An Investigation into Fe3O4 Nanoparticle-Based Composites for Enhanced Electromagnetic Radiation Shielding
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetite Nanoparticles with Chemical Condensation Method
2.2. Synthesis of Magnetite Nanoparticles Using the Liquid-Phase Combustion Method
2.3. Device Characterization
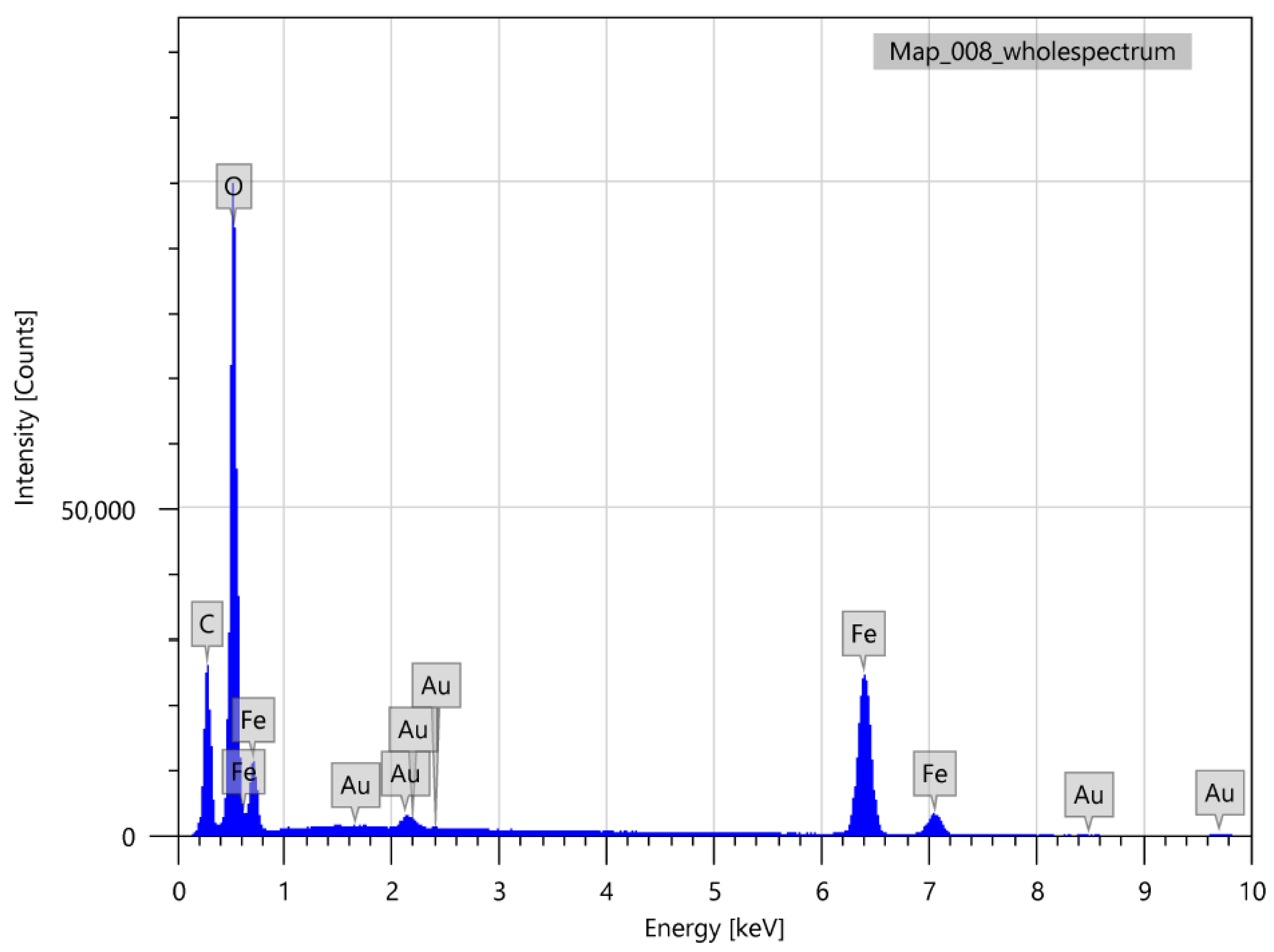
3. Results and Discussion
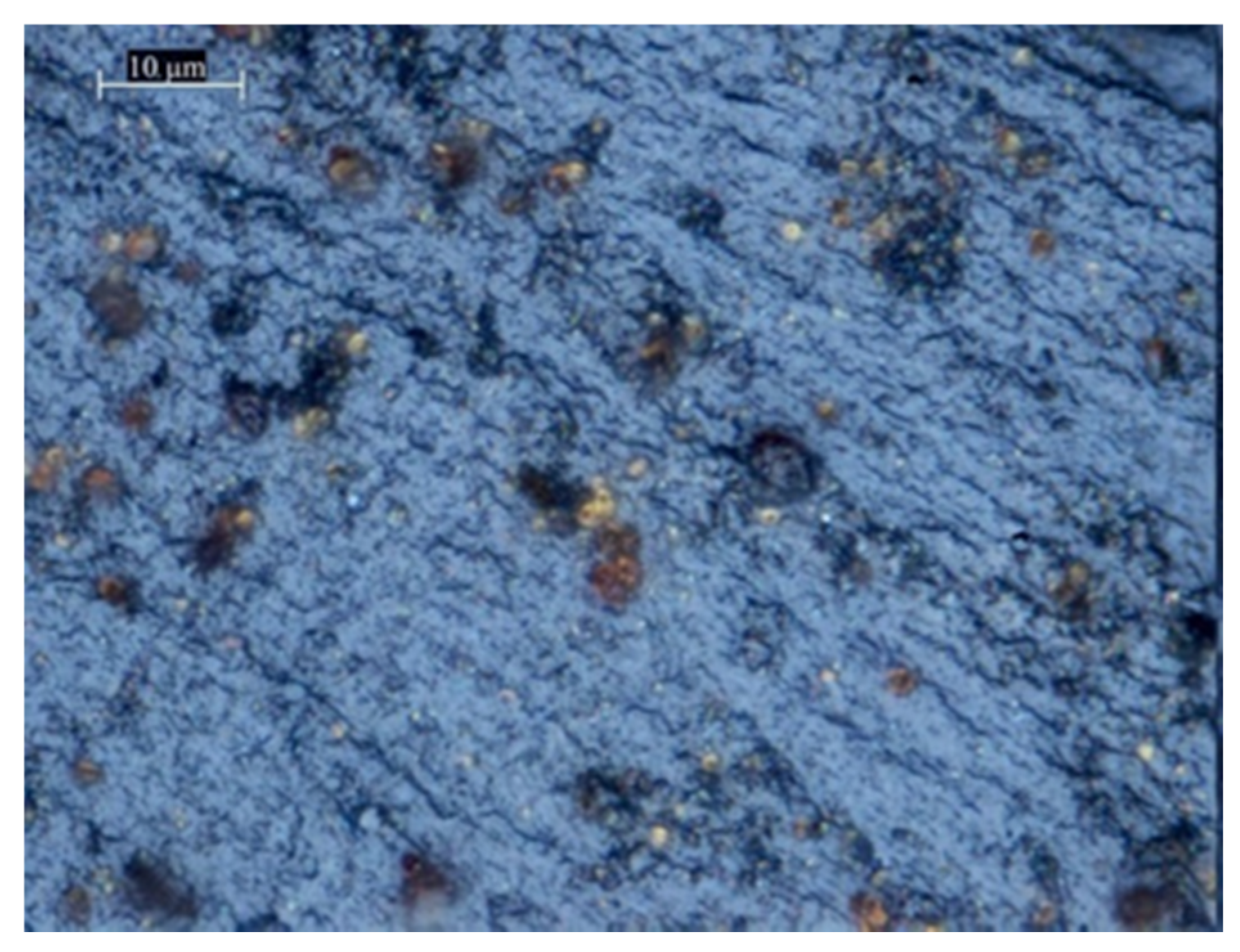
3.1. Chemical Condensation Method (CCM)
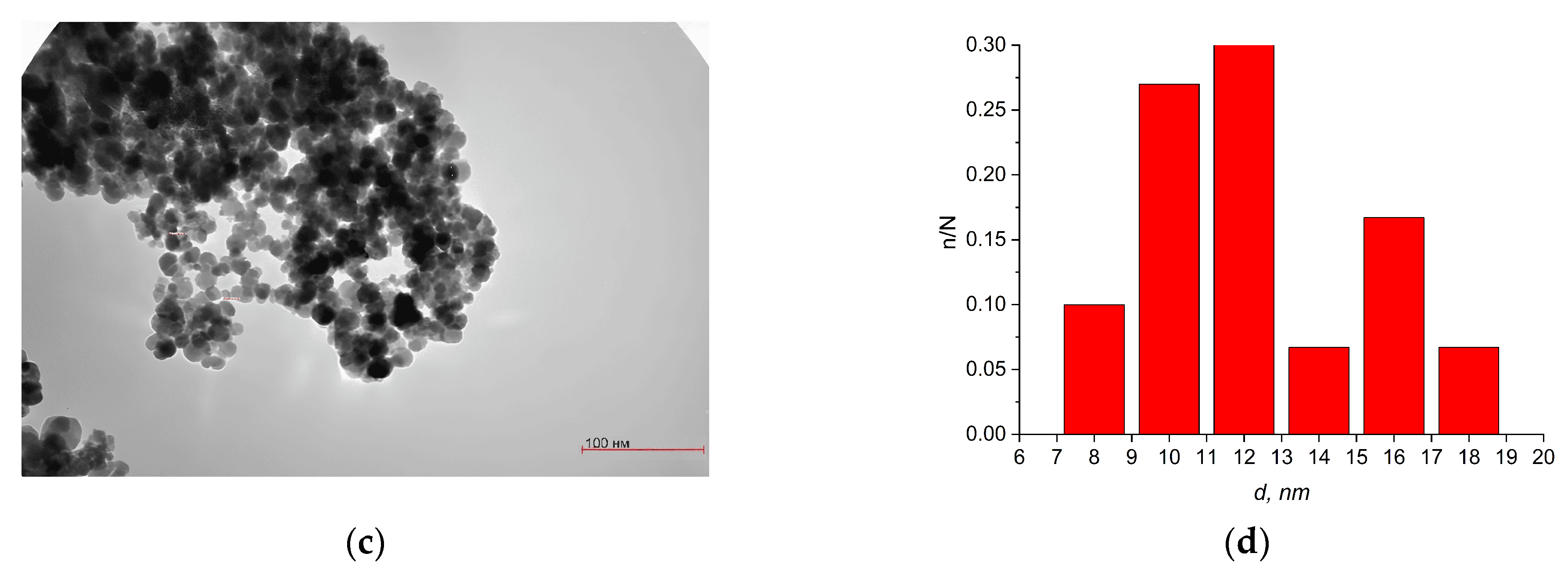
3.2. Liquid-Phase Combustion Method
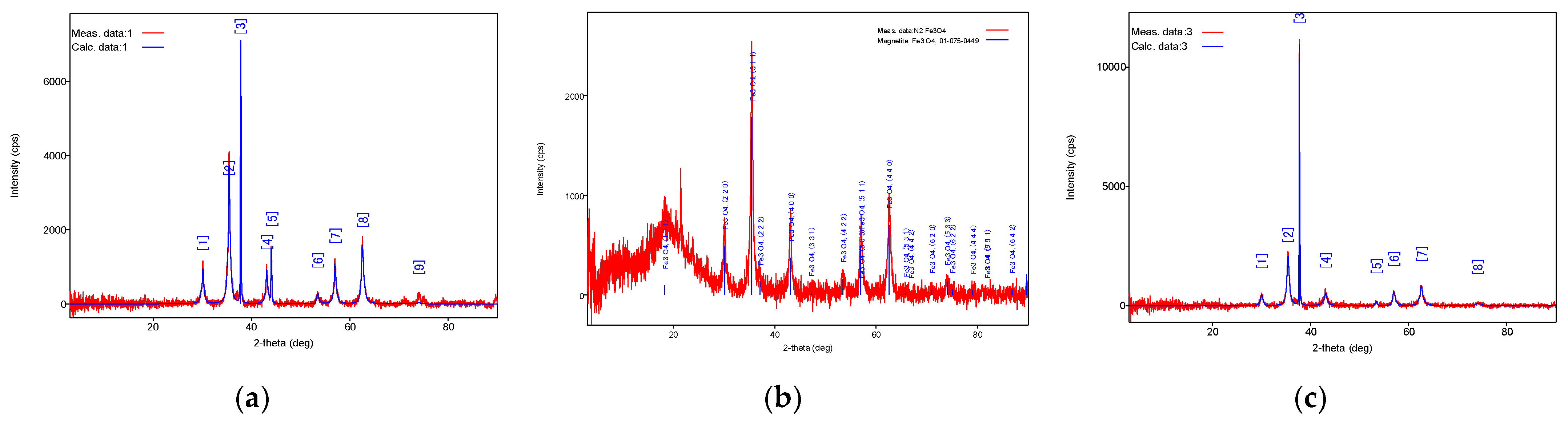
3.3. Investigation of the Shielding Characteristics of Cement-Based Materials Incorporating Magnetite Nanoparticles Synthesized Through Chemical Precipitation
3.4. Study of the Shielding Properties of Cement Stone with Additives of Magnetite Nanoparticles Synthesized with Liquid-Phase Combustion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goubau, G.; Schwering, F. On the Guided Propagation of Electromagnetic Wave Beams. IRE Trans. Antennas Propag. 1961, 9, 248–256. [Google Scholar] [CrossRef]
- Lee, J.; Kang, H.; Shin, B.G.; Song, Y.J. Cement Composites with Carbon Fiber for Electromagnetic Interference Shielding Applications. Carbon 2024, 220, 118861. [Google Scholar] [CrossRef]
- Taghipour, N.; Whitworth, G.L.; Othonos, A.; Dalmases, M.; Pradhan, S.; Wang, Y.; Kumar, G.; Konstantatos, G. Low-Threshold, Highly Stable Colloidal Quantum Dot Short-Wave Infrared Laser Enabled by Suppression of Trap-Assisted Auger Recombination. Adv. Mater. 2022, 34, 2107532. [Google Scholar] [CrossRef]
