1. Introduction
The most common building material is concrete. The first mention of it dates back to 5600 BC. In those distant times, the binder was lime. And only 200 years ago, a new unique binder appeared—Portland cement—which is produced today at more than 4 billion tons.
Over the past 100 years, concrete has revolutionized the global construction environment. All over the world, concrete structures play a key role in providing housing for an ever-growing population; providing transport on land, at sea, and in the air; supporting energy production and industry; and providing protection. Without Portland cement concrete, it would be impossible to build roads and railways, bridges and dams, high-rise buildings, and skyscrapers, and any reinforced concrete structures would not be possible. Today, concrete and cement mortars remain economical and energy-efficient building materials.
However, cement production is characterized by high emissions of CO2, a greenhouse gas, since the main raw materials for the production of cement clinker are carbonate-containing rocks, the decomposition of which releases CO2. In addition, the high temperatures of cement clinker production require high fuel consumption, the combustion of which also produces carbon dioxide.
The cement industry is estimated to account for ~7–8% of anthropogenic CO
2 emissions worldwide. With the world’s population set to grow to 9.8 billion people by 2050, the demand for cement will continue to increase since, by that time, 68% of the population will live in cities. If the world continues to strive to achieve its climate goals, the issue of reducing such emissions will become acute [
1].
Since 1 January 2019, the World Business Council for Sustainable Development (WBCSD) has transferred the Cement Sustainability Initiative (CSI) to the Global Cement and Concrete Association (GCCA). The activities of the CSI focus on the carbon footprint of the industry [
2]. All CSI members continuously reduce CO
2 emissions at their plants, measure emissions, and report them using an agreed methodology and key performance indicators (KPIs).
To support these activities, CSI has created a global database of CO2 emissions and energy efficiency reports from cement plants. This voluntary database provides standardized, accurate, and verified information to help the industry understand its current and future performance potential.
The European Cement Industry Bureau Cembureau has prepared the 2050 ROADMAP to identify the potential for reducing CO
2 emissions across the entire cement and concrete value chain. The main conclusion of the strategy is that CO
2 emissions can be reduced by acting at every stage of the value chain—clinker production, cement, concrete, construction, and (re)carbonation—to achieve net zero emissions by 2050 [
3,
4].
The following directions for reducing CO
2 emissions are presented [
5,
6,
7,
8]: savings in clinker production; savings in cement and binders; the efficiency of concrete production; carbon capture, use, and storage; the decarbonization of electricity; recarbonization—the natural process of CO
2 absorption by concrete; and efficiency of design and construction. If these steps are not taken, according to forecasts, CO
2 emissions will increase by 2050 to 3.8 Gt [
5]. The implementation of existing and development of innovative mitigation technologies will require significant funding.
Different countries are looking for different approaches to reducing CO2 emissions, but the most easily feasible technological solutions should be used first.
The Republic of Kazakhstan (RK), like the entire world community, adheres to the principles of the Paris Agreement on making a national contribution to achieving sustainable development goals and holding back the increase in global average temperatures, and aims to implement measures to fulfill state environmental policy reduce the negative impact on the environment, and preserve natural resources for future generations of the Republic of Kazakhstan [
9,
10,
11].
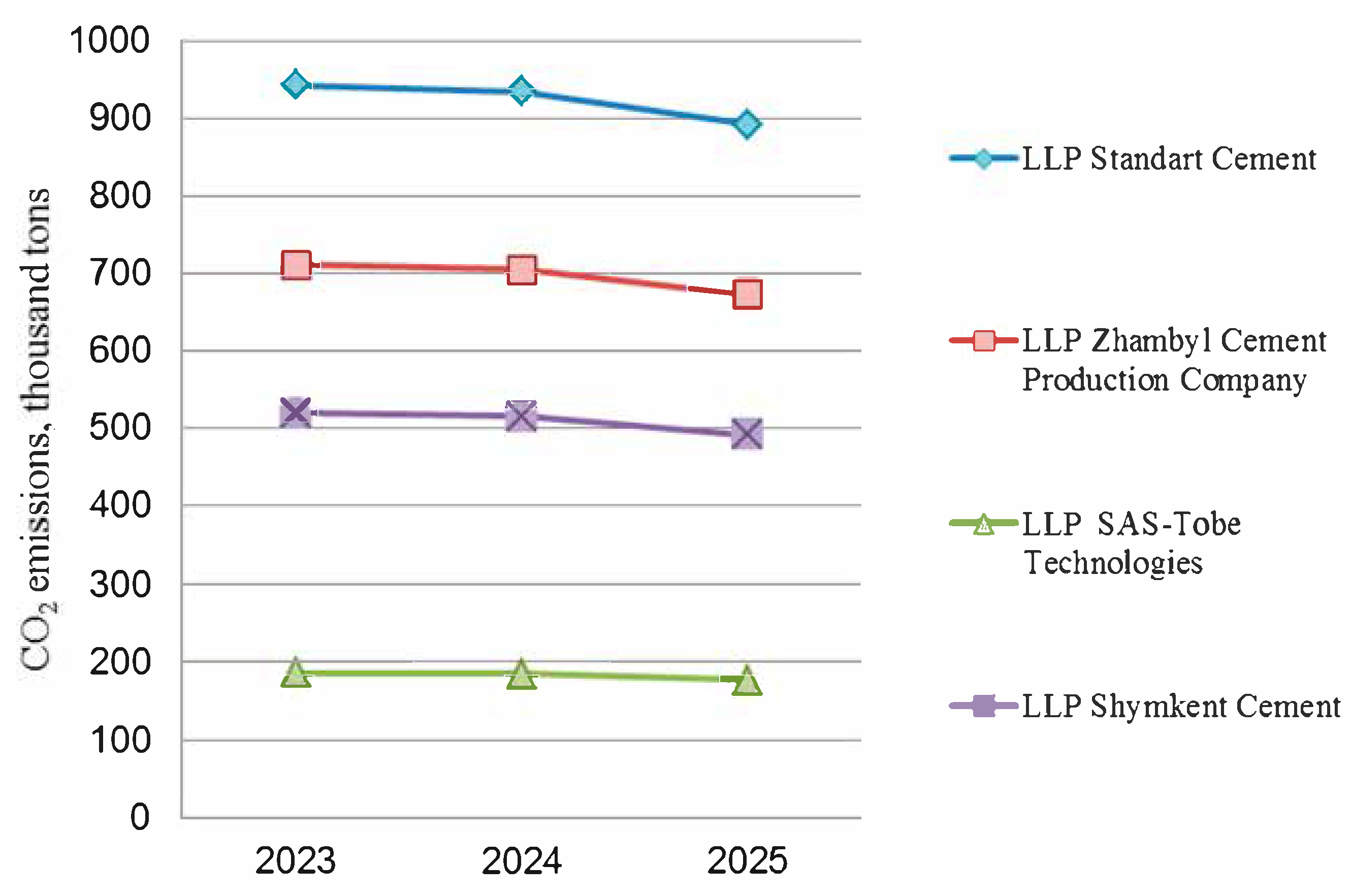
Thus, in accordance with the National Carbon Quota Plan for 2022–2025 approved by the Government of the Republic of Kazakhstan [
12] and the Environmental Code of the Republic of Kazakhstan dated 9 January 2007 (subparagraph 7; Article 16), the total volume of quotas for greenhouse gas emissions in regulated areas of activity amounts to 108.8 million tons, including for the manufacturing industry (in particular, the production of building materials—cement, lime, gypsum, and brick). Including quotas for CO
2 emissions from cement plants amount to 6.02 million tons for 2022; 5.55 million tons in 2023; 5.5 million tons in 2024; and 5.2 million tons in 2025 [
12]. The volume of quotas allocated to cement industry enterprises for 2023–2025 is presented in
Figure 1 (using four cement enterprises of the Republic of Kazakhstan as an example).
Each cement plant in the Republic of Kazakhstan has its own quotas, which are most likely determined by the level of CO2 emissions at a given plant. However, all enterprises in the industry face the task of reducing CO2 emissions during cement production in one way or another. Therefore, a relevant and inexpensive measure may be the production of composite cements with the partial replacement of the clinker share in cement to maintain and increase the annual volume of manufactured products, which are urgently needed by builders.
One of the areas of solving energy saving and ecology issues in cement production is waste recycling, replacing a portion of the clinker with a high-cost price with natural or artificial parts, i.e., natural or man-made additives, obtaining low-clinker cements [
13,
14]. Additives are not available in all regions where cement plants are located. The most accessible today are pozzolans, blast furnaces, phosphorus slags, ashes, etc. The task of researchers is to find and increase the range and volume of high-quality mineral additives. Moreover, these materials should be located near cement production in order to reduce logistic costs.
One of the ways to reduce the amount of harmful emissions released into the atmosphere, including carbon dioxide released during the production of Portland cement clinker, is to use active mineral additives, which will reduce the proportion of the clinker used in the cement. That is, it is necessary to develop effective compositions of composite cements in parts of the expensive clinker that are replaced by active mineral additives. Reducing the proportion of the clinker accordingly reduces the volume of CO
2 emissions into the atmosphere. This is a pressing global problem that is causing concern in Europe, the USA, China, and all other countries on the planet [
15,
16].
In recent years, the global cement industry has paid much attention to mineral additives. This is not a new direction because, for several decades, standards have allowed the use of limestone, slag, pozzolana, ash, and dust as additives. But the renewed interest in optimizing the use of these materials can be considered relevant today. The use of additional raw materials here—such as mineral additives—allows not only to reduce the cost of cement (in most cases, these materials are much cheaper than clinker) but also to reduce the specific amount of CO
2 emissions [
17].
To significantly reduce carbon dioxide emissions into the atmosphere, it is also possible to obtain composite cement clinkers based on various industrial wastes [
18]. Thus, phosphorus and blast furnace slag, with the partial replacement of natural raw materials in the batch for obtaining the clinker, reduce heat consumption for the decomposition of carbonates; the specific consumption of raw materials per 1 ton of clinker decreases; and the mass of material that must be heated to the sintering temperature of the clinker also decreases. As a result, the firing temperature and fuel consumption decrease, which leads to a reduction in CO
2 emissions [
19,
20].
Potapova E.N. et al. [
21] investigated the pozzolanic activity of three mineral additives—metakaolin, diatomaceous earth, and silica gel—using various methods for determining pozzolanic activity. It was found that metakaolin showed the highest activity in absorbing lime from lime mortar. The results of determining the activity of additives by the express method obtained by the authors correlated well with the results of the Frattini method. The express methods for determining activity proposed by the authors and developed by Strokova V.V. et al. can be used not only for highly active AMA (active mineral additives) but also for less active AMA. In this case, it is possible to use the classical method.
Mechai A.A. et al. proposed introducing dehydrated clay, and dolomite was proposed as a carbonate rock [
22]. The authors determined the pozzolanic activity of mineral additives from four Belarusian deposits. In order to increase pozzolanic activity, the authors carried out the heat treatment of mineral additives. As a result of the experiments, it was revealed that the types of clay from the Lukoml deposit, heat-treated at a temperature of 900 °C, have the highest pozzolanic activity.
Yakubzhanova Z.B. [
23] studied the chemical–mineralogical, physicochemical, and technological properties of glyage-like rock mass and basaltic andesite, on the basis of which the compositions of hybrid additives “glyage + phosphozol” and “glyage + basaltic andesite” with a high glyage content (up to 20%) were developed. The physicomechanical properties of Portland cement were studied with the replacement of up to 30% of clinkers with hybrid additives. The possibility of obtaining new types of composite Portland cements of the PC400-KD30 brands with hybrid additives with a high glyage content due to the targeted regulation of the hydration process, the synthesis of crystalline products, and the formation of a dense low-porosity composite were shown.
Yuldashev F.T. proposed using man-made waste, in particular, the active mineral additive “Phosphozol” and ash and slag waste up to 20% by weight, to improve the environmental situation in cement production.
Mukhiddinov D.D. proposed complex additives, including microsilica and ash-slag. At a dosage of more than 25%, the strength of the resulting cements did not decrease.
Atabaev F.B. studied the hydraulic activity of raw materials in order to determine the possibility of their use as an active mineral additive, including the partial replacement of the clinker component and improvement of the performance properties of cements. Substandard foundry slags from the machine-building and metallurgical (Bekabad metal plant) industries and small waste from soda plants obtained during the slaking of lime together with waste from marble production, used as fillers, were used as hydraulic additives. It has been established that the introduction of 10% of marble by weight into the composition of slag cement increases its strength.
