Abstract
Today, flexible and lightweight electronics are regarded as a viable alternative to conventional rigid and heavy devices in various application fields. In the optoelectronic area, organic semiconductors offer advantages such as high absorption coefficients, low processing temperatures, mechanical flexibility and compatibility with plastic substrates, while inorganic nanostructures provide good electronic properties and high thermal stability. Thus, composite films with enhanced properties can be achieved by inserting inorganic nanostructures within organic layers. In this research work, CuS nanoparticles were prepared by wet chemical precipitation and then added to an organic mixture containing poly(3-hexylthiophene) (P3HT) and N,N-bis-(1-dodecyl)perylene-3,4,9,10 tetracarboxylic diimide (AMC14), a chemically synthesized semiconductor, for fabricating hybrid composite films by matrix assisted pulsed laser evaporation (MAPLE) on indium tin oxide/poly(ethylene terephthalate) (ITO/PET) flexible substrates. A comparative assessment of the morphological, compositional, optical and electrical properties of the composite (P3HT:AMC14:CuS) and organic (P3HT:AMC14) layers was performed to evaluate their applicability in the photovoltaic cells. The transmission and emission spectra of the composite films are dominated by the optical features of AMC14, a perylene diimide derivative compound used as acceptor. In the case of devices based on MAPLE deposited composite layer fabricated on ITO/PET substrates, the electrical measurements carried under illumination revealed an improvement in the open circuit voltage parameter emphasizing their potential applications in the flexible device area.
1. Introduction
The accelerated pace of technological development in the flexible electronic area has a huge implication on our everyday lives. Devices capable of bending, folding and rolling find applications in various fields ranging from smartphones, TV displays, wearable health sensors to solar cells. In the last decade, organic photovoltaic cells (OPV) and hybrid photovoltaic cells (HPV) have been regarded as inexpensive alternative to conventional rigid technology, the most common being that based on silicon [1,2,3]. HPV systems were developed with the goal of achieving better performance by merging properties like high absorption coefficients, low processing temperatures, mechanical flexibility and compatibility with plastic substrates of the organic compounds with the good electronic properties and high thermal stability of the inorganic materials [2,4,5]. Thus, the inorganic component can be inserted either as a distinct layer or embedded as nanoparticles in an organic matrix [5], the presence of the inorganic materials augmenting the performance of the photovoltaics cells regardless of the device configuration [5,6]. The bulk heterojunction (BHJ) concept facilitated the fabrication of hybrids by dispersing inorganic nanostructures in an organic active layer, both constituents being combined in a solution and deposited as a single film coating [7]. These composite layers are featured by a large donor–acceptor (D:A) interface, which enhances the probability of generating excitons that further dissociate in free-charge carriers [7]. The inorganic nanoparticles added in D:A organic blend can also favor the transfer between the organic parts resulting in an increase in the device performance [8,9].
Poly(3-hexylthiophene) (P3HT) is the most widely used polymer as a donor in the OPV and HPV cells. P3HT is an affordable organic semiconductor characterized by a low energy gap (~1.9 eV) and a high hole mobility (exceeding 0.1 cm2/Vs) [10,11]. Usually, in blends with P3HT, fullerene derivatives such as [6,6]-phenyl-C-61-butyric acid methyl ester (PC60BM) and [6,6]-phenyl-C-71-butyric acid methyl ester (PC70BM) are applied as acceptors [12]. Lately, devices with high efficiencies (over 18%) have been obtained by mixing P3HT with non-fullerene acceptors (NFA), compounds that exhibit strong, broad absorption (from visible to near-infrared domain) and adjustable energy levels [13,14,15].
Low-cost metal chalcogenides, e.g., metal sulfides (PbS, SnS2, FeS2, Mn-doped ZnS, Cu2S and CuS) [8,9,16,17,18,19,20], metal selenides (ZnSe, CdSe and PbSe) [21,22,23] and metal tellurides (CdTe) [24] have been recently inserted in different organic compounds for developing hybrid active layers. Among metal chalcogenides, copper sulfides are regarded as promising optoelectronic materials due to their non-toxic nature, high abundance, low price, high thermal stability, and good electrical parameters [8,9,20]. In addition, the energy gap of copper sulfides (Cu2−xS) can vary from ~1.2 to ~2.8 eV depending on the stoichiometric composition (0 < x < 1) [25,26], Cu2S and CuS being the most investigated compounds. Moreover, the high extinction coefficient of the copper sulfides [27] endorses them as light absorbers in photovoltaic devices. Accordingly, Cu2S nanocrystals were embedded in blends based on poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b;4,5-b′]dithiophene-2,6-diyl-alt-(4-(2-ethylhexyl)-3-fluorothieno[3,4-b]thiophene-)-(2-carboxylate-2-6-diyl)] (PTB7-Th) and a fullerene derivative compound, phenyl-C71-butyric acid methyl ester (PC71BM), the high charge carrier mobility and high electron affinity of the semiconducting nanostructures boosting the device performance [9]. Also, Cu2S nanocrystals were added in blends based on (poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl) benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))] (PBDB-T) and a small molecule non-fullerene compound, 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene) (ITIC), the conduction band of Cu2S being situated between the lowest energy molecular orbital of donor polymer (PBDB-T) and fullerene free acceptor (ITIC) [8]. CuS has also received special attention because its bandgap can be tuned by tailoring the morphology of its 0D, 1D, 2D, and 3D structures (particle, plate, rod, disk, flower, flake, tube, sphere, wire, etc.) [28,29,30]. Despite this, few studies have focused on improving solar cell performance by embedding CuS nanoparticles or CuS nanoplates into photoactive organic blend layers based on P3HT and phenyl-C61-butyric acid methyl (PC61BM) [20,31].
The aim of this research work is to assess in a comparative manner the properties of hybrid composite and organic layers deposited by a laser technique on flexible substrates. Thus, CuS nanoparticles were prepared by wet chemical precipitation and then incorporated in an organic mixture containing P3HT and N,N′-bis-(1-dodecyl)perylene-3,4,9,10 tetracarboxylic diimide (AMC14), a chemically synthesized semiconductor, in order to obtain composite films. In the hybrid blends, P3HT and CuS nanoparticles act as donors while AMC14 is acceptor. AMC14 is a perylene diimide derivative (PDI) compound, which displays a strong absorption in the visible solar spectrum [32]. At the laboratory level, inexpensive spin-coating technique is the most broadly used in the deposition of organic and inorganic:organic films. However, this method involves (i) highly concentrated solution, most of it (~95%) being discarded during the coating procedure, (ii) substrates with particular wettability and (iii) solvent with specific thermodynamic properties, which can hinder the deposition of photoactive layers on flexible substrates [5,33,34]. Hence, in the present study, the composite films were deposited by matrix assisted pulsed laser evaporation (MAPLE) on indium tin oxide/poly(ethylene terephthalate) (ITO/PET) flexible substrates. MAPLE is derived from the pulsed laser deposition (PLD) technique for obtaining films from low amount of “soft” materials (usually 1–5% mass concentration) even on plastic flexible substrates [5,35]. In addition, the chemical structure of the organic molecules intended to be deposited as layers can be preserved by tuning the laser energy during the MAPLE process [36]. Although hybrid blends based on inorganic nanoparticles embedded in an organic matrix have been previously achieved by MAPLE on rigid glass substrates for photovoltaic devices [37,38], to our knowledge, there are no reports on the fabrication of such composite layers on flexible (plastic) substrates.
