Enhanced Stability and Adsorption of Cross-Linked Magnetite Hydrogel Beads via Silica Impregnation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation and Extraction of Silica
2.3. Characterization of Extracted Silica
2.4. Synthesis and Incorporation of Silica into Hydrogel Beads
2.5. Characterization of CMC-CS-Fe3O4-Si Hydrogel Beads
2.6. Determination of Swelling Properties of CMC-CS-Fe3O4-Si Hydrogel Beads
2.7. Assessment of Mechanical Stability of CMC-CS-Fe3O4-Si Hydrogel Beads
2.8. Assessment of Thermal Stability of CMC-CS-Fe3O4-Si Hydrogel Beads
2.9. Adsorption of Heavy Metals in Aqueous Solution
2.10. Desorption Experiment and Regeneration
2.11. Simultaneous Removal of Cr (VI) and Cu (II)
2.12. Effect of Coexisting Ions or Complex Compounds on Adsorption Efficiency
2.13. Adsorption of Methylene Blue
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Extracted Silica
3.2. Characterization of CMC-CS-Fe3O4 Hydrogel Beads Impregnated with Silica
3.3. Swelling Properties of CMC-CS-Fe3O4-Si Hydrogel Beads
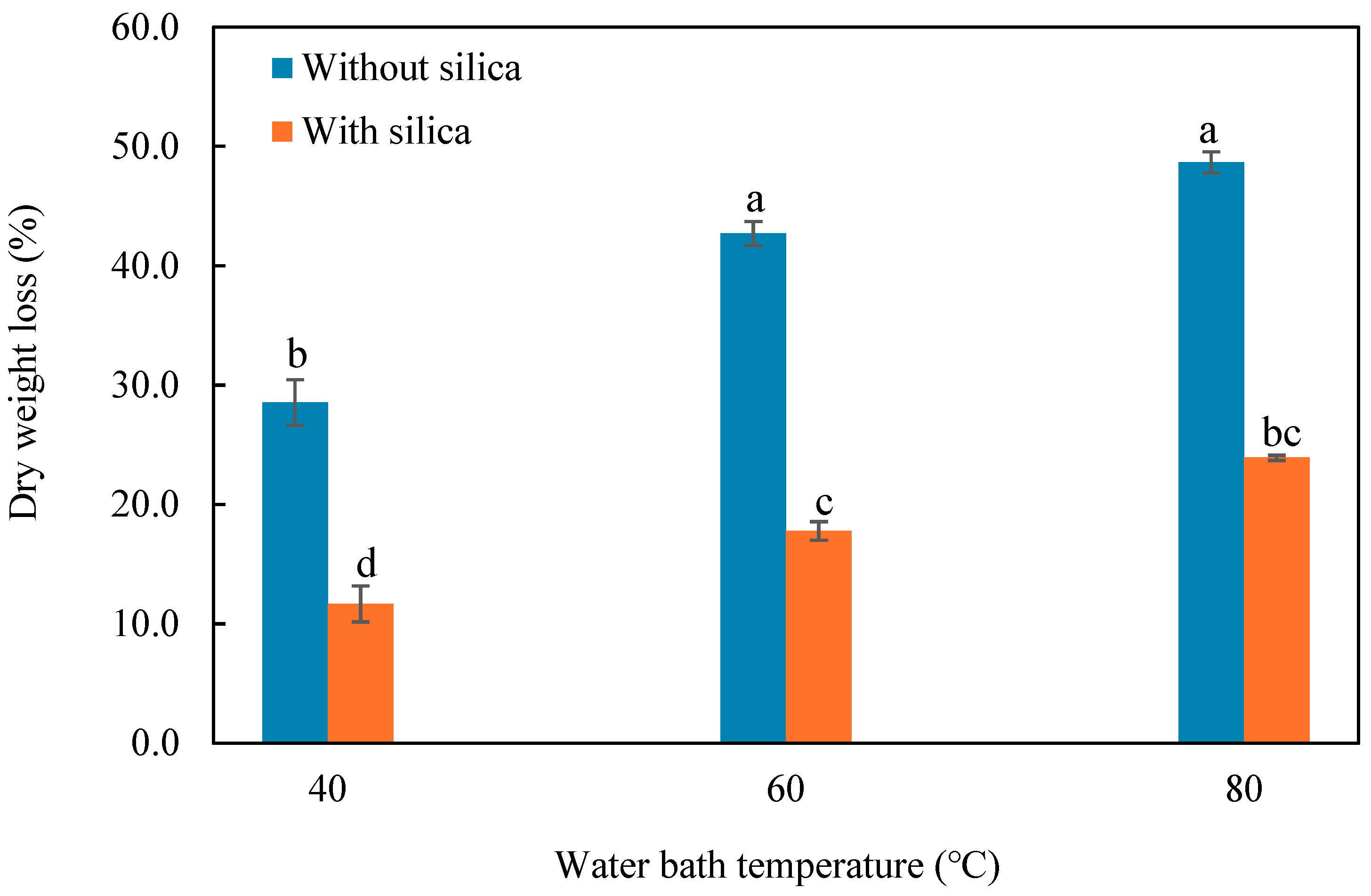
3.4. Assessment of Mechanical Stability of CMC-CS-Fe3O4-Si
3.5. Assessment of Thermal Stability of CMC-CS-Fe3O4-Si
3.6. Comparative Study on Adsorption Performance of Hydrogel After Silica Incorporation
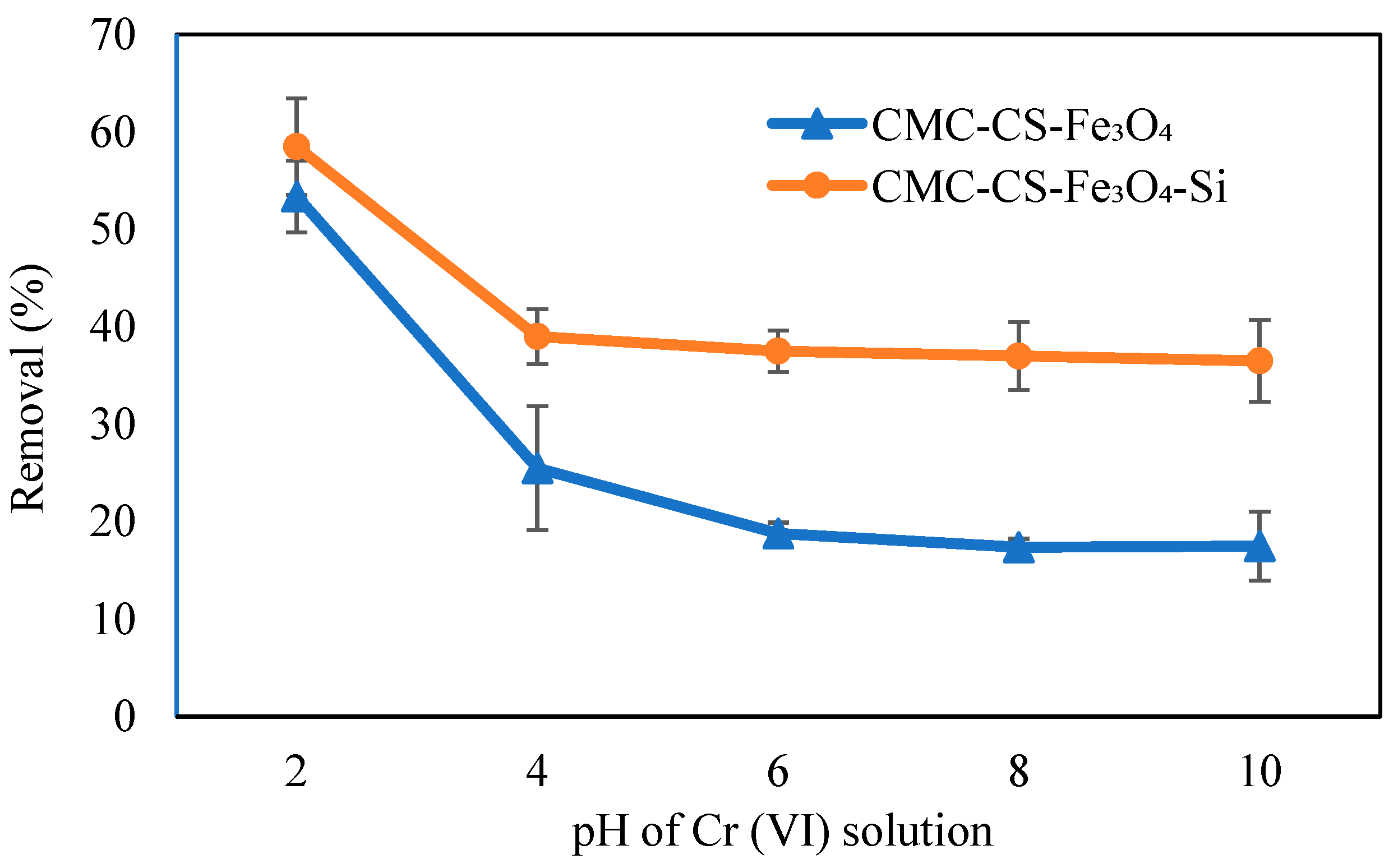
3.6.1. Influence of pH
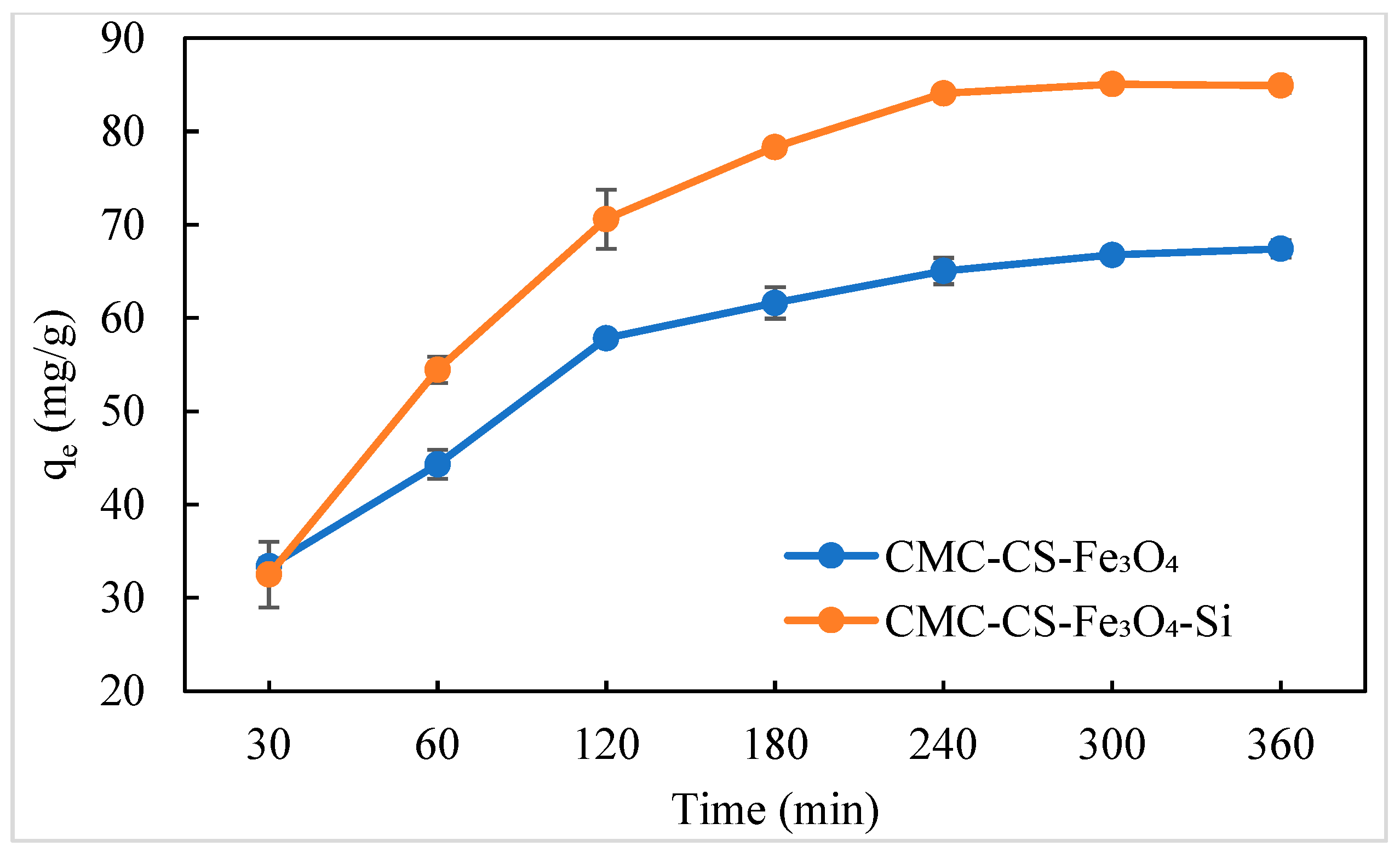
3.6.2. Influence of Contact Time
3.6.3. Effect of Initial Metal Concentration
3.7. Adsorption Kinetic and Isotherm Studies of CMC-CS-Fe3O4-Si
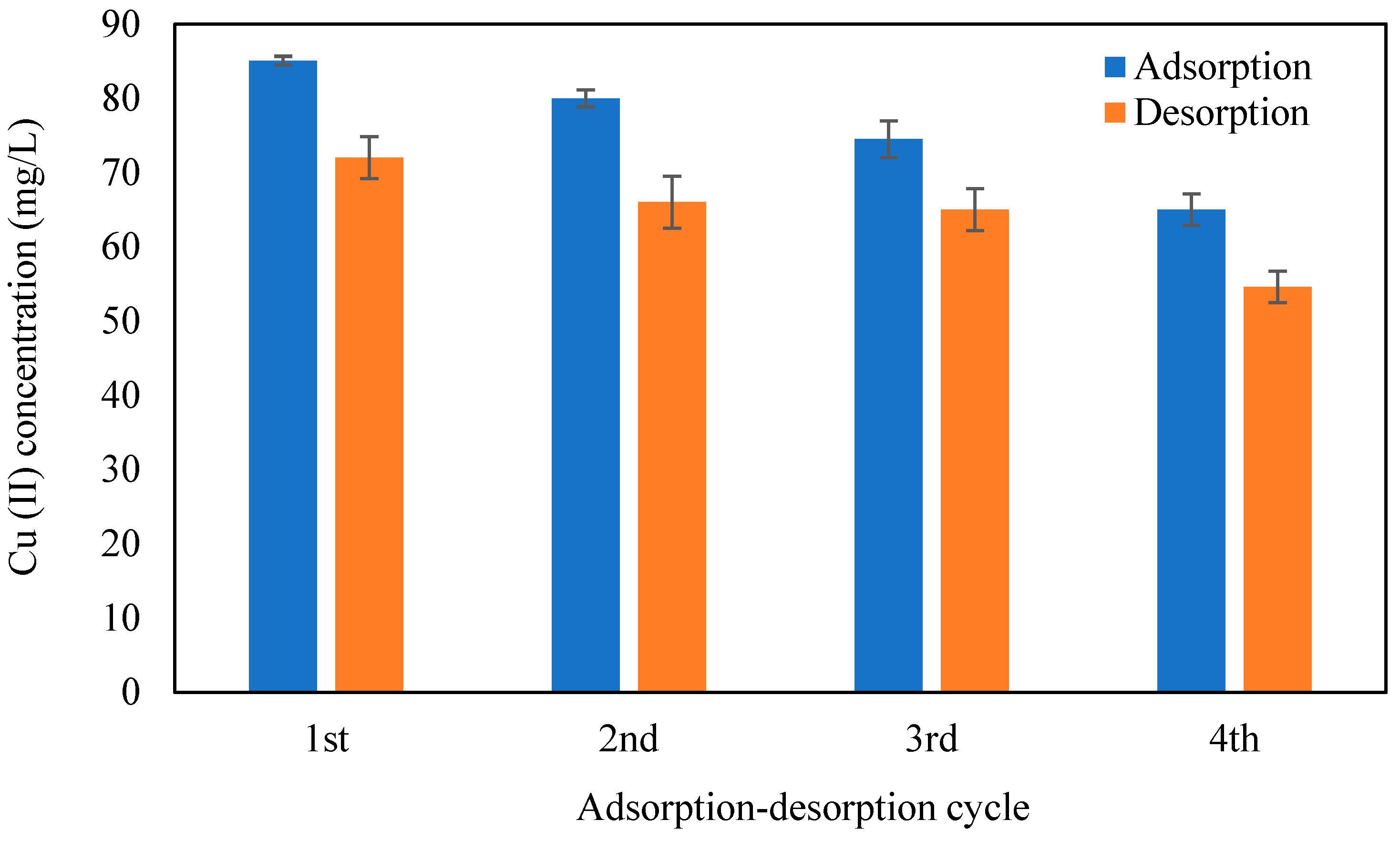
3.8. Desorption of Cr (VI) and Cu (II) and Regeneration of CMC-CS-Fe3O4-Si
3.9. Performance of CMC-CS-Fe3O4-Si for Simultaneous Adsorption of Cr (VI) and Cu (II)
3.10. Effect of Coexisting Ions and Complex Compound on Cr (VI) and Cu (II) Adsorption
3.11. Potential Application for Methylene Blue Removal
3.12. The Comparison of Adsorption Capacity with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Wang, L.; Liu, C.; Bai, Z.; Liu, X. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep. Purif. Technol. 2022, 284, 120099. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods: Isotherms, thermodynamics and kinetics study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019, 54, 89–100. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Xi, X.; Nie, S.; Ke, H.; Liu, Y.; Tong, S.; Chen, Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose 2020, 27, 915–925. [Google Scholar] [CrossRef]
