Synthesis and Characterization of MAPTAC-Modified Cationic Corn Starch: An Integrated DFT-Based Experimental and Theoretical Approach for Wastewater Treatment Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Part
2.1.1. Materials
2.1.2. Synthesis of Cationic Corn Starch
2.1.3. Characterization
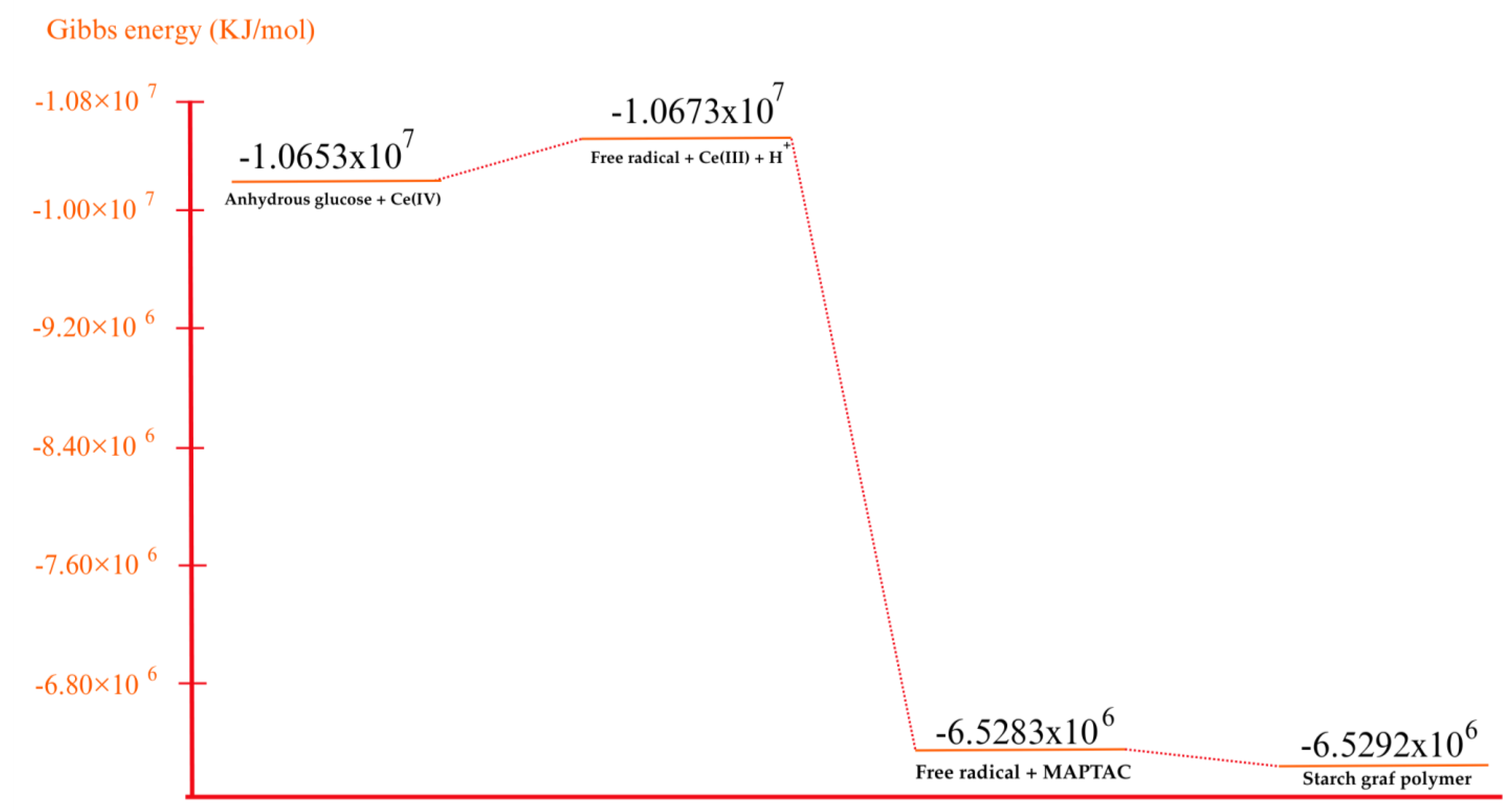
2.2. Computational Details
3. Results and Discussion
3.1. Degree of Substitution (DS)
3.2. Dependence of the Zeta Potential of Cationic Starch on pH
3.3. FTIR Spectroscopy
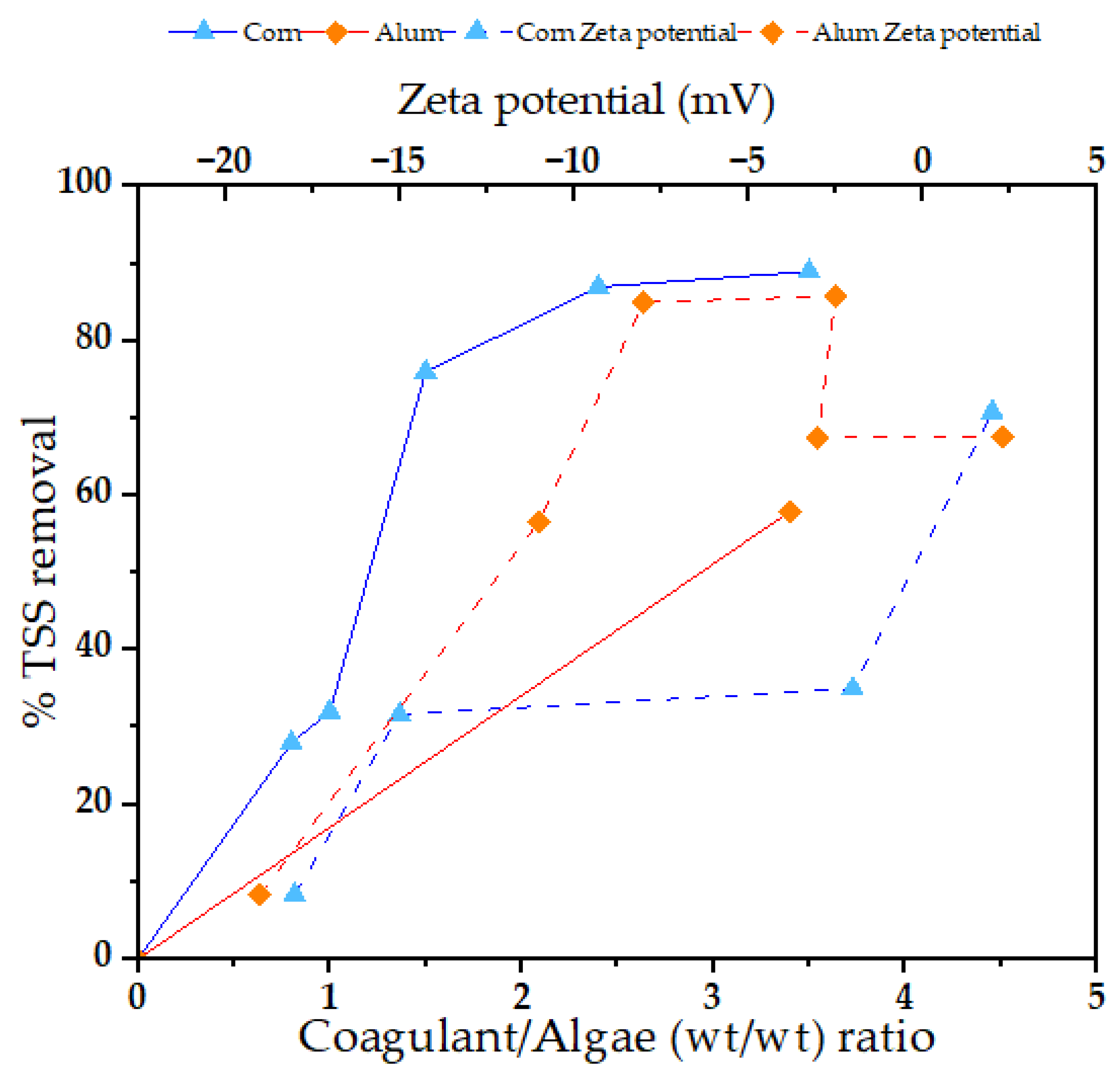
3.4. Removal of Total Suspended Solids (TSSs)
3.5. Total Phosphorus (TP) Removal
3.6. HOMO-LUMO Orbitals
3.7. Global Descriptors
3.8. Molecular Electrostatic Potential Map
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, G.; Rast, W.; Jones, R. Eutrophication of water bodies: Insights for an age-old problem [Significance of phosphate as a pollutant]. Environ. Sci. Technol. 1978, 12, 900–908. [Google Scholar] [CrossRef]
- Fink, G.; Alcamo, J.; Flörke, M.; Reder, K. Phosphorus Loadings to the World’s Largest Lakes: Sources and Trends. Glob. Biogeochem. Cycles 2018, 32, 617–634. [Google Scholar] [CrossRef]
- Khan, F.; Ansari, A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Banu, H.; Meenakshi, S. Synthesis and characterization of metal loaded chitosan-alginate biopolymeric hybrid beads for the efficient removal of phosphate and nitrate ions from aqueous solution. Int. J. Biol. Macromol. 2019, 130, 407–418. [Google Scholar] [CrossRef]
- Atnafu, T.; Leta, S. Plasticized magnetic starch-based Fe3O4 clay polymer nanocomposites for phosphate adsorption from aqueous solution. Heliyon 2021, 7, e07973. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Yuan, M.; Cai, R.; Macazo, F.; Minteer, S. Modified biochar for phosphate adsorption in environmentally relevant conditions. Chem. Eng. J. 2020, 380, 122375. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, Á.; González-Delgado, Á.; Herrera-Barros, A.; Ortega-Toro, R. Selective and Binary Adsorption of Anions onto Biochar and Modified Cellulose from Corn Stalks. Water 2023, 15, 1420. