Shining a Light on Carbon-Reinforced Polymers: Mg/MgO and TiO2 Nanomodifications for Enhanced Optical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. EPD Modified Carbon Fibers
2.2. PVD-Modified Carbon Fibers
2.2.1. Mg Film Deposition
2.2.2. TiO2 Film Deposition
2.3. Lamination Processes
3. Characterization
3.1. Atomic Force Microscopy
3.2. Raman Spectroscopy
3.3. X-Ray Diffraction
3.4. Scanning Electron Microscopy
3.5. UV/Vis Spectroscopy
4. Results and Discussion
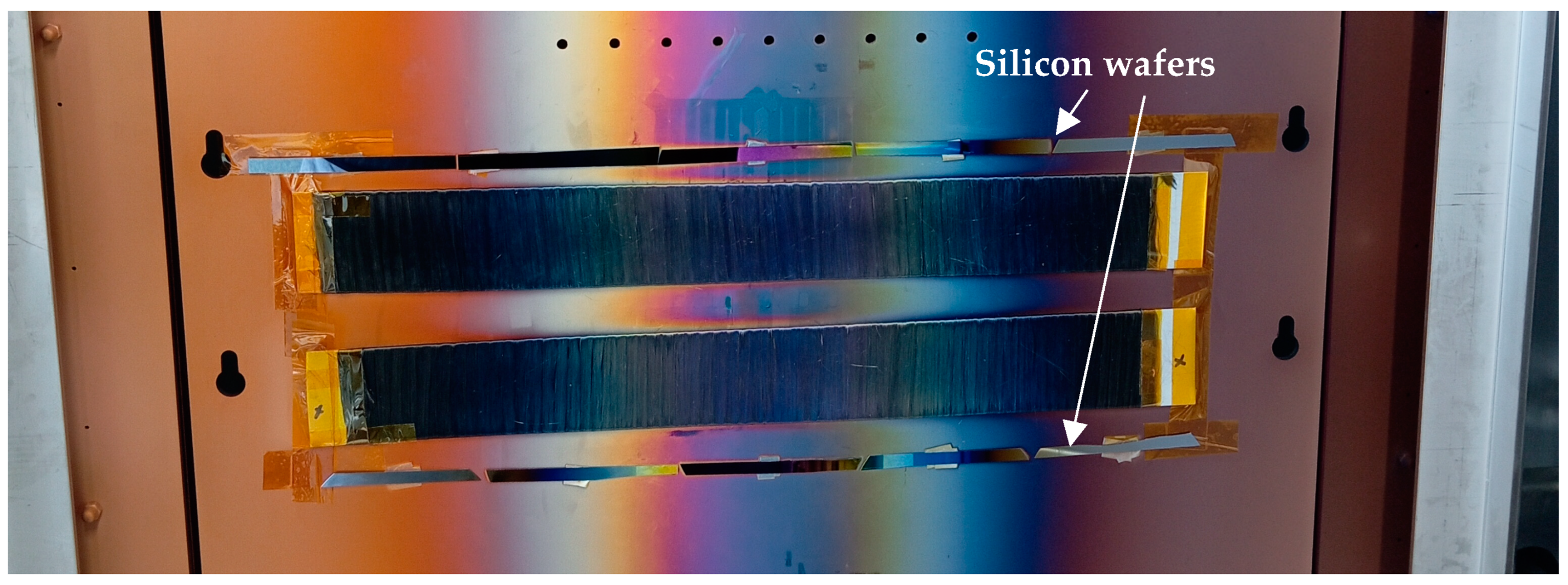
4.1. Sputtering Rate for Mg PVD-Modified Fibers
4.2. Film Thicknesses for TiO2-Modified Fibers
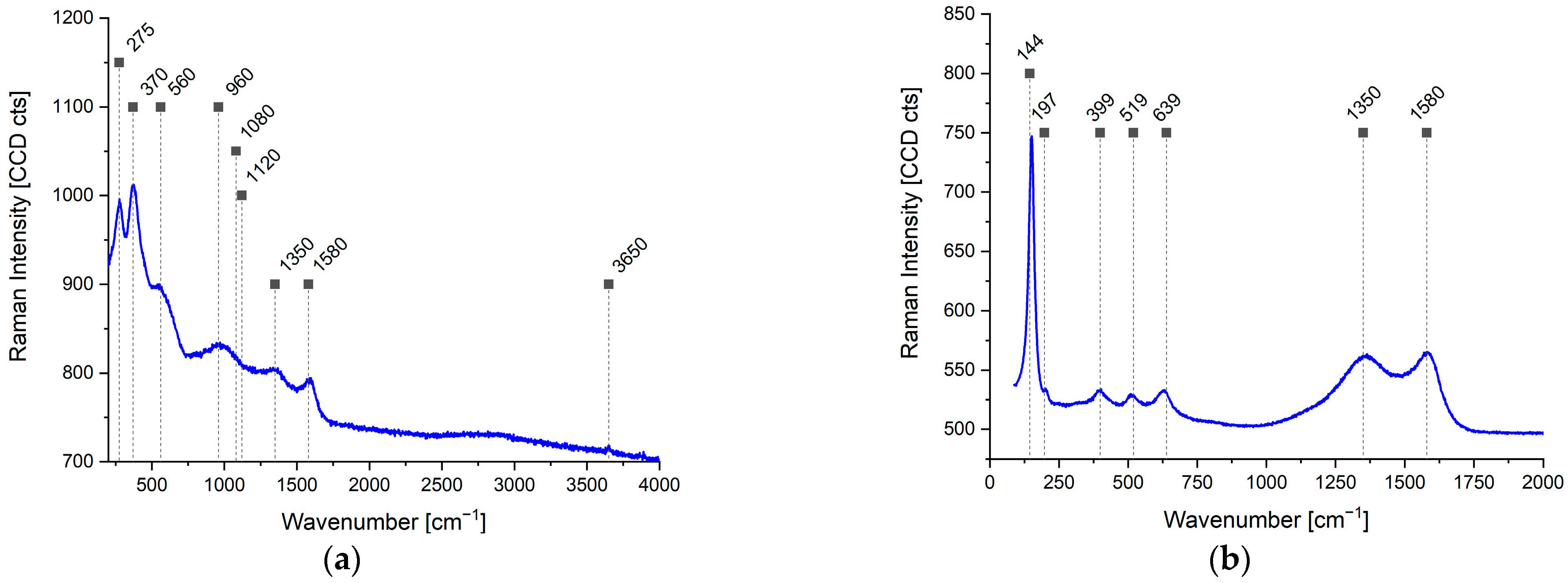
4.3. Raman Investigations
4.4. XRD Analysis
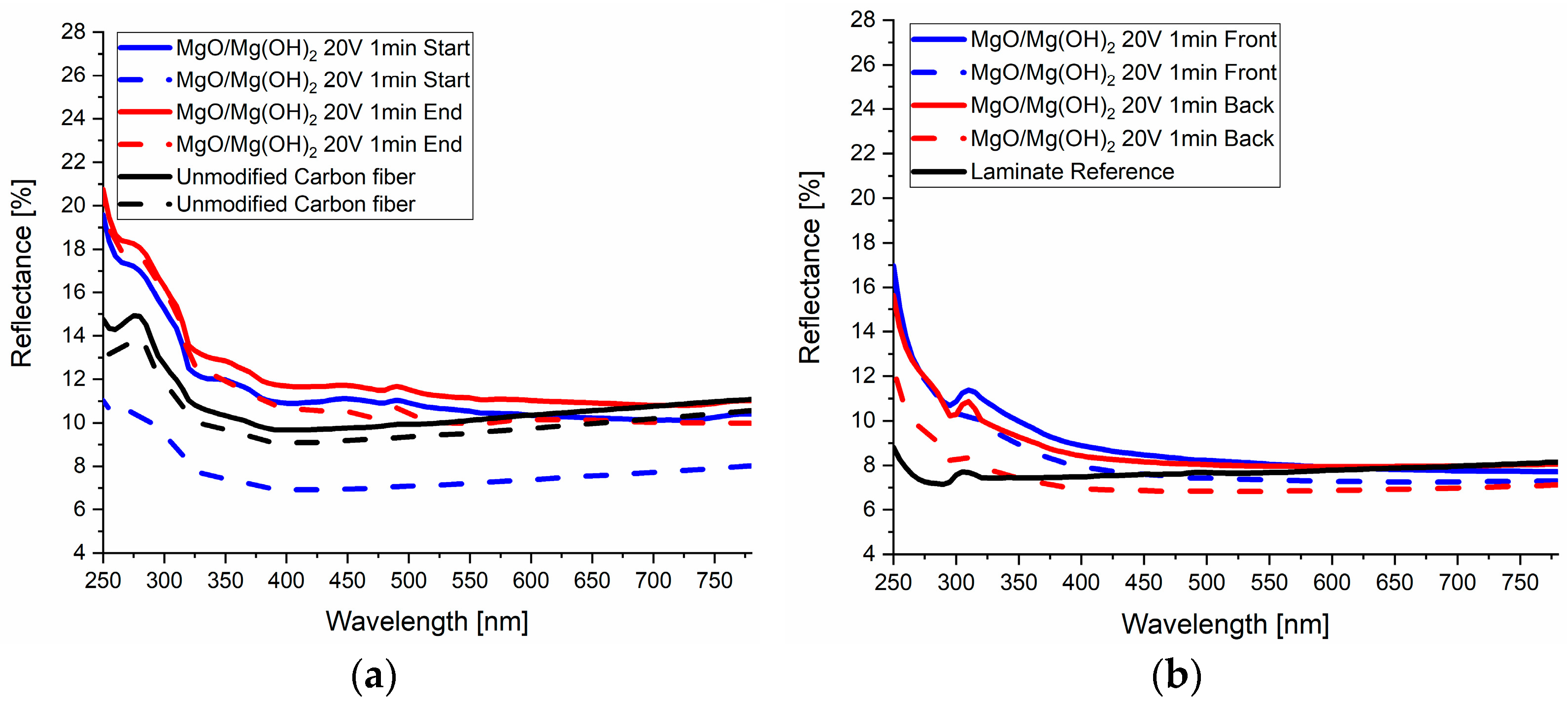
4.5. Deposition Analysis and Optical Investigations
4.5.1. Quasi-Continuous EPD Using MgO Nanoparticles
4.5.2. PVD-Based Deposition of Mg Thin Films
4.5.3. PVD-Based Deposition of TiO2 Thin Films
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Macosko, C.W. Can nanoparticle toughen fiber-reinforced thermosetting polymers? J. Mater. Sci. 2019, 54, 4471–4483. [Google Scholar] [CrossRef]
- Rashid, A.B.; Haque, M.; Islam, S.M.M.; Uddin Labib, K.M.R. Nanotechnology-enhanced fiber-reinforced polymer composites: Recent advancements on processing techniques and applications. Heliyon 2024, 10, e24692. [Google Scholar] [CrossRef]
- Böger, L.; Sumfleth, J.; Hedemann, H.; Schulte, K. Improvement of fatigue life by incorporation of nanoparticles in glass fibre reinforced epoxy. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1419–1424. [Google Scholar] [CrossRef]
- Carolan, D.; Ivankovic, A.; Kinloch, A.J.; Sprenger, S.; Taylor, A.C. Toughened carbon fibre-reinforced polymer composites with nanoparticle-modified epoxy matrices. J. Mater. Sci. 2017, 52, 1767–1788. [Google Scholar] [CrossRef]
- Rankin, S.M.; Moody, M.K.; Naskar, A.K.; Bowland, C.C. Enhancing functionalities in carbon fiber composites by titanium dioxide nanoparticles. Compos. Sci. Technol. 2021, 201, 108491. [Google Scholar] [CrossRef]
- Tang, Y.; Ye, L.; Zhang, Z.; Friedrich, K. Interlaminar fracture toughness and CAI strength of fibre-reinforced composites with nanoparticles—A review. Compos. Sci. Technol. 2013, 86, 26–37. [Google Scholar] [CrossRef]