- Sarbani, N.M.M.; Hidayat, E.; Naito, K.; Mitoma, Y.; Harada, H. Cr (VI) and Pb (II) removal using crosslinking magnetite-carboxymethyl cellulose-chitosan hydrogel beads. Gels 2023, 9, 612. [Google Scholar] [CrossRef]
- İlktaç, R.; Bayir, E. Magnetic Hydrogel Beads as a Reusable Adsorbent for Highly Efficient and Rapid Removal of Aluminum: Characterization, Response Surface Methodology Optimization, and Evaluation of Isotherms, Kinetics, and Thermodynamic Studies. ACS Omega 2023, 8, 42440–42456. [Google Scholar] [CrossRef]
- Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Wang, L.; Wu, D. Highly Elastic and Ultratough Hybrid Ionic–Covalent Hydrogels with Tunable Structures and Mechanics. Adv. Mater. 2018, 30, e1707071. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Choi, S.; Choi, Y.J.; Jang, M.; Lee, J.H.; Jeong, J.H.; Kim, J. Supertough Hybrid Hydrogels Consisting of a Polymer Double-Network and Mesoporous Silica Microrods for Mechanically Stimulated On-Demand Drug Delivery. Adv. Funct. Mater. 2017, 27, 1703826. [Google Scholar] [CrossRef]
- Xu, P.; Shang, Z.; Yao, M.; Li, X. Mechanistic insight into improving strength and stability of hydrogels via nano-silica. J. Mol. Liq. 2022, 357, 119094. [Google Scholar] [CrossRef]
- Gautam, N.; Rajesh, Y.; Kale, N.; Jagtap, M.; Chaudhari, H.; Pansare, S. Silica extraction from bamboo leaves using alkaline extraction method. Mater. Today Proc. 2023, 111, 155–160. [Google Scholar] [CrossRef]
- Azman, S.N.H.B.; Ameram, N.B.; Jaafar, H.B.; Amini, M.H.M.; Ali, A. Extraction of Silica from Bamboo Leaves Ash (Bambusoideae) Using Hydrochloric Acid and Nitric Acid. Orbital Electron. J. Chem. 2023, 15, 142–147. [Google Scholar] [CrossRef]
- Ajeel, S.A.; Sukkar, K.A.; Zedin, N.K. Extraction of high purity amorphous silica from rice husk by chemical process. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 3rd International Conference on Sustainable Engineering Techniques (ICSET 2020), Baghdad, Iraq, 15 April 2020; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 881. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Aras, A. Chitosan coated biomass waste-based magnetic hydrogel beads for the removal of methylene blue. Int. J. Phytoremediat. 2024, 26, 1500–1517. [Google Scholar]
- Widjonarko, D.M.; Jumina, J.; Kartini, I.; Nuryono, N. Nuryono Phosphonate modified silica for adsorption of Co(II), Ni(II), Cu(II), and Zn(II). Indones. J. Chem. 2014, 14, 143–151. [Google Scholar]
