A Study on the Charging–Discharging Mechanism of All Solid-State Aluminum–Carbon Composite Secondary Batteries
Abstract
1. Introduction
2. Experimental Procedure
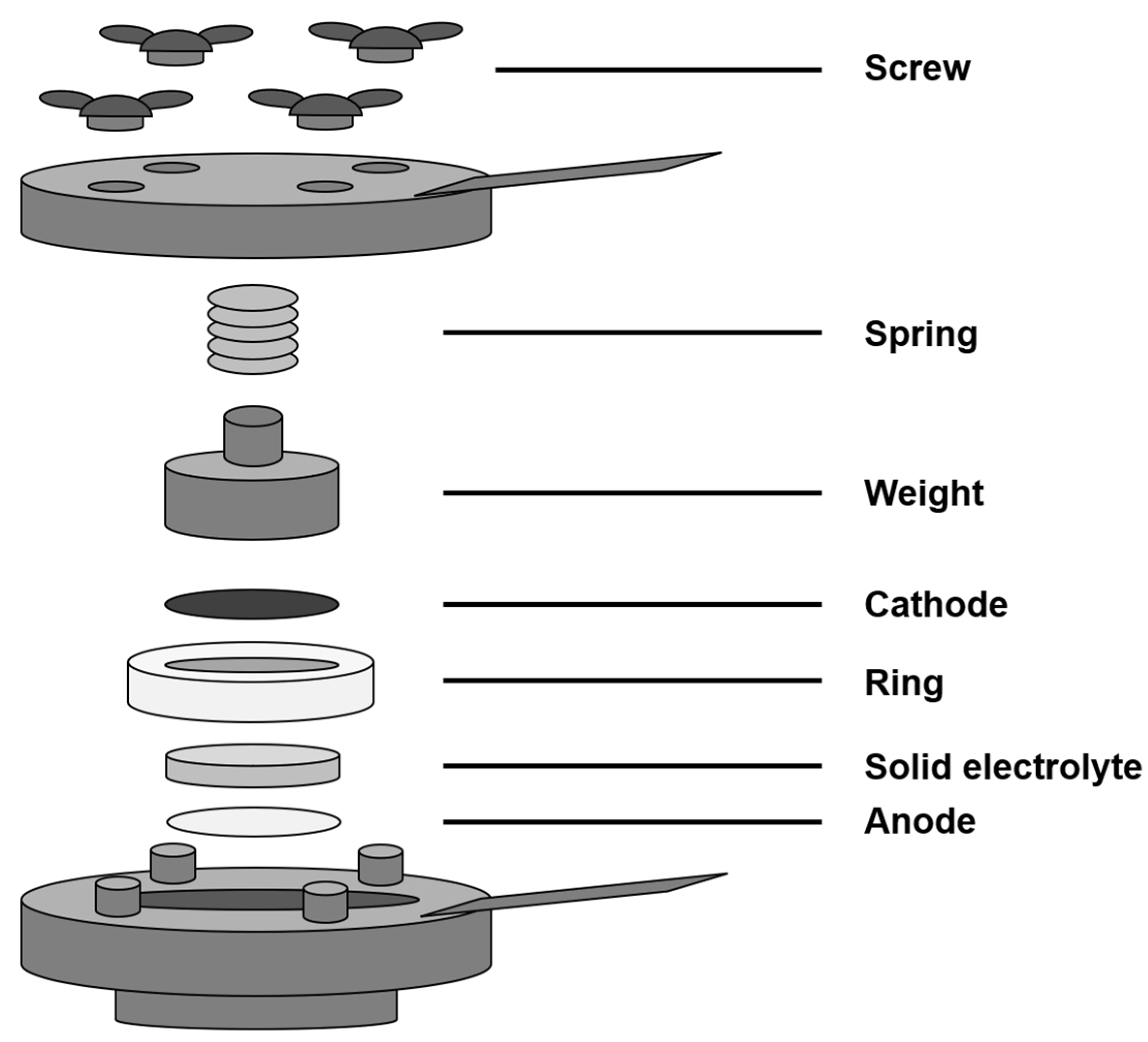
2.1. Fabrication of Electrodes
2.2. Characterization Analysis of Materials
2.3. BT Cell Charge and Discharge Test
3. Results and Discussion
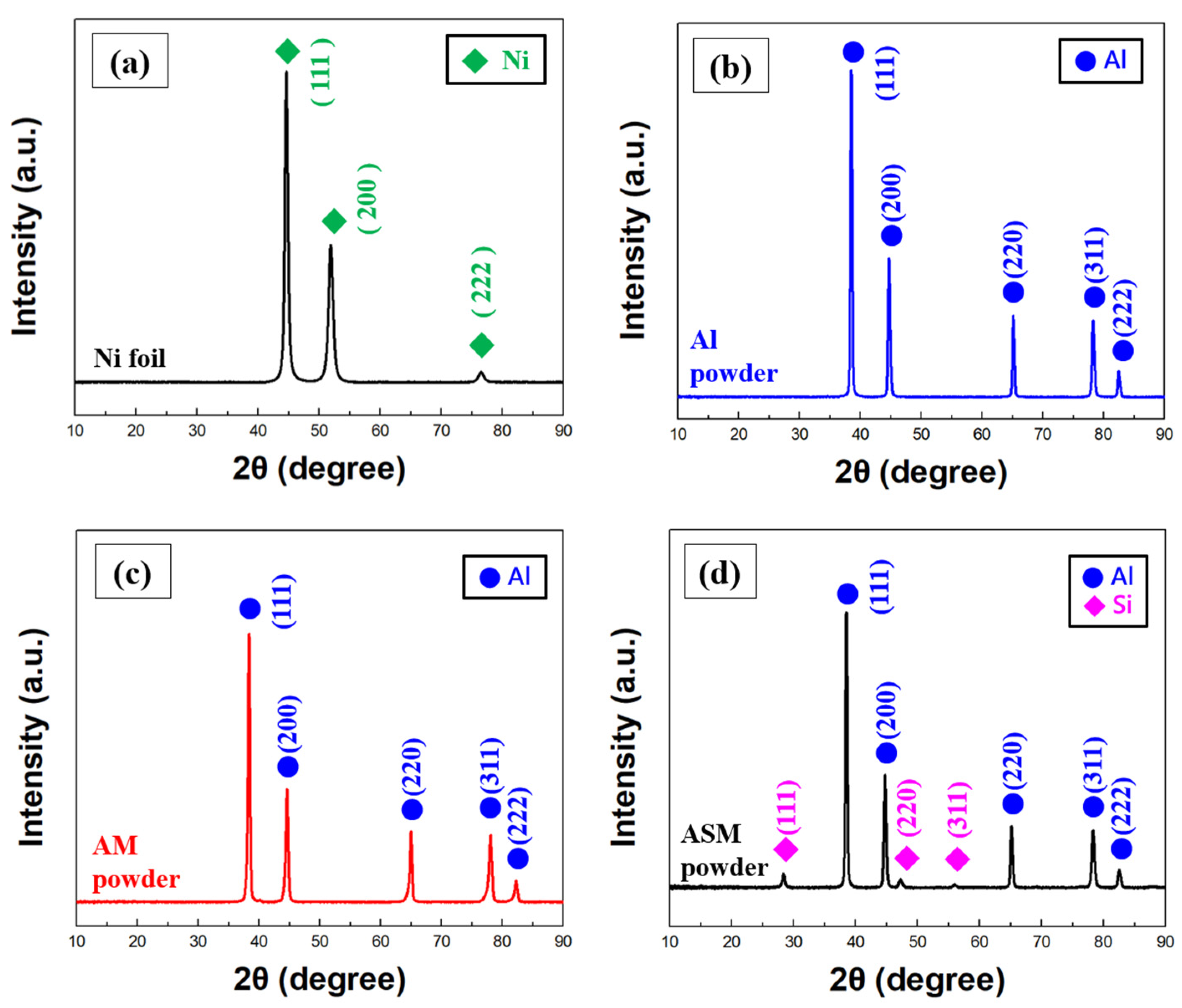
3.1. Analysis of Cathode Modification Characteristics
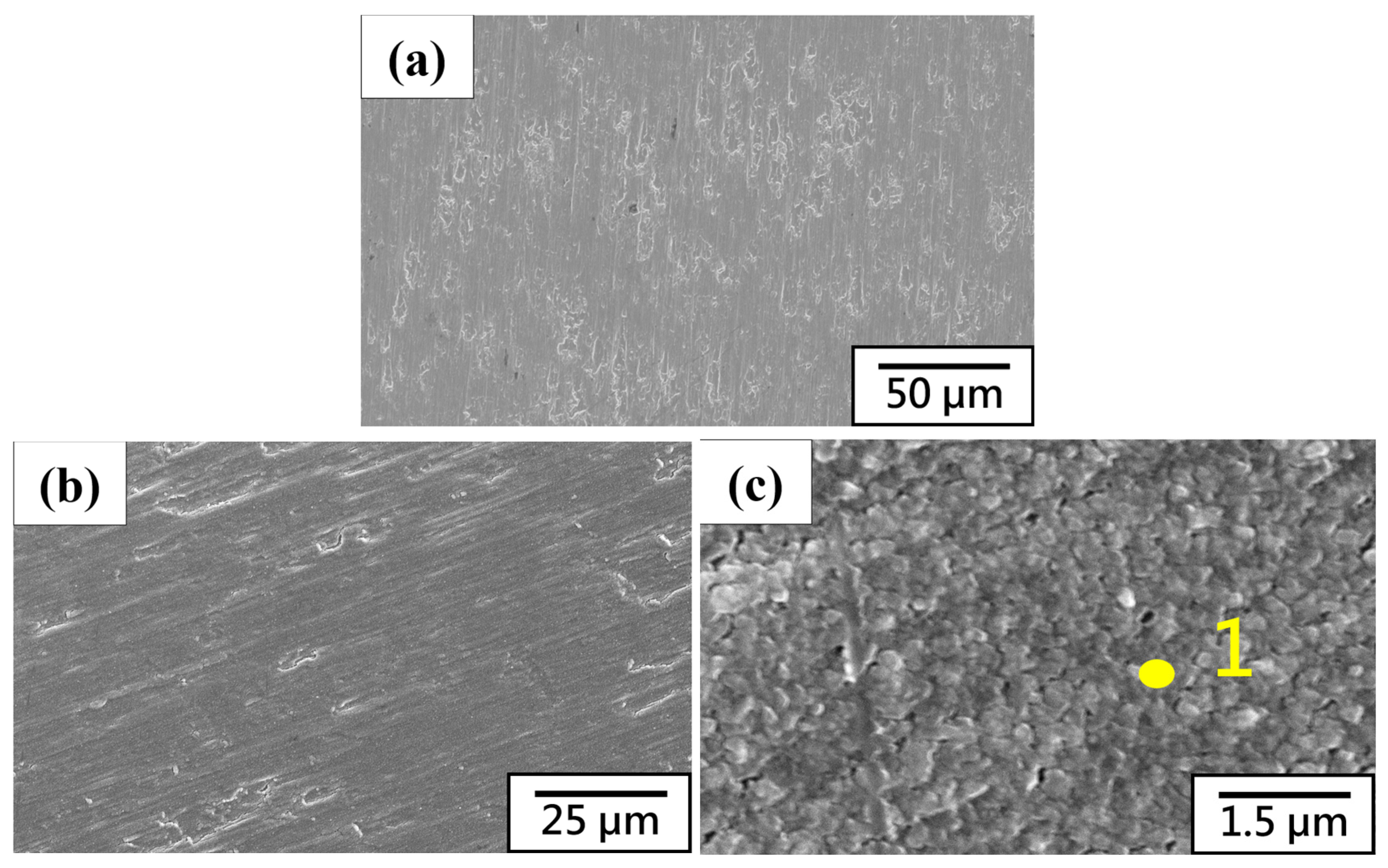
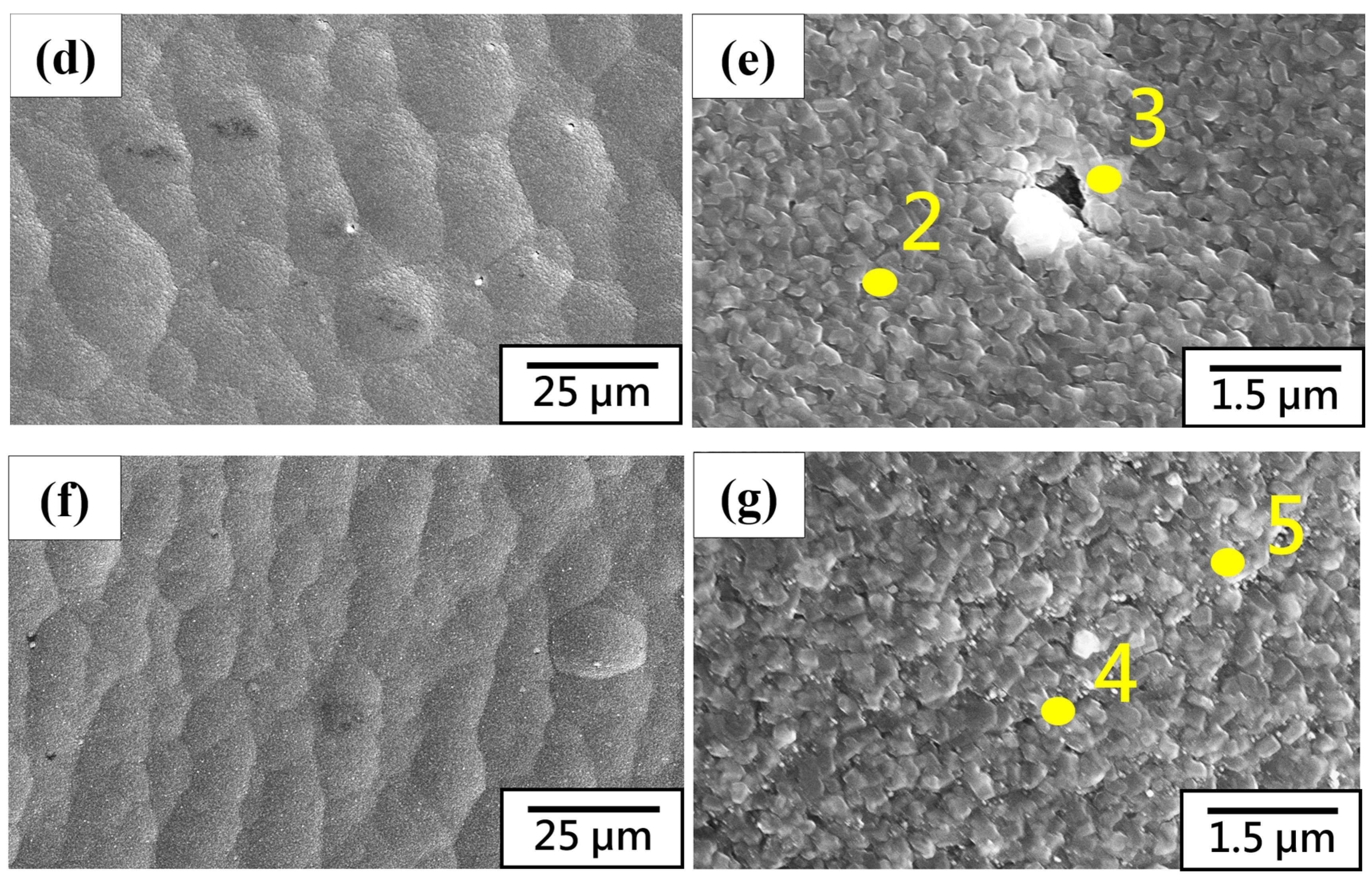
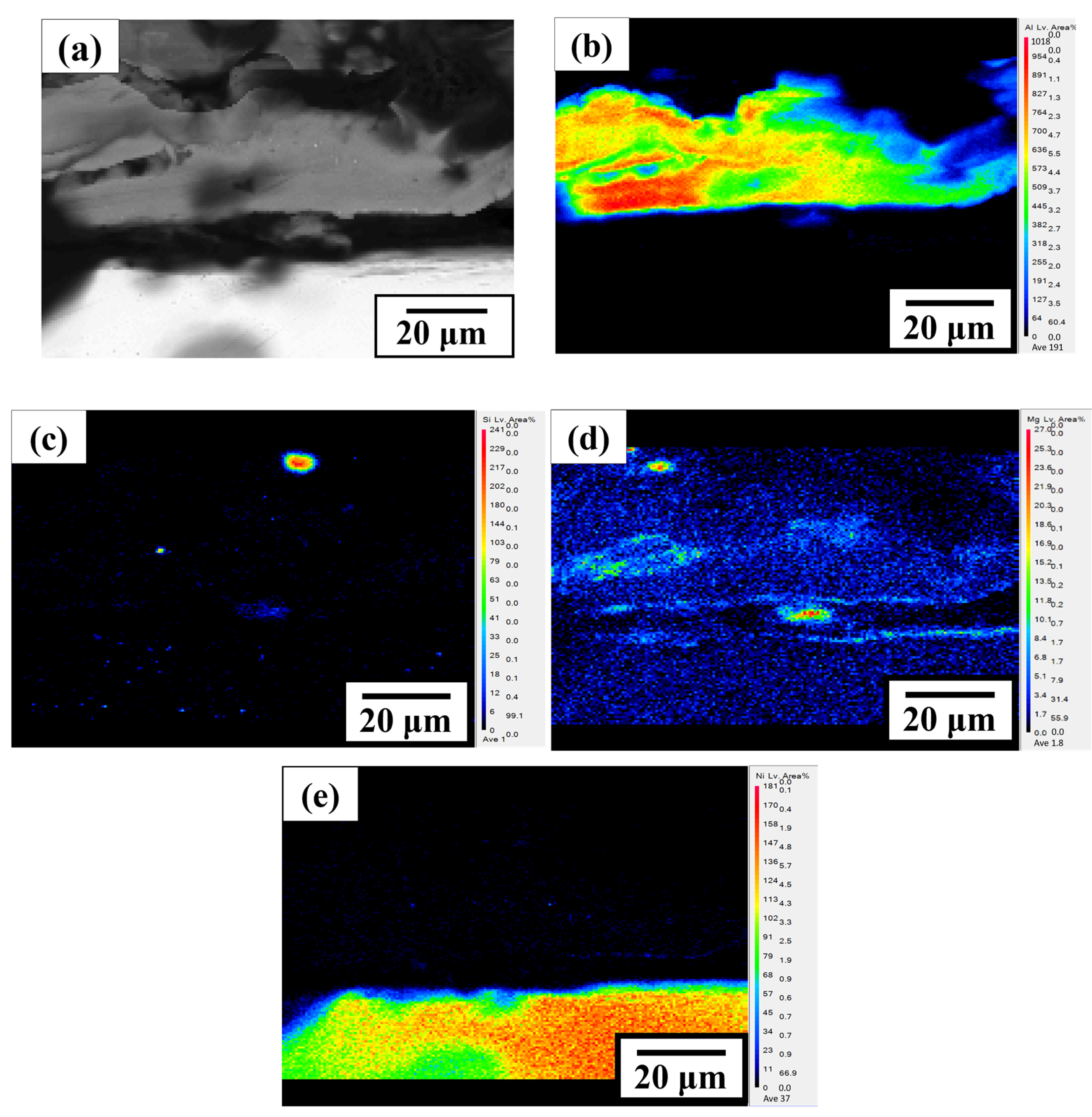
3.2. Microstructure Characteristics and Electrochemical Analysis of Thermal Evaporation Anode
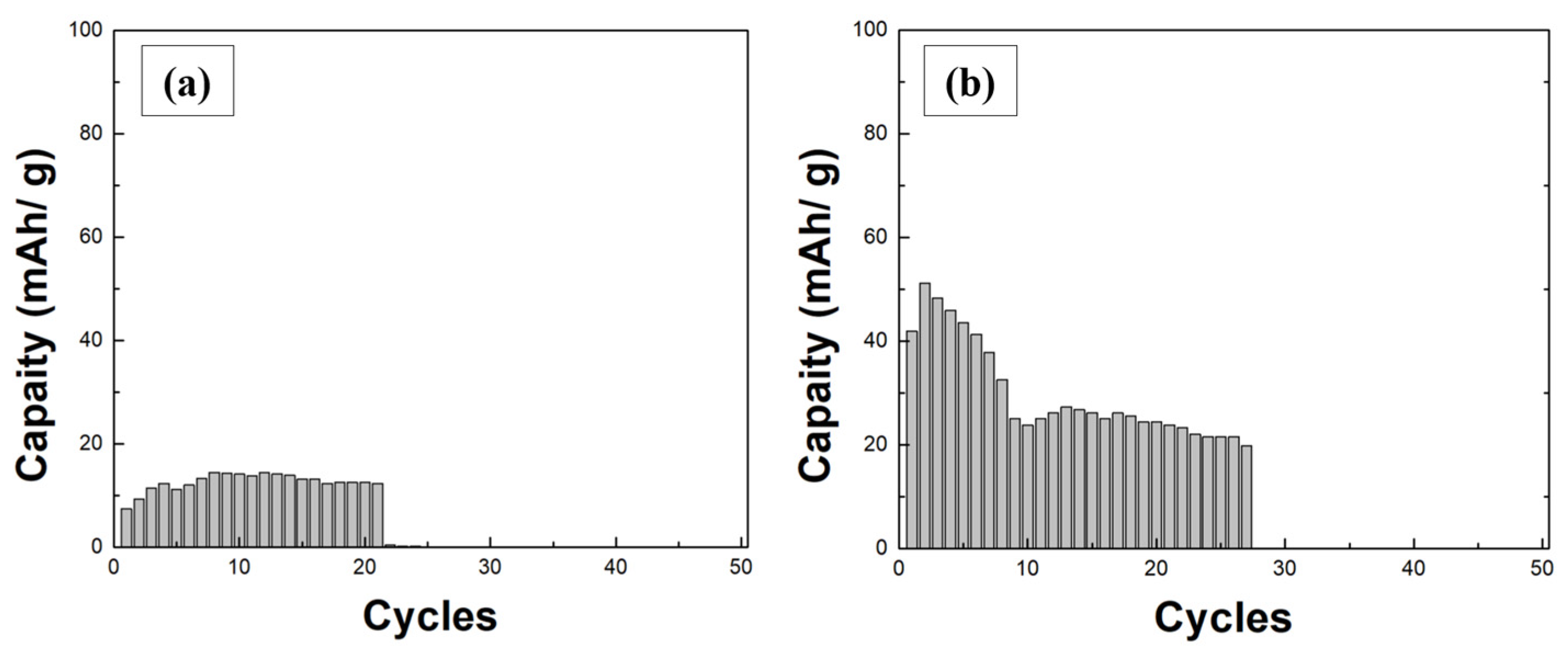
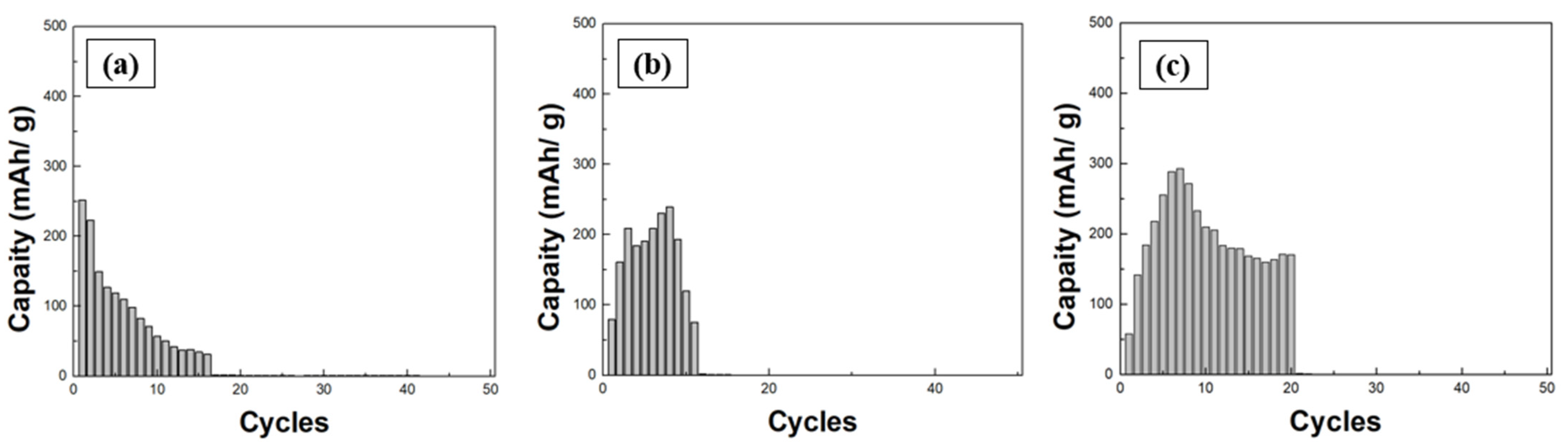
3.3. Charge–Discharge Cycle Characteristics and Ion Migration Mechanisms
- Charging process
- Negative electrode: Al(s) → Al1−x(s) + x Al3+ + 3x e−
- Positive electrode: x Al3+ + C6(s) + 3x e− → AlxC6(s)
- Full battery reaction: Al(s) + C6(s) → AlxC6(s) + Al1−x(s)