- Gangineni, P.K.; Gupta K., B.N.V.S.G.; Patnaik, S.; Prusty, R.K.; Ray, B.C. Recent advancements in interface engineering of carbon fiber reinforced polymer composites and their durability studies at different service temperatures. Polym. Compos. 2022, 43, 4126–4164. [Google Scholar] [CrossRef]
- Hashim, U.R.; Jumahat, A.; Jawaid, M.; Dungani, R.; Alamery, S. Effects of Accelerated Weathering on Degradation Behavior of Basalt Fiber Reinforced Polymer Nanocomposites. Polymers 2020, 12, 2621. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L. Damage Monitoring of Structural Resins Loaded with Carbon Fillers: Experimental and Theoretical Study. Nanomaterials 2020, 10, 434. [Google Scholar] [CrossRef]
- Michael, M. On “Aesthetic Publics”. Sci. Technol. Hum. Values 2018, 43, 1098–1121. [Google Scholar] [CrossRef]
- Perovich, D.K. Light reflection and transmission by a temperate snow cover. J. Glaciol. 2007, 53, 201–210. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Ravi, R.P.; Udoh, I.I.; Mathew, G.M.; Rajan, T.P.D. Polymer-Based Coating for Steel Protection, Highlighting Metal–Organic Framework as Functional Actives: A Review. Corros. Mater. Degrad. 2023, 4, 284–316. [Google Scholar] [CrossRef]
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings: Science and Technology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9781119026891. [Google Scholar]
- Khanna, A.S. High-Performance Organic Coatings; Woodhead Publishing Limited: Cambridge, UK, 2008; ISBN 9781845694739. [Google Scholar]
- Meyer, R.W. Handbook of Polyester Molding Compounds and Molding Technology; Springer: Boston, MA, USA, 1987; ISBN 9781461319610. [Google Scholar]
- Pomázi, Á.; Toldy, A. Multifunctional Gelcoats for Fiber Reinforced Composites. Coatings 2019, 9, 173. [Google Scholar] [CrossRef]
- Lackner, J.; Waldhauser, W.; Major, L.; Kot, M. Tribology and Micromechanics of Chromium Nitride Based Multilayer Coatings on Soft and Hard Substrates. Coatings 2014, 4, 121–138. [Google Scholar] [CrossRef]
- Major, L.; Janusz, M.; Kot, M.; Lackner, J.M.; Major, B. Development and complex characterization of bio-tribological Cr/CrN + a-C:H (doped Cr) nano-multilayer protective coatings for carbon–fiber-composite materials. RSC Adv. 2015, 5, 9405–9415. [Google Scholar] [CrossRef]
- Kozlova, A.A.; Kondrashov, E.K.; Deev, I.S. Protective properties of paint and lacquer coatings based on a fluorine-containing film-forming material. Prot. Met. Phys. Chem. Surf. 2016, 52, 1181–1186. [Google Scholar] [CrossRef]