- Dhaneswara, D.; Fatriansyah, J.F.; Situmorang, F.W.; Haqoh, A.N. Haqoh Synthesis of Amorphous Silica from Rice Husk Ash: Comparing HCl and CH3COOH Acidification Methods and Various Alkaline Concentrations. Int. J. Technol. 2020, 11, 200–208. [Google Scholar] [CrossRef]
- Onikeku, O.; Shitote, S.M.; Mwero, J.; Adedeji, A.A. Evaluation of Characteristics of Concrete Mixed with Bamboo Leaf Ash. Open Constr. Build. Technol. J. 2019, 13, 67–80. [Google Scholar] [CrossRef]
- He, X.; Xu, H.; Li, H. Cr(VI) Removal from Aqueous Solution by Chitosan/Carboxylmethyl Cellulose/Silica Hybrid Membrane. World J. Eng. Technol. 2015, 3, 234–240. [Google Scholar] [CrossRef]
- Benghanem, S.; Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Grafting of oxidized carboxymethyl cellulose with hydrogen peroxide in presence of Cu(II) to chitosan and biological elucidation. Biocybern. Biomed. Eng. 2017, 37, 94–102. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical investigations of chitosan-based hydrogels containing Aloe vera designed for biomedical use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Pu, S.; Ma, H.; Zinchenko, A.; Chu, W. Novel highly porous magnetic hydrogel beads composed of chitosan and sodium citrate: An effective adsorbent for the removal of heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. Int. 2017, 24, 16520–16530. [Google Scholar] [CrossRef]
- Hsiao, M.-H.; Tung, T.-H.; Hsiao, C.-S.; Liu, D.-M. Nano-hybrid carboxymethyl-hexanoyl chitosan modified with (3-aminopropyl)triethoxysilane for camptothecin delivery. Carbohydr. Polym. 2012, 9, 632–639. [Google Scholar] [CrossRef]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous magnetic cellulose-chitosan composite microspheres: Preparation, characterization, and application for Cu(II) adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Nasaj, M.; Farmany, A.; Shokoohizadeh, L.; Jalilian, F.A.; Mahjoub, R.; Roshanaei, G.; Nourian, A.; Shayesteh, O.H.; Arabestani, M. Vancomycin and nisin-modified magnetic Fe3O4@SiO2 nanostructures coated with chitosan to enhance antibacterial efficiency against methicillin resistant Staphylococcus aureus (MRSA) infection in a murine superficial wound model. BMC Chem. 2024, 18, 43. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Pawlak, F.; Tomaszewska, J.; Jesionowski, T. Preparation and Characterization of Novel PVC/Silica–Lignin Composites. Polym. 2015, 7, 1767–1788. [Google Scholar] [CrossRef]
- Parfenyuk, E.V.; Dolinina, E.S. Silica hydrogel composites as a platform for soft drug formulations and cosmetic compositions. Mater. Chem. Phys. 2022, 287, 126160. [Google Scholar] [CrossRef]
