. 2018, 1097, 012009–012017. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, G.; Bai, Y.; Yan, D.; Yu, C.; Wan, N.; Zhang, W. Al-doped SnO2 hollow sphere as a novel anode material for lithium-ion battery. Solid State Ion. 2015, 272, 133–137. [Google Scholar] [CrossRef]
- Wang, E.; Chen, P.; Yin, X.; Wu, Y.; Que, W. Tailoring electronic properties of SnO2 quantum dots via aluminum addition for high-efficiency perovskite solar cells. Solar RRL. 2019, 3, 1900041–1900410. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, J.; Fu, N.; Fang, Y.; Liu, T.; Zhang, Q.; Zhou, X.; Lin, Y.; Pan, F. Enhancing the performance of dye-sensitized solar cells: Doping SnO2 photoanodes with Al to simultaneously improve conduction band and electron lifetime. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 3066–3073. [Google Scholar] [CrossRef]
- Soussi, A.; Haounati, R.; Aithssi, A.; Taoufiq, M.; Baoubih, S.; Jellil, Z.; El hankari, S.; Elfanaoui, A.; Markazi, R.; Ihlal, A. Investigating structural, morphological, electronic, and optical properties of SnO2 and Al-doped SnO2: A combined DFT calculation and experimental study. Phy. B Cond. Matt. 2024, 690, 416242–416251. [Google Scholar] [CrossRef]
- Bouabdalli, E.; El Jouad, M.; Garmim, T.; Louardi, A.B.; Hartiti, B.; Monkade, M.; Touhtouh, S.; Hajjaji, A. Elaboration and characterization of Ni and Al co-doped SnO2 thin films prepared by spray pyrolysis technique for photovoltaic applications. Mat. Sci. Eng. B 2022, 286, 116044–116053. [Google Scholar] [CrossRef]
- Suvarna, T.; Reddy, K.G.; Mohan, V.M.; Lavanya, G.; Ramana Reddy, M.V.R.; Vardhani, C.P. Investigation on Sb-doped SnO2 as an efficient sensor for the detection of formaldehyde. Inorg. Chem. Comm. 2024, 167, 112748–112759. [Google Scholar] [CrossRef]
- Abvarza, B.; Alehdaghia, H.; Ziraka, M. Preparation of Al-doped SnO2 thin films via ultrasonic mist vapor deposition method: Optimization the growth parameters towards a superhydrophobic layer. Phy. B Cond. Matt. 2025, 712, 417240–417251. [Google Scholar] [CrossRef]