Cherkasov V.D. et al. developed effective active mineral additives based on chemically modified diatomite and studied the processes of hydration and structure formation of cement composites with the addition of modified diatomite.
Konan K.L. et al. studied the processes of obtaining the hydration and hardening of composite cements with the addition of metakaolin, kaolin, etc.
The presented results show that composite cements are quite widespread in many countries of the world; their use allows manufacturers to effectively save on expensive clinkers while reducing carbon dioxide emissions. In this regard, the following can be noted:
One of the most relevant and widespread areas today to ensure the sustainable production of Portland cement and energy conservation, solving environmental problems, is the replacement of part of the clinker in Portland cement with natural active mineral additives;
Portland cement with active mineral additives is an easily produced and in-demand material today;
Research on the development of composite cements is aimed not only at reducing the cost of Portland cement and improving environmental conditions but also at ensuring the high quality of the resulting products and using local reserves of available raw materials;
Studies on the use of clay shales are isolated and contradictory.
At the cement plants of Kazakhstan LLP “Gezhuba Shieli Cement”, LLP “Semey Cement”, LLP “Caspian Cement”, and others, when producing composite cements, CEM II/A-K, CEM II/V-K, granulated slags, shale, limestone, and fly ash are used. Clay shales are quite common in the territory of the Republic of Kazakhstan, but at the moment, they are used as raw material (aluminosilicate) components in the production of Portland cement clinker.
Clay shales are rocks of metamorphic origin. The structure is shale. They consist of very small particles of clay minerals and quartz impurities. Distinguishing features are easy for separation into long individual columns and tiles under mechanical impact. The texture is shale with a parallel arrangement of shells and embroidered minerals. Rocks crystallize partially or completely with a change in mineral composition and without changes. In weakly metamorphosed rocks, hidden crystalline or transitional structures are found, including relict ones, which are subject to metamorphism.
Since clay shales, according to their chemical and mineralogical composition, are aluminosilicates, it can be assumed that during their thermal activation, like clays, amorphous aluminum and silicon oxides are formed, which are capable of reacting with the portlandite of cement stone to form an additional amount of calcium hydrosilicates and hydroaluminates.
Heat-treated clay shale is quite effective and is used as an active mineral additive in some foreign plants. AMA replaces clinkers, increases the strength of Portland cement, and optimally affects the processes of grinding clinkers and the formation of the structure of hardening cement stone.
3. Results and Discussion
3.1. Composition of Materials Used
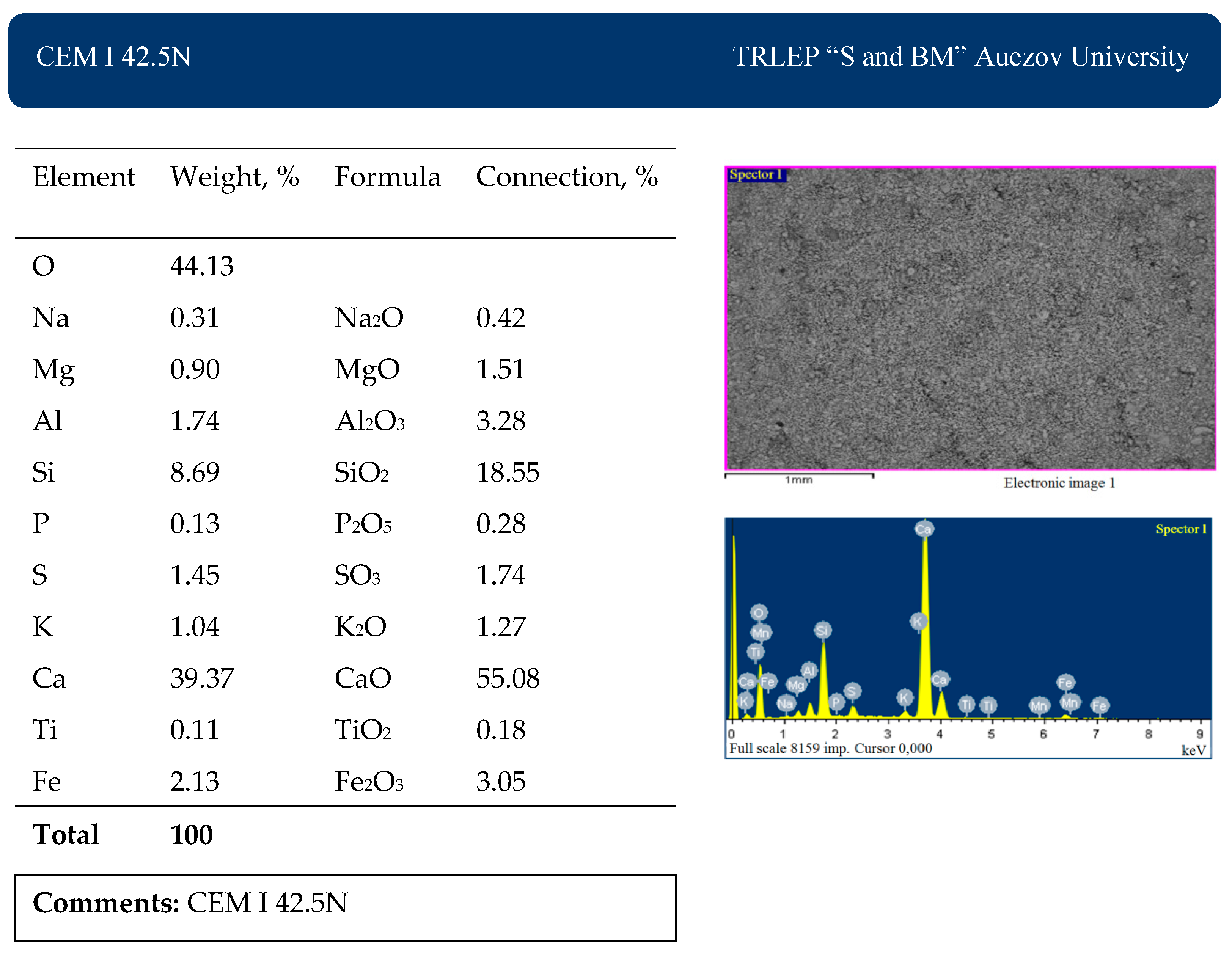
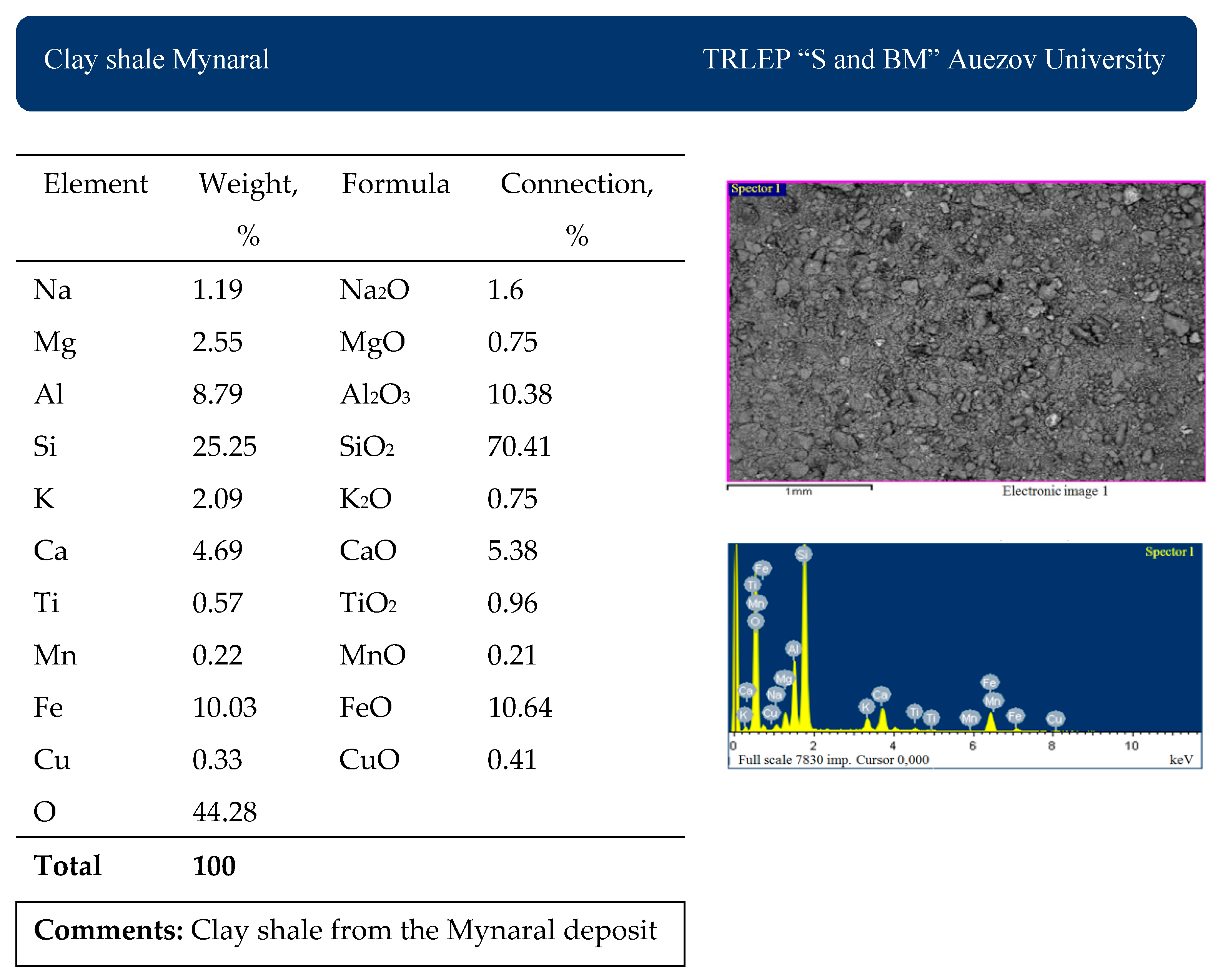
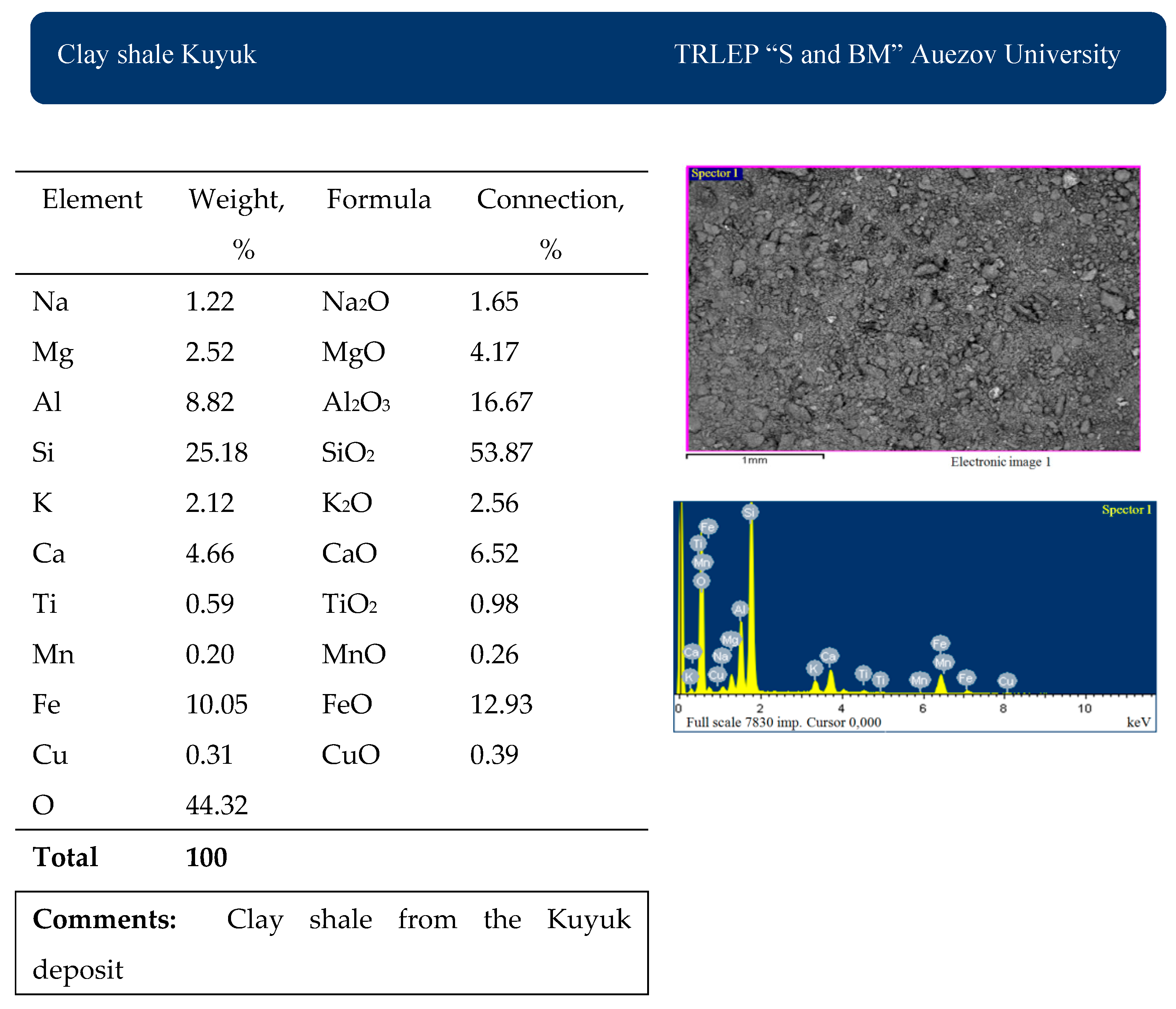
The chemical analysis of the average composition of the materials used was studied in the accredited laboratory of Testing Regional Laboratory—IRLIP. The results are presented in
Table 1.