2. Experimental
2.1. Materials
ITO/PET flexible substrates (14 Ω/sq resistivity) were acquired from Solaronix. Prior coating, ITO/PET substrates were cleaned with Hellmanex (Ossila). P3HT (97.3%) was bought from Ossila (Sheffield, UK). The organic reagents used in the chemical synthesis of AMC14, i.e., perylene-3,4,9,10-tetracarboxylic dianhydride (97%), dodecylamine (99%), acetic acid (99.5%) and N-methyl-2-pyrrolidone anhydrous (99.5%) were provided by Sigma-Aldrich (St. Louis, MO, USA). The chlorobenzene (99.8%) used as solvent in the fabrication of the MAPLE frozen target was also furnished by Sigma-Aldrich. The copper and sulfur source precursors involved in the chemical synthesis of CuS nanoparticles, i.e., copper acetate (98%) and sodium thiosulfate (98%) were purchased from Merck (Darmstadt, Germany) and used without further purification.
2.2. Methods
2.2.1. Chemical Synthesis of the AMC14 (Perylene Diimide Derivative Compound) and CuS Nanoparticles
AMC14 was synthetized via a chemical route detailed in the reference [32]. Accordingly, the chemical reaction takes place between perylene-3,4,9,10-tetracarboxylic dianhydride (2 g, 0.005 mol) and dodecylamine (2.07 g, 0.01 mol) in the presence of 1 mL acetic acid and 30 mL N-methyl-2-pyrrolidone. The synthesis was carried out in a round-bottomed flask under magnetic stirring at 85 °C for 8 h in argon flow, the mixture being then cooled at room temperature. The bordeaux-red precipitate was separated by filtration, washed with methanol and dried in vacuum.
CuS nanoparticles were prepared by modifying the wet chemical precipitation approach given in the reference [39]. The reaction between Cu(CH3COO)2 and Na2S2O3 with 1:7.5 weight ratio was performed in water under vigorous stirring at 90 °C for 6 h. The black precipitate was collected by centrifugation, washed with distilled water and absolute ethanol and finally dried at room temperature.
2.2.2. Fabrication of the Flexible Heterostructures Based on MAPLE Deposited Organic (P3HT:AMC14) and Composite (P3HT:AMC14:CuS) Layers
The organic (P3HT:AMC14) and hybrid composite (P3HT:AMC14:CuS) films were deposited by MAPLE using a KrF* excimer laser source (Coherent, CompexPro 205, λ = 248 nm, τFWHM ~25 ns) working at room temperature and the following experimental parameters: 300 mJ/cm2 laser fluence, 20 Hz frequency, 10−4 mbar residual pressure in the deposition chamber, 5 cm target–substrate distance and 25 k laser pulses. More information on the MAPLE technique can be found in the references [40,41]. For a thorough assessment, the layers were deposited on flexible (ITO/PET) and rigid (glass and silicon) substrates in the same deposition cycle being rotated to improve the thickness homogeneity. Both organic materials (P3HT and AMC14) exhibit good solubility in chlorobenzene (CB). Therefore, this chlorinated aromatic compound was used as solvent to obtain the frozen targets (4.5% w/v) used in the MAPLE process. Although P3HT is mainly soluble in chloroform and CB, according to the literature, CB favors the preparation of films with adequate morphology [42]. Another criterion for choosing CB is correlated to the laser wavelength: usually, the laser energy is mostly absorbed by the solvent during the MAPLE procedure [43]. The amount of CuS nanoparticles added to the active layer was selected taking into consideration the very few studies previously reported on the deposition of blend films based on P3HT and metal sulfide nanostructures for photovoltaic applications [20,31,44,45]. Hence, the best device parameters were achieved by adding 1% CuS nanoplates with diameters between 30 and 680 nm in P3HT:PCBM (1:1 weight ratio) [31], 1% FeS2 nanoparticles with sizes between 20 and 30 nm in P3HT [44], 3% CuS nanoparticles with different shapes and sizes in P3HT:PCBM (1:1 weight ratio) [20] or 10% ZnS nanowires with diameters about 10 ± 20 nm and length up to 100 ± 150 nm in P3HT [45]. As a consequence, the frozen targets involved in the MAPLE process were prepared by freezing in liquid nitrogen the mixtures obtained in CB based on P3HT:AMC14 (1:1 weight ratio) or P3HT:AMC14:CuS (1:1:0.1 weight ratio). Further, metallic electrodes (Al, 100 nm) were deposited by thermal vacuum evaporation using a Tecuum AG (Winterthur, Switzerland), VCM600-V3-80 setup (2Å/s deposition rate) on the organic and hybrid MAPLE layers fabricated on ITO/PET flexible substrates. Figure 1 presents the schematic representation of the stages involved in the procedure for getting the MAPLE frozen target (Figure 1a) and the configuration of the developed heterostructures based on hybrid composite layers (Figure 1b).
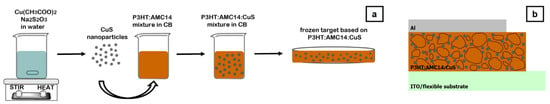
Figure 1.
Schematic representation of the stages implicated in the fabrication of the MAPLE frozen target (a) and the developed heterostructures based on MAPLE hybrid composite layers (b).
2.3. Characterization Techniques
The thickness of the MAPLE layers on ITO/PET substrates was evaluated as the average of three scans in various points employing an Ambios Technology XP 100 profilometer (Ambios Technology, Inc., SantaCruz, CA, USA).
The diffraction (XRD) pattern of the chemically synthesized copper sulfide nanoparticles was collected between 15° and 70° by a Bruker D8 Advance setup with a Bragg–Brentano geometry and having Cu Kα1 (λ = 1.54056 Å) monochromatized radiation.
