Abstract
This paper reviews the performance of low-grade calcined clay as a partial substitute for Portland cement in concrete, emphasizing its potential to enhance sustainability in construction. Thermal treatment of naturally occurring clays at optimal temperatures produces amorphous siliceous materials with pozzolanic properties. Clays with substantial kaolinite content exhibit significant pozzolanic reactivity when calcined at temperatures between 700 and 850 °C, with effective firing possible up to 1000 °C. Research shows that replacing Portland cement with calcined clays improves the mechanical and durability properties of concrete, with replacement levels ranging from 10% to 60%, depending on factors such as chemical composition, mineralogy, and reactivity. This paper synthesizes recent findings on low-grade calcined clays with 60–80% purity, which are more abundant, cost-effective, and easier to produce, particularly in developing regions lacking the resources and technology to process high-purity clays (>95% purity). Key aspects explored include calcination methods, optimal firing temperatures, and their effects on particle size distribution and pozzolanic activity. This study also examines the impact of low-grade calcined clay on fresh and hardened concrete and the durability properties of concrete and mortar. By providing a comprehensive analysis, this review highlights the potential of low-grade calcined clays to contribute to more sustainable and durable concrete production, emphasizing the need to optimize calcination processes and fully harness their pozzolanic properties.
1. Introduction
Sustainability has become a global priority due to the ongoing depletion of the ozone layer and the resulting challenges of global warming and climate change. In response, the traditional linear economy model of “make, use, dispose” is being replaced by the circular economy, which focuses on keeping resources in use for as long as possible while maximizing their value. To contribute to this global effort against climate change, the construction industry has increasingly embraced green and eco-friendly materials for housing and infrastructure applications.
There is an ever-growing need for infrastructure due to population growth and urbanization and this has necessitated an increase in cement production over the years. Cement production, however, has been known to contribute greenhouse gases directly to the environment due to the decomposition of calcium carbonate to produce lime and carbon dioxide [1,2]. It is an established fact that carbon dioxide emissions originating from cement production account for about 8% of total global anthropogenic CO2 release [3,4,5] In the clinkerization process, CO2 mainly originates from raw material decomposition (CaCO3) and to a lesser extent from fuel burning. Both processes contribute 60–70% and 30–40%, respectively, to the direct CO2 emissions from clinkerization [6]. Consequently, the use of supplementary cementitious materials (SCMs) has gained considerable popularity and attention over the years due to their technical advantages and, largely, environmental benefits [7,8]. Figure 1 presents the global cement production between 2008 and 2022 produced from data published by the International Energy Agency (IEA) [9].
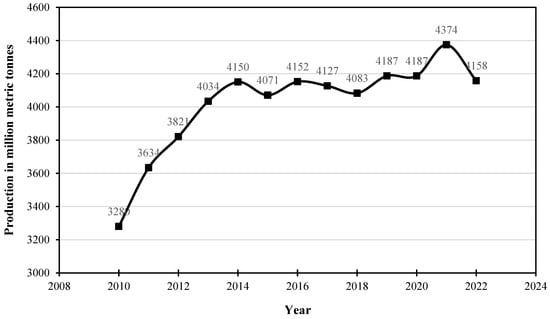
Figure 1.
Global production of cement from 2010–2022.
Supplementary cementitious materials (SCMs) are soluble siliceous, aluminosiliceous, or calcium aluminosiliceous powders used as partial replacements of clinker in cements or as partial replacements of Portland cement in concrete mixtures [10,11,12] They usually comprise natural materials or industrial and agricultural waste. Examples of such SCMs are pulverized fly ash obtained from coal-fired power plants, silica fume obtained as a by-product from the production of elemental silica, calcined clays obtained from the burning of natural clays, steel slag obtained as a by-product of steel production [13], and rice husk ash, also obtained as agricultural waste [14]. These materials have been known to improve concrete properties such as compressive strength, durability, and impermeability through hydraulic or pozzolanic activity [14,15,16,17].
One SCM that has garnered significant attention in recent years is calcined clay. Calcined clays, prepared with clays with high kaolinite [18,19], have been used to substitute cement in concrete over the years due to their excellent reactivity and refinement of microstructure, resulting in enhanced durability and mechanical performance [20]. Nevertheless, the accessibility of high-grade kaolinitic clays poses a challenge as they are confined to certain geographical areas worldwide. Additionally, there is an increasing need for kaolinite-rich clays in the manufacturing of diverse industrial products. This rivalry adds to the elevated expense associated with the utilization of kaolinite-rich clays as SCMs for concrete applications [21,22].
Clays are typically categorized based on their internal structure into two main groups: 1:1 clay minerals (e.g., kaolinite) and 2:1 clay minerals (e.g., illite and montmorillonite). They are predominantly composed of fine mineral particles with sizes generally below 20 µm. In nature, clay deposits are often found as intricate mixtures of clay minerals and non-clay minerals, reflecting their complex geological origins [23]. Clay, when thermally activated at the right temperature, is widely used as a pozzolanic material. Clays having a significant proportion of kaolinite have proven to be highly pozzolanic if calcined between about 600 and 850 °C [18,24], although it can be exceptionally fired up to 1000 °C [25,26]. Kaolinite undergoes thermal activation to form metakaolin, a highly reactive pozzolanic material. This transformation occurs through the dehydroxylation of kaolinite at elevated temperatures, resulting in an amorphous aluminosilicate phase that readily participates in pozzolanic reactions with calcium hydroxide to form additional cementitious compounds [27]. This process significantly enhances the material’s contribution to the strength and durability of blended cementitious systems. The pozzolanic activity of calcined clay is associated with the removal of structural water from the clay layers [26]. The successful calcination of clays offers significant potential for sustainable construction practices. By utilizing abundant and naturally occurring clay minerals, calcined clays can serve as a low-carbon alternative to traditional clinker in cement production. This approach aligns with global efforts to reduce greenhouse gas emissions in the cement and concrete industry. The combination of optimized thermal activation parameters and advanced grinding techniques ensures that calcined clays can meet the performance demands of modern construction materials.
Several researchers [27,28,29,30,31,32,33] have studied and proven that the partial replacement of Portland cement with calcined clays improves the mechanical and durability properties of mortar and concrete. The extent of the performance of calcined clay in concrete has been known to depend on chemical and mineralogical factors, as well as the pozzolanic reactivity, degree of replacement, and other external factors. Several materials have been published about the calcination processes, pozzolanic reactivity, and strength and durability properties of calcined clay concrete [34,35]. The innovation of this review paper lies in its focused evaluation of calcined impure kaolinitic clay as a sustainable and technically viable partial replacement for Portland cement, addressing both performance attributes and practical implementation challenges. Unlike previous reviews that broadly discuss calcined clays, this paper uniquely examines the role of kaolinite content, their calcination optimization, and the synergistic effects with clinker hydration mechanisms. Furthermore, it integrates recent advancements in characterization techniques, durability assessments, and real-world applicability—bridging the gap between laboratory research and industrial-scale adoption.
2. Calcination of Clays
Clays need to be thermally activated to unlock their pozzolanic properties, which are essential for their application in cementitious materials. This activation is achieved by altering the crystalline structure of clay minerals through a process called thermal activation, commonly referred to as calcination. Calcination involves heating clay minerals to specific temperatures, resulting in dehydroxylation. Dehydroxylation refers to the removal of molecular water, which can either be adsorbed onto the surface, trapped within the pores or channels, or associated with interlayer cations commonly found in 2:1 clay minerals [23,36]. The extent and temperature range at which dehydration occurs are influenced by various factors, including the storage environment, the degree of crystallinity of the clay minerals, and the specific characteristics of the cations present in the interlayer spaces. The transformation enhances the material’s reactivity and pozzolanic behavior, making it suitable for use in cement and concrete applications [37,38].