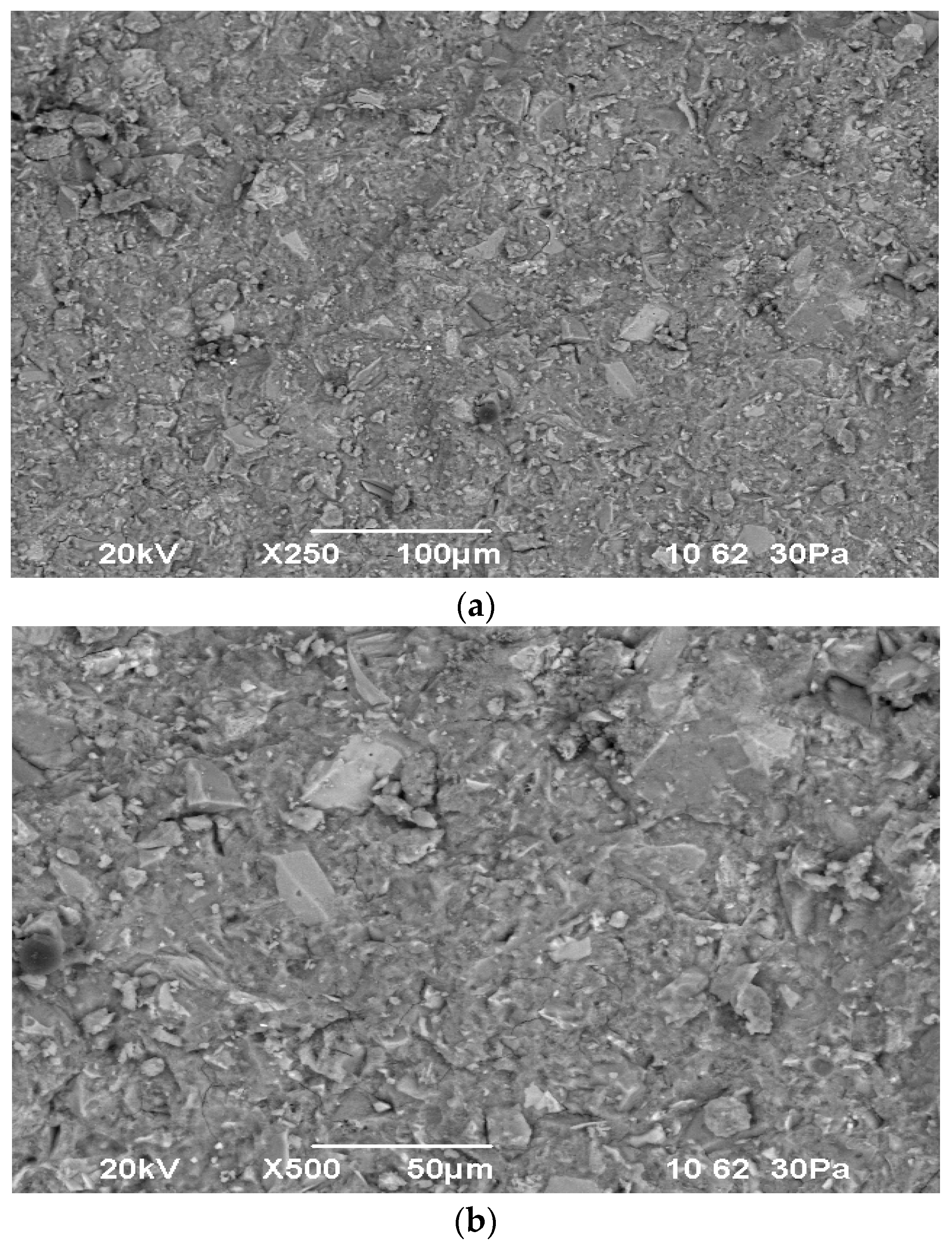
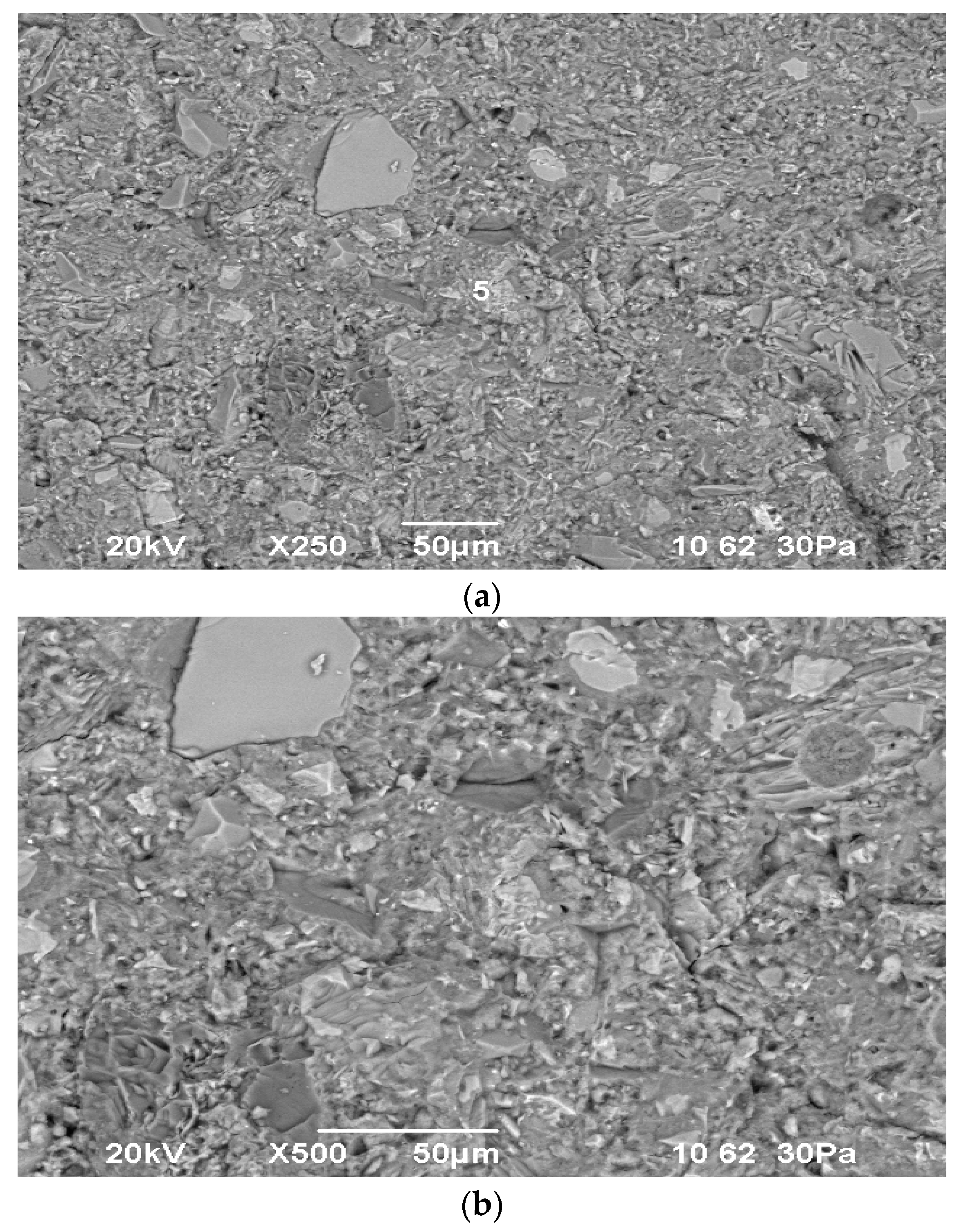
The results of scanning electron microscopy and X-ray fluorescence analysis of the materials used are presented in
Figure 2,
Figure 3 and
Figure 4.
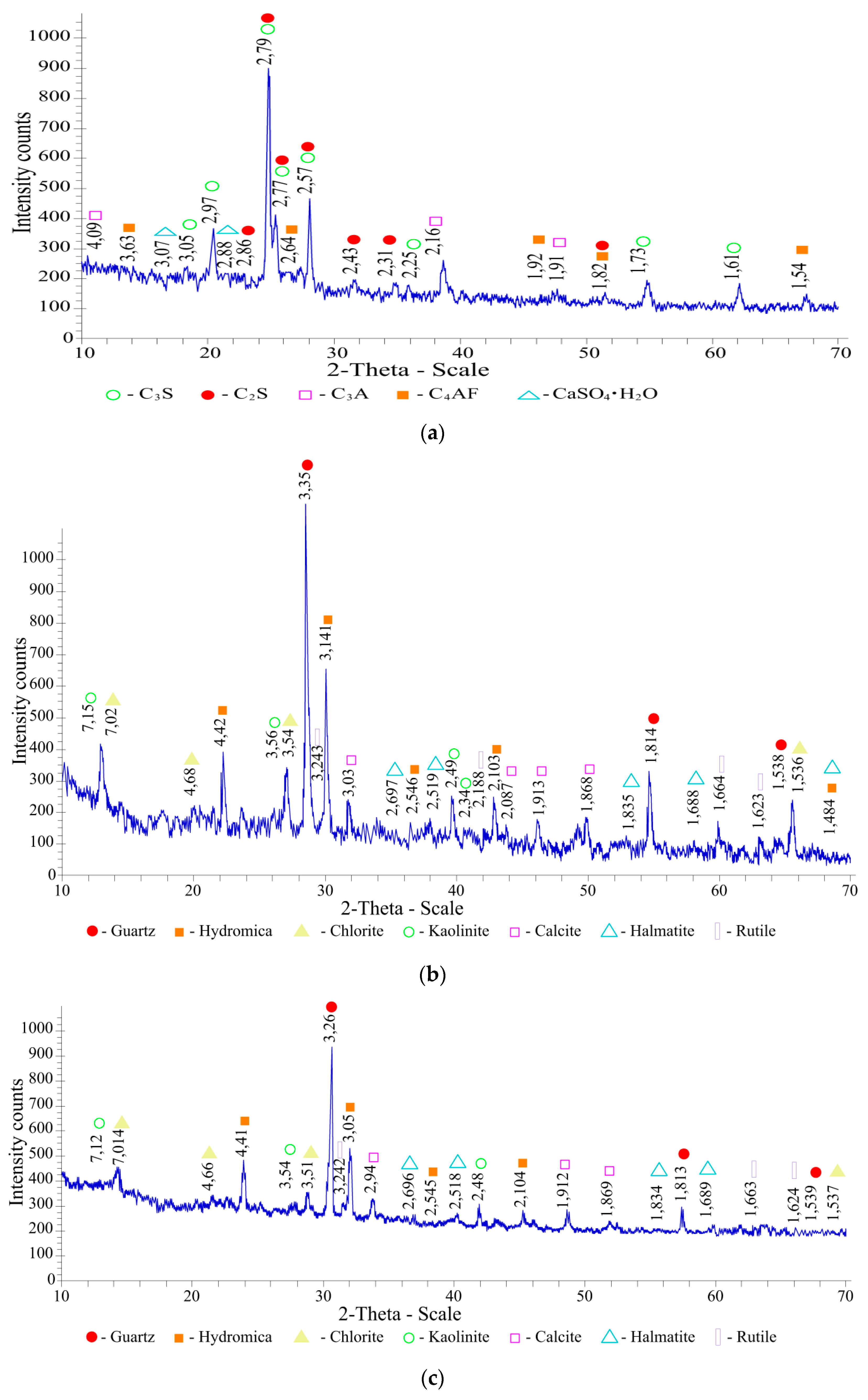
The X-ray phase analysis of Portland cement CEM I 42.5N (
Figure 5a) confirmed that it contains the following main minerals and dihydrate gypsum: alite 3CaO·SiO
2:d/n—2.79; 2.77; 2.57; 2.25; 2.97; 1.73; 3.05 Å; belite 2CaO·SiO
2:d/n—2.79; 2.77; 2.31; 2.43; 2.86; 1.82; Å; tetracalcium aluminoferrite 4CaO·Al
2O
3·Fe
2O
3:d/n—2.64; 1.82; 1.54; 1.92 Å; tricalcium aluminate 3CaO·Al
2O
3:d/n—2.16; 4.09; 1.91 Å; and gypsum CaSO
4·2H
2O: d/n –2.88; 3.07; 2.69 Å.