The vibrational and optical properties of the MAPLE films on various substrates (silicon, glass and ITO/PET) were analyzed. The infrared (FTIR) spectra were recorded between 3100 cm−1 and 500 cm−1 using a Shimadsu IRTracer 100 spectrometer (Shimadzu, Kyoto, Japan). The transmission (UV–Vis) spectra were acquired between 200 nm and 1100 nm on a Thermo Scientific Evolution 220 spectrophotometer (Thermo Scientific, Schwerte, Germany). The photoluminescence (PL) spectra were obtained between 450 nm and 850 nm using 435 nm excitation wavelength with an FL 920 Edinburgh Instruments spectrometer (Edinburgh Instruments Ltd., Livingston, UK) having a 450 W Xe lamp excitation source and double monochromators on both excitation and emission.
The surface morphology and the elemental composition of the MAPLE films on silicon were investigated by a Zeiss Merlin Compact field emission scanning electron microscope (FESEM) and a Zeiss Gemini SEM 500 field emission scanning electron microscope (FESEM) (Zeiss, Oberkochen, Germany) having an energy dispersive X-ray analysis (EDX) Quantax Bruker XFlash detector 610 M (Billerica, MA, USA) as accessory. Also, the topography and the surface roughness parameters of the MAPLE layers on ITO/PET substrates were evaluated with a Multiview 4000 Nanonics system (Nanonics, Jerusalem, Israel) atomic force microscope (AFM) working in the intermittent mode.
The electrical measurements were carried out on heterostructures based on MAPLE layers fabricated on ITO/PET flexible substrates (see Figure 1b), the current-voltage (J-V) characteristics being recorded at room temperature under illumination using an experimental setup consisting in a LOT-Oriel solar simulator (AM 1.5), a Newport Oriel monochromator and a Keithley SourceMeter 2400 model (Cleveland, OH, USA).
3. Results and Discussion
3.1. Morphological and Structural Properties of the CuS Nanoparticles
Copper sulfide synthesized by wet chemical precipitation was investigated from morphological and structural point of view. The FESEM images (Figure 2a,b) reveal that the prepared black powder consists in nanoparticles with sizes below 100 nm and agglomerated nanoparticles. The XRD pattern (Figure 2c) shows the main peaks associated with the Miller indexes of the reflecting planes attributed to the CuS hexagonal covellite phase (ICDD 00-006-0464). The crystallite size (D) of the CuS nanoparticles was calculated to be around 23 nm based on Debye–Scherrer formula D = Kλ/βcosθ, where K is the shape factor (0.9), λ is the wavelength of the incident radiation (0.154 nm), β is the full width at half maximum (FWHM) of the most intense diffraction peak, and θ is the Bragg angle.
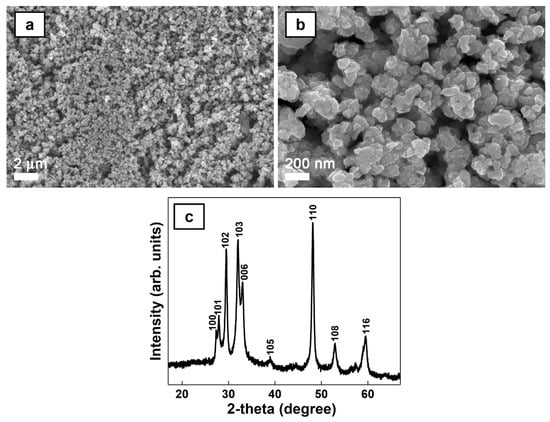
Figure 2.
FESEM images at two magnifications (a,b) and XRD pattern (c) of the chemically synthetized CuS nanoparticles.
3.2. Characterization of the MAPLE Deposited Films
The CuS nanoparticles were further embedded in the P3HT:AMC14 blends to achieve the P3HT:AMC14:CuS composite layers using the MAPLE technique. Concerning the thickness values of the MAPLE films, as was expected the P3HT:AMC14:CuS samples (690 nm) are thicker than the P3HT:AMC14 samples (610 nm). It must be mentioned that in the case of PDI compounds like AMC14, the molecules prone to be disposed tilted rather than parallel to the surface of the substrate [46].
3.2.1. Vibrational, Transmission and Photoluminescence Properties
FTIR spectroscopy was used to confirm the preservation of the chemical structure of the organic molecules during the laser deposition. The vibrational signature of the P3HT and AMC14 can be recognized in the FTIR spectra of the organic blend and composite layers (Figure 3). The distinctive absorption bands of the P3HT are observed: thiophene ring C–H out-of-plane bending vibration at 830 cm−1, C–C ring stretching at 1453 cm−1, quinoid unit C=C vibration at 1511 cm−1 [47,48]. The specific absorption bands of the AMC14 are remarked: C-H bend at 809 cm−1, C-C bend at 1250 cm−1 and 1076 cm−1, C-N stretching at 1348 cm−1, aromatic C=C stretching at 1592 cm−1, C=O imide in-plane and out-of-plane asymmetric stretching at 1695 cm−1 and 1655 cm−1 [49,50]. The higher-frequency bands assigned to the common functional groups like C–H, the thienyl ring aromatic stretching vibration at 3055 cm−1, –CH3 and –CH2– stretching vibrations at 2930 cm−1 and 2851 cm−1 can be related to both organic compounds, P3HT and AMC14. CuS vibrational fingerprint located at 663 cm−1, which is associated with the Cu–S bond stretching mode [51] is visible in the case of hybrid composite sample. Usually, the solvent used to prepare the frozen target is pumped out during MAPLE deposition. However, in the present case, the CB fingerprint is identified in the investigated samples through the particular C-Cl vibration bands situated at 700–760 cm−1 [52]. Considering that in a study previously reported, no solvent traces were evidenced for P3HT films deposited by MAPLE from toluene [53], the traces of CB in the MAPLE samples can be linked to the lower vapor pressure of CB (1.2 kPa at 25 °C) compared to that of the toluene (2.9 kPa at 25 °C) [54].
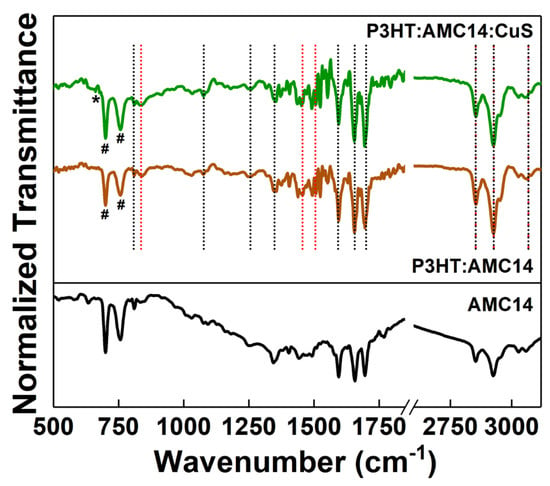
Figure 3.