- Discharging process
- Negative electrode: AlxC6(s) → x Al3+ + C6(s) + 3x e−
- Positive electrode: Al1−x(s) + x Al3+ + 3x e− → Al(s)
- Full battery reaction: AlxC6(s) + Al1−x(s) → Al(s) + C6(s)

3.4. Large-Scale Module Battery and Motor–Fan Unit
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ma, J.; Wang, J.; Zhang, X.; Zhou, G.; Liang, Z. Progress, Key Issues, and Future Prospects for Li-Ion Battery Recycling. Glob. Chall. 2022, 6, 2200067. [Google Scholar]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.M.; Li, F. The rechargeable aluminum battery: Opportunities and challenges. Angew. Chem. Int. Ed. 2019, 58, 11978–11996. [Google Scholar]
- Wang, D.Y.; Wei, C.Y.; Lin, M.C.; Pan, C.J.; Chou, H.L.; Chen, H.A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar]
- Jiang, M.; Fu, C.; Meng, P.; Ren, J.; Wang, J.; Bu, J.; Dong, A.; Zhang, J.; Xiao, W.; Sun, B. Challenges and strategies of low-cost aluminum anodes for high-performance Al-based batteries. Adv. Mater. 2022, 34, 2102026. [Google Scholar]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar]