The effectiveness of calcination is influenced by several critical parameters, including the heating rate, target temperature, duration of temperature exposure, cooling rate, and surrounding atmospheric conditions. These factors determine the extent of structural modification and influence the final pozzolanic reactivity of the calcined clay. Notably, the calcination process requires careful temperature control to achieve dehydroxylation while avoiding temperatures high enough to induce recrystallization, which can reduce pozzolanic reactivity [39,40]. For most clay minerals, the optimal calcination temperature lies in the range of 600–900 °C, depending on the specific mineral composition.
From a practical standpoint, calcined clay pozzolan is typically produced by grinding heat-treated clays into a fine powder with a mean particle size ranging up to 20 µm [41,42,43]. This finely ground material is characterized by its plate-like, angular, and porous particle morphology, which contributes to its high surface area and enhanced reactivity. The specific gravity of calcined clays typically ranges between 2.0 and 2.5, making them relatively lightweight compared to other supplementary cementitious materials such as slag or fly ash [44,45]. Figure 2 shows the production process of calcined clay.

Figure 2.
Calcined clay production.
2.1. Calcination Process
The firing or heat-treatment of clays to a specified temperature (usually between 600 °C and 900 °C) causes the octahedral layer to undergo dehydroxylation in a process known as calcination [22]. The exact nature of the structural transformation depends on many factors, including the heating rate, holding temperature and time, atmosphere (oxidizing or reducing), and cooling rate [37]. The calcination of clay is mostly done using a flash, rotary, or simple laboratory kiln or furnace [28]. This review focuses on laboratory methods used in the calcination of clays.
Msinjili et al. [46] conducted a comparative analysis of calcined illitic clays (brick clays) and low-grade kaolinitic clays as supplementary cementitious materials. The thermal activation process involved placing 50 g of raw clay into platinum crucibles within a muffle furnace. The furnace was heated at a rate of 10 °C/min to the specified temperature, maintained for 3 h. Subsequently, the samples were cooled on a laboratory bench for 30 min before being stored in a desiccator at 23 °C with silica gel to prevent excessive water absorption and potential rehydroxylation. In contrast, Du and Pang [47] dried their clay samples at 50 °C for 72 h with air recirculation and then ground them in a ball mill for 30 min. The calcination process involved placing the clay-filled crucibles in a furnace, heating from room temperature to the desired temperature at a rate of 10 °C/min, maintaining the temperature for 1 h, and then quickly removing the crucibles to cool on a metal plate at ambient temperature.
Investigating the pozzolanic activity of thermally treated excavated waste clays, Yanguatin et al. [48] dried the clays in an oven at 100 °C for 24 h and then calcined them at 550 °C, 650 °C, and 750 °C for residence times of 1 and 3 h using a programmable laboratory furnace with a fixed bed technique, followed by cooling at room temperature.
Dixit et al. [49] dried the clay at 105 °C for 48 h and ground it in a ball mill for 40 min. The resulting powdered clays were calcined in a laboratory furnace at 700 °C with a heating rate of 10 °C/min and a residence time of 1 h. The clays were then left to cool naturally within the furnace to room temperature. Zhou et al. [50] employed a different approach by forming 5 mm diameter balls from the clay prior to calcination. These clay balls were calcined in a kiln for 2 h and the resulting calcined nodules were milled to achieve cement fineness. This variation in methodology underscores the diverse techniques used to optimize the pozzolanic activity of calcined clays.
The calcination method employed by Zhou et al. [50] differs notably from those used by other researchers, who typically calcined clay in its powdered form. Zhou’s approach, involving the formation of 5 mm diameter clay balls prior to calcination, ostensibly facilitates uniform heat transfer throughout the clay. However, their findings revealed that the inner portions of the nodules were charred, likely due to inadequate heat penetration to the core. This uneven heat distribution could adversely affect the pozzolanic reactivity and other critical properties of the calcined clay, consequently impacting the performance of the resultant concrete. To enhance the efficacy of the calcination process, the introduction of an inert material into the clay nodules prior to calcination may be beneficial. This modification could improve heat transfer throughout the clay nodules, potentially allowing for calcination at lower temperatures and shorter durations while achieving comparable results. Such an approach could optimize the thermal activation process, thereby improving the quality and performance of the calcined clay as a supplementary cementitious material.
2.2. Calcination Temperature
It is crucial to calcine clay at a suitable temperature because of its effect on the hydration kinetics, pozzolanic reactivity, formation of relevant phases, and ultimately the serviceability of concrete. The composition and structure of clay minerals determine the ideal temperatures of calcination at which dihydroxylation takes place [31]. Many researchers have examined how clay calcination temperature affects the pozzolanic reactivity and other properties of concrete. The temperature that can be used to derive maximum pozzolanic reactivity has shown several irregularities. Different temperatures have been reported by other researchers. Table 1 presents some results from these studies.

Table 1.
A summary of the calcination temperatures that other studies have utilized.
2.3. Clay Mineral Transformation After Calcination
Metakaolin is generated through the heat-treatment of kaolinitic clays within a temperature interval spanning from 600 °C to 800 °C [54]. This thermal treatment process is relevant to producing a substance exhibiting elevated pozzolanic reactivity. Dehydroxylation takes place as moisture is extracted from the clay, which causes the collapse of the material’s structure and leads to the creation of an amorphous alumino-silicate known as metakaolin or metakaolinite [54].
Based on investigations conducted by various researchers into the transformation of kaolin into metakaolinite, the key factors influencing the dehydroxylation process predominantly include the calcination temperature, duration, cooling rate, and surrounding environmental conditions. The change in weight due to dihydroxylation of the kaolinite mineral at room temperature is approximately 14% [55]. Figure 3 and Figure 4 respectively present the particle morphology of raw and calcined clay (at 800 °C), as examined by the scanning electron microscope (SEM) at 1500 magnification.
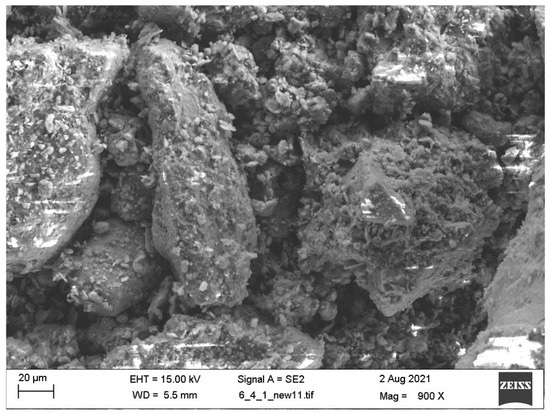
Figure 3.
SEM of raw clay: Reprinted with permission from [36].

Figure 4.
SEM of calcined clay: Reprinted with permission from [36].
3. Low-Grade Calcined Clays
Calcined kaolinitic clays, prepared with clays with high kaolinite [18,19], have been used to substitute cement in concrete over the years due to their excellent reactivity and refinement of microstructure [20]. Ultimately, this results in enhanced durability and mechanical performance. Nevertheless, the accessibility of high-grade kaolinitic clays poses a hurdle as they are confined to certain geographical areas worldwide. Additionally, there is an increasing need for kaolinite-rich clays in the manufacturing of diverse industrial products. This rivalry adds to the elevated expense associated with the utilization of kaolinite-rich clays as SCMs for concrete applications [21,22].
It is therefore suggested that naturally occurring clays having a relatively lower kaolinite content, if fired at the right temperature, have the potential to produce an amorphous siliceous material which possesses pozzolanic characteristics. The potential of heat-treating and converting low-grade clays into pozzolans is a possibility due to the availability of large low-grade clay deposits in every region of the world and their economic viability [22]. While the term “impure clays” has not been standardized, low-grade clays are frequently thought to contain less than 40% kaolinite [21,47,50,56,57].