FTIR spectra of the organic and composite layers prepared by MAPLE on silicon substrates. Absorption bands attributed to the P3HT (red vertical dot lines), AMC14 (black vertical dot lines), CuS (*) and CB (#).
The optical properties of the organic and hybrid composite films deposited by MAPLE were analyzed by UV–Vis and PL (Figure 4). To avoid the influence of the ITO/PET substrate on the optical properties of the MAPLE layers, the measurements were initially carried out on samples prepared on glass (transmission spectra) and silicon (PL spectra) substrates. It can be noted that the shape of the UV–Vis and PL spectra of the organic blend and composite films resembles, most probably due to the smaller amount of CuS nanoparticles. Thus, the UV–Vis spectra of the mixed layers (Figure 4a) are dominated by the characteristic absorption bands of the AMC14. Accordingly, the UV–Vis spectra show the absorption bands due to the 0→0 transition at ~540 nm, 0→1 transition at ~495 nm and 0→2 transition at ~470 nm, which can be correlated with individual molecules and π–π stacking interactions inside the PDI assembly [55,56,57]. Regarding the P3HT, this compound exhibits a large absorption band between 400 nm and 650 nm with two maxima at ~605 nm and ~560 nm [58]. In the UV–Vis spectra of the mixed layers, the P3HT absorption is not observed, most likely being hidden by the strong visible absorption of the AMC14. However, the presence of the P3HT can modify the molecular packing conformation of the PDI compound, resulting in an attenuation and shift towards lower wavelengths of the specific absorption bands of the AMC14. According to the literature, the PDI compounds tend to form aggregates, the process being strongly influenced by the type of organic solvent used in their dissolution [57,59].
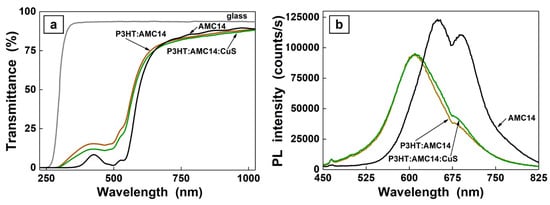
Figure 4.
UV–Vis (a) and PL (b) spectra of the organic and composite layers prepared by MAPLE on glass (UV–Vis) and silicon (PL) substrates.
In the PL spectra of the mixed layers (Figure 4b), the typical emission band of the AMC14 with maxima at ~650 nm and ~690 nm [60] can be clearly recognized. Similar to the UV–Vis spectra, the emission band of the P3HT with a maximum at ~650 nm and a shoulder at ~700 nm [48] can be hidden by the large and intense emission band of the AMC14. Yet, the presence of the P3HT in the mixed layers can lead to changes in the intensity ratio of the two maxima, the enlargement, the blue shift and the quenching of the emission band of the AMC14. The films based on PDI compounds can disclose a blue-shift effect, which is usually linked to the formation of some J-aggregates due to the “head-to-tail” configuration of the transition dipoles, i.e., the chromophores from the neighboring molecules tend to interact and lead to specific aggregation patterns [61]. The quenching process suggests that an efficient charge transfer appears between the donor (P3HT) and the acceptor (PDI derivative), this effect being in accordance with those previously reported on blends based on P3HT and other PDI compounds [62,63].
3.2.2. Morphological, Compositional and Surface Topography Properties
The FESEM images and the EDX spectra of the MAPLE mixed layers (Figure 5) reveal almost the same morphology consisting in the appearance of some particles on the surface of the prepared films, this being the particular feature of the MAPLE deposition method [64]. The EDX data display the signals corresponding to C and O (P3HT and AMC14), N (AMC14), S (P3HT and CuS nanoparticles), Cl (CB traces—in agreement with FTIR spectra), Cu (CuS nanoparticles) and Si (substrate) confirming the presence of the inorganic nanoparticles in the composite films.
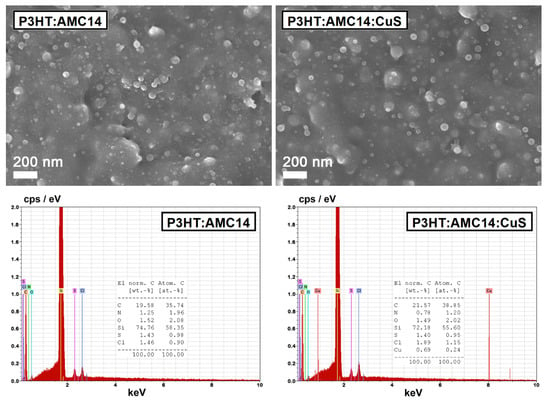
Figure 5.
FESEM images and EDX spectra of the organic and composite layers prepared by MAPLE on silicon substrates. The weight and the atomic percentages of the elements from the corresponding samples are also provided.
Based on the AFM images recorded on the MAPLE deposited mixed layers (Figure 6), the roughness parameters (root mean square—RMS/roughness average—Ra) were assessed at 58 nm/45 nm for P3HT:AMC14 organic film and 58 nm/46 nm for P3HT:AMC14:CuS composite film. As can be remarked, both types of samples present the same RMS values (58 nm) and very close Ra values (45 and 46 nm, respectively). Thus, the estimated values suggest that the influence of the CuS nanoparticles embedded in the organic blend is negligible in respect to the arrangement of the organic molecules on the substrate surface. A similar result obtained for the MAPLE hybrid coatings based on CoPc:C60:ZnO [65] emphasizes that the addition of a small amount of inorganic nanoparticles into an organic matrix does not affect the roughness parameters.
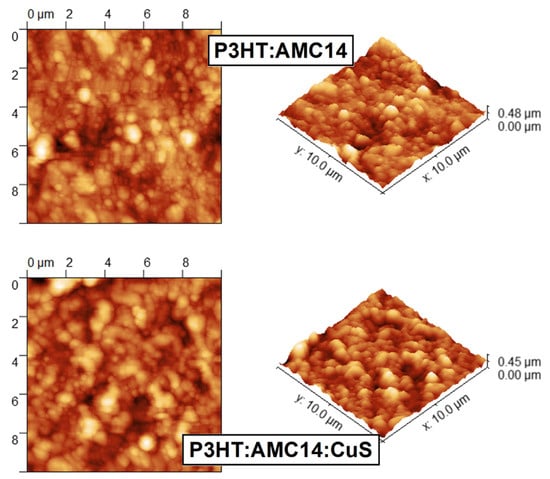
Figure 6.
AFM images (2D and 3D) of the organic and composite layers prepared by MAPLE on ITO/PET substrates.