- Lapinsky, S.E.; Easty, A.C. Electromagnetic Interference in Critical Care. J. Crit. Care 2006, 21, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Wanasinghe, D.; Aslani, F.; Ma, G.; Habibi, D. Review of Polymer Composites with Diverse Nanofillers for Electromagnetic Interference Shielding. Nanomaterials 2020, 10, 541. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Shen, B.; Zheng, W. Evaluation, Fabrication and Dynamic Performance Regulation of Green EMI-Shielding Materials with Low Reflectivity: A Review. Compos. Part B Eng. 2022, 233, 109652. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Khadheer Pasha, S.K. Recent Advances in Electromagnetic Interference Shielding Properties of Metal and Carbon Filler Reinforced Flexible Polymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Rubežienė, V.; Varnaitė-Žuravliova, S.; Sankauskaitė, A.; Pupeikė, J.; Ragulis, P.; Abraitienė, A. The Impact of Structural Variations and Coating Techniques on the Microwave Properties of Woven Fabrics Coated with PEDOT:PSS Composition. Polymers 2023, 15, 4224. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, M.; Ge, S.; Li, J.; Alshammari, A.S.; Luo, J.; Amin, M.A.; Qiu, H.; Jiang, J.; Asiri, Y.M.; et al. Advanced Functional Electromagnetic Shielding Materials: A Review Based on Micro-Nano Structure Interface Control of Biomass Cell Walls. Nano-Micro Lett. 2025, 17, 3. [Google Scholar] [CrossRef]