The following diffraction maxima were recorded in the X-ray diffraction pattern of the Mynaral clay shale (
Figure 5b): quartz-SiO
2: d/n—3.35; 1.814; 1.538 Å; hydromica (K
<1Al
2[OH]
2(AlSi
3O
10)·nH
2O): d/n—3.141; 4.42; 1.484; 2.103; 2.546 Å; chlorite (Mg,Fe)
6−2x(Al, Fe)
2x[OH]
8(Si
4−2xAl
2x)O
10:d/n—7.02; 3.54; 4.68; 1.536 Å; kaolinite (Al
2[OH]
4Si
2O
5):d/n—7.15; 3.56; 2.49; 2.34 Å; calcite CaCO
3:d/n—3.03; 1.868; 1.913; 2.087 Å; hematite-Fe
2O
3:d/n– 2697; 2519; 1.835; 1.688; 1.484 Å; and rutile TiO
2: d/n—2.188; 1.664; 3.243; 1.623 Å. The obtained X-ray diffraction pattern indicates the polymineral composition of clay shales. The mineralogical composition of clay shale, wt.%, is as follows: quartz—26.52, kaolinite—12, calcite—7.23, chlorite—15, hydromica—39.09, rutile—0.16.
The following diffraction maxima were recorded in the X-ray diffraction pattern of the Kuyuk clay shale as follows (
Figure 5c): quartz-SiO
2: d/n—3.26; 1.813; 1.539 Å; hydromica (K
<1Al
2[OH]
2(AlSi
3O
10)·nH
2O): d/n—3.05; 4.41; 1.483; 2.104; 2.545 Å; chlorite (Mg,Fe)
6−2x(Al, Fe)
2x[OH]
8(Si
4−2xAl
2x)O
10:d/n—7.04; 3.54; 4.71; 1.538 Å; kaolinite (Al
2[OH]
4Si
2O
5) d/n—7.14; 3.54; 2.47; 2.35 Å; calcite CaCO
3: d/n—3.028; 1.868; 1.913; Å; hematite-Fe
2O
3: d/n– 2.697; 2.519; 1.835; 1.687 Å; and rutile TiO
2: d/n– 3.242; 2.187; 1.687; 1.624 Å. Shale is a polymineral rock containing the following (%): quartz—27.29, kaolinite—13, calcite—6.49, chlorite—16, hydromica—36.26, and rutile—0.96.
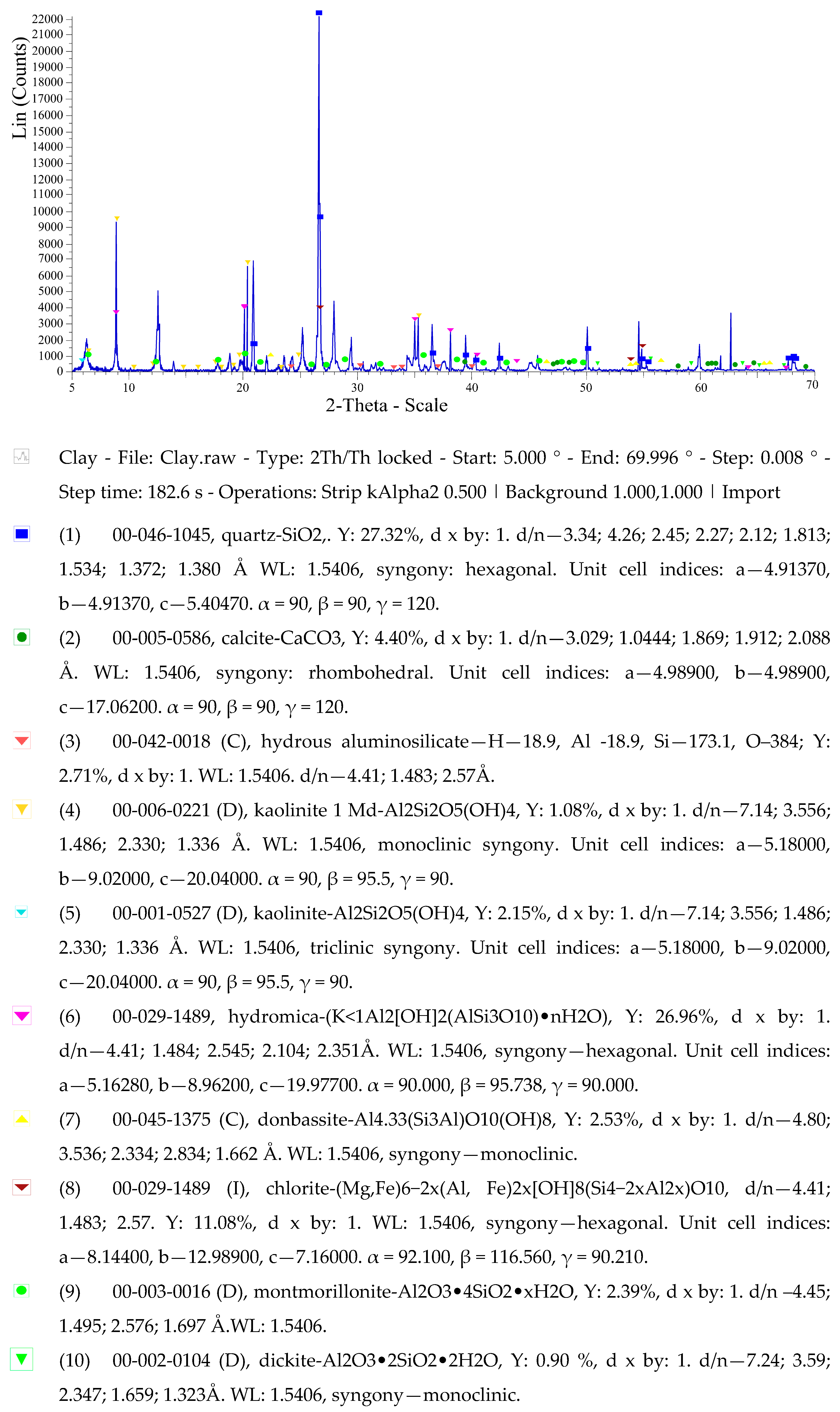
The diffraction pattern of clay shale from Kuyuk (
Figure 6), obtained on an X-ray Diffractometry device with a Bruker AXS D8 attachment, shows the following minerals:
3.2. Thermal Activation of Clay Shales
The experimental part of this work presents the main physicochemical changes occurring during the firing of clay shales at temperatures of 700–900 °C. For this purpose, the clay shales were pre-crushed and then abraded on a disk abrader to a particle size of 2–5 mm. Then, the material was dried in a SNOL-3.5/3m drying cabinet at a temperature of 90 °C for 1 h.
The firing of clay shales was carried out in an SNOL 7.2/1100 electric furnace at temperatures of 700 °C, 800 °C, and 900 °C. Isothermal holding at these temperatures was 1 h.
The cements were obtained by the joint grinding of the starting materials for 30 min to a specific surface area of 4100 to 4200 cm2/g. The amount of burnt clay shale introduced during grinding ranged from 5 to 15%.
The normal density of cement pastes without shale additives, with the addition of 5–15% Mynaral shale, was 27–28%, and 27.3% with Kuyuk shale. With an increase in the shale dosage to 15%, the water requirement of the paste increased to 27.5%.
An X-ray analysis of the burnt clay shale was performed. The main physicochemical changes occurring during shale firing were studied using X-ray analysis.
The studies were carried out using the equipment of the Central Laboratory of Certification Testing of Building Materials, Kazakhstan, Almaty—TselSIM (
Figure 7a) and the Center for Collective Use of the Mendeleyev Russian Chemical Technical University, Moscow—RCTU (
Figure 7b).
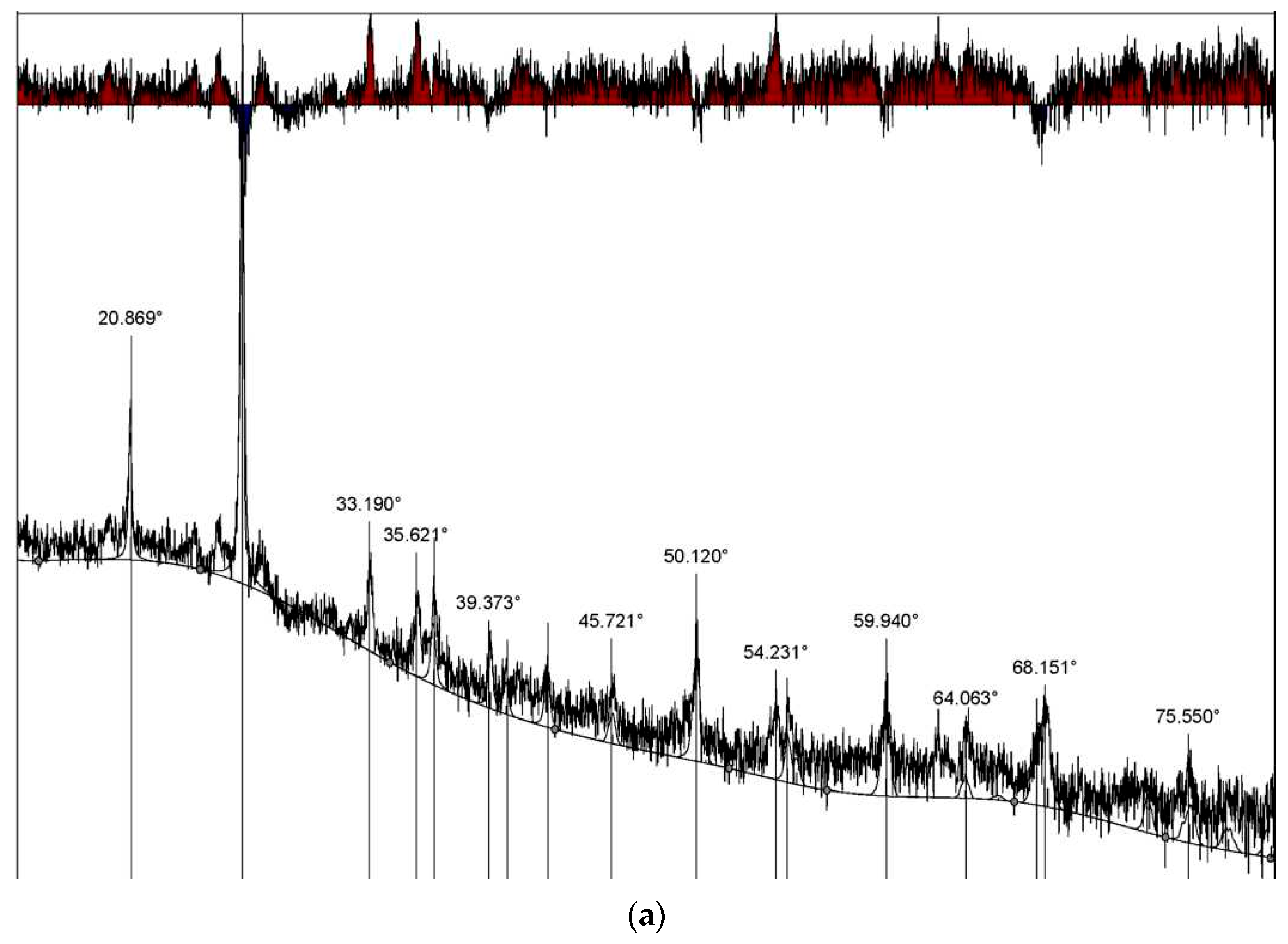
The X-ray diffraction pattern of Mynaral clay shales fired at 900 °C (
Figure 7a,b) shows the diffraction maxima of the following minerals: β-quartz-SiO
2 d/n—4.27; 3.34; 2.458; 2.282; 1.817, 1.543Å; anhydrous mica (K
<1Al
2(AlSi
3O
10) d/n—3.13; 4.51; 1.4834; 2.132; 2.52 Å; chlorite (Mg, Fe)
6−2x(Al, Fe)
2x(Si
4−2xAl
2x)O
10 d/n—3.4571; 1.543 Å; hematite-Fe
2O
3—2.7004; 1.8211; 1.693; 1.483 Å; and rutile TiO
2 d/n—2.132; 1.693; 3.241Å.