3.3. Electrical Assessment of the Flexible Devices Based on MAPLE Deposited Films
The UV–Vis spectra of the organic and hybrid active films obtained by MAPLE on ITO/PET flexible substrates, the J-V characteristics plotted under illumination for the heterostructures containing MAPLE deposited layers and the schematic energy level diagram of the compounds used in their fabrication were presented in Figure 7. In the UV–Vis spectra of the MAPLE films deposited on ITO/PET (Figure 7a), the typical absorption bands of the acceptor compound (AMC14) can be identified. In addition, it can be noted the impact of the substrate transmittance on the optical properties of the mixed films taken into consideration that the layers were prepared on both substrates, glass (Figure 4a) and ITO/PET (Figure 7a) in the same experimental cycle. In terms of electrical performances, both heterostructures present photovoltaic effect (Figure 7b). The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) levels of the third component (CuS) should be positioned between the main donor (P3HT) and acceptor (AMC14) in order to achieve a cascade energy level configuration, as can be seen in the schematic energy level diagram of all compounds from the investigated structure (Figure 7c).
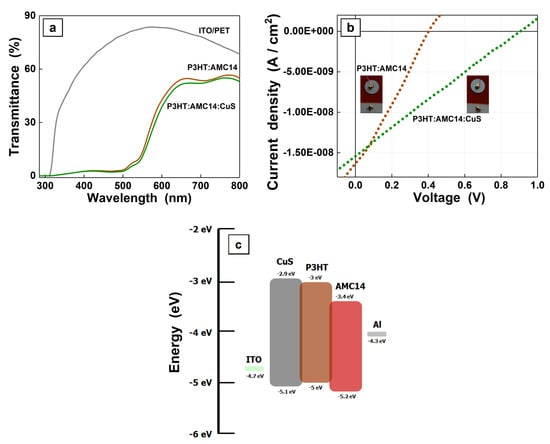
Figure 7.
UV–Vis spectra of the active layers prepared by MAPLE on ITO/PET substrates (a), J-V characteristics recorded under illumination of the flexible heterostructures based on MAPLE deposited organic and composite layers (b) and schematic energy level diagram of all components from the investigated composite structure (c).
Thus, from energetic point of view, HOMO and LUMO of the components from the composite samples are the follows: HOMOP3HT = 5 eV and LUMOP3HT = 3 eV [45], HOMOAMC14 = 5.2 eV and LUMOAMC14 = 3.4 eV [66] and HOMOCuS = 5.1 eV and LUMOCuS = 2.9 eV [67]. As can be remarked, the position of the HOMO levels of the compounds favors an efficient hole transport. Considering the position of the LUMO levels, the donor P3HT can transfer an electron either toward the acceptor AMC14 or toward the third component CuS. Further, CuS can transfer an electron toward the acceptor AMC14 while a hole is transferred toward the P3HT donor [68]. A substantial improvement was observed in the open circuit voltage (VOC) value, i.e., VOC = 0.9 V for structure based on P3HT:AMC14:CuS composite layer in comparison to VOC = 0.4 eV for structure based on P3HT:AMC14 organic blend layer.
Therefore, it can be assumed that CuS plays a significant role in the charge transfer mechanism, an effective charge transfer taking place between the organic compounds and inorganic nanoparticles, assuring the charge carrier path towards electrodes and the electrical performances of the photovoltaic structures. Also, a potential contribution to the enhancement of the VOC value can be attributed to the slightly increased of the CuS/acceptor bandgap (ELUMO(AMC14)-EHOMO(CuS) = 1.7 eV) compared with the D/A bandgap (ELUMO(AMC14)-EHOMO(P3HT) = 1.6 eV) because a higher bandgap value can be correlated with a decrease in losses determined by the recombination currents.
It has to be noted that a similar result, meaning higher VOC value for a specific amount of inorganic nanostructures embedded in an organic matrix, was reported for structures based on composite films containing (i) P3HT:PCBM:CuS nanoparticles (VOC = 0.57 V) [20], (ii) P3HT:FeS2 nanoparticles (VOC = 0.23 V) [44] and (iii) P3HT:ZnS nanowires (VOC = 0.63 V) [45], but these layers were made by spin-coating on rigid glass substrates. In addition, some studies show that a higher concentration of inorganic nanostructures in the composite layers can lower the electrical parameters of the fabricated devices due to an aggregation effect [44,45]. Consequently, the addition of a small amount of CuS p-type semiconductor in the P3HT:AMC14 blend allows the preparation of composite films with suitable properties for the targeted application, i.e., photovoltaic devices. Furthermore, the results acquired in this study endorse the potential use of the MAPLE hybrid composite films deposited on ITO/PET substrates in the flexible device area.
4. Conclusions
Films based on P3HT:AMC14:CuS and P3HT:AMC14 were deposited by MAPLE on ITO/PET flexible substrates and thoroughly characterized. Thus, hexagonal covellite CuS nanoparticles (crystallite size of ~23 nm) were obtained by wet chemical precipitation and subsequently added in an organic blend containing P3HT and AMC14, a chemically synthesized semiconductor compound. Further, the organic and composite layers were deposited by MAPLE from frozen targets by using chlorobenzene as solvent and a weight ratio of 1:1 for P3HT:AMC14 and 1:1:0.1 for P3HT:AMC14:CuS. The FESEM images of the MAPLE deposited mixed films evidenced a typical MAPLE morphology consisting in the formation of some particles on the surface of the prepared layers. The EDX data revealed the signal of Cu element confirming the presence of the CuS nanoparticles in the composite films. The UV–Vis and PL spectra were dominated by the optical features of the AMC14, the PDI compound being used as acceptor in the active layers. The electrical measurements performed under illumination evidenced that the heterostructures based on MAPLE composite films developed on flexible substrates exhibit photovoltaic effect. The J-V characteristics showed that the VOC value of the fabricated device can be enhanced by embedding a small amount of CuS nanoparticles in the P3HT:AMC14 blend. Thus, this study emphasizes that organic:inorganic composite layers based on inexpensive and relative easily synthetized materials can be deposited by MAPLE on ITO/PET flexible substrates with potential applications in the field of flexible electronics.