- Wanasinghe, D.; Aslani, F.; Ma, G.; Habibi, D. Advancements in Electromagnetic Interference Shielding Cementitious Composites. Constr. Build. Mater. 2020, 231, 117116. [Google Scholar] [CrossRef]
- Peng, M.; Qin, F. Clarification of Basic Concepts for Electromagnetic Interference Shielding Effectiveness. J. Appl. Phys. 2021, 130, 225108. [Google Scholar] [CrossRef]
- Lan, C.; Zou, L.; Wang, N.; Qiu, Y.; Ma, Y. Multi-Reflection-Enhanced Electromagnetic Interference Shielding Performance of Conductive Nanocomposite Coatings on Fabrics. J. Colloid Interface Sci. 2021, 590, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, S.-H.; Wang, H.-Y.; Wang, G.-S.; Yin, P.-G. Excellent Electromagnetic Wave Absorbing Properties of Two-Dimensional Carbon-Based Nanocomposite Supported by Transition Metal Carbides Fe3C. Carbon 2020, 162, 438–444. [Google Scholar] [CrossRef]
- Kaidar, B.; Imash, A.; Smagulova, G.; Keneshbekova, A.; Kazhdanbekov, R.; Yensep, E.; Akalim, D.; Lesbayev, A. Magnetite-Incorporated 1D Carbon Nanostructure Hybrids for Electromagnetic Interference Shielding. Nanomaterials 2024, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, F.; Liu, J.; Ma, Q.; Qiao, R.; Xu, Z. Synthesis of MWCNTs/Si3N4 Nanocomposites Via Click Chemistry and Electromagnetic Wave-Absorption Properties. J. Electron. Mater. 2025, 54, 1228–1244. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Wang, Y.; Zong, M.; Zhu, Y. Construction of Polymer-Derived Double Transition Metal Compound/Carbon Matrix Nanocomposites for Efficient Electromagnetic Wave Absorber. Ceram. Int. 2024, 50, 45416–45426. [Google Scholar] [CrossRef]