- Henke, M.; Lis, B.; Krystofiak, T. Mechanical and Chemical Resistance of UV Coating Systems Prepared under Industrial Conditions Using LED Radiation. Polymers 2023, 15, 4550. [Google Scholar] [CrossRef]
- Chandrababu, V.; Parameswaranpillai, J.; Gopi, J.A.; Pathak, C.; Midhun Dominic, C.D.; Feng, N.L.; Krishnasamy, S.; Muthukumar, C.; Hameed, N.; Ganguly, S. Progress in food packaging applications of biopolymer-nanometal composites—A comprehensive review. Biomater. Adv. 2024, 162, 213921. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Hu, Z.; Wu, D.; Chen, P. The influence of UV radiation and moisture on the mechanical properties and micro-structure of single Kevlar fibre using optical methods. Polym. Degrad. Stab. 2012, 97, 1755–1761. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Sampers, J.; Hutten, E.; Gijsman, P. Accelerated weathering of unsaturated polyester resins. Aspects of appearance change. Polym. Test. 2015, 44, 208–223. [Google Scholar] [CrossRef]
- Rumchev, K.; Brown, H.; Spickett, J. Volatile organic compounds: Do they present a risk to our health? Rev. Environ. Health 2007, 22, 39–55. [Google Scholar] [CrossRef]
- Pandey, P.; Yadav, R. A Review on Volatile Organic Compounds (VOCs) as Environmental Pollutants: Fate and Distribution. IJPE 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Di Tomasso, C.; József Gombos, Z.; Summerscales, J. Styrene emissions during gel-coating of composites. J. Clean. Prod. 2014, 83, 317–328. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Kumar, A.; Singh, V.; Chakraborty, B.; Kumar, R.; Min, L. Recent Advancement in Organic Aerosol Understanding: A Review of Their Sources, Formation, and Health Impacts. Water Air Soil Pollut 2023, 234, 750. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, X.; Yang, F.; Ye, J. Preparation and Characterization of Waterborne UV Lacquer Product Modified by Zinc Oxide with Flower Shape. Polymers 2020, 12, 668. [Google Scholar] [CrossRef]
- Pieters, K.; Mekonnen, T.H. Progress in waterborne polymer dispersions for coating applications: Commercialized systems and new trends. RSC Sustain. 2024, 2, 3704–3729. [Google Scholar] [CrossRef]