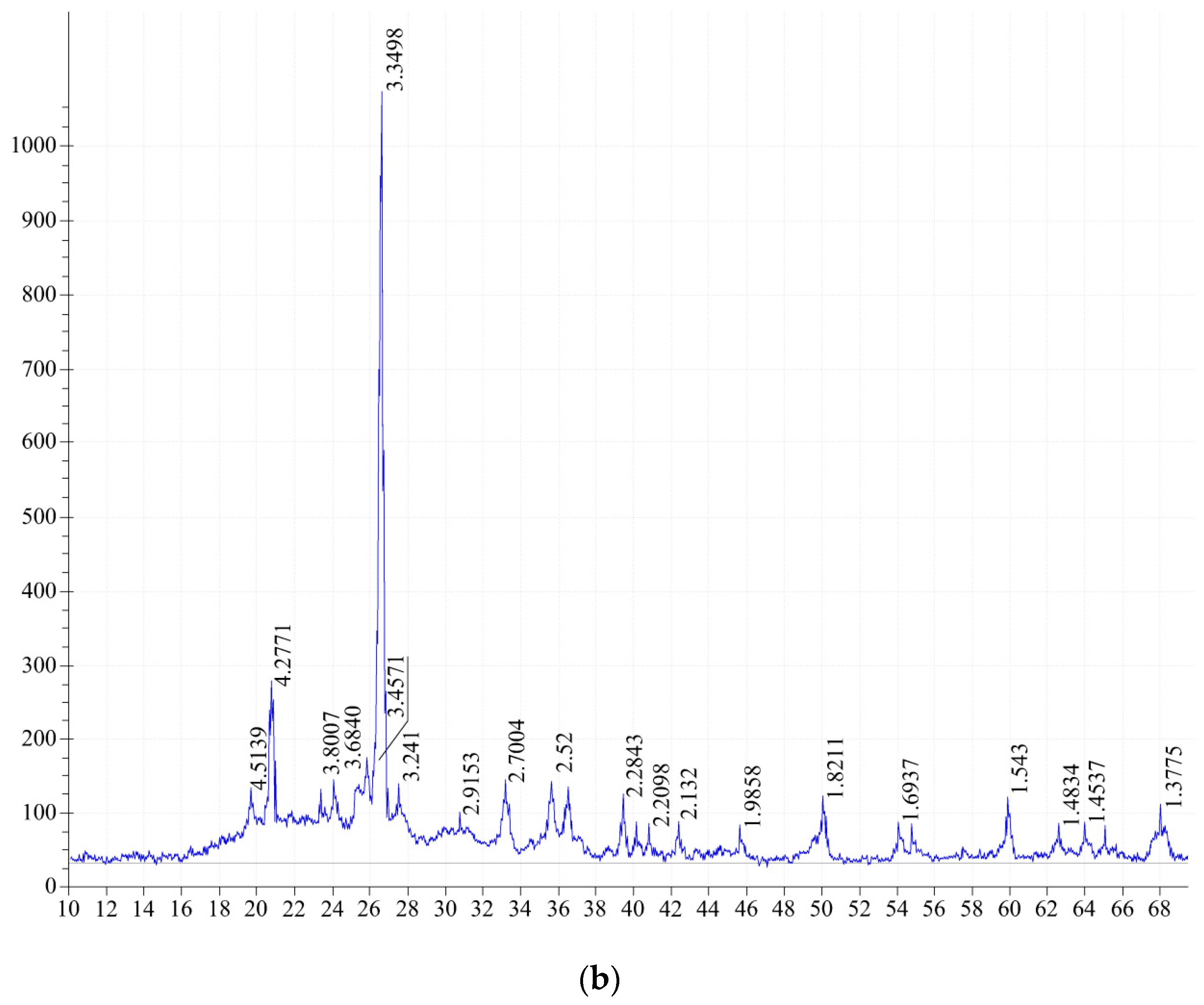
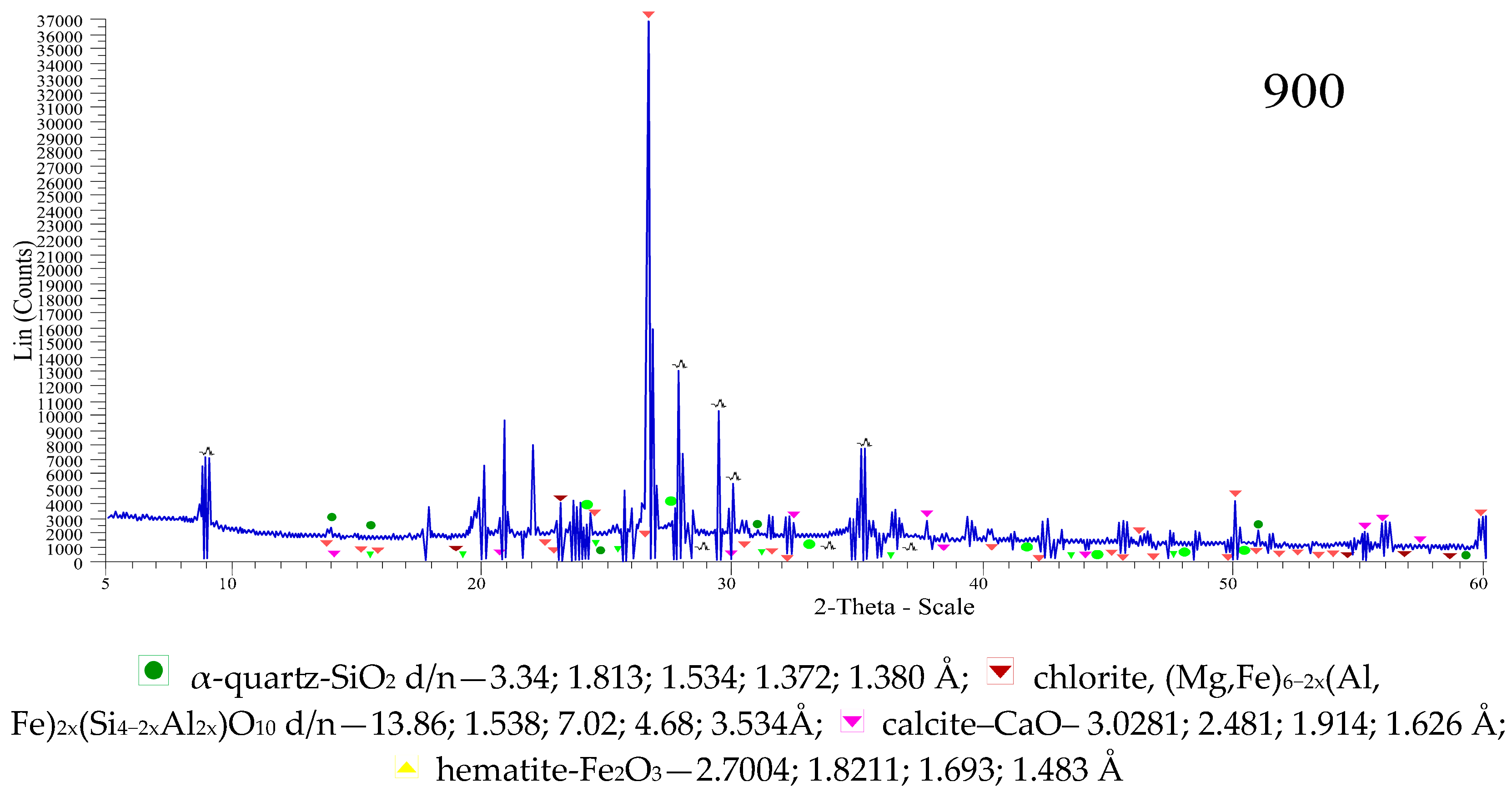
The X-ray diffraction pattern of the Kuyuk clay shale (
Figure 8) fired at 900 °C shows diffraction maxima of the following minerals: α-quartz-SiO
2 d/n—3.34; 1.813; 1.534; 1.372; 1.380 Å, chlorite, (Mg,Fe)
6−2x(Al, Fe)
2x(Si
4−2xAl
2x)O
10 d/n—13.86; 1.538; 7.02; 4.68; 3.534 Å.
3.3. Pozzolanic Activity
The pozzolanic activity of thermally activated clay shales was determined by the absorption of Ca(OH)
2. It was found that at temperatures below 700 °C, heat-treated clay shales did not have pozzolanic activity. It makes no sense to fire shales above 900 °C since, at this temperature, the crystalline phase of mullite begins to form. The results obtained are shown in
Figure 9 and
Figure 10.