3.1. Calcined Low-Grade Clays in Cementitious Systems
3.1.1. Effect of Fineness
Sieve analysis, over the years, has been the easiest method for the determination of the fineness of pozzolanic materials. This is because sophisticated tools are not needed unlike other methods such as Brunauer–Emmett–Teller (BET) and Blaine [58]. The BET model, lately, is seen to be a better approach for analyzing the fineness of SCMs than the air permeability method, which has been the standard for the determination of the fineness of Portland cement [59]. The fineness and extent of substitution are essential factors that require meticulous consideration because of their impact on the characteristics of the resulting concrete.
Yao et al. [60] observed alterations in the structural and morphological attributes influencing the pozzolanic reactivity of muscovite when subjected to varying degrees of mechanical activation. The mechanically activated muscovite exhibited potential for reacting with portlandite. Similarly, Ma et al. [61] investigated the impact of waste brick powder (WBP) on the workability and compressive strength of mortar. The pulverized brick was subjected to milling and sieving processes to achieve fineness levels of 42 µm, 18 µm, 12 µm, and 6 µm. The results indicated that an increasing fineness led to decreased workability and water demand, which is because of the disruption of original pores in the powder due to continuous milling, resulting in an improved microstructure.
Contrary to Ma et al.’s [61] findings, previous studies have shown that an increased fineness of pozzolans can elevate water demand and subsequently reduce workability and slump using a constant w/b ratio. This effect is often attributed to the particle size and internal porosity of the pozzolan. In some cases, it is linked to the agglomeration of pozzolan particles, wherein the agglomerated particles restrict some of the water, rendering it unavailable for enhancing workability [59,62]. Figure 5 shows the influence of fineness on workability.
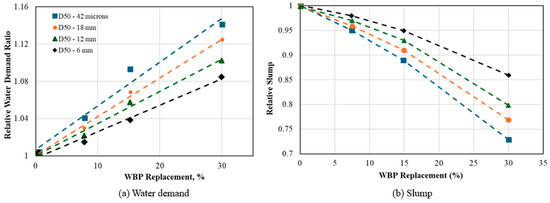
Figure 5.
Effect of fineness on workability: Adapted with permission from [61].
3.1.2. Effect of Particle Size
The particle size distribution (PSD) of low-grade clays plays a pivotal role in their performance when used as supplementary cementitious materials (SCMs) in mortar and concrete. Unlike high-purity kaolinitic clays, low-grade clays often contain a mix of clay and non-clay minerals, which significantly influences their PSD and reactivity after calcination. Advanced techniques like laser diffraction have largely replaced traditional sieving methods for PSD measurement, offering greater precision and the ability to capture finer particle sizes. However, the inherent heterogeneity of low-grade clays introduces unique challenges in optimizing their PSD for use in construction applications [60].
Yanguatin et al. [48] explored the impact of calcining low-grade clays at temperatures up to 650 °C and reported notable changes in PSD. At 550 °C, the agglomeration of kaolinite particles during dehydroxylation caused an increase in particle size, with a shift in the d50 value (the particle size at which 50% of the material is finer). This agglomeration reduced the fineness of the calcined clay, which could negatively impact its performance in cementitious systems. When the temperature was raised to 650 °C, further coalescence of particles was observed, but the PSD began to stabilize, indicating a critical range where particle size distribution is more predictable and manageable.
Similarly, Liu et al. [13] found that the PSD of low-grade calcined clays was influenced not only by the calcining temperature but also by the duration of thermal treatment. Prolonged calcination at 750 °C increased particle agglomeration, reducing the surface area and, consequently, the pozzolanic reactivity of the clay. This highlights the interplay between calcination parameters and the mineralogical characteristics of low-grade clays, where excessive heat treatment can diminish their performance due to sintering effects.
Zheng et al. [57] investigated montmorillonite-rich low-grade clays calcined at temperatures ranging from 600 to 1000 °C. The study revealed that calcination at 800–850 °C resulted in a more refined PSD, improving the reactivity and bonding properties of the clay when used in concrete. This refinement was linked to the breakdown of clay mineral structures, enhancing the availability of reactive silica and alumina. However, at temperatures exceeding 900 °C, the PSD widened again due to particle sintering, leading to reduced surface area and diminished pozzolanic activity. The optimal calcining temperature for low-grade clays was determined to be within the 800–850 °C range, consistent with findings for higher-purity clays.
Ferreiro et al. [63] provided additional insights into the PSD of low-grade clays, noting significant differences between uncalcined and calcined materials. Uncalcined low-grade clays exhibited a broad PSD, with particles ranging from 1 to 110 μm. Upon calcination at 850 °C, the PSD narrowed significantly and the material displayed improved packing density and reactivity. However, further heating to 925 °C caused a re-expansion of the PSD due to phase changes and the dissolution of agglomerated particles. This underscores the delicate balance required to optimize calcination parameters for low-grade clays to achieve desirable PSD and reactivity characteristics.
Recent research also emphasizes the role of mineral impurities in low-grade clays in influencing PSD and its evolution during calcination. Zhou et al. [50] observed that the non-clay mineral fraction, such as quartz and feldspar, remains largely unaffected by calcination, contributing to a bimodal PSD in calcined low-grade clays. This bimodality can be leveraged in concrete mix designs to improve particle packing and reduce porosity, provided the reactive clay fraction is adequately activated.
The PSD of low-grade clays is a key determinant of their performance in cementitious systems. Calcination significantly alters the PSD, with optimal temperatures generally falling within the range of 800–850 °C. However, the complex mineralogical composition of low-grade clays necessitates careful control of calcining parameters to avoid undesirable effects such as particle agglomeration or excessive sintering. Future research should focus on understanding the interactions between PSD, mineral composition, and calcination parameters to maximize the potential of low-grade clays as sustainable SCMs. The PSD of calcined and uncalcined clays is shown in Figure 6.
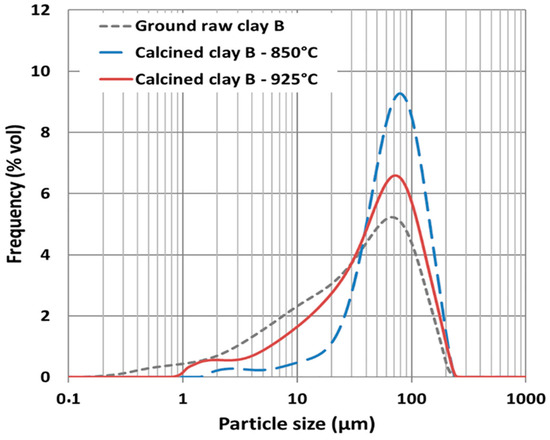
Figure 6.
PSD of raw and calcined clays. Reprinted with permission from [63].
3.1.3. Effect on Hydration and Pozzolanic Reactivity
The pozzolanic activity of clay is primarily measured by its ability to react with calcium hydroxide (Ca(OH)2), a by-product of Portland cement hydration, to form calcium silicate hydrate (C-S-H), which is responsible for the development of strength in concrete [20,55,64]. Several established methods, such as the Frattini test, Chapelle test, strength activity index (SAI), portlandite consumption, and the R3 (rapid, relevant, and reliable) approach, are commonly used to assess pozzolanic reactivity, each offering insights into the material’s potential performance in concrete [55].
Kaminskas et al. [26] investigated the pozzolanic reactivity of smectite clay fired at various temperatures (500–900 °C) using the Chapelle test. Their findings revealed that pozzolanic reactivity increased with the temperature up to 700 °C but declined once the temperature exceeded this threshold. This suggests that calcination temperatures above 700 °C may lead to a loss of reactivity due to the onset of undesirable structural changes, such as sintering or the collapse of the clay mineral structure [65]. In contrast, Shafiq et al. [65] observed enhanced pozzolanic reactivity at 800 °C, with a corresponding increase in compressive strength. This suggests that calcining at higher temperatures may be beneficial for certain types of clay, provided the temperature is controlled to avoid detrimental effects.