- Li, S.; Meng Lin, M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Haiden, L.; Brunner, A.J.; Pansare, A.V.; Feuchter, M.; Pinter, G. Tailoring the optical and UV reflectivity of CFRP-epoxy composites: Approaches and selected results. Sci. Eng. Compos. Mater. 2023, 30, 20220175. [Google Scholar] [CrossRef]
- Haiden, L.; Feuchter, M.; Brunner, A.J.; Pansare, A.; Barbezat, M.; Ravindran, B.; Terziyska, V.; Pinter, G. Altering the optical appearance of carbon reinforced polymers on an intrinsic level using Ag and SiO2. Proc. Inst. Mech. Eng. Part L 2025, 239, 733–745. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, H.; Tzounis, L.; Santagiuliana, G.; Bilotti, E.; Papageorgiou, D.G. Multifunctional epoxy nanocomposites reinforced by two-dimensional materials: A review. Carbon 2021, 185, 57–81. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Strømme, M.; Welch, K. Enhanced UV protection and water adsorption properties of transparent poly(methyl methacrylate) films through incorporation of amorphous magnesium carbonate nanoparticles. J. Polym. Res. 2021, 28, 281. [Google Scholar] [CrossRef]
- Stankic, S.; Müller, M.; Diwald, O.; Sterrer, M.; Knözinger, E.; Bernardi, J. Size-dependent optical properties of MgO nanocubes. Angew. Chem. Int. Ed. Engl. 2005, 44, 4917–4920. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, J. On the synthesis and optical absorption studies of nano-size magnesium oxide powder. J. Phys. Chem. Solids 2008, 69, 2764–2772. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Microstructural, optical and magnetic properties study of nanocrystalline MgO. Mater. Res. Express 2014, 1, 25026. [Google Scholar] [CrossRef]
- Zwijnenburg, M.A. The effect of particle size on the optical and electronic properties of magnesium oxide nanoparticles. Phys. Chem. Chem. Phys. 2021, 23, 21579–21590. [Google Scholar] [CrossRef]
- Reiling, G.H.; Hensley, E.B. Fundamental Optical Absorption in Magnesium Oxide. Phys. Rev. 1958, 112, 1106–1111. [Google Scholar] [CrossRef]
- Roessler, D.M.; Walker, W.C. Electronic Spectrum and Ultraviolet Optical Properties of Crystalline MgO. Phys. Rev. 1967, 159, 733–738. [Google Scholar] [CrossRef]
- Chayed, N.F.; Badar, N.; Rusdi, R.; Kamarudin, N.; Kamarulzaman, N. Optical Band Gap Energies of Magnesium Oxide (MgO) Thin Film and Spherical Nanostructures. AIP Conf. Proc. 2011, 1400, 328–332. [Google Scholar] [CrossRef]
- Townsend, J.R. Solid-State Absorption Spectra of Mg and MgO. Phys. Rev. 1953, 92, 556–560. [Google Scholar] [CrossRef]