- Razaz, G.; Arshadirastabi, S.; Blomquist, N.; Örtegren, J.; Carlberg, T.; Hummelgård, M.; Olin, H. Aluminum alloy anode with various iron content influencing the performance of aluminum-ion batteries. Materials 2023, 16, 933. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Yang, W.; Gao, D.; Zhang, Z.; Dong, X. Laser-sintering fabrication of integrated Al/Ni anodes for lithium-ion batteries. RSC Adv. 2022, 12, 13168–13179. [Google Scholar] [CrossRef] [PubMed]
- Doche, M.L.; Novel-Cattin, F.; Durand, R.; Rameau, J.J. Characterization of different grades of aluminum anodes for aluminum/air batteries. J. Power Sources 1997, 65, 197–205. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xue, J. The role of micro-naoscale AlSb precipitates in improving the discharge performance of Al-Sb alloy anodes for Al-air batteries. J. Power Sources 2019, 425, 186–194. [Google Scholar] [CrossRef]
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Moghanni-Bavil-Olyaei, H.; Arjomandi, J. Performance of Al–1Mg–1Zn–0.1Bi–0.02In as anode for the Al–AgO battery. RSC Adv. 2015, 5, 91273–91279. [Google Scholar] [CrossRef]
- Jeffrey, P.W.; Wojciech, H.; Smith, F.N. Aluminum Anode Alloy. U.S. Patent No. 4,751,086, 14 June 1988. [Google Scholar]
- Gao, J.; Fan, H.; Wang, E.; Song, Y.; Sun, G. Exploring the effect of magnesium content on the electrochemical performance of aluminum anodes in alkaline batteries. Electrochim. Acta 2020, 353, 136497. [Google Scholar] [CrossRef]
- Zhou, W.; Upreti, S.; Whittingham, M.S. Electrochemical performance of Al–Si–graphite composite as anode for lithium–ion batteries. Electrochem. Commun. 2011, 13, 158–161. [Google Scholar] [CrossRef]