The reactivity of low-grade clays was further investigated by Yanguatin et al. [48] using the Frattini test, which measures the solubility of calcium hydroxide. At 550 °C, calcined clay showed no reactivity over the first seven days, with the calcium hydroxide concentration remaining above the solubility curve. However, after 28 days, the reactivity of the calcined clay became evident, with a significant reduction in Ca(OH)2, suggesting the onset of pozzolanic reactions as the clay absorbed the available calcium hydroxide. This delayed reactivity pattern is characteristic of certain low-grade clays, where the pozzolanic activity becomes more pronounced over time, especially in hydrated environments [50].
Zhou et al. [50] used the portlandite consumption method to assess the pozzolanic reactivity of calcined clays after 28 days of hydration. Their results indicated that portlandite consumption increased with curing time, stabilizing after 28 days. This behavior was further corroborated by the strength activity index (SAI), where calcined clay samples exhibited increased pozzolanic reactivity over time, contributing to greater compressive strength in blended cement pastes. These results underline the importance of curing time in fully activating the pozzolanic potential of calcined clays.
Du and Pang [47] investigated the pozzolanic reactivity of impure clay containing 19.5% kaolinite using thermogravimetric analysis (TGA). They observed that pastes containing calcined marine clay at temperatures ranging from 600 to 800 °C showed reduced portlandite content compared to conventional Portland cement pastes, indicating significant pozzolanic activity. The reduction in portlandite was attributed to both pozzolanic reactions and cement dilution effects, confirming that calcined clays enhance cement hydration and contribute to strength development. Similarly, Zheng et al. [57] found that only samples containing 10% and 20% calcined clay met the minimum SAI requirement of 75% after 28 and 90 days of curing. This highlights the role of calcining temperature in optimizing the pozzolanic reactivity of low-grade clays and improving their performance in concrete applications.
Alujas et al. [18] explored the pozzolanic reactivity of low-grade calcined clays by measuring the heat of hydration. The results indicated that calcining temperature significantly influenced the cumulative heat released during hydration, with higher temperatures leading to a greater amount of heat evolution compared to the reference Portland cement. This increase in heat evolved was attributed to the accelerated pozzolanic reactions occurring at elevated temperatures, demonstrating that calcined clays enhance cement hydration and strength development. The reduction in the induction period further suggests that higher calcination temperatures promote more rapid pozzolanic reactions, which could improve the early strength of cementitious systems [66].
Msinjili et al. [66] conducted a study on the hydration of blended cement pastes containing calcined brick clay, using TGA to assess phase changes over 28 days. The results revealed that blended pastes had a lower content of Ca(OH)2 and higher quantities of C-S-H and AFm phases, which are indicative of pozzolanic activity. The reference cement paste contained 11.5% Ca(OH)2, while the blended cement pastes had between 6.8% and 8.8%, confirming that the calcined clay promoted the formation of beneficial hydration products and increased the reactivity of the blended paste.
The pozzolanic reactivity of calcined clays is largely driven by the dissolution of alumina and silica in an alkaline environment, which leads to the formation of calcium silicate hydrates (C-S-H) when in contact with portlandite (CH). According to Du and Pang (2018) [47], the X-ray diffraction (XRD) patterns of pastes containing calcined marine clay revealed a significant reduction in portlandite peaks after 28 days of hydration, indicating the consumption of Ca(OH)2 due to pozzolanic reactions. Zheng et al. [57] observed a similar trend in the hydration of low-grade calcined clay blends, further supporting the idea that the calcination process enhances the pozzolanic reactivity by increasing the availability of reactive alumina and silica.
From these studies, calcination temperature plays a pivotal role in determining the hydration and pozzolanic reactivity of clays, influencing both the physical properties and performance of cementitious materials. The optimal calcining temperature is typically in the range of 600 to 800 °C, depending on the clay mineral composition. Higher calcination temperatures generally lead to an increased rate of pozzolanic reactions, improved strength development, and enhanced overall reactivity, although excessive temperatures may cause undesirable effects such as sintering, which can reduce reactivity. Further studies on the long-term hydration behavior and the interaction between calcined clays and Portland cement are necessary to fully understand the potential of calcined clays as sustainable SCMs in concrete applications. Table 2 presents a summary of the effect of low-grade calcined clay on pozzolanic reactivity.

Table 2.
Summary of the effect of low-grade calcined clay on pozzolanic reactivity.
3.1.4. Workability
Workability is a critical property of freshly prepared concrete, reflecting its ability to be mixed, compacted, and shaped for its intended purpose while maintaining uniformity and cohesion [69]. The workability of fresh concrete is often assessed using the slump test, as prescribed by EN 12350-2, which serves as a measure of flowability. Factors influencing workability include fineness, chemical composition, porosity of the material, and the water-to-binder (w/b) ratio [70]. The incorporation of calcined clay as a SCM significantly affects these parameters, primarily due to its fineness and increased water demand. Cai et al. [71] reported that the water requirement for achieving adequate workability increased with the level of cement replacement by calcined clay, which can be attributed to the higher surface area and porous structure of calcined clays.
Zhou et al. [50] investigated the effect of calcined waste clays on the workability of concrete at varying water-to-cement (w/c) ratios. Their results demonstrated a decrease in workability with a lower w/c ratio; however, the replacement of Portland cement with calcined clay did not consistently impact the slump. In fact, in some cases, such as with 10 wt.% replacement, an improvement in slump was observed. This finding suggests that calcined clays might interact differently depending on their calcination temperature, composition, and replacement levels, and further studies are needed to elucidate these mechanisms.
Jagtap et al. [72] and Lenka and Panda [73] examined the effect of calcined clay content on workability at w/b ratios of 0.42 and 0.43, respectively. Both studies reported a consistent reduction in workability as the calcined clay (metakaolin) content increased, highlighting the influence of its fineness and surface characteristics. Jagtap et al. [72] attributed this decline to the higher fineness modulus of calcined clay, which reduced the availability of cement paste required to lubricate aggregates and improve flow. In contrast, Zhou et al. [50] observed no consistent trends in slump reduction with calcined clay replacement and even reported higher slump values in some cases, particularly with lower replacement levels. This discrepancy could stem from differences in calcining temperatures and clay mineralogy, which influence particle packing, water absorption, and dispersion.
Cardinaud et al. [21], Akasha [74], and Gobinath et al. [75] similarly noted that the surface area and particle size distribution of calcined clays significantly impacted workability. Fine calcined clays with high surface areas generally exhibited higher water demands, resulting in lower slump values when used in constant w/b ratio mixes. Conversely, Msinjili et al. [66] observed that blends with carefully optimized calcined clays and chemical admixtures achieved workability comparable to Portland cement concrete. Such findings indicate that the calcining temperature, which governs the reactivity and particle morphology of calcined clays, plays a pivotal role in determining their effect on workability.
Gunjal and Kondraivendhan [76] and Ng et al. [77] also highlighted that the reduction in workability with increasing calcined clay content is directly correlated with the material’s specific surface area and porosity. These characteristics result in increased water adsorption and reduced free water availability for lubrication. Parashar et al. [78] and Zhou et al. [50] emphasized the need to interpret increases in slump observed in some concrete mixes containing calcined clay, as this behavior may depend on unique interactions between calcined clays and the cementitious matrix.
In summary, while calcined clays generally reduce the workability of mortar and concrete due to their fineness and water demand, the extent of this effect depends on calcining temperature, replacement levels, and mineralogical characteristics. Optimal performance can be achieved by tailoring mix designs and w/b ratios and incorporating chemical admixtures to mitigate workability issues. Further research is needed to understand the interplay between calcining conditions, clay mineralogy, and fresh concrete properties to enable the broader use of calcined clays as sustainable SCMs. Figure 7 presents the influence of calcined clay on workability.