- Blachnio, M.; Zienkiewicz-Strzalka, M. Evaluation of the Dye Extraction Using Designed Hydrogels for Further Applications towards Water Treatment. Gels 2024, 10, 159. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Zhu, X.X.; Talbi, M.; Berrada, M. Synthesis, Characterization, and Swelling Properties of a New Highly Absorbent Hydrogel Based on Carboxymethyl Guar Gum Reinforced with Bentonite and Silica Particles for Disposable Hygiene Products. ACS Omega 2022, 7, 39002–39018. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, D.; Hu, Y.; Kim, Y.; Hong, I.K.; Kim, M.S.; Jung, S. pH-responsive succinoglycan-carboxymethyl cellulose hydrogels with highly improved mechanical strength for controlled drug delivery systems. Polymers 2021, 13, 3197. [Google Scholar] [CrossRef]
- Song, H.; Zheng, L. Nanocomposite films based on cellulose reinforced with nano-SiO2: Microstructure, hydrophilicity, thermal stability, and mechanical properties. Cellulose 2013, 20, 1737–1746. [Google Scholar] [CrossRef]
- Sethy, T.R.; Sahoo, P.K. Highly toxic Cr (VI) adsorption by (chitosan-g-PMMA)/silica bionanocomposite prepared via emulsifier-free emulsion polymerisation. Int. J. Biol. Macromol. 2019, 122, 1184–1190. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose–Silica Nanocomposite Aerogels by In Situ Formation of Silica in Cellulose Gel. Angew. Chemie 2012, 124, 2118–2121. [Google Scholar] [CrossRef]
- Ji, D.; Kim, J. Recent Strategies for Strengthening and Stiffening Tough Hydrogels. Adv. NanoBiomed Res. 2021, 1, 2100026. [Google Scholar] [CrossRef]
- Zaragoza, J.; Babhadiashar, N.; O’brien, V.; Chang, A.; Blanco, M.; Zabalegui, A.; Lee, H.; Asuri, P. Experimental Investigation of Mechanical and Thermal Properties of Silica Nanoparticle-Reinforced Poly(acrylamide) Nanocomposite Hydrogels. PLoS ONE 2015, 10, e0136293. [Google Scholar] [CrossRef]
- FitzSimons, T.M.; Anslyn, E.V.; Rosales, A.M. Effect of pH on the Properties of Hydrogels Cross-Linkedvia Dynamic Thia-Michael Addition Bonds. ACS Polym. Au 2022, 2, 129. [Google Scholar] [CrossRef]
- Guo, H.; Ge, J.; Li, L.; Zhang, G.; Li, Z.; Wang, W.; Liu, M. New Insights and Experimental Investigation of High-Temperature Gel Reinforced by Nano-SiO2. Gels 2022, 8, 362. [Google Scholar] [CrossRef]