- Chang, K.C.; Zhao, J.R.; Hung, F.Y. Effects of hyper-high-temperature solid-solution treatment on microstructure evolution and nanoprecipitation of the Al-Ni-Cu-Fe-Zr-Sc alloy manufactured by selective laser melting. J. Alloys Compd. 2021, 883, 160781. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hung, F.Y.; Zhao, J.R. A new iron battery technology: Charge-discharge mechanism of ferrous chloride and ferric oxide electrolyte in all solid-state iron-graphite batteries. Mater. Sci. Eng. B 2024, 303, 117305. [Google Scholar] [CrossRef]
- Lim, C.H.; Jung, Y.H.; Yeom, S.J.; Lee, H.W.; Kim, D.K. Encapsulation of lithium vanadium phosphate in reduced graphene oxide for a lithium-ion battery cathode with stable elevated temperature performance. Electrochim. Acta 2017, 253, 208–217. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar]
- Adhitama, E.; Dias Brandao, F.; Dienwiebel, I.; Bela, M.M.; Javed, A.; Haneke, L.; Stan, M.C.; Winter, M.; Gomez-Martin, A.; Placke, T. Pre-lithiation of silicon anodes by thermal evaporation of lithium for boosting the energy density of lithium ion cells. Adv. Funct. Mater. 2022, 32, 2201455. [Google Scholar]