Author Contributions
Conceptualization, M.S. and N.P.; Writing—original draft, M.S and N.P.; Investigation: M.S., N.P., A.C., G.P., G.P.-P., S.I., A.S. (Andrei Stochioiu), A.M.C. and G.S.; Validation: N.P., A.S., and G.S.; Resources, M.S.; Data curation, G.P., G.P.-P., S.I., A.S. (Andrei Stochioiu), A.M.C.; Visualisation, G.P., G.P.-P., S.I., A.S. (Andrei Stochioiu), A.M.C.; Writing—review and editing, N.P., A.S. (Anca Stanculescu) and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Ministry of Research, Innovation and Digitalization, CNCS-UEFISCDI, project number PN-IV-P2-2.1-TE-2023-0909, the core program of NIMP under contract 28N/2023 and the core program of INFLPR under contract no. 30N/2023.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solak, E.K.; Irmak, E. Advances in Organic Photovoltaic Cells: A Comprehensive Review of Materials, Technologies, and Performance. RSC Adv. 2023, 13, 12244–12269. [Google Scholar] [CrossRef] [PubMed]
- Devasahayam, S.; Hussain, C.M. Thin-Film Nanocomposite Devices for Renewable Energy Current Status and Challenges. Sustain. Mater. Technol. 2020, 26, e00233. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, B.; Sun, R.; Yang, X.; Zhang, M.; Gao, Y.; Xiao, B.; Papkovskaya, E.D.; Luponosov, Y.; Brabec, C.J.; et al. 19.46%-Efficiency all-polymer organic solar cells with excellent outdoor operating stability enabled by active layer reconstruction. Energy Environ. Sci. 2025, 18, 1812–1823. [Google Scholar] [CrossRef]
- Raza, M.A.; Rehman, Z.U.; Tanvir, M.G.; Maqsood, M.F. Metal oxide-conducting polymer-based composite electrodes for energy storage applications. In Renewable Polymers and Polymer-Metal Oxide Composites Synthesis, Properties, and Applications; Haider, S., Haider, A., Eds.; Elsevier: Cambridge, MA, USA, 2022; pp. 195–252. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N. Hybrid Nanocomposite Thin Films for Photovoltaic Applications: A Review. Nanomaterials 2021, 11, 1117. [Google Scholar] [CrossRef]
- Sorrentino, R.; Kozma, E.; Luzzati, S.; Po, R. Interlayers for non-fullerene-based polymer solar cells: Distinctive features and challenges. Energy Environ. Sci. 2021, 14, 180–223. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells—Enhanced Efficiencies Via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Lakhotiya, G.; Belsare, N.; Rana, A.; Gupta, V. Cu2S nanocrystals incorporated highly efficient non-fullerene ternary organic solar cells. Curr. Appl. Phys. 2019, 19, 394–399. [Google Scholar] [CrossRef]
- Lakhotiya, G.; Belsare, N.; Arbuj, S.; Kale, B.; Rana, A. Enhanced performance of PTB7-Th:PCBM based active layers in ternary organic solar cells. RSC Adv. 2019, 9, 7457–7463. [Google Scholar] [CrossRef]
- Pascual-San-José, R.; Rodríguez-Martínez, X.; Adel-Abdelaleim, R.; Stella, M.; Martínez-Ferrero, E.; Campoy-Quiles, M. Blade coated P3HT:non-fullerene acceptor solar cells: A high-throughput parameter study with a focus on up-scalability. J. Mater. Chem. C 2019, 7, 20369–20382. [Google Scholar] [CrossRef]
- Berger, P.R.; Kim, M. Polymer solar cells: P3HT:PCBM and beyond. J. Renew. Sustain. Energy 2018, 10, 013508. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuo, Z.; Zhang, J.; Wang, X.; Xu, X.; Wang, Z.; Xin, Y.; Wang, J.; Wang, J.; Tang, W.; et al. Influence of PC60BM or PC70BM as electron acceptor on the performance of polymer solar cells. Sol. Energy Mater. Sol. Cells 2012, 97, 71–77. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Zhong, W.; Leng, S.; Zhou, G.; Zou, Y.; Su, X.; Ding, H.; Gu, P.; Liu, F.; et al. Progress and Prospects of the Morphology of Non-Fullerene Acceptor Based High-Efficiency Organic Solar Cells. Energy Environ. Sci. 2021, 14, 4341–4357. [Google Scholar] [CrossRef]
- Xiao, Z.; Jia, X.; Ding, L. Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 2017, 62, 1562–1564. [Google Scholar] [CrossRef]
- Chang, L.; Sheng, M.; Duan, L.; Uddin, A. Ternary organic solar cells based on non-fullerene acceptors: A review. Org. Electron. 2021, 90, 106063. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Gallagher, A.; Liu, Z.K.; Sun, Y.X.; Ma, W.L. High-efficiency polymer-PbS hybrid solar cells via molecular engineering. J. Mater. Chem. A 2015, 3, 2572–2579. [Google Scholar] [CrossRef]
- Tan, F.R.; Qu, S.C.; Wu, J.; Liu, K.; Zhou, S.Y.; Wang, Z.G. Preparation of SnS2 colloidal quantum dots and their application in organic/inorganic hybrid solar cells. Nanoscale Res. Lett 2011, 6, 298. [Google Scholar] [CrossRef]
- Yu, P.; Qu, S.; Jia, C.; Liu, K.; Tan, F. Modified synthesis of FeS2 quantum dots for hybrid bulk-heterojunction solar cells. Mater. Lett. 2015, 157, 235–238. [Google Scholar] [CrossRef]
- Jabeen, U.; Adhikari, T.; Shah, S.M.; Pathak, D.; Nunzi, J.-M. Synthesis, characterization and photovoltaic performance of Mn-doped ZnS quantum dots-P3HT hybrid bulk heterojunction solar cells. Opt. Mater. 2017, 73, 754–762. [Google Scholar] [CrossRef]
- Hamed, M.S.G.; Mola, G.T. Copper sulphide as a mechanism to improve energy harvesting in thin film solar cells. J. Alloys Compd. 2019, 802, 252–258. [Google Scholar] [CrossRef]
- Benchaabane, A.; Hamed, Z.B.; Telfah, A.; Sanhoury, M.A.; Kouki, F.; Zellama, K.; Bouchriha, H. Effect of OA-ZnSe nanoparticles incorporation on the performance of PVK organic photovoltaic cells. Mater. Sci. Semicond. Process. 2017, 64, 115–123. [Google Scholar] [CrossRef]
- Yang, X.-K.; Qiao, J.-W.; Chen, Z.-H.; Wen, Z.-C.; Yin, H.; Hao, X.-T. CdSe quantum dot organic solar cells with improved photovoltaic performance. J. Phys. D Appl. Phys. 2021, 54, 115504. [Google Scholar] [CrossRef]
- Tan, Z.N.; Zhang, W.Q.; Qian, D.P.; Zheng, H.; Xiao, S.Q.; Yang, Y.P.; Zhu, T.; Xu, J. Efficient hybrid infrared solar cells based on P3HT and PbSe nanocrystal quantum dots. Mater. Sci. Forum 2011, 685, 38–43. [Google Scholar] [CrossRef]
- Yao, S.; Chen, Z.; Li, F.; Xu, B.; Song, J.; Yan, L.; Jin, G.; Wen, S.; Wang, C.; Yang, B.; et al. High-efficiency aqueous-solutionprocessed hybrid solar cells based on P3HT dots and CdTe nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 7146–7152. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Kim, J.; Hong, K.; Oh, E.; Yang, Y.; Yu, D. Photocurrent and photovoltaic characteristics of copper sulfide nanowires grown by a hydrothermal method. Mater. Lett. 2014, 133, 132–134. [Google Scholar] [CrossRef]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified Copper Sulfide (Cu2-XS) Micro-/Nanostructures: A Comprehensive Review on Synthesis, Modifications and Applications. Nanoscale 2017, 9, 11357–11404. [Google Scholar] [CrossRef]