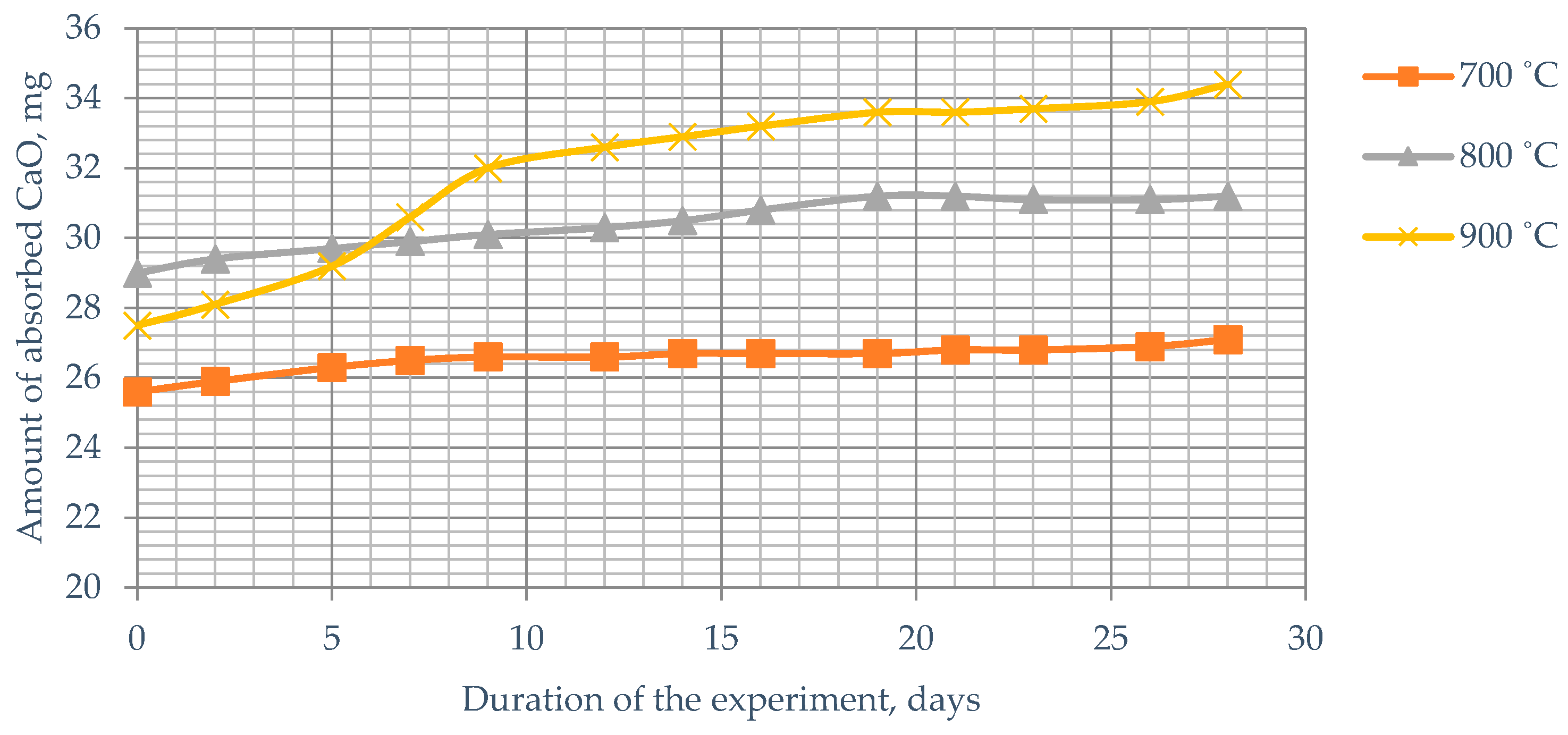
As can be seen from the presented data, during the firing of Mynaral shale at 700 °C, its activity changed from 26 to 27 mg; when fired at 800 °C, its activity changed to 31–31.5 mg; and at a temperature of 900 °C, its activity changed up to 34.4 mg CaO (
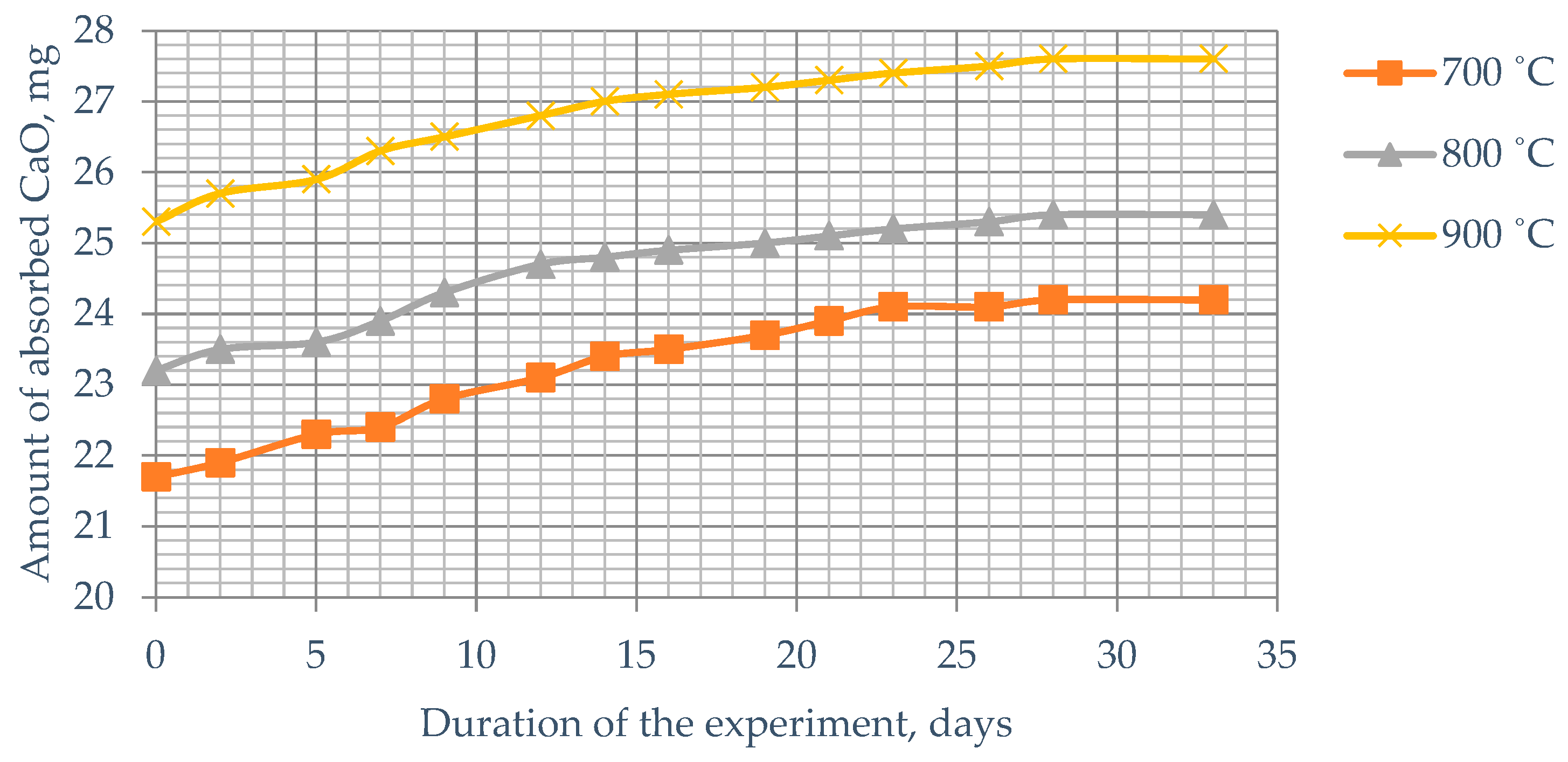
Figure 9). Burnt Kuyuk shale, fired at 900 °C, absorbs up to 27–28.2 mg CaO (
Figure 10). It is evident that at any temperature of heat treatment for Kuyuk shale, the amount of absorbed lime does not change—the curve reaches a plateau, while for Mynaral clay shale, processed at 900 °C, the absorption curve tends to increase.
3.4. Properties of Blended Cements
The effect of thermally activated clay shale additives on cement properties was investigated. The shale content varied from 5 to 25%, replacing the corresponding portion of cement with it.
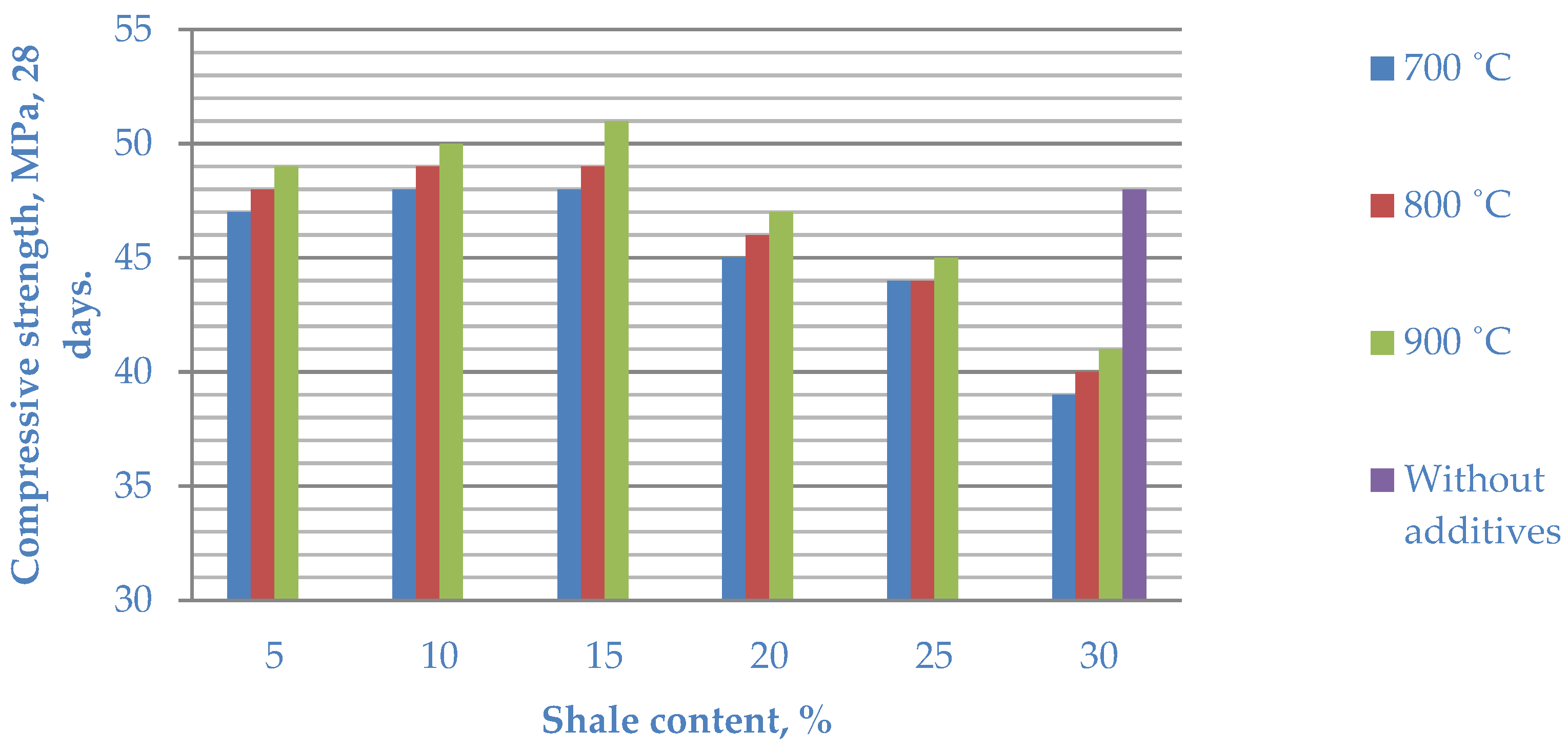
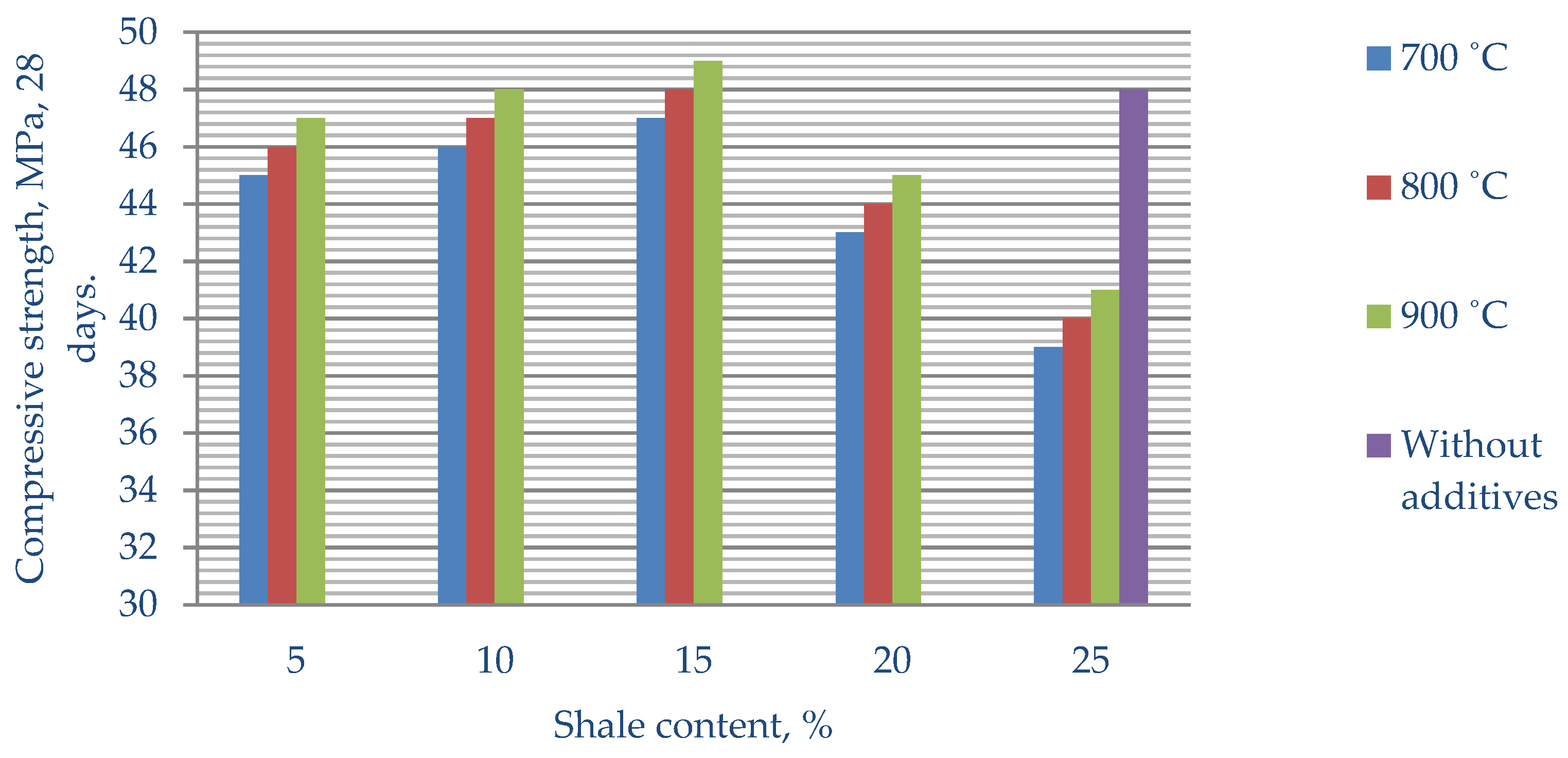
The compressive strength of cement without additives (control) for 28 days was 48 MPa. The introduction of heat-treated clay shale practically does not change the water–cement ratio but leads to an insignificant increase in both the beginning and end of the setting time of the cement mortar. At the same time, the strength properties of the composite cement are somewhat improved. The maximum compressive strength was shown by cement with the 15% Mynaral additive—51 MPa—and the maximum strength value of the fired clay shale Kuyuk was 49 MPa.
