Preliminary Study on Syngas Production from a CO2 and CH4 Mixture via Non-Thermal Dielectric Barrier Discharge Plasma Incorporated with Metal–Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-8, NH2-UiO-66, and NH2-MIL-53(Al) Catalysts
2.3. Characterization of ZIF-8, NH2-UiO-66, and NH2-MIL-53(Al) Catalysts
2.4. Experimental Runs for Non-Thermal DBD Plasma DRM
3. Results and Discussion
3.1. Characterization Study of ZIF-8, NH2-UiO-66, and NH2-MIL-53(Al) Catalysts
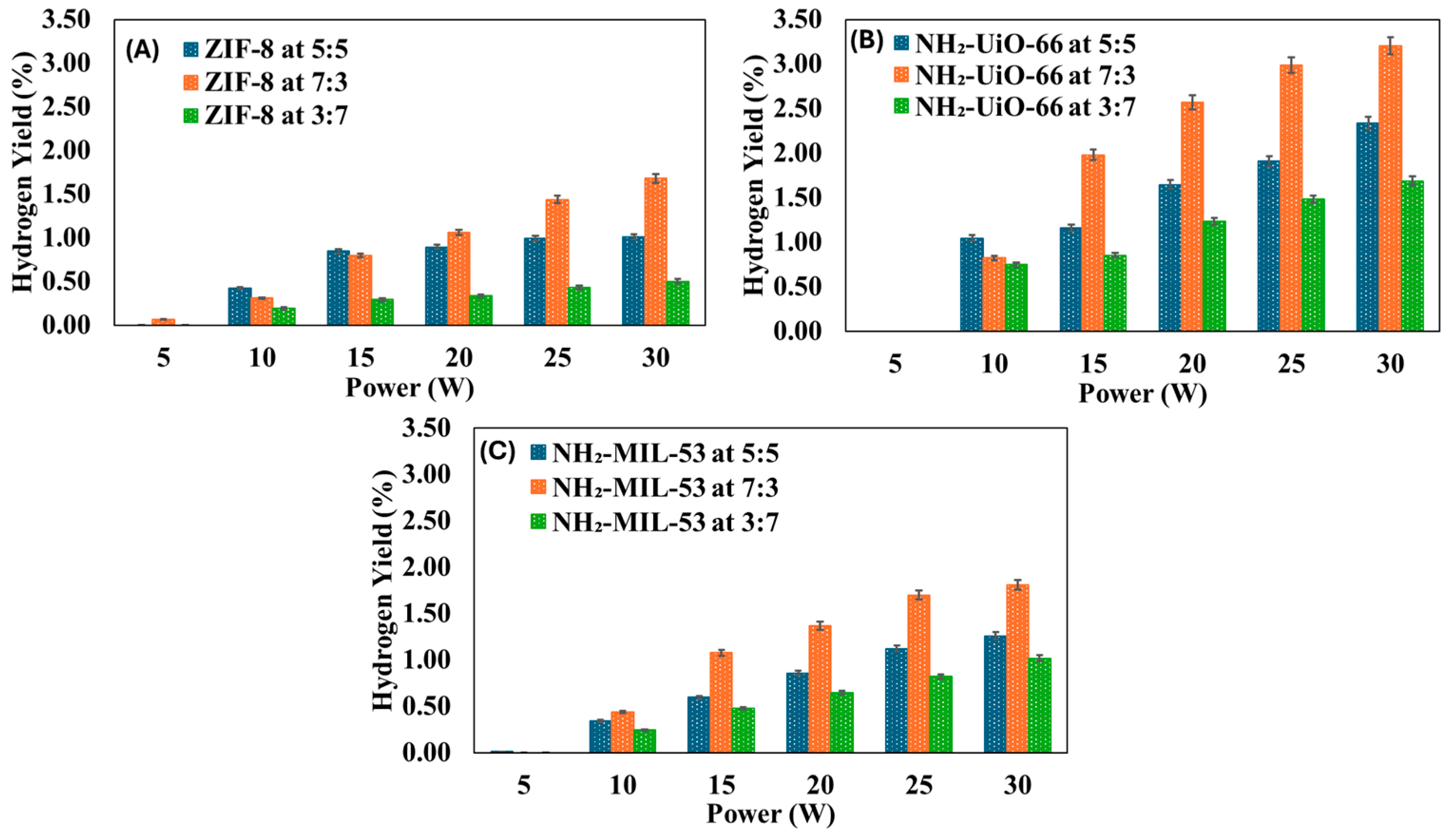
3.2. Effect of CO2:CH4 Molar Ratio on Syngas Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. CO2 Emissions in 2022; IEA: Paris, France, 2023. [Google Scholar]
- Li, D.; Rohani, V.; Fabry, F.; Parakkulam Ramaswamy, A.; Sennour, M.; Fulcheri, L. Direct conversion of CO2 and CH4 into liquid chemicals by plasma-catalysis. Appl. Catal. B Environ. 2020, 261, 118228. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil kumar, P.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Anantha Narayanan, V.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology—A novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef]
- Rahim, I.; Nomura, S.; Mukasa, S.; Toyota, H. Decomposition of methane hydrate for hydrogen production using microwave and radio frequency in-liquid plasma methods. Appl. Therm. Eng. 2015, 90, 120–126. [Google Scholar] [CrossRef]
- D’Angola, A.; Colonna, G.; Kustova, E. Editorial: Thermal and Non-Thermal Plasmas at Atmospheric Pressure. Front. Phys. 2022, 10, 852905. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts. Appl. Catal. B Environ. 2020, 260, 118195. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Trinh, Q.H.; Hossain, M.M.; Lee, W.G.; Mok, Y.S. Enhancement of plasma-assisted catalytic CO2 reforming of CH4 to syngas by avoiding outside air discharges from ground electrode. Int. J. Hydrogen Energy 2020, 45, 18519–18532. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Malik, M.I.; Achouri, I.E.; Abatzoglou, N.; Gitzhofer, F. Intensified performance of methane dry reforming based on non-thermal plasma technology: Recent progress and key challenges. Fuel Process. Technol. 2023, 245, 107748. [Google Scholar] [CrossRef]
- Lai, L.S.; Yeong, Y.F.; Ani, N.C.; Lau, K.K.; Shariff, A.M. Effect of Synthesis Parameters on the Formation of Zeolitic Imidazolate Framework 8 (ZIF-8) Nanoparticles for CO2 Adsorption. Part. Sci. Technol. 2014, 32, 520–528. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Li, Q.; Guan, K.; Li, Y.; Jin, W. UiO-66-polyether block amide mixed matrix membranes for CO2 separation. J. Membr. Sci. 2016, 513, 155–165. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Chew, T.L.; Lau, K.K. Optimization of spinning parameters on the fabrication of NH2-MIL-53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding Applications of Metal–Organic Frameworks: Zeolite Imidazolate Framework ZIF-8 as an Efficient Heterogeneous Catalyst for the Knoevenagel Reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Ge, J.; Liu, L.; Shen, Y. Facile synthesis of amine-functionalized UiO-66 by microwave method and application for methylene blue adsorption. J. Porous Mater. 2016, 24, 647–655. [Google Scholar] [CrossRef]