- Singh, A.; Manivannan, R.; Victoria, S.N. Simple one-pot sonochemical synthesis of copper sulphide nanoparticles for solar cell applications. Arab. J. Chem. 2019, 12, 2439–2447. [Google Scholar] [CrossRef]
- Shaikh, G.Y.; Nilegave, D.S.; Girawale, S.S.; Kore, K.B.; Newaskar, S.R.; Sahu, S.A.; Funde, A.M. Structural, Optical, Photoelectrochemical, and Electronic Properties of the Photocathode CuS and the Efficient CuS/CdS Heterojunction. ACS Omega 2022, 7, 30233–30240. [Google Scholar] [CrossRef]
- Shamraiz, U.; Hussain, R.A.; Badshah, A. Fabrication and applications of copper sulfide (CuS) nanostructures. J. Solid State Chem. 2016, 238, 25–40. [Google Scholar] [CrossRef]
- Sangeetha, C.K.; Kusuma, J.; Himanshu, B.; Yogesh, J.; Sachin, R.R. Unveiling Electronic Structure, Band Alignment, and Charge Carrier Kinetics of Copper Sulfide. Phys. Chem. C 2024, 128, 20205–20214. [Google Scholar] [CrossRef]
- Kim, Y.; Heyne, B.; Abouserie, A.; Pries, C.; Ippen, C.; Günter, C.; Taubert, A.; Wedel, A. CuS nanoplates from ionic liquid precursors—Application in organic photovoltaic cells. J. Chem. Phys. 2018, 148, 193818. [Google Scholar] [CrossRef]
- Breazu, C.; Girtan, M.; Stanculescu, A.; Preda, N.; Rasoga, O.; Costas, A.; Catargiu, A.M.; Socol, G.; Stochioiu, A.; Popescu-Pelin, G.; et al. MAPLE-Deposited Perylene Diimide Derivative Based Layers for Optoelectronic Applications. Nanomaterials 2024, 14, 1733. [Google Scholar] [CrossRef]
- Mishra, A.; Bhuyan, N.N.; Xu, H.; Sharma, G.D. Advances in layer-by-layer processing for efficient and reliable organic solar cells. Mater. Adv. 2023, 4, 6031–6063. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Caricato, A.P. MAPLE and MALDI: Theory and Experiments. In Lasers in Materials Science, 1st ed.; Castillejo, M., Ossi, P., Zhigilei, L., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 295–323. [Google Scholar] [CrossRef]
- Prodana, M.; Stoian, A.B.; Ionita, D.; Brajnicov, S.; Boerasu, I.; Enachescu, M.; Burnei, C. In-Depth Characterization of Two Bioactive Coatings Obtained Using MAPLE on TiTaZrAg. Materials 2024, 17, 2989. [Google Scholar] [CrossRef]
- Ge, W.; Atewologun, A.; Stiff-Roberts, A.D. Hybrid nanocomposite thin films deposited by emulsion-based resonant infrared matrix-assisted pulsed laser evaporation for photovoltaic applications. Org. Electron. 2015, 22, 98–107. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Costas, A.; Breazu, C.; Stanculescu, A.; Rasoga, O.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G. Hybrid organic-inorganic thin films based on zinc phthalocyanine and zinc oxide deposited by MAPLE. Appl. Surf. Sci. 2020, 503, 144317. [Google Scholar] [CrossRef]
- Yadav, S.; Bajpai, P. Synthesis of copper sulfide nanoparticles: Ph dependent phase stabilization. Nano-Struct. Nano-Objects 2017, 10, 151–158. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Socol, G. Organic Thin Films Deposited by Matrix-Assisted Pulsed Laser Evaporation (MAPLE) for Photovoltaic Cell Applications: A Review. Coatings 2021, 11, 1368. [Google Scholar] [CrossRef]
- Caricato, A.P.; Ge, W.; Stiff-Roberts, A.D. UV- and RIR-MAPLE: Fundamentals and Applications, 1st ed.; Springer Series in Materials Science; Springer Nature Switzerland: Cham, Switzerland, 2018; pp. 275–308. [Google Scholar] [CrossRef]
- Wu, M.-C.; Wu, Y.-J.; Yen, W.-C.; Lo, H.-H.; Lin, C.-F.; Su, W.-F. Correlation between nanoscale surface potential and power conversion efficiency of P3HT/TiO2 nanorod bulk heterojunction photovoltaic devices. Nanoscale 2010, 2, 1448. [Google Scholar] [CrossRef]
- Patz, T.M.; Doraiswamy, A.; Narayan, R.J.; Menegazzo, N.; Kranz, C.; Mizaikoff, B.; Zhong, Y.; Bellamkonda, R.; Bumgardner, J.D.; Elder, S.H.; et al. Matrix assisted pulsed laser evaporation of biomaterial thin films. Mater. Sci. Eng. C 2007, 27, 514–522. [Google Scholar] [CrossRef]
- Matus-Arrambide, A.; Mendoza-Jiménez, R.A.; Moure-Flores, F.; Mayén-Hernández, S.A.; Olvera-Amador, M.L.; Arenas-Arrocena, M.C.; Santos Cruz, J. Poly-3-hexylthiophene doped with iron disulfide nanoparticles for hybrid solar cells. Int. J. Energy Res. 2019, 43, 3723–3731. [Google Scholar] [CrossRef]
- Matras-Postołek, K.; Żaba, A.; Nowak, E.; Dąbczyński, P.; Rysz, J.; Sanetra, J. Formation and characterization of one-dimensional ZnS nanowires for ZnS/P3HT hybrid polymer solar cells with improved efficiency. Appl. Surf. Sci. 2018, 451, 180–190. [Google Scholar] [CrossRef]
- Kampen, T.U.; Salvan, G.; Paraian, A.; Himcinschi, C.; Kobitski, A.Y.; Friedrich, M.; Zahn, D.R.T. Orientation of perylene derivatives on semiconductor surfaces. Appl. Surf. Sci. 2013, 212–213, 501–507. [Google Scholar] [CrossRef]
- Barrett, H.P.; Kennedy, W.J.; Boucher, D.S. Spectroscopic characterization of P3HT/SWNT composites synthesized usingin situ GRIM methods: Improved polymer ordering via nanoscaffolding. J. Polym. Sci. Part B Polym. Phys. 2013, 52, 310–320. [Google Scholar] [CrossRef]
- Motaung, D.E.; Malgas, G.F.; Arendse, C.J. Comparative study: The effects of solvent on the morphology, optical and structural features of regioregular poly(3-hexylthiophene):fullerene thin films. Synth. Met. 2010, 160, 876–882. [Google Scholar] [CrossRef]
- Asir, S.; Demir, A.S.; Icıl, H. The synthesis of novel, unsymmetrically substituted, chiral naphthalene and perylene diimide: Photophysical, electrochemical, chiroptical and intramolecular charge transfer properties. Dye. Pigment. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Meena, S.; Chhillar, P.; Pathak, S.; Roose, B.; Jacob, J. Perylene diimide based low band gap copolymers: Synthesis, characterization and their applications in perovskite solar cells. J. Polym. Res. 2020, 27, 226. [Google Scholar] [CrossRef]
- Raj, S.I.; Jaiswal, A.; Uddin, I. Ultrasmall aqueous starch-capped CuS quantum dots with tunable localized surface plasmon resonance and composition for the selective and sensitive detection of mercury(ii) ions. RSC Adv. 2020, 10, 14050–14059. [Google Scholar] [CrossRef]
- Available online: https://docbrown.info/page06/spectra/chlorobenzene-ir.htm (accessed on 16 December 2024).