The results of the physical and mechanical tests of composite cements with additives of thermally activated Mynaral shale are shown in
Table 2 and
Figure 11, and cements with additives of thermally activated Kuyuk shale are shown in
Table 3 and
Figure 12.
The introduction of 5–15% of Mynaral shale fired at 700–900 °C into composite cement increases the strength of the cement stone at all stages of hardening. The best strength indicators are demonstrated by cements with the addition of 15% of Mynaral shale fired at 900 °C. The strength at the age of 3–28 days is 5–6.2% higher than that of the control samples. This can be explained by the compaction of the cement stone structure due to the formation of an additional amount of calcium hydrosilicates and hydroaluminates during the interaction of fired shale minerals with Ca(OH)2.
This is proven, among other things, by the high activity of Mynaral shale fired at 900 °C as a mineral additive by the absorption method with lime from lime mortar. The amount of absorbed CaO at the age of 30 days was 33.0–34.4 mg.
As can be seen from the data in
Table 3, the normal density of the cement paste is 27–28%. The setting times of all cements meet the requirements of GOST**. Increasing the dosage of burnt Kuyuk shale from 5 to 15% slightly slows down the beginning and end of the setting of the cement paste. The strength of composite cements depends both on the firing temperature of the Kuyuk clay shale and on the dosage of the burnt additive. With an increase in the shale dosage, the average density of the cement stone decreases monotonously from the initial 2250 g/cm
3 to 2145—2200 g/cm
3. Increasing the firing temperature of the Kuyuk shale clearly increases the strength of the cement stone. However, the strength of the samples with the addition of burnt shale is slightly lower than that of the control samples. The best strength indicators at 28 days of age are shown by cements with the introduction of 15% burnt Kuyuk shale compared to samples containing 5 and 10% of the additive.
The lower strength of cements with Kuyuk shale additives is explained by the lower absorption of CaO from the lime solution. According to our data, it was 27.4–28.2 mg CaO after 30 days, which is significantly less than that of Mynaral shale (33–34.3 mg).
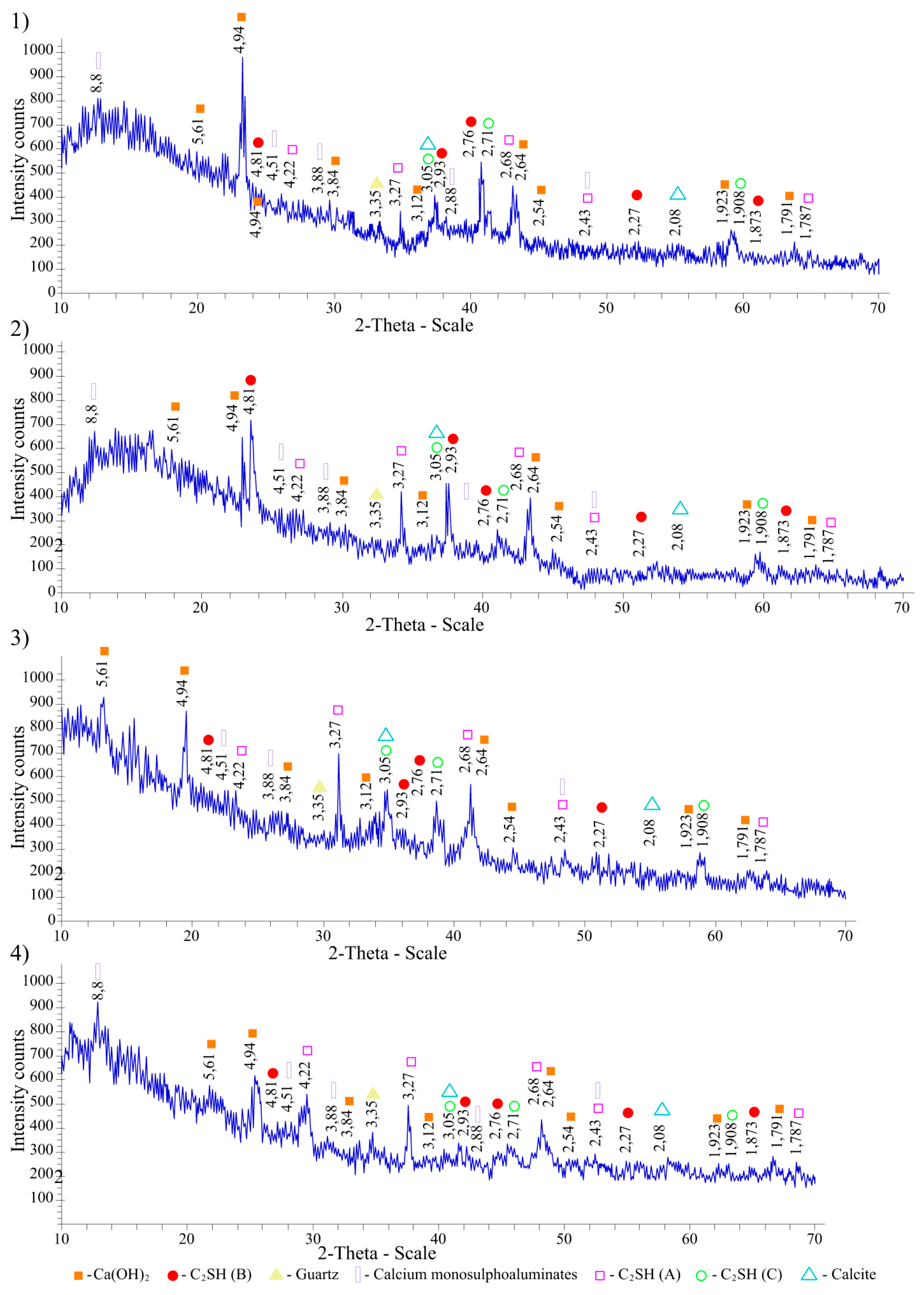
X-ray diffraction patterns of cement stone hardened for 28 days, both without additives and with 15% Mynaral clay shale, fired at 700–900 °C, are shown in
Figure 13.
The additive-free cement stone is represented by hardened hydrosilicate gel and hydroaluminates of various compositions. Ettringite is not observed, and calcium monosulfohydroaluminate is formed. Portlandite crystals and the original unreacted phases of belite and alite are present, as well as single crystals of dicalcium hydrosilicates of type A.
Cement stone containing 10% of Mynaral clay shales fired at a temperature of 700 °C contains a smaller amount of Ca(OH)2, and the content of calcium hydrosilicates increases. For a composition containing 10% of shales thermally activated at 800 and 900 °C, the amount of portlandite is even lower.
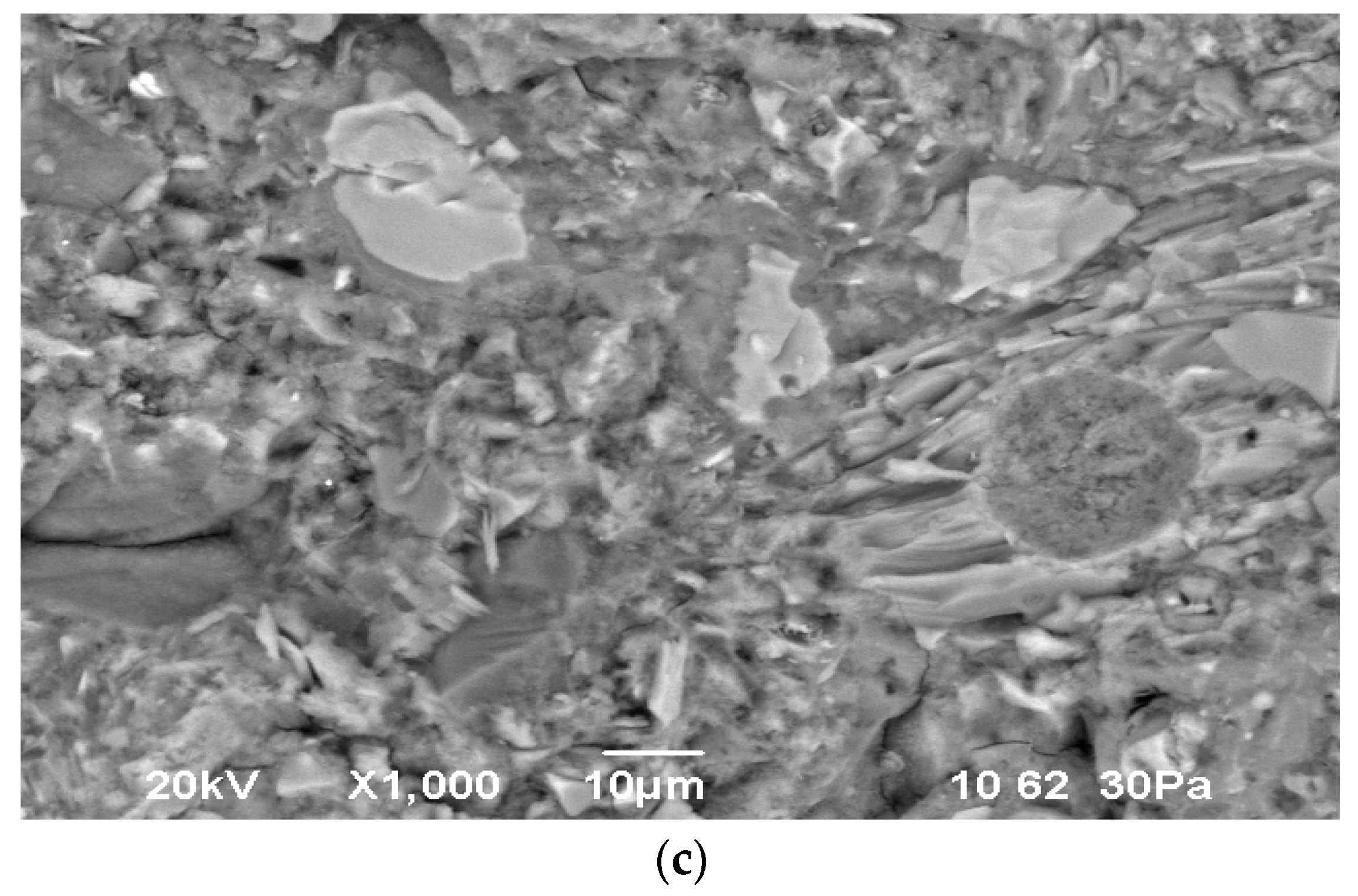
3.5. Scanning Electron Microscopy (SEM)
Using a scanning electron microscope, a study was conducted on the microstructure of hydrated cement stone without additives and with additives of 5–15% Mynaral shale, fired at 700–900 °C (
Figure 14 and
Figure 15).
The physical structure of hardened cement stone is a dense mass of hydrated solid phases with pores of various sizes. The solid mass of hydrates consists of crystals of various shapes, amorphous granules, and cement stone and also contains particles of unhydrated clinkers. Individual crystals in the mass of cement stone can form crystalline growths, which are especially noticeable within the section of the cement stone filler and in the pores.