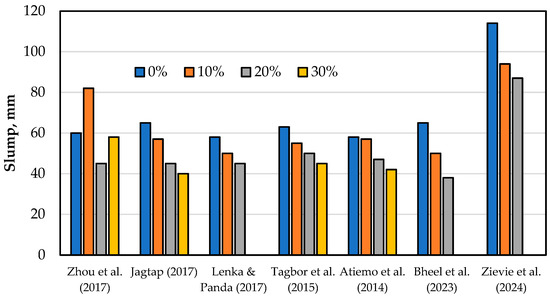
Figure 7.
Effect of calcined clay on concrete workability [16,50,72,73,79,80,81].
3.1.5. Effect on Mechanical Properties
The mechanical properties of mortar and concrete are critical determinants of their structural performance, durability, and overall suitability for construction applications. Compressive strength, flexural strength, and tensile strength are among the most important parameters, as they directly influence a structure’s ability to withstand applied loads and resist cracking or deformation over time. Enhancing these properties while maintaining sustainability and cost-effectiveness is a key focus in the development of advanced cementitious materials.
Pozzolans generally contribute to improved long-term strength through their reactivity but often result in reduced early strength [26]. The improved microstructure of the cement matrix blended with calcined clay is primarily attributed to pozzolanic reactions, which enhance its mechanical properties over time. Zheng et al. [57] reported a reduction in the early compressive strength of blended cement mortar incorporating low-grade calcined clay. At 3 and 7 days, compressive strength declined as the proportion of calcined clay increased, with an optimal substitution level identified at 10%. Similar trends were noted for flexural strength, underscoring the balance required in optimizing substitution levels to mitigate early strength losses while capitalizing on long-term gains.
Further investigations by Munduli and Mukherjee [82] explored the flexural strength of concrete containing recycled aggregates and calcined clay. Their findings indicated a decline in flexural strength with increasing recycled aggregate content. However, the addition of low-grade calcined clay enhanced flexural strength by up to 15%, demonstrating the potential of calcined clays to compensate for strength losses in more sustainable concrete mixtures. Similarly, Yaba et al. [83] reported consistent improvements in flexural strength across all substitution levels of calcined clay, emphasizing the importance of material synergy in achieving superior mechanical performance.
Salimi et al. [84] examined the tensile strength of concrete containing low-grade calcined clay and observed an increase as the water-to-binder (w/b) ratio decreased. Interestingly, the lowest w/b ratio yielded the highest tensile strength, highlighting the critical role of mixture design in optimizing the performance of calcined clay concretes. Gobinath et al. [75] corroborated these findings by demonstrating that compressive strength increased with curing age, although the reference cement consistently outperformed blended systems. This disparity highlights the slower pozzolanic reaction kinetics of calcined clays, which can be mitigated through chemical or mechanical activation to enhance early strength development [85].
Schulze and Rickert [32] studied strength development in Portland cement partially replaced with various calcined clays. While early strength was generally lower for all clay types compared to the reference cement, kaolinitic clays outperformed the control at later ages, consistent with their high reactivity. Conversely, chloritic clays exhibited inferior performance, emphasizing the need to tailor the calcined clay selection to project requirements. Kaminskas et al. [26] observed a strength reduction beyond 15% substitution, which was attributed to the dilution effect of less reactive smectite clays. Enhancing the pozzolanic reactivity of these clays through advanced activation techniques could potentially overcome such limitations.
Zhou et al. [50] presented intriguing results by varying the w/b ratio from 0.3 to 0.5 in calcined clay concretes. Concrete prepared with a 0.3 w/b ratio exhibited poor workability, as indicated by a 0 mm slump, yet achieved remarkable compressive strength. This phenomenon was attributed to the unique properties of calcined clays and their ability to interact chemically with OPC to form C-S-H at a faster rate. Interestingly, the low-grade calcined clay concrete’s early strength at 3 and 7 days was comparable to, or even exceeded, the control’s strength at 14, 28, and 90 days. These results challenge the conventional understanding of calcined clays as slow-reacting pozzolans and warrant further investigation to elucidate the mechanisms driving such performance.
Figure 8 illustrates the 28-day compressive strength of mortar and concrete prepared with 20% low-grade calcined clay. The data show variations in strength depending on calcination temperature, highlighting the importance of optimizing thermal treatment to maximize pozzolanic reactivity and strength development.
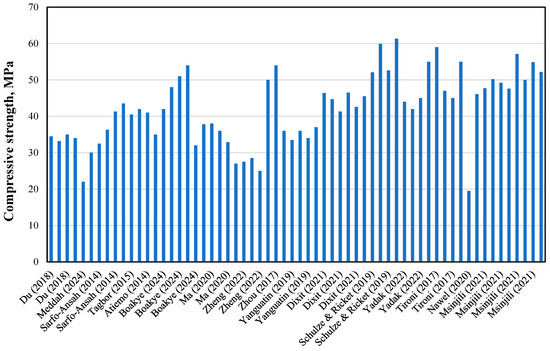
Figure 8.
Compressive strength of mortar and concrete blended with low-grade calcined clay [8,16,27,32,47,48,50,51,52,53,57,61,66,67,68,79,86].
The influence of low-grade calcined clays on the mechanical properties of mortar and concrete is multifaceted, depending on factors such as clay type, calcination conditions, substitution levels, and mixture design. While early strength reductions are common, long-term enhancements in compressive and flexural strength are achievable through proper material selection and activation techniques. Future research should focus on optimizing calcination processes, investigating synergistic effects with other SCMs, and understanding the microstructural evolution of low-grade calcined clay concretes to fully realize their potential in sustainable construction practices.
4. Durability Properties of Calcined Clay Blended Systems
Durability is the capacity of a material to resist abrasion, chemical erosion, weathering, and other service conditions. The capacity of chlorides and sulphates to penetrate concrete through capillary action, permeation, or diffusion has a major impact on the durability of the concrete [87,88,89]. Concrete is used extensively in the construction sector, and as such it is exposed to a range of environmental conditions that can affect its longevity. Enforcing concrete structures’ durability in these hostile settings could guarantee their longevity and lower the frequency of repairs and maintenance. If the flow of water and dissolved ions into the concrete is restricted, then this can happen [90]. The phenomenon known as “permeability” is most often used to describe the movement of water as well as ions into concrete [86].
Concrete’s resistance to ion infiltration and water is frequently evaluated using proven accelerated procedures. This could be by rapid chloride permeability test, electrical resistivity and conductivity, sorptivity, salt ponding, or sulphate resistance. Different methods of durability measurements were compared by Bermúdez Odriozola and Alaejos Gutiérrez [91] to determine the permeability properties of concrete. According to the report, techniques like oxygen permeability were unable to identify the impact of additional elements on the durability. However, techniques such as water accessibility and penetration depth testing have shown to be useful in determining how durable concrete is.
Water permeability has been shown to be a poor indicator of how durable concrete is against ion transport, which runs counter to the conclusions of [91]. When concrete’s resistance to chloride ion transport was assessed using electrical resistance and water penetration tests, similar outcomes were seen. The accuracy of durability measurements of blended cement concrete using electrical resistivity was, however, found to be doubtful [92].
The chemical and physical behaviors of cement mixed with low-grade calcined clay vary based on the calcining circumstances, cement replacement percentages, and the mineralogical and chemical constituents of the clay. Disparities in the durability outcome of calcined clay concrete are caused by these and other parameters. The disparity in durability performance of blended cement concrete and plain concrete against extreme environmental conditions largely depends on the concentration of chlorides or sulphates as well as the total time of exposure [93]. Below is an evaluation of the durability of blended cement concrete that contains calcined clay under various environmental circumstances and with varied application methods.
4.1. Chloride Resistance
The migration of chloride into concrete structures is one of the main reasons steel-reinforced concretes deteriorate. There is widespread agreement that adding calcined kaolinite clay to concrete strengthens its resistance to chloride because it creates reactive aluminates that promote Friedel’s salt production, enhances the arrangement of pores, and boosts chloride binding capacity [93]. Several studies have shown that blended cement concrete containing calcined clay has been found to significantly resist the ingress of chlorides [94,95,96,97,98].