- Socol, M.; Preda, N.; Breazu, C.; Petre, G.; Stanculescu, A.; Stavarache, I.; Popescu-Pelin, G.; Stochioiu, A.; Socol, G.; Iftimie, S.; et al. Effects of Solvent Additive and Micro-Patterned Substrate on the Properties of Thin Films Based on P3HT:PC70BM Blends Deposited by MAPLE. Materials 2023, 16, 144. [Google Scholar] [CrossRef]
- Ge, W.; Li, N.K.; McCormick, R.D.; Lichtenberg, E.; Yingling, Y.G.; Stiff-Roberts, A.D. Emulsion-based RIR-MAPLE deposition of conjugated polymers: Primary solvent effect and its implications on organic solar cell performance. ACS Appl. Mater. Interfaces 2016, 8, 19494–19506. [Google Scholar] [CrossRef]
- Wei, W.; Ouyang, S.; Zhang, T. Perylene diimide self-assembly: From electronic structural modulation to photocatalytic applications. J. Semicond. 2020, 41, 091708. [Google Scholar] [CrossRef]
- Balambiga, B.; Dheepika, R.; Devibala, P.; Imran, P.M.; Nagarajan, S. Picene and PTCDI based solution processable ambipolar OFETs. Sci. Rep. 2020, 10, 22029. [Google Scholar] [CrossRef]
- Mohamad, D.K.; Fischereder, A.; Yi, H.; Cadby, A.J.; Lidzey, D.G.; Iraqi, A. A Novel 2,7-Linked Carbazole Based “Double Cable” Polymer with Pendant Perylene Diimide Functional Groups: Preparation, Spectroscopy and Photovoltaic Properties. J. Mater. Chem. 2011, 21, 851–862. [Google Scholar] [CrossRef]
- Erdmann, T.; Back, J.; Tkachov, R.; Ruff, A.; Voit, B.; Ludwigs, S.; Kiriy, A. Dithienosilole-based all-conjugated block copolymers synthesized by a combination of quasi-living Kumada and Negishi catalyst-transfer polycondensations. Polym. Chem. 2014, 5, 5383–5390. [Google Scholar] [CrossRef]
- Lucenti, E.; Botta, C.; Cariati, E.; Righetto, S.; Scarpellini, M.; Tordin, E.; Ugo, R. New organic-inorganic hybrid materials based on perylene diimide-polyhedral oligomeric silsesquioxane dyes with reduced quenching of the emission in the solid state. Dye. Pigment. 2013, 96, 748–755. [Google Scholar] [CrossRef]
- Stanculescu, A.; Breazu, C.; Socol, M.; Rasoga, O.; Preda, N.; Petre, G.; Solonaru, A.M.; Grigoras, M.; Stanculescu, F.; Socol, G.; et al. Effect of ITO electrode patterning on the properties of organic heterostructures based on non-fullerene acceptor prepared by MAPLE. Appl. Surf. Sci. 2020, 509, 145351. [Google Scholar] [CrossRef]
- Farr, E.P.; Fontana, M.T.; Zho, C.C.; Wu, P.; Li, Y.P.; Knutson, N.; Rubin, Y.; Schwartz, B. Bay-Linked Perylenediimides are Two Molecules in One: Insights from Ultrafast Spectroscopy, Temperature Dependence, and Time-Dependent Density Functional Theory Calculations. J. Phys. Chem. C 2019, 123, 2127–2138. [Google Scholar] [CrossRef]
- Kozma, E.; Kotowski, D.; Luzzati, S.; Catellani, M.; Bertini, F.; Famulari, A.; Raos, G. Improving the efficiency of P3HT:perylene diimide solar cells via bay-substitution with fused aromatic rings. RSC Adv. 2013, 3, 9185. [Google Scholar] [CrossRef]
- Karak, S.; Ray, S.K.; Dhar, A. Photoinduced charge transfer and photovoltaic energy conversion in self-assembled N, N′-dioctyl-3,4,9,10-perylenedicarboximide nanoribbons. Appl. Phys. Lett. 2010, 97, 043306. [Google Scholar] [CrossRef]
- Stanculescu, F.; Rasoga, O.; Catargiu, A.M.; Vacareanu, L.; Socol, M.; Breazu, C.; Preda, N.; Socol, G.; Stanculescu, A. MAPLE prepared heterostructures with arylene based polymer active layer for photovoltaic applications. Appl. Surf. Sci. 2015, 336, 240–248. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Costas, A.; Borca, B.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G.; Stanculescu, A. Thin Films Based on Cobalt Phthalocyanine:C60 Fullerene:ZnO Hybrid Nanocomposite Obtained by Laser Evaporation. Nanomaterials 2020, 10, 468. [Google Scholar] [CrossRef]
- Boobalan, G.; Imran, K.M.; Manoharan, C.; Nagarajan, S. Fabrication of highly fluorescent perylene bisimide nanofibers through interfacial self-assembly. J. Colloid Interface Sci. 2013, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiu, T.; Tao, G.-H.; Wang, G.; Sun, C.; Li, P.; Fang, J.; He, L. Manipulating surface ligands of Copper Sulfide nanocrystals: Synthesis, characterization, and application to organic solar cells. J. Colloid Interface Sci. 2014, 419, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Irshad, Z.; Hussain, R.; Lee, W.; Kim, M.; Lim, J. Efficient ternary active layer materials for organic photovoltaics. Sol. Energy 2023, 257, 324–343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).