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, X.; Zhang, H.; Li, Q.; Mo, J.; Xing, M.; Dong, B.; Zhu, H. Phosphate Removal from Polluted Water via Lanthanum-Modified Sludge Biochar. Sustainability 2024, 16, 5667. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.; Ok, Y.; Tsang, D.; Tsang, Y.; Giri, B.; Singh, R. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616–617, 1242–1260. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, J.; Chi, T.; Wang, P.; Yang, B. Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 2015, 9, 1066–1075. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhang, Z.; Liu, Q.; Jiang, K.; Tian, J.; Zhang, Y.; Ni, F. Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J. Environ. Chem. Eng. 2021, 9, 105079. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Du, Q.; Shi, J. Adsorption of different forms of phosphorus on modified corn bracts. Water Environ. Res. 2019, 91, 748–755. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.; Bagheri, F. Optimization of the lead removal from aqueous solution using two starch based adsorbents: Design of experiments using response surface methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 102793. [Google Scholar] [CrossRef]
- Mittal, H.; Alhassan, S.; Ray, S. Efficient organic dye removal from wastewater by magnetic carbonaceous adsorbent prepared from corn starch. J. Environ. Chem. Eng. 2018, 6, 7119–7131. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef]
- Rott, E.; Nouri, M.; Meyer, C.; Minke, R.; Schneider, M.; Mandel, K.; Drenkova-Tuhtan, A. Removal of phosphonates from synthetic and industrial wastewater with reusable magnetic adsorbent particles. Water Res. 2018, 145, 608–617. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.; Santos, S.; Boaventura, R.; Botelho, C. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Ibáñez, C.; Peñuelas, J. Changing nutrients, changing rivers. Science 2019, 365, 637–638. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.; Paerl, H.; Dodds, W. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Kundu, S.; Coumar, M.V.; Rajendiran, S.; Rao, A.; Rao, A.S. Phosphates from Detergents and Eutrophication of Surface Water Ecosystem in India. Curr. Sci. 2015, 108, 1320–1325. [Google Scholar]