- Kaseem, M.; Rehman, Z.U.; Hossain, S.; Singh, A.K.; Dikici, B. A Review on Synthesis, Properties, and Applications of Polylactic Acid/Silica Composites. Polymers 2021, 13, 3036. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, K.; Li, P.; He, X.; Li, W.; Wu, Y. Effect of nano-SiO2 on the compatibility interface and properties of polylactic acid-grafted-bamboo fiber/polylactic acid composite. Int. J. Biol. Macromol. 2020, 157, 177–186. [Google Scholar] [CrossRef]
- Li, S.-S.; Wang, X.-L.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Cui, L.; Li, Z.-C. Upon designing carboxyl methylcellulose and chitosan-derived nanostructured sorbents for efficient removal of Cd(II) and Cr(VI) from water. Int. J. Biol. Macromol. 2020, 143, 640–650. [Google Scholar] [CrossRef]
- Alsamman, M.T.; Sánchez, J. Adsorption of Copper and Arsenic from Water Using a Semi-Interpenetrating Polymer Network Based on Alginate and Chitosan. Polym. 2023, 15, 2192. [Google Scholar] [CrossRef]
- Samani, M.; Ahlawat, Y.K.; Golchin, A.; Alikhani, H.A.; Fathi-Gerdelidani, A.; Ahlawat, U.; Malik, A.; Panwar, R.; Maan, D.S.; Ahmed, M.; et al. Nano silica-mediated stabilization of heavy metals in contaminated soils. Sci. Rep. 2024, 14, 20496. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.D.; Yi, C.H.; Zhang, H.Y.; Qiu, K.Y.; Wang, Y.Y. Stabilization of Cd-contaminated agricultural soils by modified nano-silica. Environ. Chem. 2019, 38, 1106–1112. [Google Scholar]
- Park, J.E.; Shin, J.-H.; Oh, W.; Choi, S.-J.; Kim, J.; Kim, C.; Jeon, J. Removal of hexavalent chromium (VI) from wastewater using chitosan-coated iron oxide nanocomposite membranes. Toxics 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Omer, A.; Hu, Z.; Yang, L.-Y.; Ji, C.; Ouyang, X.-K. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, J.; Wang, X.; Li, Y.; Lin, S.; Han, J. Adsorption removal of copper (II) and chromium (VI) from wastewater by Fe3O4-loaded granular activated carbon. Water Pract. Technol. 2024, 19, 99–112. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Adamczuk, A.; Kołodyńska, D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 2015, 274, 200–212. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Galhoum, A.A.; Alshahrie, A.; Al-Turki, Y.A.; Al-Amri, A.M.; Wageh, S. Wageh Superparamagnetic Multifunctionalized Chitosan Nanohybrids for Efficient Copper Adsorption: Comparative Performance, Stability, and Mechanism Insights. Polymers 2023, 15, 1157. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Tijani, J.O.; Jacob, J.O.; Suleiman, M.A.T. Simultaneous removal of Cu(II) and Cr(VI) ions from petroleum refinery wastewater using ZnO/Fe3O4 nanocomposite. J. Environ. Sci. Health Part A 2023, 57, 1146–1167. [Google Scholar] [CrossRef]