Dixit et al. [52] studied the resistance chemical transport in concrete containing calcined clay with varying kaolinite content as shown in Figure 9. The clay was sourced from five different locations, calcined, and replaced with OPC by 30%. Comparing blended and reference mortars, the study found that charge passed during the Rapid Chloride Penetration Test (RCPT) decreased by 20–60%, while electrical resistance increased by 50–200%. The resistivity value and RCPT revealed a notable increase up to 91 days, in contrast to the compressive strength, indicating that additional pore refinement was observed in blended mixes.
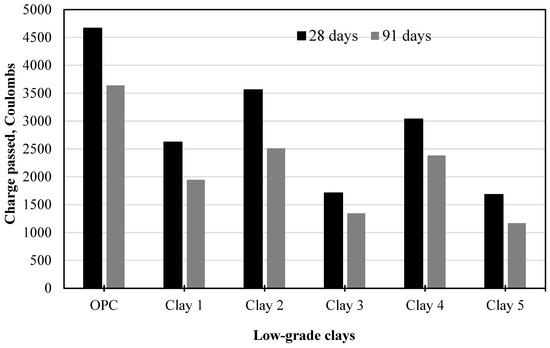
Figure 9.
Resistance of calcined clay concrete against chloride ingress: Adapted with permission from [52].
Nas and Kurbetci [99] also noted that as the metakaolin replacement ratio spiked there was a discernible drop in the fast chloride ion permeability. The reference concrete, which ASTM C1202 would have categorized as highly permeable, became a very low permeability class with 20% MK. Badogiannis et al. [100] also used a basic model that took into account the processes of binding and diffusion (adsorption and desorption) to examine how metakaolin affected chloride resistance. For the metakaolins under study, the efficiency factors (k-values) were ascertained. Depending on the active silica and alumina concentrations, both metakaolins demonstrated a notable resistance to chloride penetration. When added, however, chloride penetration is greatly reduced, improving the durability of concrete.
Following the quick chloride migration test, Nawel et al. [51] also found that lightweight, regular concrete containing 5% and 20% calcined clay had lower effective chloride ion diffusion coefficients than unmodified concrete. This was confirmed by Jafari and Rajabipour [101] when they replaced Portland cement with calcined clay. At 56 days, the coulomb values for the reference mix and 20% calcined clay combinations showed a similar trend, at 3752 and 3497, respectively. Adding calcined clay to concrete decreased the incidence of chloride intrusion by a total of 7% to 20%.
The function of calcined kaolinitic clays in relation to chloride transport in concrete structures is well researched and reported. There are not, however, many investigations on calcined clays with different mineralogy, especially low-grade clays. The impressive chloride resistance of blended cement concrete containing calcined clay has been assigned to the complex pore structure within the concrete [95]. Additionally, some studies have attributed it to the pore solution’s conduction [98,102].
4.2. Sulphate Resistance
Sulphate attack remains a critical durability challenge for concrete in sulphate-rich environments such as soils, seawater, industrial effluents, and decaying organic matter [103]. The attack results from the ingress of sulphate ions (SO42−) into hardened concrete, leading to expansive reactions involving calcium aluminate monophases (AFm) and portlandite with sulphate to form ettringite (3CaO·Al2O3·3CaSO4·32H2O), which compromises structural integrity [104]. To mitigate this issue, the inclusion of calcined clays in cementitious systems has been increasingly studied, with recent research highlighting their potential to enhance sulphate resistance.
Aramburo et al. [105] demonstrated that the use of higher proportions of calcined clay in blended cements reduced sulphate-induced expansion. This improvement was attributed to a lower concentration of portlandite throughout the test duration, which restricted the formation of expansive ettringite. Additionally, calcined clays contributed to pozzolanic reactions, consuming portlandite and enhancing the refinement of the pore structure, thereby reducing sulphate ingress.
Hu and He [106] further explored the role of metakaolin in sulphate resistance using cement pastes exposed to a 5% sodium sulphate environment. Their study revealed that while a 10% metakaolin dosage optimized compressive strength, a higher dosage of 20% was required to improve sulphate resistance significantly. The increased dosage led to enhanced portlandite consumption and stabilized the C-(A)-S-H gel, effectively reducing the formation of secondary ettringite. This was primarily due to the migration of aluminum from raw materials into the C-(A)-S-H structure rather than forming ettringite, particularly at the higher metakaolin content [106].
Cordoba and Irassar [107] investigated the performance of calcined illitic shales in mortar bars exposed to sulphate attack. Their results showed that incorporating calcined shale reduced sulphate expansion by refining the pore structure and depleting portlandite availability. The refined pore network limited sulphate ion ingress, thereby mitigating damage from ettringite formation.
Boakye and Khorami [29] studied the mass loss and compressive strength degradation in low-grade calcined clay concrete exposed to a 5% sodium sulphate solution for 90 days. They reported significant improvements in sulphate resistance with higher calcined clay contents. Compared to the control specimens, which experienced 13.3% mass loss and 10% compressive strength loss, blended cement specimens with calcined clay exhibited reduced deterioration. This resistance was attributed to the reduced portlandite content and the formation of denser hydration products, which limited sulphate interactions and secondary ettringite formation.
More recent research highlights similar trends. For instance, Ding et al. [108] investigated blended cements with metakaolin in sulphate-rich environments and found that metakaolin dosages above 15% effectively delayed sulphate ingress and reduced expansion by improving the microstructure and reducing permeability. Similarly, Khan et al. [109] observed that calcined clays significantly enhanced the durability of concrete exposed to magnesium sulphate, where calcined clay’s pozzolanic activity contributed to the depletion of calcium hydroxide and the densification of the cement matrix. A relationship between mass loss and strength loss is presented in Figure 10.
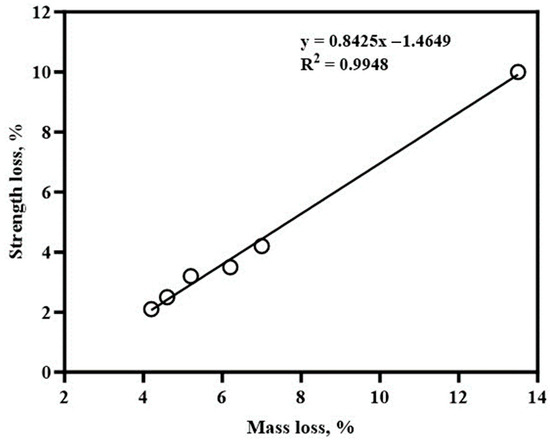
Figure 10.
Relationship between mass loss and strength loss: Adapted with permission from [29].
4.3. Alkali Silica Reaction
When concrete is prepared with alkali-sensitive aggregates, it is subject to the risk of being damaged by the alkali–silica reaction (ASR). This effect can be evaluated by measuring the expansion of concrete samples [110]. Sarfo-Ansah et al. [8] studied the potential mitigation of the ASR using calcined clay pozzolan and reported that the highest expansion was recorded for reference bars with no pozzolan, reaching a maximum of 0.35% at 42 days, whilst the expansion was reduced by between 42.5% and 107.8% at 14 days and between 9.4% and 16.4% at 84 days with increasing calcined clay pozzolan content. Mortar bars with 25% pozzolan were the least expansive, recording an expansion less than 0.1% at all test ages. X-ray diffractometry of the hydrated blended cement paste powders showed the formation of stable calcium silicates in increasing quantities, whilst the presence of expansive alkali–silica gel, responsible for ASR expansion, decreased as pozzolan content increased.