- Haque, S. How Effective Are Existing Phosphorus Management Strategies in Mitigating Surface Water Quality Problems in the U.S.? Sustainability 2021, 13, 6565. [Google Scholar] [CrossRef]
- Acelas, N.; Martin, B.; López, D.; Jefferson, B. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 2015, 119, 1353–1360. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, Y.; Xiao, S. Adsorption behaviors and mechanisms of Al-Fe dual-decorated biochar adsorbent for phosphate removal from rural wastewater. J. Dispers. Sci. Technol. 2022, 44, 2520–2531. [Google Scholar] [CrossRef]
- Mancuso, C.; Jamison, M.; Zaporski, J.; Yang, Z. Effects of coagulant morphology and chemical properties on soluble reactive phosphate removal in corn ethanol wastewater. Water Environ. Res. 2021, 93, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wang, Y. Application of Magnesium Modified Corn Biochar for Phosphorus Removal and Recovery from Swine Wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Farag, A.; Sokker, H.; Zayed, E.; Eldien, F.; Alrahman, N. Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int. J. Biol. Macromol. 2018, 120 Pt B, 2188–2199. [Google Scholar] [CrossRef]
- Eltaweil, A.; El-Monaem, E.; Elshishini, H.; El-Aqapa, H.; Hosny, M.; Abdelfatah, A.; Ahmed, M.; Hammad, E.; El-Subruiti, G.; Fawzy, M.; et al. Recent developments in alginate-based adsorbents for removing phosphate ions from wastewater: A review. RSC Adv. 2022, 12, 8228–8248. [Google Scholar] [CrossRef]
- Schweizer, S.; Taubert, A. Polymer-controlled, bio-inspired calcium phosphate mineralization from aqueous solution. Macromol. Biosci. 2007, 7, 1085–1099. [Google Scholar] [CrossRef]
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2018, 294, 2180–2190. [Google Scholar] [CrossRef]
- Sun, S.; Gao, M.; Wang, Y.; Qiu, Q.; Han, J.; Qiu, L.; Feng, Y. Phosphate removal via biological process coupling with hydroxyapatite crystallization in alternating anaerobic/aerobic biofilter reactor. Bioresour. Technol. 2021, 326, 124728. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Wang, X.; Zheng, S.; Yang, L. Highly effective wastewater phosphorus removal by phosphorus accumulating organism combined with magnetic sorbent MFC@La(OH)3. Chem. Eng. J. 2018, 335, 443–449. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Y.; Huang, H.; Tan, Z.; Sun, Y.; Liu, H. Simultaneous nitrogen and phosphorus removal by interactions between phosphate accumulating organisms (PAOs) and denitrifying phosphate accumulating organisms (DPAOs) in a sequencing batch reactor. Sci. Total Environ. 2020, 744, 140852. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.; Aziz, A.; Farooqi, I.; Basheer, F. Development of denitrifying phosphate accumulating and anammox micro-organisms in anaerobic hybrid reactor for removal of nutrients from low strength domestic sewage. Bioresour. Technol. 2018, 267, 149–157. [Google Scholar] [CrossRef]
- Zong, E.; Wang, C.; Yang, J.; Zhu, H.; Jiang, S.; Liu, X.; Song, P. Preparation of TiO2/cellulose nanocomposites as antibacterial bio-adsorbents for effective phosphate removal from aqueous medium. Int. J. Biol. Macromol. 2021, 182, 434–444. [Google Scholar] [CrossRef]
- Frisch, M.J. Revisión B. 01; Gaussiano. Inc.: Wallingford, CT, USA, 2018. [Google Scholar]
- Fernández, J.H.; Guerra, Y.; Cano, H. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules 2022, 27, 4832. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Puello-Polo, E.; Castro-Suarez, J.R. Characterization of the Morphological and Chemical Profile of Different Families of Microplastics in Samples of Breathable Air. Molecules 2023, 28, 1042. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Ortega-Toro, R.; Castro-Suarez, J.R. Theoretical–Experimental Study of the Action of Trace Amounts of Formaldehyde, Propionaldehyde, and Butyraldehyde as Inhibitors of the Ziegler–Natta Catalyst and the Synthesis of an Ethylene–Propylene Copolymer. Polymers 2023, 15, 1098. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano-Cuadro, H.; Puello-Polo, E. Emission of Bisphenol A and Four New Analogs from Industrial Wastewater Treatment Plants in the Production Processes of Polypropylene and Polyethylene Terephthalate in South America. Sustainability 2022, 14, 10919. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Trilleras, J. Characterization of Microplastics in Total Atmospheric Deposition Sampling from Areas Surrounding Industrial Complexes in Northwestern Colombia. Sustainability 2022, 14, 13613. [Google Scholar] [CrossRef]