Since the strength of cement stone is determined by its physical structure, its strength characteristics correlate with the hydration of the solid phase or the porosity of the material.
The structure is dense, with low porosity. The porosity content in the obtained samples of cement stone is 3%. A structure with the densest mass is formed, in which the contacts between particles are high. The main binding function here is performed by hydrosilicate gel.
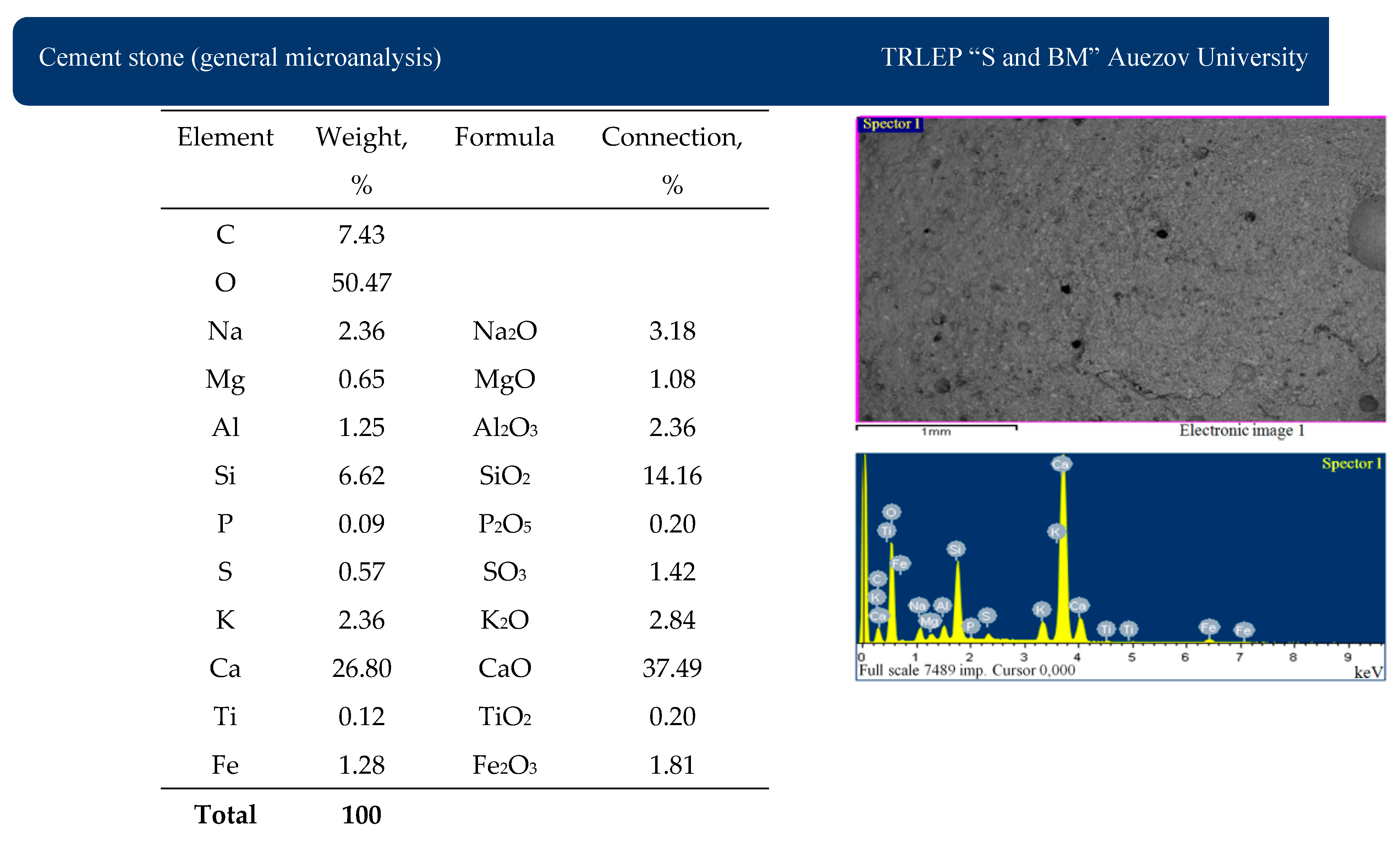
In the micrograph of cement stone without additives (
Figure 16), there are crystals of portlandite Ca(OH)
2 in the form of colorless crystals. The main mass is represented by hardened hydrosilicate gel. In small quantities, in cement stone without additives, there is dicalcium hydrosilicate-type C
2SH (A) in the form of colorless rhombic prisms. The microstructure of cement stone without additives of the resulting mixture is dense and uniform.
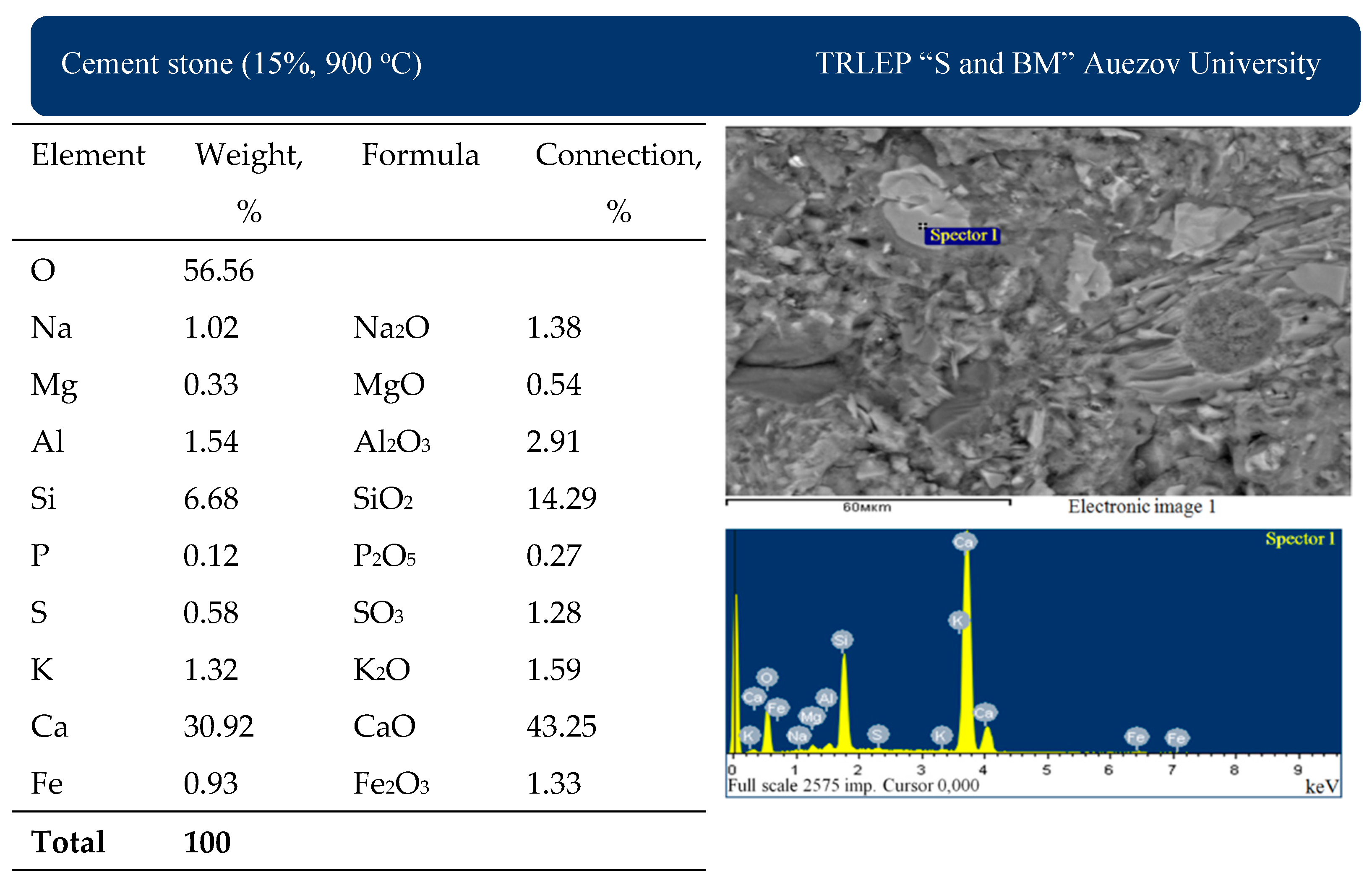
As can be seen in the micrograph (
Figure 17), the main mass of cement stone containing 15% of Mynaral clay shale fired at 900 °C is dense hydrosilicate. The content of C
2SH (A) is low compared to cement stone without additives. This cement stone has the highest indicator in the process of determining the compressive strength, which is confirmed by the optimality of the structure from the point of view of microscopic studies.
Cement stone with the addition of 15% Mynaral clay shale, fired at 900 °C, contains a very small amount of calcium monosulphate hydrosulphoaluminate in the form of colorless hexagonal plates, 3CaO·CaSO4·12H2O, of ettringite in the form of colorless long needle-shaped crystals, 3CaO·Al2O3·3CaSO4·31H2O, and white unfermented calcite, CaCO3. Visible (on the right) is the dissolution of a large crystal of portlandite due to the interaction of Ca(OH)2 with amorphous silicon and aluminum oxides from thermally activated shale.
3.6. Approximate Calculation of Reduction in CO2 Emissions from Production of 1 Ton of Composite Cement with Addition of 15% Burnt Shale
The production of composite cements with burnt shale is planned to be implemented at Shymkentcement LLP. According to calculations at this plant, when producing 1 ton of clinker, carbon dioxide emissions amount to 776 kg of CO2. The introduction of 15% of the active mineral additive of burnt clay shale from Mynaral during the grinding of composite cement allows the clinker component to be reduced in the production of this cement by 15%.
Thus, a reduction in the proportion of the clinker in composite cement by 15% due to the introduction of a mineral additive allows CO2 emissions into the atmosphere to be reduced by the same amount.
When producing 1 ton of composite cement with the addition of 15% burnt shale, the emissions are as follows:
776 kg − (776 × 0.15) = 659.6 kg of CO2 per ton of composite cement.
The reduction in emissions due to the use of the 15% mineral additive developed is the following:
776 − 659.6 = 116.4 kg per ton of cement.
With an annual production of, for example, 1,000,000 tons of composite cement, the reduction in emissions is the following:
1,000,000 × 0.1164 = 116,400 tons of CO2.
4. Conclusions
The chemical and mineralogical composition of the raw materials used in the research work was fully studied using the physicochemical methods of SEM, XRD, DTA, and IR. The clay shales of the Mynaral deposit and the Kuyuk deposit consist mainly of quartz, clay minerals (hydromica, chlorite, kaolinite), small amounts of calcite, hematite, and rutile minerals. The Mynaral clay shales contain 70.41% SiO2, 10.38% Al2O3, 10.64% FeO, and 5.38% CaO and, by their chemical composition, are suitable for use as an active mineral additive to clinker in cement production.
During the firing of Mynaral shale at 700 °C, its activity increases to 26–27 mg at a firing temperature of 800 °C to 31–31.5 mg and at a temperature of 900 °C up to 34.4 mg CaO. The fired shale of the Kuyuk deposit absorbs up to 27–28.2 mg CaO.
The introduction of Mynaral shale fired at 700–900 °C in an amount of 5–15% into composite cement increases the strength of cements at the age of 3–28 days by 5–6.2%. This can be explained by the compaction of the structure of the cement stone due to the formation of an additional amount of calcium hydrosilicates during the interaction of the minerals of the fired shale with Ca(OH)2.
The thermal activation of mineral additives does not require significant additional fuel and energy costs. The thermal treatment of shale can be carried out by introducing finely crushed shale onto a clinker layer in the kiln cooler. The result is an active mineral additive with low cost and high quality.
Reducing the proportion of the clinker in composites of Portland cement by 15% will reduce CO2 emissions into the atmosphere. With the production of 1 million tons of cement, CO2 emissions will decrease by 116,400 tons.
Heat-treated clay shales at a temperature of 900 °C have high pozzolanic activity.
The obtained physical and mechanical strength indicators of cement stone with additives of 15% heat-treated Mynaral shale at a temperature of 900 °C correspond to the material composition of general construction cements according to the GOST 31108–2020 [
32] General construction cements CEM II/A-Sl.