- Huang, B.C.; Fan, W.C.; Hung, F.Y. Study on microstructure and charge–discharge mechanism of all solid-state indium-graphite batteries. Energy Storage 2024, 6, e544. [Google Scholar]
- Swain, T. Synthesis, characterization and thermal property of {Cu3(PO4)2·2H2O.; Na3PO4; NaHSO4·H2O}. Sol. Energy 2018, 159, 369–374. [Google Scholar]
- Okamoto, H. Al-Mg (aluminum-magnesium). J. Phase Equilibria Diffus. 1998, 19, 598. [Google Scholar] [CrossRef]
- Abu Nayem, S.M.; Ahmad, A.; Shaheen Shah, S.; Saeed Alzahrani, A.; Saleh Ahammad, A.J.; Aziz, M.A. High Performance and Long-cycle Life Rechargeable Aluminum Ion Battery: Recent Progress, Perspectives and Challenges. Chem. Rec. 2022, 22, e202200181. [Google Scholar]









| Material | C (wt. %) | O (wt. %) | Na (wt. %) | P (wt. %) |
|---|---|---|---|---|
| GF | 100.0 | 0.0 | 0.0 | 0.0 |
| GFN | 14.5 | 41.7 | 26.1 | 17.7 |
| Electrode | Resistivity (Ω × cm) |
|---|---|