- Alejandro, H.F.J.; Alfonso, P.P.J.; Ortega-Toro, R. Application of computational studies using density Functional Theory (DFT) to evaluate the catalytic degradation of polystyrene. Polymers 2025, 17, 923. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.A.H.; Palomo, J.A.P.; Ortega-Toro, R. Application of DFT and Experimental Tests for the Study of Compost Formation Between Chitosan-1,3-dichloroketone with Uses for the Removal of Heavy Metals in Wastewater. J. Compos. Sci. 2025, 9, 91. [Google Scholar] [CrossRef]






| Name | HOMO | LUMO | Chemical Potential (μ) | Ionization Potential (I) | Electronegativity (χ) | Electronic Affinity (A) | Electrophilicity (ω) | Hardness (η) |
|---|---|---|---|---|---|---|---|---|
| Starch | −0.24911 | −0.04027 | −0.20884 | 0.24911 | 0.14469 | 0.04027 | 0.00228 | 0.10442 |
| Modified starch | −0.15611 | −0.06840 | −0.08771 | 0.15611 | 0.11226 | 0.06840 | 0.00017 | 0.04386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, J.A.H.; Palomo, J.A.P. Synthesis and Characterization of MAPTAC-Modified Cationic Corn Starch: An Integrated DFT-Based Experimental and Theoretical Approach for Wastewater Treatment Applications. J. Compos. Sci. 2025, 9, 240. https://doi.org/10.3390/jcs9050240
Fernández JAH, Palomo JAP. Synthesis and Characterization of MAPTAC-Modified Cationic Corn Starch: An Integrated DFT-Based Experimental and Theoretical Approach for Wastewater Treatment Applications. Journal of Composites Science. 2025; 9(5):240. https://doi.org/10.3390/jcs9050240
Chicago/Turabian StyleFernández, Joaquín Alejandro Hernández, and Jose Alfonso Prieto Palomo. 2025. "Synthesis and Characterization of MAPTAC-Modified Cationic Corn Starch: An Integrated DFT-Based Experimental and Theoretical Approach for Wastewater Treatment Applications" Journal of Composites Science 9, no. 5: 240. https://doi.org/10.3390/jcs9050240
APA StyleFernández, J. A. H., & Palomo, J. A. P. (2025). Synthesis and Characterization of MAPTAC-Modified Cationic Corn Starch: An Integrated DFT-Based Experimental and Theoretical Approach for Wastewater Treatment Applications. Journal of Composites Science, 9(5), 240. https://doi.org/10.3390/jcs9050240