- Pan, J.; Gao, B.; Guo, K.; Gao, Y.; Xu, X.; Yue, Q. Insights into selective adsorption mechanism of copper and zinc ions onto biogas residue-based adsorbent: Theoretical calculation and electronegativity difference. Sci. Total Environ. 2022, 805, 150413. [Google Scholar] [CrossRef]
- Pei, Y.; Li, M.; Li, W.; Su, K.; Chen, J.; Yang, H.; Hu, D.; Zhang, S. Cr(VI) removal by cellulose-based composite adsorbent with a double-network structure. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126963. [Google Scholar] [CrossRef]
- Rahman, A.; Lamb, D.; Rahman, M.M.; Bahar, M.; Sanderson, P. Adsorption-Desorption Behavior of Arsenate Using Single and Binary Iron-Modified Biochars: Thermodynamics and Redox Transformation. ACS Omega 2022, 7, 101–117. [Google Scholar] [CrossRef]
- Ling, C.; Liu, F.; Pei, Z.; Zhang, X.; Wei, M.; Zhang, Y.; Zheng, L.; Zhang, J.; Li, A.; Xing, B. Citric Acid Enhanced Copper Removal by a Novel Multi-amines Decorated Resin. Sci. Reports 2015, 5, 9944. [Google Scholar] [CrossRef]
- Huber, M.; Hilbig, H.; Badenberg, S.C.; Fassnacht, J.; Drewes, J.E.; Helmreich, B. Heavy metal removal mechanisms of sorptive filter materials for road runoff treatment and remobilization under de-icing salt applications. Water Res. 2016, 102, 453–463. [Google Scholar] [CrossRef] [PubMed]
- El-Hefnawy, M.E.; Selim, E.M.; Assaad, F.F.; Ismail, A.I. The effect of chloride and sulfate ions on the adsorption of Cd2+ on clay and sandy loam Egyptian soils. Sci. World J. 2014, 2014, 806252. [Google Scholar] [CrossRef]
- Zhong, C.; Lee, D.H.; Kim, J.H.; Kang, J.-H. Adsorption of dissolved copper and zinc on sand and iron oxide-coated sand (IOCS) for urban stormwater treatment: Effects of pH, chloride, and sulfate. Desalination Water Treat. 2021, 219, 319–326. [Google Scholar] [CrossRef]
- Sabar, S.; Aziz, H.A.; Yusof, N.; Subramaniam, S.; Foo, K.; Wilson, L.; Lee, H. Preparation of sulfonated chitosan for enhanced adsorption of methylene blue from aqueous solution. React. Funct. Polym. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Shah, S.S.; Ramos, B.; Teixeira, A.C.S.C. Adsorptive Removal of Methylene Blue Dye Using Biodegradable Superabsorbent Hydrogel Polymer Composite Incorporated with Activated Charcoal. Water 2022, 14, 3313. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, W.; Tu, W.; Zhu, M. Facile Cross-Link Method to Synthesize Magnetic Fe3O4@SiO2-Chitosan with High Adsorption Capacity toward Hexavalent Chromium. J. Chem. Eng. Data 2019, 64, 226–233. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Nguyen, M.-D.; Nguyen, Q.H.; Do, T.-T.; Luu, T.-T.; Luu, A.T.; Tap, T.D.; Ho, T.-H.; Phan, T.P.; Nguyen, T.D.; et al. Chitosan-MnO2 nanocomposite for effective removal of Cr (VI) from aqueous solution. Chemosphere 2020, 257, 127147. [Google Scholar] [CrossRef]