- Cohen, M.L.; Lin, P.J.; Roessler, D.M.; Walker, W.C. Ultraviolet Optical Properties and Electronic Band Structure of Magnesium Oxide. Phys. Rev. 1967, 155, 992–996. [Google Scholar] [CrossRef]
- de Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M.N. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium Oxide (MgO) Nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Han, S.; Zhang, J.; Qi, Y. A novel comprehensive composite material for auxiliary solar photovoltaic double-sided power generation: Combining reflective cooling, flame retardant, and smoke suppressant properties. Mater. Today Commun. 2025, 44, 112087. [Google Scholar] [CrossRef]
- Sun, H.; Qi, Y.; Zhang, J. Effect of magnesium hydroxide as a multifunctional additive on high solar reflectance, thermal emissivity, and flame retardancy properties of PP/SEBS/oil composites. Polym. Compos. 2020, 41, 4010–4019. [Google Scholar] [CrossRef]
- Hagemann, H.-J.; Gudat, W.; Kunz, C. Optical constants from the far infrared to the x-ray region: Mg, Al, Cu, Ag, Au, Bi, C, and Al2O3. J. Opt. Soc. Am. 1975, 65, 742–744. [Google Scholar] [CrossRef]
- Kroger, H.; Tomboulian, D.H. Far Ultraviolet Absorption Spectrum of Magnesium. Phys. Rev. 1963, 130, 152–154. [Google Scholar] [CrossRef]
- Sabine, G.B. Reflectivities of Evaporated Metal Films in the Near and Far Ultraviolet. Phys. Rev. 1939, 55, 1064–1069. [Google Scholar] [CrossRef]
- Vidal-Dasilva, M.; Aquila, A.L.; Gullikson, E.M.; Salmassi, F.; Larruquert, J.I. Optical constants of magnetron-sputtered magnesium films in the 25–1300 eV energy range. J. Appl. Phys. 2010, 108, 063517. [Google Scholar] [CrossRef]
- O’Bryan, H.M. The Optical Constants of Several Metals in Vacuum*. J. Opt. Soc. Am. 1936, 26, 122–127. [Google Scholar] [CrossRef]
- Nam, K.H.; Han, J.G. Microstructure and optical properties of MgO films synthesized by closed-field unbalanced magnetron sputtering with additional electron emission. Surf. Coat. Technol. 2003, 171, 51–58. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Ab Aziz, S.; Abbas, Z.; Obaiys, S.J.; Khamis, A.M.; Hussain, I.R.; Zaid, M.H.M. Preparation of a Chemically Reduced Graphene Oxide Reinforced Epoxy Resin Polymer as a Composite for Electromagnetic Interference Shielding and Microwave-Absorbing Applications. Polymers 2018, 10, 1180. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Schönberger, W.; Bartzsch, H.; Schippel, S.; Bachmann, T. Deposition of rutile TiO2 films by pulsed and high power pulsed magnetron sputtering. Surf. Coat. Technol. 2016, 293, 16–20. [Google Scholar] [CrossRef]