- Rakhshani, N.; Hassanzadeh Nemati, N.; Saadatabadi, A.R.; Sadrnezhaad, S.K. Fabrication of novel poly(N-vinylcaprolactam)-coated UiO-66-NH2 metal organic framework nanocarrier for the controlled release of doxorubicin against A549 lung cancer cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102881. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53(Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Miao, Y.; Yokochi, A.; Jovanovic, G.; Zhang, S.; von Jouanne, A. Application-oriented non-thermal plasma in chemical reaction engineering: A review. Green Energy Resour. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.; Qin, W.; Wu, H.; Liu, B.; Li, S.; Bogaerts, A. Plasma-catalytic dry reforming of CH4: Effects of plasma-generated species on the surface chemistry. Chem. Eng. J. 2024, 498, 155847. [Google Scholar] [CrossRef]
- Kim, J.; Jeoung, J.; Jeon, J.; Kim, J.; Mok, Y.S.; Ha, K.-S. Effects of dielectric particles on non-oxidative coupling of methane in a dielectric barrier discharge plasma reactor. Chem. Eng. J. 2019, 377, 119896. [Google Scholar] [CrossRef]
- Ozkan, A.; Dufour, T.; Arnoult, G.; De Keyzer, P.; Bogaerts, A.; Reniers, F. CO2–CH4 conversion and syngas formation at atmospheric pressure using a multi-electrode dielectric barrier discharge. J. CO2 Util. 2015, 9, 74–81. [Google Scholar] [CrossRef]
- Feng, J.; Sun, X.; Li, Z.; Hao, X.; Fan, M.; Ning, P.; Li, K. Plasma-Assisted Reforming of Methane. Adv. Sci. 2022, 9, 2203221. [Google Scholar] [CrossRef] [PubMed]




| Catalysts | BET Surface Area (m2/g) |
|---|---|
| ZIF-8 | 1157.92 |
| NH2-UiO-66(Zr) | 527.74 |
| NH2-MIL-53(Al) | 239.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunder, N.; Yin Fong, Y.; Mun, S.L.S. Preliminary Study on Syngas Production from a CO2 and CH4 Mixture via Non-Thermal Dielectric Barrier Discharge Plasma Incorporated with Metal–Organic Frameworks. J. Compos. Sci. 2025, 9, 148. https://doi.org/10.3390/jcs9040148
Sunder N, Yin Fong Y, Mun SLS. Preliminary Study on Syngas Production from a CO2 and CH4 Mixture via Non-Thermal Dielectric Barrier Discharge Plasma Incorporated with Metal–Organic Frameworks. Journal of Composites Science. 2025; 9(4):148. https://doi.org/10.3390/jcs9040148
Chicago/Turabian StyleSunder, Naveen, Yeong Yin Fong, and Serene L. S. Mun. 2025. "Preliminary Study on Syngas Production from a CO2 and CH4 Mixture via Non-Thermal Dielectric Barrier Discharge Plasma Incorporated with Metal–Organic Frameworks" Journal of Composites Science 9, no. 4: 148. https://doi.org/10.3390/jcs9040148
APA StyleSunder, N., Yin Fong, Y., & Mun, S. L. S. (2025). Preliminary Study on Syngas Production from a CO2 and CH4 Mixture via Non-Thermal Dielectric Barrier Discharge Plasma Incorporated with Metal–Organic Frameworks. Journal of Composites Science, 9(4), 148. https://doi.org/10.3390/jcs9040148