- Chen, D.; Li, W.; Wu, Y.; Zhu, Q.; Lu, Z.; Du, G. Preparation and characterization of chitosan/montmorillonite magnetic microspheres and its application for the removal of Cr (VI). Chem. Eng. J. 2013, 221, 8–15. [Google Scholar] [CrossRef]
- Shahnaz, T.; Sharma, V.; Subbiah, S.; Narayanasamy, S. Multivariate optimisation of Cr (VI), Co (III) and Cu (II) adsorption onto nanobentonite incorporated nanocellulose/chitosan aerogel using response surface methodology. J. Water Process Eng. 2020, 36, 101283. [Google Scholar] [CrossRef]
- Wang, H.; Fang, S.; Zuo, M.; Li, Z.; Yu, X.; Tang, X.; Sun, Y.; Yang, S.; Zeng, X.; Lin, L. Removal of copper ions by cellulose nanocrystal-based hydrogel and reduced adsorbents for its catalytic properties. Cellulose 2022, 29, 4525–4537. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Microcrystalline cellulose (MCC) as an adsorbent in copper removal from aqueous solution. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 32nd Symposium of Malaysian Chemical Engineers (SOMChE2021), Kuching, Sarawak, 15-16 July 2021; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 1195, p. 012022. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Influence of pH on Cr(VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 1st International Symposium on Green Technology for Value Chains, Tangerang, Indonesia, 3–5 October 2016; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 60, p. 012010. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Synthesis, Characterization, Kinetics, and Thermodynamics of EDTA-Modified Chitosan-Carboxymethyl Cellulose as Cu(II) Ion Adsorbent. ACS Omega 2019, 4, 17425–17437. [Google Scholar] [CrossRef] [PubMed]
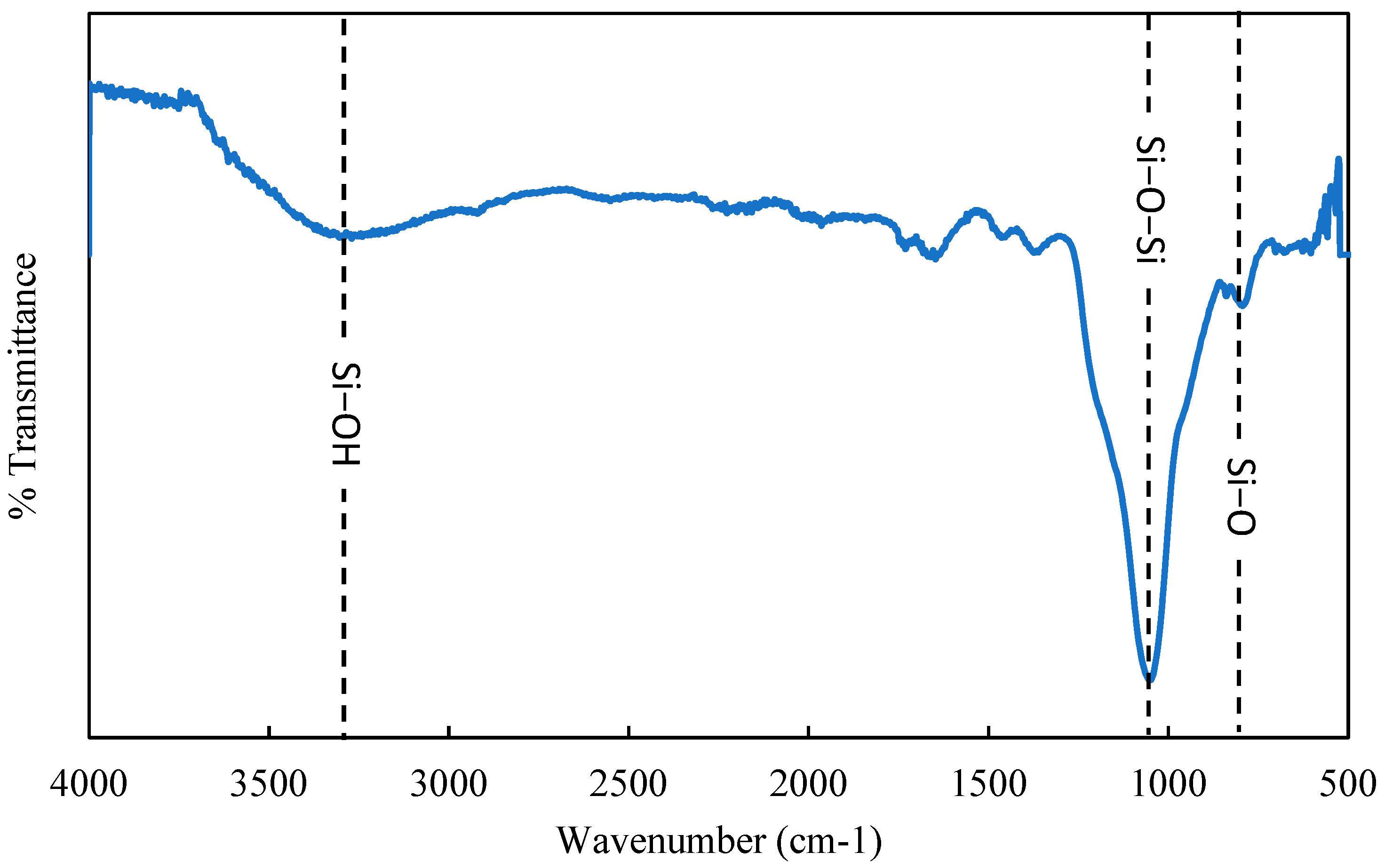


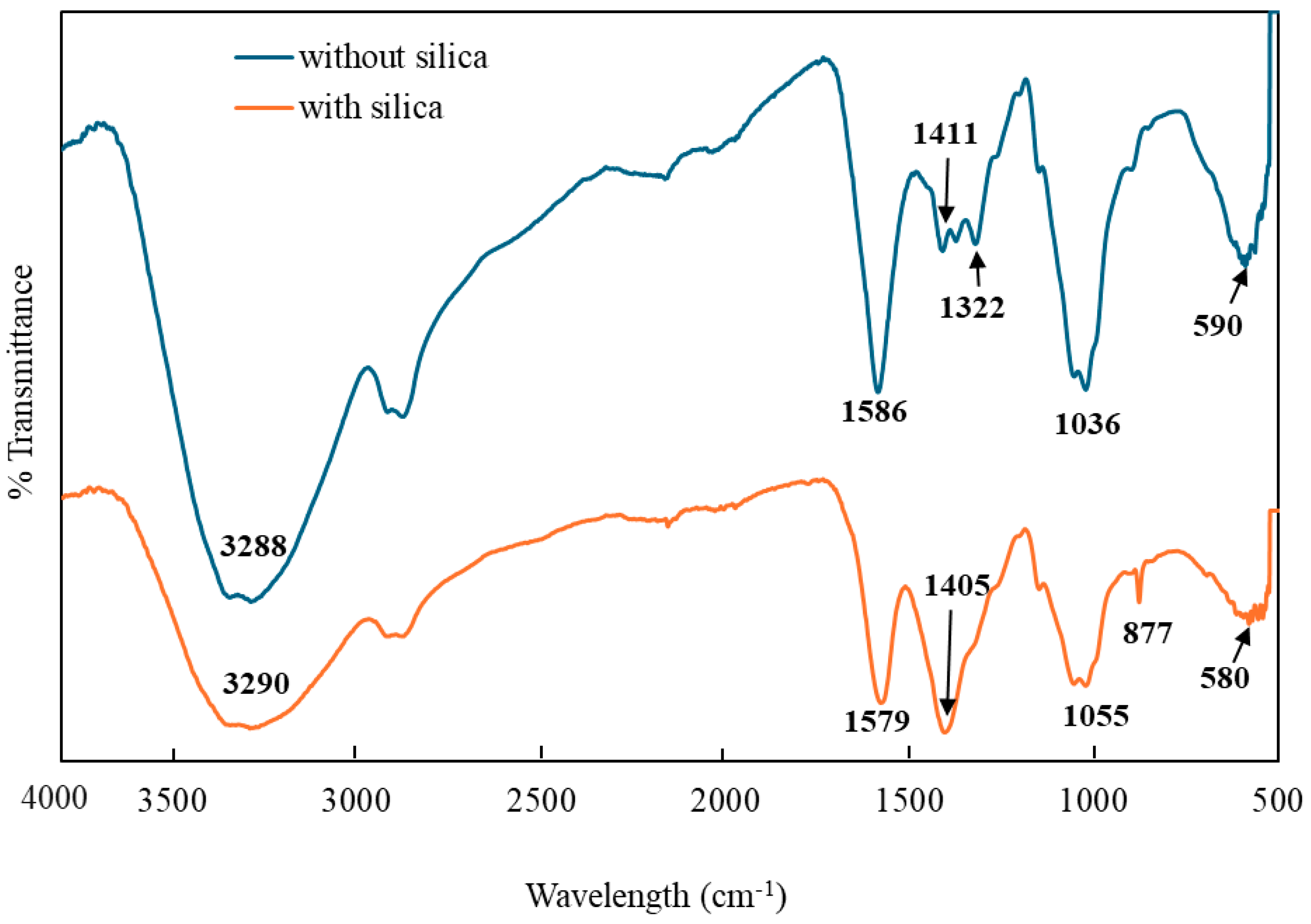
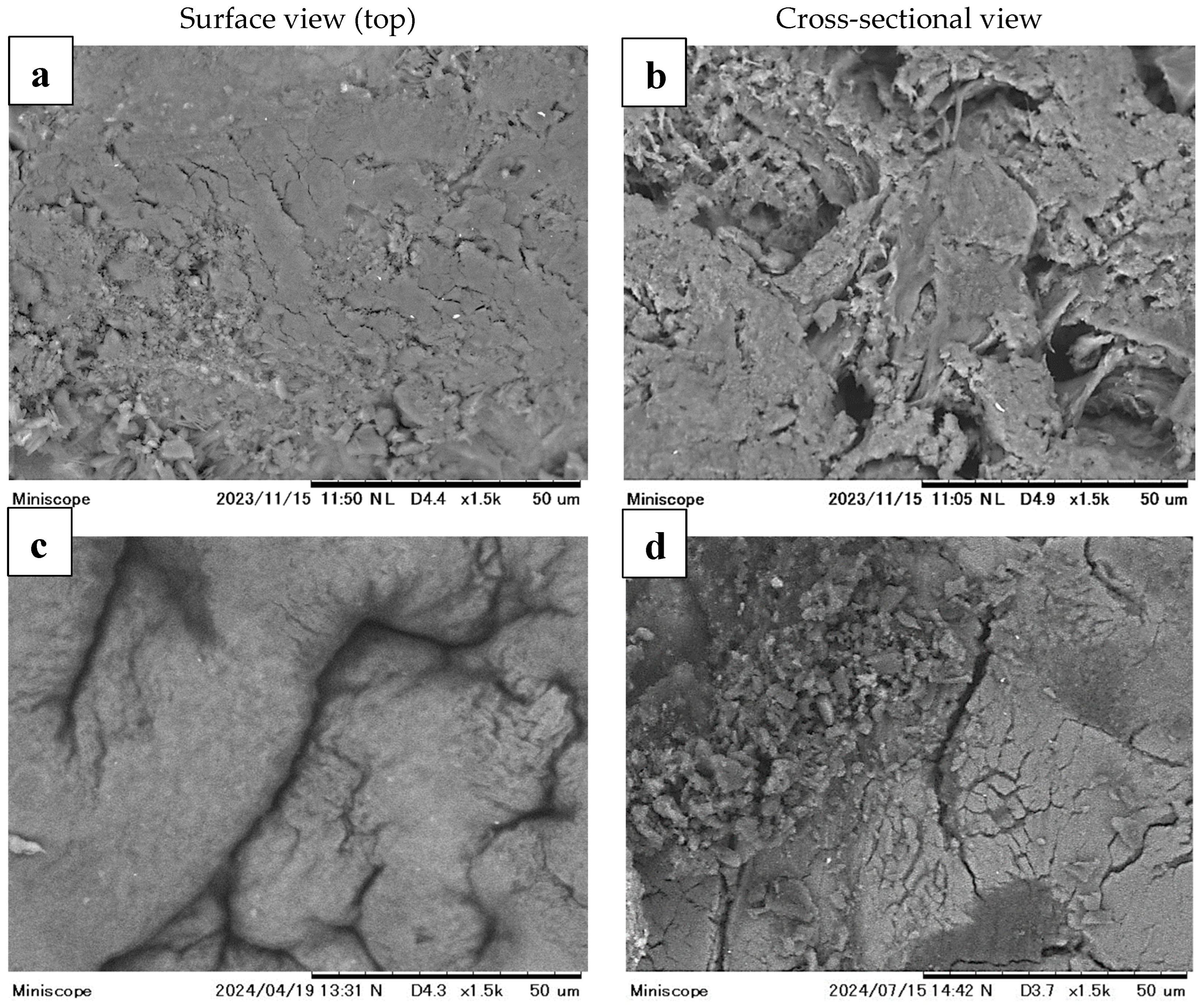
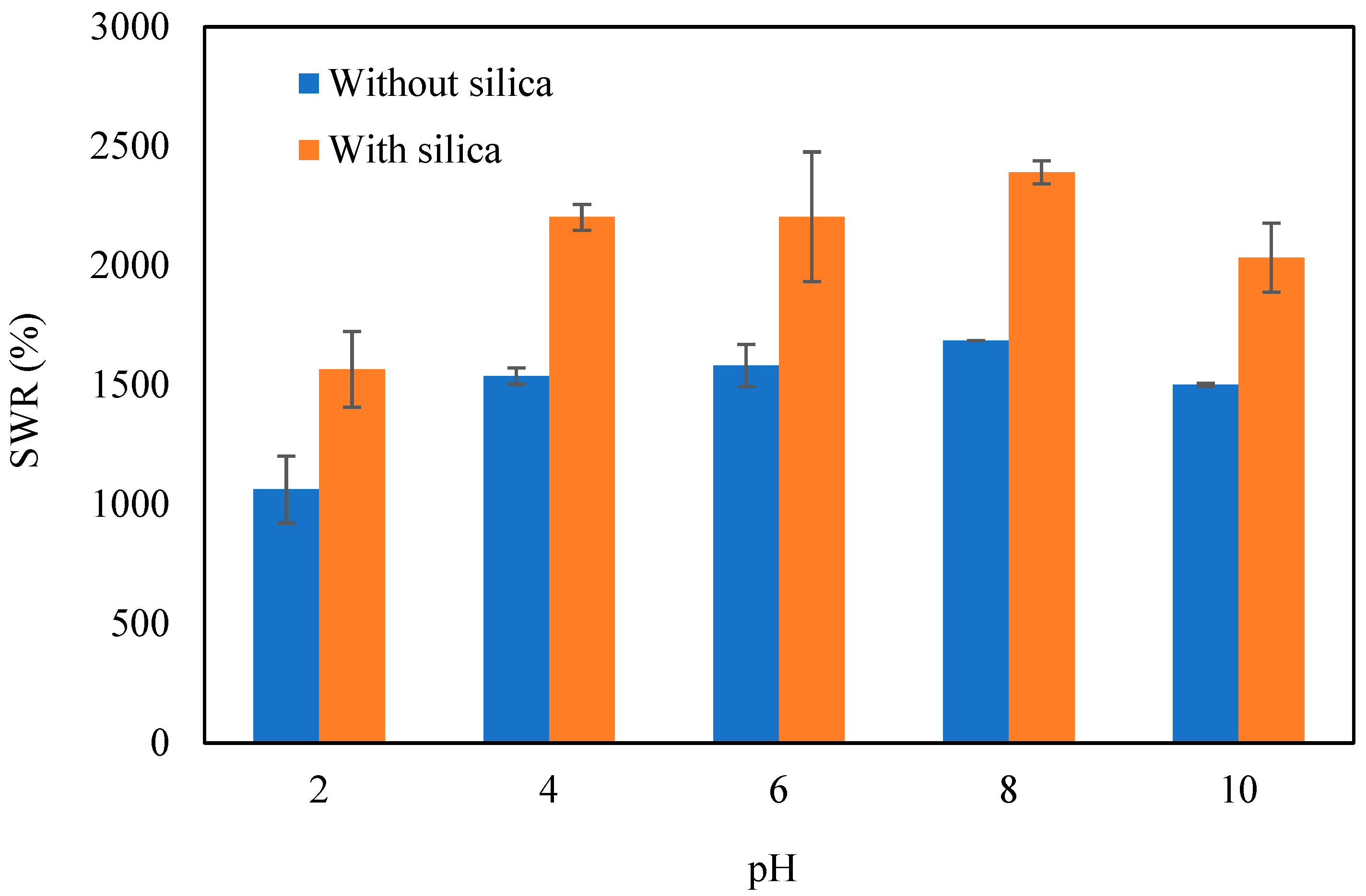
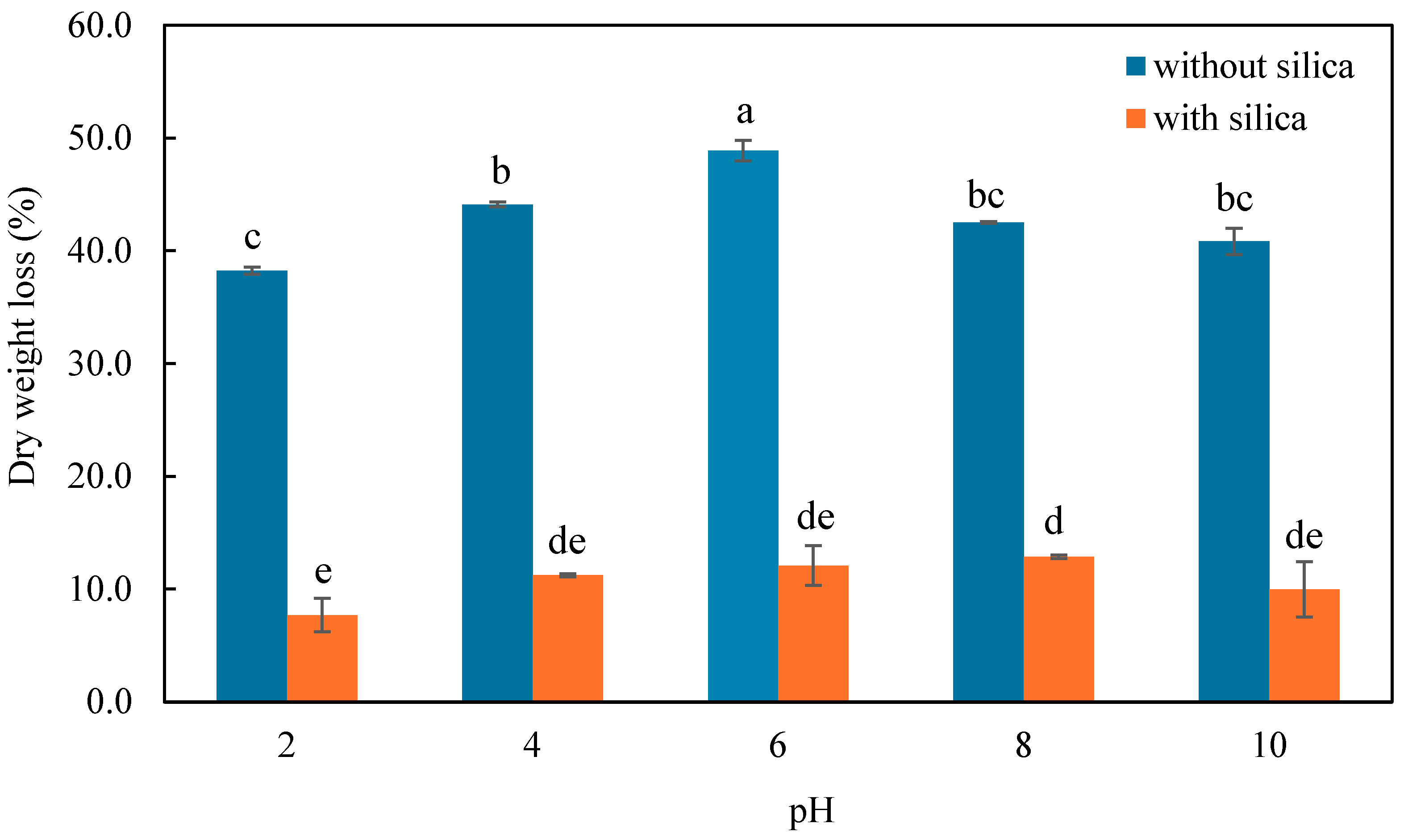










| Elements | Mass % | Atom % |
|---|---|---|
| Si | 47.47 | 33.99 |
| O | 52.53 | 66.01 |
| Adsorption Kinetic Models | Parameters | Cr (VI) | Cu (II) |
|---|---|---|---|
| Pseudo-first-order | 25.9119 | 63.1684 | |
| 0.0078 | 0.0081 | ||
| R2 | 0.9341 | 0.9678 | |
| Pseudo-second-order | 57.8035 | 103.0928 | |