Jafari and Rajabipour [101] again experimented with the performance of calcined clay in mitigating the ASR using highly reactive (R2) aggregate and moderately reactive (R1) aggregate. It was observed that the 14-day ASR expansion was reduced from 0.33% in the control mixture to 0.26%, 0.13%, and 0.08% in mortars containing 10%, 20%, and 30% calcined clay, respectively. Using 30% calcined clay was sufficient to suppress the ASR of the highly reactive aggregates below the ASTM innocuous limit of 0.10%. It was clear that for the moderately reactive aggregates, a lower calcined clay dosage of 15% was sufficient to mitigate the ASR.
The potential to suppress the ASR using metakaolin was also investigated by Bakera and Alexander [111]. It was concluded from the study that metakaolin has excellent potential in mitigating ASR expansion, although the optimum replacement rate depended significantly on the nature of the reactive aggregate. The study recommended that before using metakaolin to suppress the ASR, an effective replacement level must be established in combination with the given aggregate. In studying the effect of a calcined Westerwald bentonite as supplementary cementitious material on the long-term performance of concrete, Trumer et al. [110] observed that while the reference OPC sample missed the limits claimed for uncritical aggregates, which was purposed by the material choice, the mortar with calcined clay did not undergo any effective macroscopic changes under the same conditions.
Wei et al. [112] also experimented with the effectiveness of metakaolin against the ASR in concrete. The incorporation of metakaolin and bentonite in cement mitigated the expansion of mortar bars. With 10% cement substitution, the expansion of mortars at an early age (first 10 days) could be effectively suppressed, but the slow reaction did not stop. With the increasing incorporation of metakaolin and bentonite, the expansion of mortars decreased to an innocuous level. When 50% cement was replaced with metakaolin, the expansion was suppressed to a very low level (<0.025%). It was found that with the replacement of metakaolin by bentonite, the expansion of mortar was further decreased. Sarfo-Ansah et al. [113] compared the influence of calcined clay, steel slag, and granite dust on the mitigation of the ASR. The findings showed that resistance to expansion in mortar containing 25% calcined clay was about 10% and 25% better than that of those containing steel slag and granite dust, respectively. Boakye and Khorami [29] reported a progressive increase in expansion in mortar bars incorporating calcined clay as the curing age advanced (Figure 11). The expansion peaked at 0.55% after 35 days when the calcined clay content was 15%. Similarly, the reference mortar bars exhibited a significant increase in expansion, reaching a maximum of 0.35% at 35 days. Notably, the expansion of all mortar bar samples exceeded the maximum threshold of 0.1% specified in ASTM C1260, highlighting the potential susceptibility of these materials to deleterious expansion.
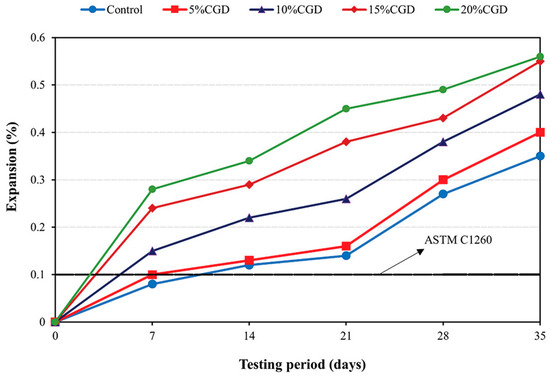
Figure 11.
Expansion of calcined clay mortars due to alkali–silica reaction: Adapted with permission from [29].
5. Conclusions, Perspectives and Recommendations
The growing emphasis on sustainable development necessitates the adoption of eco-friendly construction materials that reduce CO2 emissions while maintaining high performance. Calcined clay offers a promising alternative to Portland cement by lowering the clinker factor—reducing reliance on clinker, a CO2-intensive component of cement. Substituting cement with calcined clay enhances sustainability, aligning with global climate change mitigation efforts. Despite these advantages, research on calcined clays has primarily focused on high-kaolinite clays, leaving significant knowledge gaps regarding the performance of low-grade clays with a kaolinite content below 20%.
Calcination conditions, particularly temperature and duration, are crucial for optimizing pozzolanic reactivity, hydration, and strength development. Each clay type requires specific heat-treatment parameters to achieve optimal reactivity, yet comprehensive studies on the calcination of less reactive clays remain scarce. Furthermore, durability studies have predominantly examined high-reactivity clays, with limited data on the resistance of low-grade calcined clays to chloride ingress and other environmental stressors. Understanding how calcined clays modify concrete microstructures to enhance durability requires further investigation.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohammed, S. Processing, effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Constr. Build. Mater. 2017, 140, 10–19. [Google Scholar]
- Teklay, A.; Yin, C.; Rosendahl, L.; Køhler, L.L. Experimental and modeling study of flash calcination of kaolinite rich clay particles in a gas suspension calciner. Appl. Clay Sci. 2015, 103, 10–19. [Google Scholar]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar]
- Najimi, M.; Ghafoori, N.; Sharbaf, M. Alkali-activated natural pozzolan/slag mortars: A parametric study. Constr. Build. Mater. 2018, 164, 625–643. [Google Scholar]
- Li, R.; Lei, L.; Sui, T.; Plank, J. Effectiveness of PCE superplasticizers in calcined clay blended cements. Cem. Concr. Res. 2021, 141, 106334. [Google Scholar]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 12, 1244–1256. [Google Scholar]
- Sarfo-Ansah, J.; Atiemo, E.; Boakye, K.; Adjei, D.; Adjaottor, A. Calcined Clay Pozzolan as an Admixture to Mitigate the Alkali-Silica Reaction in Concrete. J. Mater. Sci. Chem. Eng. 2014, 20–26. [Google Scholar]
- IEA. Global Cement Production in the Net Zero Scenario, 2010–2030; IEA: Paris, France, 2022. [Google Scholar]
- Aprianti S, E. A huge number of artificial waste material can be supplementary cementitious material (SCM) for concrete production—A review part II. J. Clean. Prod. 2017, 142, 4178–4194. [Google Scholar]
- Babbar, S.; Behara, R.S.; Koufteros, X.A.; Wong, C.W.Y. Charting leadership in SCM research from Asia and Europe. Int. J. Prod. Econ. 2018, 203, 350–378. [Google Scholar]
- Samad, S.; Shah, A. Role of binary cement including Supplementary Cementitious Material (SCM), in production of environmentally sustainable concrete: A critical review. Int. J. Sustain. Built Environ. 2017, 2, 663–674. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Duan, W.; Sanaei Ataabadi, H.; Jia, Q.; Zeng, J.; Yoo, D.-Y. Innovative self healing composites using steel slag and chitosan. Cem. Concr. Compos. 2024, 152, 105652. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Nehdi, M.L. Clay in cement-based materials: Critical overview of state-of-the-art. Constr. Build. Mater. 2014, 51, 372–382. [Google Scholar] [CrossRef]
- Tagbor, T.A.; Boakye, K.A.; Sarfo-Ansah, J.; Atiemo, E. A study of the pozzolanic properties of Anfoega Kaolin. Int. J. Eng. Res. Appl. 2015, 5, 28–33. [Google Scholar]
- Wee, J. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay. Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 37, 303–318. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Cardinaud, G.; Rozière, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined clay—Limestone cements: Hydration processes with high and low-grade kaolinite clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar]
- Hanein, T.; Thienel, K.C.; Zunino, F.; Marsh, A.T.M.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.G.; Ben Haha, M.; Avet, F.; et al. Clay calcination technology: State-of-the-art review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 3. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 1, 113–122. [Google Scholar]