| GF | 7.813 × 10−4 |
| GFN | 6.970 × 10−4 |
| Ni | 5.361 × 10−5 |
| TE Al/ Ni | 6.898 × 10−4 |
| TE AM/ Ni | 1.568 × 10−3 |
| TE ASM/ Ni | 7.813 × 10−5 |
| Element | Al | Si | Mg | O | Ni |
|---|---|---|---|---|---|
| 1 (wt. %) | 58.2 | 0.0 | 0.0 | 18.9 | 22.9 |
| 2 (wt. %) | 72.4 | 0.0 | 12.4 | 15.2 | 0.0 |
| 3 (wt. %) | 77.1 | 0.0 | 7.7 | 15.2 | 0.0 |
| 4 (wt. %) | 74.2 | 0.0 | 6.4 | 19.4 | 0.0 |
| 5 (wt. %) | 76.6 | 0.0 | 5.1 | 18.2 | 0.0 |
| Element | C | O | P | Na | Mg | Al | Si | Ni |
|---|---|---|---|---|---|---|---|---|
| 1 (wt. %) | 98.2 | 1.2 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2 (wt. %) | 11.2 | 62.5 | 11.8 | 1.6 | 0.7 | 11.0 | 1.3 | 0.0 |
| 3 (wt. %) | 7.9 | 7.2 | 0.0 | 0.6 | 0.0 | 0.5 | 0.0 | 83.8 |
| 4 (wt. %) | 0.0 | 41.8 | 0.0 | 0.0 | 0.0 | 15.6 | 0.0 | 41.6 |
| Cycles | Duration Time |
|---|---|
| First | 5 min 27 s |
| Second | 4 min 18 s |
| Third | 4 min 25 s |
| Fourth | 4 min 57 s |
| Fifth | 4 min 26 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-Y.; Wu, B.-D.; Hung, F.-Y. A Study on the Charging–Discharging Mechanism of All Solid-State Aluminum–Carbon Composite Secondary Batteries. J. Compos. Sci. 2025, 9, 166. https://doi.org/10.3390/jcs9040166
Lin J-Y, Wu B-D, Hung F-Y. A Study on the Charging–Discharging Mechanism of All Solid-State Aluminum–Carbon Composite Secondary Batteries. Journal of Composites Science. 2025; 9(4):166. https://doi.org/10.3390/jcs9040166
Chicago/Turabian StyleLin, Jia-Ying, Bo-Ding Wu, and Fei-Yi Hung. 2025. "A Study on the Charging–Discharging Mechanism of All Solid-State Aluminum–Carbon Composite Secondary Batteries" Journal of Composites Science 9, no. 4: 166. https://doi.org/10.3390/jcs9040166
APA StyleLin, J.-Y., Wu, B.-D., & Hung, F.-Y. (2025). A Study on the Charging–Discharging Mechanism of All Solid-State Aluminum–Carbon Composite Secondary Batteries. Journal of Composites Science, 9(4), 166. https://doi.org/10.3390/jcs9040166