- Xia, X.; Xiao, Q. Electromagnetic Interference Shielding of 2D Transition Metal Carbide (MXene)/Metal Ion Composites. Nanomaterials 2021, 11, 2929. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhou, B.; He, C.; Wang, B.; Feng, Y.; Liu, C. Synergistically Enhancing Electromagnetic Interference Shielding Performance and Thermal Conductivity of Polyvinylidene Fluoride-Based Lamellar Film with MXene and Graphene. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106945. [Google Scholar] [CrossRef]
- Cheng, M.; Xu, X.; Zhang, H.; Kong, S.; Feng, Z.; Meng, F.; Wang, Y. Self-Assembling, Flexible and Stable Aramid Nanofiber/Polypyrrole/MXene Composite Film for Efficient Electromagnetic Interference Shielding, Dual-driven Heating and Infrared Thermal Camouflage. Chem. Eng. J. 2025, 505, 159112. [Google Scholar] [CrossRef]
- Katheria, A.; Das, P.; Nayak, J.; Roy, B.; Pal, A.; Biswas, S.; Das, N.C. MXene and Fe3O4 Decorated G-C3N4 Incorporated High Flexible Hybrid Polymer Composite for Enhanced Electrical Conductivity, EMI Shielding and Thermal Conductivity. Next Mater. 2025, 6, 100292. [Google Scholar] [CrossRef]
- Oraby, H.; Tantawy, H.R.; Correa-Duarte, M.A.; Darwish, M.; Elsaidy, A.; Naeem, I.; Senna, M.H. Tuning Electro-Magnetic Interference Shielding Efficiency of Customized Polyurethane Composite Foams Taking Advantage of RGO/Fe3O4 Hybrid Nanocomposites. Nanomaterials 2022, 12, 2805. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Li, R.; Wang, Z.; Lou, Z.; Li, Y. Facile Synthesis of Ultralight and Porous Melamine-Formaldehyde (MF) Resin-Derived Magnetic Graphite-Like C3N4/Carbon Foam with Electromagnetic Wave Absorption Behavior. Crystals 2020, 10, 656. [Google Scholar] [CrossRef]