| 0.0007 | 0.0017 | ||
| R2 | 0.9988 | 0.9977 |
| Adsorption Isotherm | Parameters | Cr (VI) | Cu (II) |
|---|---|---|---|
| Langmuir | 94.3396 | 113.6364 | |
| 0.0241 | 0.0260 | ||
| 0.2932 | 0.2780 | ||
| R2 | 0.9298 | 0.6420 | |
| Freundlich | 3.721 | 1.9519 | |
| 1/n | 0.6877 | 1.3324 | |
| R2 | 0.9798 | 0.9592 |
| Adsorbents | Adsorbate | Adsorption Capacity | References |
|---|---|---|---|
| Magnetic magnetite nanoparticle | Chromium (VI) Copper (II) | 8.67 mg/g 18.61 mg/g | [69] |
| Magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel | Copper (II) | 56.8 mg/g | [54] |
| Fe3O4@SiO-CS composite | Chromium (VI) | 96.2 mg/g | [70] |
| Chitosan-manganese oxide nanocomposite | Chromium (VI) | 61.56 mg/g | [71] |
| Chitosan/Montmorillonite-Fe3O4 microsphere | Chromium (VI) | 58.8 mg/g | [72] |
| Nanobentonite/Nanocellulose/Chitosan aerogel | Chromium (VI) | 98.9 mg/g | [73] |
| Cellulose nanocrystal/carboxymethylcellulose-sodium/polyvinyl alcohol hydrogel | Copper (II) | 108 mg/g | [74] |
| Microcrystalline cellulose (MCC) | Copper (II) | 0.44 mg/g | [75] |
| Carboxymethyl cellulose-graft-poly(acrylic acid) | Chromium (VI) | 6.53 mg/g | [76] |
| Carboxymethyl cellulose/chitosan/silica cross-linked GPTMS | Chromium (VI) | 16.08 mg/g | [28] |
| EDTA-modified chitosan-carboxymethyl cellulose | Copper (II) | 142.95 mg/g | [77] |
| CMC-Cs-Fe3O4-Si | Chromium (VI) Copper (II) | 53.00 mg/g 85.06 mg/g | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Sarbani, N.M.; Hidayat, E.; Naito, K.; Aoyagi, M.; Harada, H. Enhanced Stability and Adsorption of Cross-Linked Magnetite Hydrogel Beads via Silica Impregnation. J. Compos. Sci. 2025, 9, 152. https://doi.org/10.3390/jcs9040152
Mohamad Sarbani NM, Hidayat E, Naito K, Aoyagi M, Harada H. Enhanced Stability and Adsorption of Cross-Linked Magnetite Hydrogel Beads via Silica Impregnation. Journal of Composites Science. 2025; 9(4):152. https://doi.org/10.3390/jcs9040152
Chicago/Turabian StyleMohamad Sarbani, Nur Maisarah, Endar Hidayat, Kanako Naito, Mitsuru Aoyagi, and Hiroyuki Harada. 2025. "Enhanced Stability and Adsorption of Cross-Linked Magnetite Hydrogel Beads via Silica Impregnation" Journal of Composites Science 9, no. 4: 152. https://doi.org/10.3390/jcs9040152
APA StyleMohamad Sarbani, N. M., Hidayat, E., Naito, K., Aoyagi, M., & Harada, H. (2025). Enhanced Stability and Adsorption of Cross-Linked Magnetite Hydrogel Beads via Silica Impregnation. Journal of Composites Science, 9(4), 152. https://doi.org/10.3390/jcs9040152