- Radhakrishnan, T. The optical properties of titanium dioxide. Proc. Indian Acad. Sci. (Math. Sci.) 1952, 35, 117–125. [Google Scholar] [CrossRef]
- Soussi, A.; Ait Hssi, A.; Boujnah, M.; Boulkadat, L.; Abouabassi, K.; Asbayou, A.; Elfanaoui, A.; Markazi, R.; Ihlal, A.; Bouabid, K. Electronic and Optical Properties of TiO2 Thin Films: Combined Experimental and Theoretical Study. J. Electron. Mater. 2021, 50, 4497–4510. [Google Scholar] [CrossRef]
- Zywitzki, O.; Modes, T.; Sahm, H.; Frach, P.; Goedicke, K.; Glöß, D. Structure and properties of crystalline titanium oxide layers deposited by reactive pulse magnetron sputtering. Surf. Coat. Technol. 2004, 180–181, 538–543. [Google Scholar] [CrossRef]
- Skowronski, L.; Chodun, R.; Zdunek, K. TiO2—based decorative interference coatings produced at industrial conditions. Thin Solid Film. 2020, 711, 138294. [Google Scholar] [CrossRef]
- Skowroński, Ł.; Trzcinski, M.; Antończak, A.J.; Domanowski, P.; Kustra, M.; Wachowiak, W.; Naparty, M.K.; Hiller, T.; Bukaluk, A.; Wronkowska, A.A. Characterisation of coloured TiO /Ti/glass systems. Appl. Surf. Sci. 2014, 322, 209–214. [Google Scholar] [CrossRef]
- Lin, Y.-C. Novel Optical Thin Film Color Filter: Simulation and Experiment. Chin. J. Physic 2012, 50, 643–651. [Google Scholar]
- Soled, S.; Wachter, W.; Wo, H. Use of zeta potential measurements in catalyst preparation. In Studies in Surface Science and Catalysis: Scientific Bases for the Preparation of Heterogeneous Catalysts; Gaigneaux, E.M., Devillers, M., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 101–107. ISBN 0167-2991. [Google Scholar]
- Lin, J.X.; Wang, L. Adsorption of dyes using magnesium hydroxide-modified diatomite. Desalination Water Treat. 2009, 8, 263–271. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R.; Sarkar, P.; Nicholson, P.S. Electrophoretic deposition of MgO from organic suspensions. J. Eur. Ceram. Soc. 2000, 20, 99–106. [Google Scholar] [CrossRef]
- Robinson, M.; Pask, J.A.; Fuerstenau, D.W. Surface Charge of Alumina and Magnesia in Aqueous Media. J. Am. Ceram. Soc. 1964, 47, 516–520. [Google Scholar] [CrossRef]