- Michałowska, A.; Kudelski, A. The First Silver-Based Plasmonic Nanomaterial for Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy with Magnetic Properties. Molecules 2022, 27, 3081. [Google Scholar] [CrossRef]
- Yang, C.; Li, K.; Hu, M.; Li, X.; Li, M.; Hu, X.; Li, Y.; Huang, Z.; Meng, G. Flexible ZrO2/ZrB2/C Nanofiber Felt with Enhanced Microwave Absorption and Ultralow Thermal Conductivity. J. Mater. 2025, 11, 100988. [Google Scholar] [CrossRef]
- Wu, F.; Hu, P.; Hu, F.; Tian, Z.; Tang, J.; Zhang, P.; Pan, L.; Barsoum, M.W.; Cai, L.; Sun, Z. Multifunctional MXene/C Aerogels for Enhanced Microwave Absorption and Thermal Insulation. Nano-Micro Lett. 2023, 15, 194. [Google Scholar] [CrossRef]
- Hou, T.; Wang, B.; Jia, Z.; Wu, H.; Lan, D.; Huang, Z.; Feng, A.; Ma, M.; Wu, G. A Review of Metal Oxide-Related Microwave Absorbing Materials from the Dimension and Morphology Perspective. J. Mater. Sci. Mater. Electron. 2019, 30, 10961–10984. [Google Scholar] [CrossRef]
- Lesbayev, A.B.; Smagulova, G.T.; Kim, S.; Prikhod’ko, N.G.; Manakov, S.M.; Guseinov, N.; Mansurov, Z.A. Solution-Combustion Synthesis and Characterization of Fe3O4 Nanoparticles. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 195–197. [Google Scholar] [CrossRef]