- Mallick, H.K.; Zhang, Y.; Pradhan, J.; Sahoo, M.P.K.; Pattanaik, A.K. Influence of particle size and defects on the optical, magnetic and electronic properties of Al doped SnO2 nanoparticles. J. Alloys Compd. 2021, 854, 156067–156077. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Rouf, H.K. Al-doped SnO2 thin films: Impacts of high temperature annealing on the structural, optical and electrical properties. J. Mat. Res. And Tech. 2021, 15, 3409–3425. [Google Scholar] [CrossRef]
- Ausama, I.K.; Attarid, M.O. Influence of the aluminum doping on the physical and gas sensing properties of SnO2 for H2 gas detection. Sens. Actuators B Chem. 2021, 340, 129633–129640. [Google Scholar]
- Kar, A.; Kundu, S.; Patra, A. Surface Defect-Related Luminescence Properties of SnO2 Nanorods and Nanoparticles. J. Phys. Chem. 2011, 115, 118–124. [Google Scholar] [CrossRef]
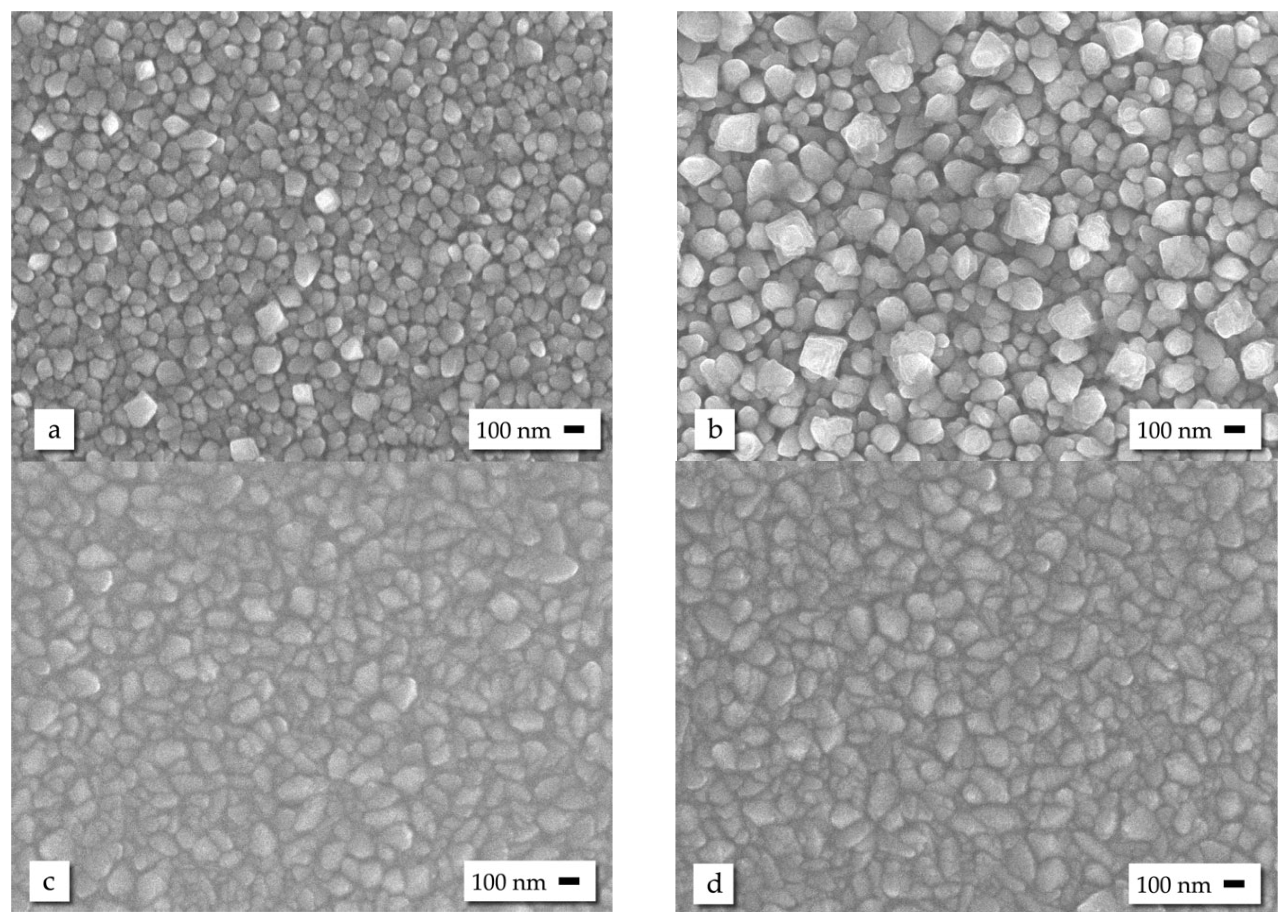
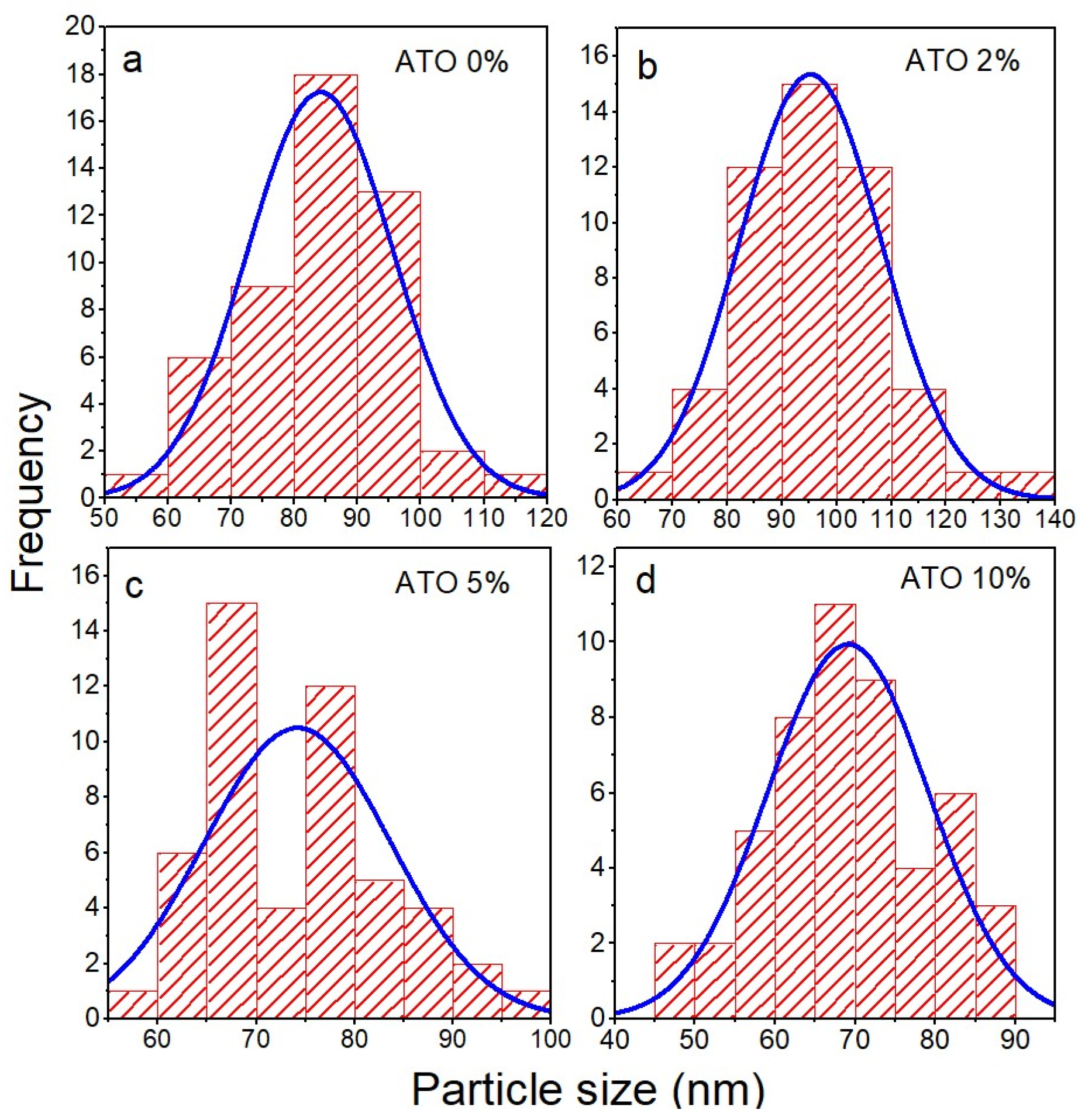
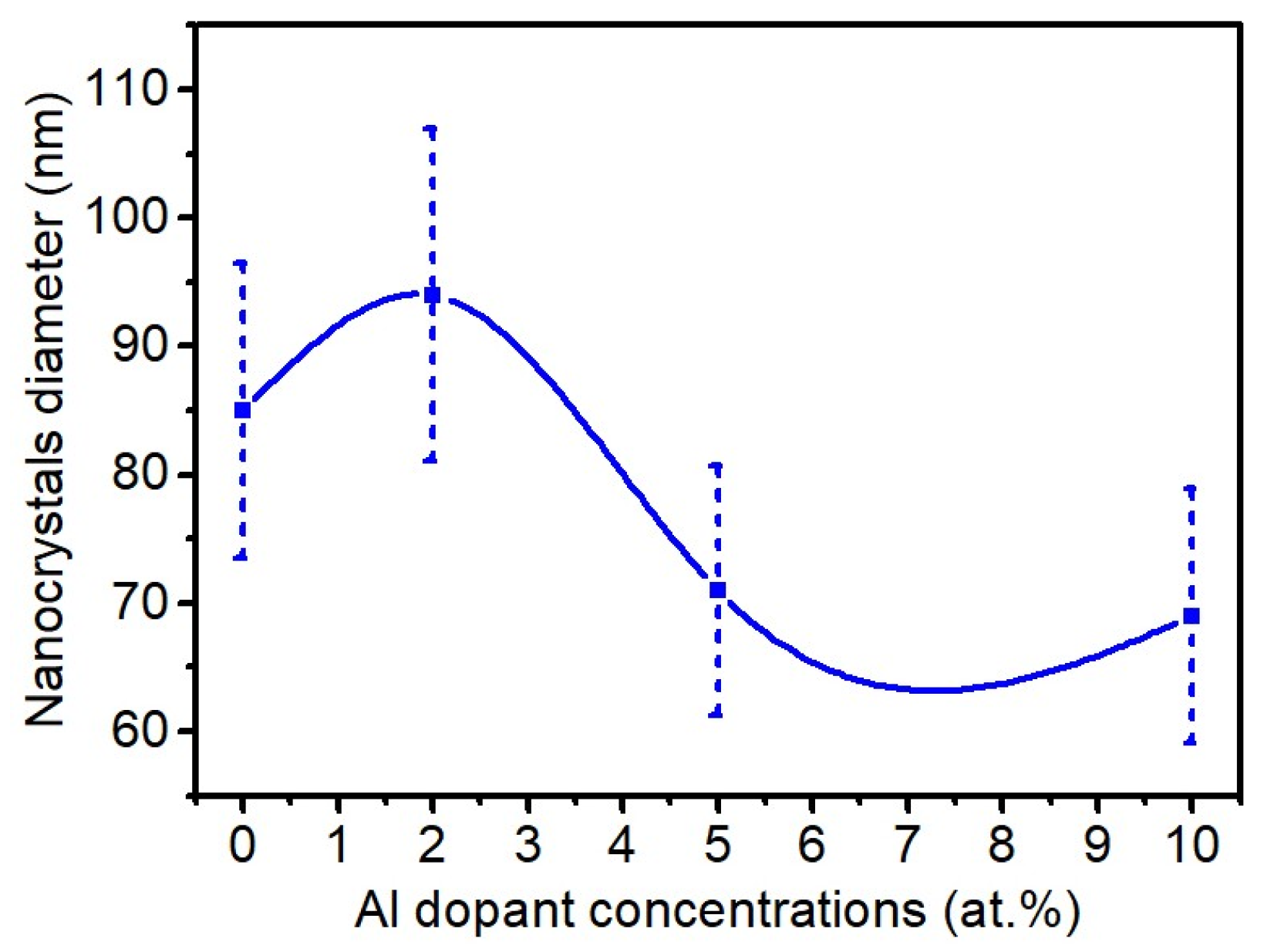
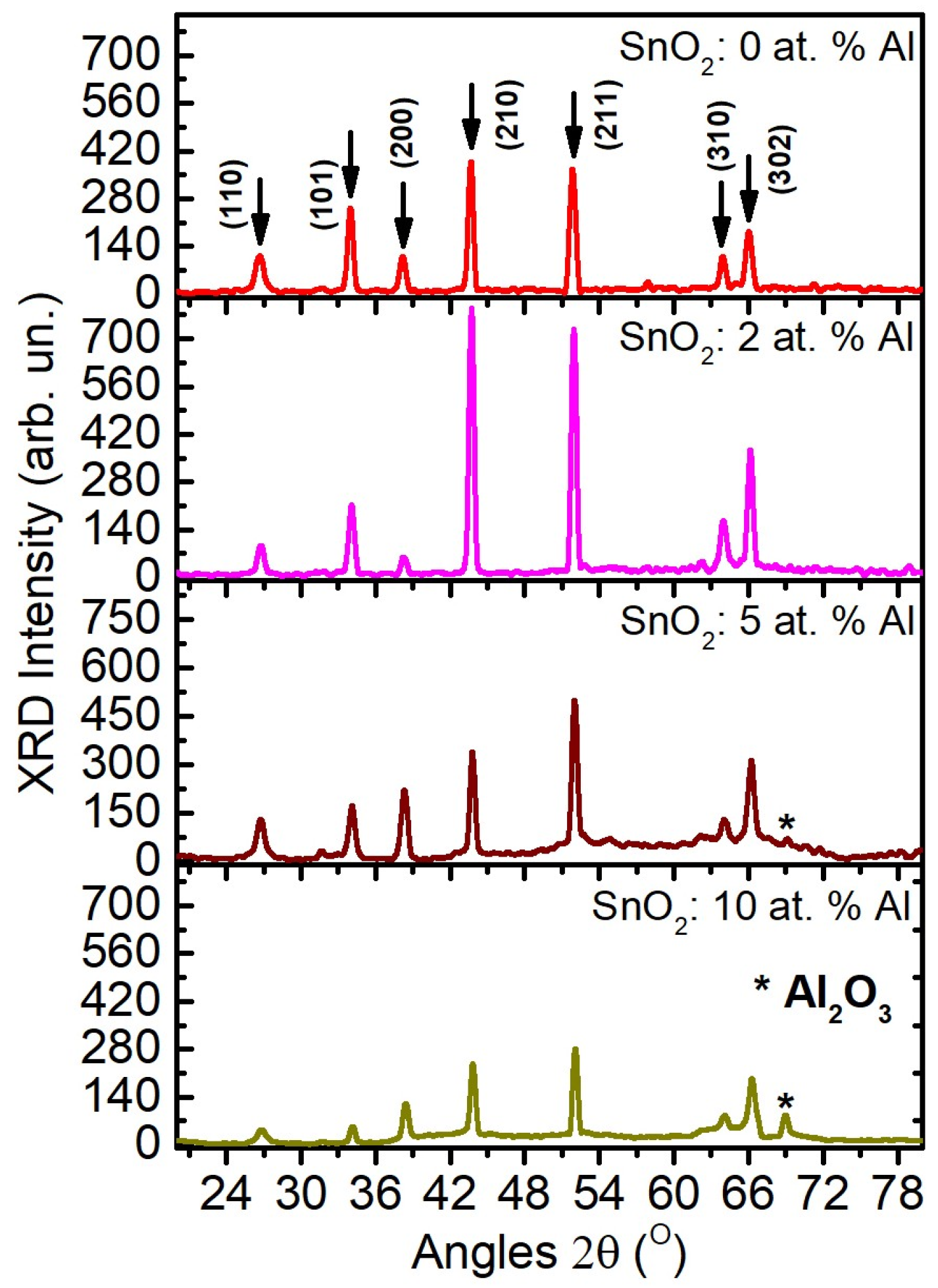
| Sample | (110) | (101) | (200) | (210) | (211) | (310) | (302) | a (Å) | c (Å) |
|---|---|---|---|---|---|---|---|---|---|
| ATO:0.0Al | 26.66 | 33.98 | 38.24 | 43.65 | 51.84 | 63.94 | 66.04 | 4.724 | 3.176 |
| ATO:2.0Al | 26.75 | 34.08 | 38.28 | 43.73 | 51.96 | 64.00 | 66.17 | 4.709 | 3.168 |
| ATO:5.0Al | 26.78 | 34.12 | 38.36 | 43.84 | 52.03 | 64.12 | 66.28 | 4.704 | 3.164 |
| ATO:10.0Al | 26.84 | 34.18 | 38.45 | 43.85 | 52.13 | 64.14 | 66.32 | 4.693 | 3.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballardo Rodriguez, I.C.; Garduño Terán, U.; Díaz Cano, A.I.; El Filali, B.; Badaoui, M. The Effect of Doping with Aluminum on the Optical, Structural, and Morphological Properties of Thin Films of SnO2 Semiconductors. J. Compos. Sci. 2025, 9, 358. https://doi.org/10.3390/jcs9070358
Ballardo Rodriguez IC, Garduño Terán U, Díaz Cano AI, El Filali B, Badaoui M. The Effect of Doping with Aluminum on the Optical, Structural, and Morphological Properties of Thin Films of SnO2 Semiconductors. Journal of Composites Science. 2025; 9(7):358. https://doi.org/10.3390/jcs9070358
Chicago/Turabian StyleBallardo Rodriguez, Isis Chetzyl, U. Garduño Terán, A. I. Díaz Cano, B. El Filali, and M. Badaoui. 2025. "The Effect of Doping with Aluminum on the Optical, Structural, and Morphological Properties of Thin Films of SnO2 Semiconductors" Journal of Composites Science 9, no. 7: 358. https://doi.org/10.3390/jcs9070358
APA StyleBallardo Rodriguez, I. C., Garduño Terán, U., Díaz Cano, A. I., El Filali, B., & Badaoui, M. (2025). The Effect of Doping with Aluminum on the Optical, Structural, and Morphological Properties of Thin Films of SnO2 Semiconductors. Journal of Composites Science, 9(7), 358. https://doi.org/10.3390/jcs9070358