- Swann, S. Film thickness distribution in magnetron sputtering. Vacuum 1988, 38, 791–794. [Google Scholar] [CrossRef]
- Ozimek, M.; Wilczyński, W.; Szubzda, B. Magnetic thin film deposition with pulsed magnetron sputtering: Deposition rate and film thickness distribution. IOP Conf. Ser. Mater. Sci. Eng. 2016, 113, 12009. [Google Scholar] [CrossRef]
- Dekermenjian, M.; Ruediger, A.P.; Merlen, A. Raman spectroscopy investigation of magnesium oxide nanoparticles. RSC Adv. 2023, 13, 26683–26689. [Google Scholar] [CrossRef]
- Morozov, I.; Sathasivam, S.; Belousova, O.V.; Parkin, I.P.; Kuznetcov, M.V. Effect of synthesis conditions on room-temperature ferromagnetic properties of Mg-O nanoparticles. J. Alloys Compd. 2018, 765, 343–354. [Google Scholar] [CrossRef]
- Brubaker, Z.E.; Langford, J.J.; Kapsimalis, R.J.; Niedziela, J.L. Quantitative analysis of Raman spectral parameters for carbon fibers: Practical considerations and connection to mechanical properties. J. Mater. Sci. 2021, 56, 15087–15121. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haiden, L.; Feuchter, M.; Brunner, A.J.; Barbezat, M.; Pansare, A.; Ravindran, B.; Terziyska, V.; Pinter, G. Shining a Light on Carbon-Reinforced Polymers: Mg/MgO and TiO2 Nanomodifications for Enhanced Optical Performance. J. Compos. Sci. 2025, 9, 187. https://doi.org/10.3390/jcs9040187
Haiden L, Feuchter M, Brunner AJ, Barbezat M, Pansare A, Ravindran B, Terziyska V, Pinter G. Shining a Light on Carbon-Reinforced Polymers: Mg/MgO and TiO2 Nanomodifications for Enhanced Optical Performance. Journal of Composites Science. 2025; 9(4):187. https://doi.org/10.3390/jcs9040187
Chicago/Turabian StyleHaiden, Lukas, Michael Feuchter, Andreas J. Brunner, Michel Barbezat, Amol Pansare, Bharath Ravindran, Velislava Terziyska, and Gerald Pinter. 2025. "Shining a Light on Carbon-Reinforced Polymers: Mg/MgO and TiO2 Nanomodifications for Enhanced Optical Performance" Journal of Composites Science 9, no. 4: 187. https://doi.org/10.3390/jcs9040187
APA StyleHaiden, L., Feuchter, M., Brunner, A. J., Barbezat, M., Pansare, A., Ravindran, B., Terziyska, V., & Pinter, G. (2025). Shining a Light on Carbon-Reinforced Polymers: Mg/MgO and TiO2 Nanomodifications for Enhanced Optical Performance. Journal of Composites Science, 9(4), 187. https://doi.org/10.3390/jcs9040187