- Lesbayev, A.; Elouadi, B.; Borbotko, T.; Manakov, S.; Smagulova, G.; Boiprav, O.; Prikhodko, N. Influence of Magnetite Nanoparticles on Mechanical and Shielding Properties of Concrete. Eurasian Chem. J. 2017, 19, 223. [Google Scholar] [CrossRef]
- Boiprav, O.V.; Bogush, V.A. Lightweight microwave absorbers with loop-like surface irregularities based on foiled materials for anechoic chambers. MOMENTO 2024, 1–16. [Google Scholar] [CrossRef]
- Boiprav, O.V.; Bogush, V.A. Advanced Layered Flexible Radio-Absorbing Materials Based on Powdered Charcoal. Inorg. Mater. Appl. Res. 2024, 15, 280–288. [Google Scholar] [CrossRef]
- Hasanov, M. The development of a piezoelectric defect detection device through mathematical modeling applied to polymer composite materials. New Mater. Compd. Appl. 2024, 8, 62–74. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Liu, D.; Wang, Y.; Zhang, X.; Wen, G.; Zhang, X.; Huang, X. Carbon-Coated Octahedral Fe3O4/Fe2O3 Nanocomposites for Enhanced Electromagnetic Wave Absorption. ACS Appl. Nano Mater. 2025, 8, 2952–2964. [Google Scholar] [CrossRef]
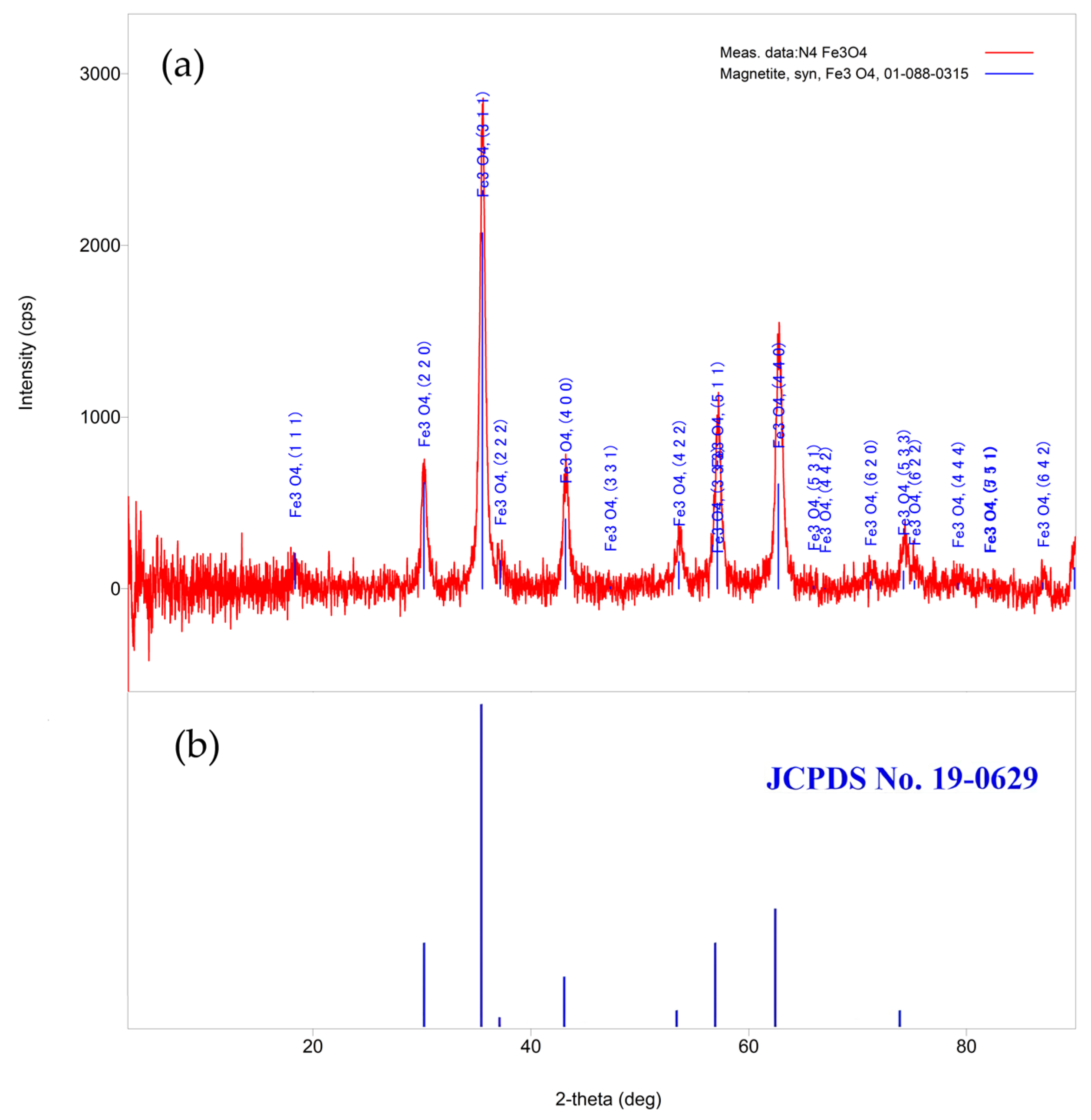






| 2-Theta (deg) | D (ang.) | Height (cps) | FWHM (deg) | Int. I (cps deg) | Phase Name |
|---|---|---|---|---|---|
| 18.50(4) | 4.791(11) | 71(24) | 0.65(14) | 65(14) | Fe3O4(111) |
| 30.08(3) | 2.969(3) | 481(63) | 0.58(3) | 354(22) | Fe3O4(2.2.0) |
| 35.521(14) | 2.5252(9) | 1876(125) | 0.569(13) | 1530(25) | Fe3O4(3.1.1) |
| 43.21(4) | 2.0922(18) | 474(63) | 0.56(4) | 364(21) | Fe3O4(4.0.0) |
| 53.60(10) | 1.708(3) | 201(41) | 0.72(10) | 220(23) | Fe3O4(4.2.2) |
| 57.15(4) | 1.6104(10) | 701(76) | 0.61(3) | 641(20) | Fe3O4(5.1.1) |
| 62.69(3) | 1.4809(6) | 1008(92) | 0.68(2) | 943(24) | Fe3O4(4.4.0) |
| 71.30(7) | 1.3217(12) | 66(23) | 0.9(4) | 101(24) | Fe3O4(6.2.0) |
| 74.16(12) | 1.2776(18) | 214(42) | 0.78(19) | 326(30) | Fe3O4(5.3.3) |
| Stoichiometric Ratio | LXRD, nm | SBET (m2/g) | DBET, nm | Carbon Content, % |
|---|---|---|---|---|
| Fe(NO3)/C6H8O7, 1:1 | 20 | 72.203 | 16 ± 1 | 24.37 |
| Fe(NO3)/C6H8O7, 1:1.5 | 18 | 22.240 | 51 ± 2 | 32.70 |
| Fe(NO3)/C6H8O7, 1:2 | 13 | 9.204 | 125 ± 4 | 38.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesbayev, A.; Akalim, D.; Kalauov, B.; Yerezhep, D. An Investigation into Fe3O4 Nanoparticle-Based Composites for Enhanced Electromagnetic Radiation Shielding. J. Compos. Sci. 2025, 9, 226. https://doi.org/10.3390/jcs9050226
Lesbayev A, Akalim D, Kalauov B, Yerezhep D. An Investigation into Fe3O4 Nanoparticle-Based Composites for Enhanced Electromagnetic Radiation Shielding. Journal of Composites Science. 2025; 9(5):226. https://doi.org/10.3390/jcs9050226
Chicago/Turabian StyleLesbayev, Aidos, Doszhan Akalim, Bakhytzhan Kalauov, and Darkhan Yerezhep. 2025. "An Investigation into Fe3O4 Nanoparticle-Based Composites for Enhanced Electromagnetic Radiation Shielding" Journal of Composites Science 9, no. 5: 226. https://doi.org/10.3390/jcs9050226
APA StyleLesbayev, A., Akalim, D., Kalauov, B., & Yerezhep, D. (2025). An Investigation into Fe3O4 Nanoparticle-Based Composites for Enhanced Electromagnetic Radiation Shielding. Journal of Composites Science, 9(5), 226. https://doi.org/10.3390/jcs9050226