- He, C.; Makovicky, E.; Osbæck, B. Thermal stability and pozzolanic activity of raw and calcined mixed-layer mica/smectite. Appl. Clay Sci. 2000, 3, 141–161. [Google Scholar]
- Kaminskas, R.; Kubiliute, R.; Prialgauskaite, B. Smectite clay waste as an additive for Portland cement. Cem. Concr. Compos. 2020, 113, 103710. [Google Scholar]
- Sarfo-Ansah, J.; Atiemo, E.; Boakye, K.A.; Momade, Z. Comparative study of chemically and mechanically clay pozzolana. J. Mater. Sci. Appl. 2014, 5, 86–94. [Google Scholar] [CrossRef]
- Almenares, R.S.; Vizcaíno, L.M.; Damas, S.; Mathieu, A.; Alujas, A.; Martirena, F. Industrial calcination of kaolinitic clays to make reactive pozzolans. Case Stud. Constr. Mater. 2017, 6, 225–232. [Google Scholar]
- Boakye, K.; Khorami, M. Influence of Calcined Clay Pozzolan and Aggregate Size on the Mechanical and Durability Properties of Pervious Concrete. J. Compos. Sci. 2023, 5, 182. [Google Scholar]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132–133, 552–560. [Google Scholar]
- Neißer-Deiters, A.; Scherb, S.; Beuntner, N.; Thienel, K. Influence of the calcination temperature on the properties of a mica mineral as a suitability study for the use as SCM. Appl. Clay Sci. 2019, 179, 105168. [Google Scholar]
- Schulze, S.E.; Rickert, J. Suitability of natural calcined clays as supplementary cementitious material. Cem. Concr. Compos. 2019, 95, 92–97. [Google Scholar] [CrossRef]
- Zhao, D.; Khoshnazar, R. Microstructure of cement paste incorporating high volume of low-grade metakaolin. Cem. Concr. Compos. 2020, 106, 103453. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl. Clay. Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- Tregger, N.A.; Pakula, M.E.; Shah, S.P. Influence of clays on the rheology of cement pastes. Cem. Concr. Res. 2010, 3, 384–391. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M.; Saidani, M.; Ganjian, E.; Dunster, A.; Ehsani, A.; Tyrer, M. Mechanochemical Characterisation of Calcined Impure Kaolinitic Clay as a Composite Binder in Cementitious Mortars. J. Compos. Sci. 2022, 6, 134. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Pontikes, Y.; Elsen, J.; Cizer, Ö. Comparing the reactivity of different natural clays under thermal and alkali activation. RILEM Tech. Lett. 2019, 4, 74–80. [Google Scholar] [CrossRef]
- Seiffarth, T.; Hohmann, M.; Posern, K.; Kaps, C. Effect of thermal pre-treatment conditions of common clays on the performance of clay-based geopolymeric binders. Appl. Clay Sci. 2013, 73, 35–41. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Chapter 10.2—Thermally Modified Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 411–433. [Google Scholar]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Mineral. Geochem. 2012, 1, 211–278. [Google Scholar] [CrossRef]
- Al-Akhras, N.M. Durability of metakaolin concrete to sulfate attack. Cem. Concr. Res. 2006, 9, 1727–1734. [Google Scholar] [CrossRef]
- Ambroise, J.; Maximilien, S.; Pera, J. Properties of metakaolin blended cements. Adv. Cem. Based Mater. 1994, 4, 161–168. [Google Scholar] [CrossRef]
- Tafraoui, A.; Escadeillas, G.; Vidal, T. Durability of the Ultra High Performances Concrete containing metakaolin. Constr. Build. Mater. 2016, 112, 980–987. [Google Scholar]
- Mardani-Aghabaglou, A.; Sezer, G.İ.; Ramyar, K. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures view point. Constr. Build. Mater. 2014, 70, 17–25. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Bahrami Jovein, H. Influence of metakaolin as supplementary cementing material on strength and durability of concretes. Constr. Build. Mater. 2012, 30, 470–479. [Google Scholar]
- Msinjili, N.S.; Gluth, G.J.G.; Sturm, P.; Vogler, N.; Kune, H. Comparison of calcined illitic clays (brick clays) and lowgrade kaolinitic clays as supplementary cementitious materials. Mater. Struct. 2019, 52, 94. [Google Scholar]
- Du, H.; Pang, S.D. Value-added utilization of marine clay as cement replacement for sustainable concrete production. J. Clean. Prod. 2018, 198, 867–873. [Google Scholar] [CrossRef]
- Yanguatin, H.; Ramírez, J.H.; Tironi, A.; Tobón, J.I. Effect of thermal treatment on pozzolanic activity of excavated waste clays. Constr. Build. Mater. 2019, 168, 814–823. [Google Scholar]
- Dixit, A.; Du, H.; Pang, S.D. Marine clay in ultra-high performance concrete for filler substitution. Constr Build Mater 2020, 263, 120250. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, R.; Tyrer, M.; Wong, H.; Cheeseman, C. Sustainable infrastructure development through use of calcined excavated waste clay as a supplementary cementitious material. J. Clean. Prod. 2017, 168, 1180–1192. [Google Scholar]
- Nawel, S.; Mounir, L.; Hedi, H. Mechanical and durability study of Tunisian calcined clay in lightweight concrete of expanded clay. Eur. J. Environ. Civ. Eng. 2019, 25, 2257–2276. [Google Scholar]
- Dixit, A.; Du, H.; Pang, S.D. Performance of mortar incorporating calcined marine clays with varying kaolinite content. J. Clean. Prod. 2021, 282, 124513. [Google Scholar]
- Boakye, K.; Khorami, M.; Saidani, M.; Ganjian, E.; Dunster, A.; Tyrer, M.; Ehsani, A. Influence of Calcining Temperature on the Mineralogical and Mechanical Performance of Calcined Impure Kaolinitic Clays in Portland Cement Mortars. J. Mater. Civ. Eng. 2024, 36, 04024040. [Google Scholar] [CrossRef]
- Khatib, J.M.; Baalbaki, O.; ElKordi, A.A. Metakaolin. In Waste and Supplementary Cementitious Materials in Concrete; Elsevier: Amsterdam, The Netherlands, 2018; pp. 493–511. [Google Scholar]
- Fabbri, B.; Gualtieri, S.; Leonardi, C. Modifications induced by the thermal treatment of kaolin and determination of reactivity of metakaolin. Appl. Clay. Sci. 2013, 73, 2–10. [Google Scholar] [CrossRef]
- Malacarne, C.S.; Longhi, M.A.; Silva, M.R.C.; Gonçalves, J.P.; Rodríguez, E.D.; Kirchheim, A.P. Influence of low-grade materials as clinker substitute on the rheological behavior, hydration and mechanical performance of ternary cements. Case Stud. Constr. Mater. 2021, 15, e00776. [Google Scholar] [CrossRef]
- Zheng, D.; Liang, X.; Cui, H.; Tang, W.; Liu, W.; Zhou, D. Study of performances and microstructures of mortar with calcined low-grade clay. Constr. Build. Mater. 2022, 327, 126963. [Google Scholar]
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; De Belie, N. Physical characterization methods for supplementary cementitious materials. Mater. Struct. 2014, 48, 3675–3686. [Google Scholar]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar]
- Yao, G.; Zang, H.; Wang, J.; Wu, P.; Qiu, J.; Lyu, X. Effect of mechanical activation on the pozzolanic activity of muscovite. Clays Clay Miner. 2019, 67, 2019–2216. [Google Scholar]
- Ma, Z.; Tang, Q.; Wu, H.; Xu, J.; Liang, C. Mechanical properties and water absorption of cement composites with various fineness and contents of waste brick powder from C&D waste. Cem. Concr. Compos. 2020, 114, 103758. [Google Scholar]
- Kong, D.; Du, X.; Wei, S.; Zhang, H.; Yang, Y.; Shah, S.P. Influence of nano-silica agglomeration on microstructure and properties of the hardened cement-based materials. Constr. Build. Mater. 2012, 37, 707–715. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.M.C.; Lund, J.; Herfort, D. Influence of fineness of raw clay and calcination temperature on the performance of calcined clay-limestone blended cements. Appl. Clay. Sci. 2019, 169, 81–90. [Google Scholar]
- Paiva, H.; Silva, A.S.; Velosa, A.; Cachim, P.; Ferreira, V.M. Microstructure and hardened state properties on pozzolan-containing concrete. Constr. Build. Mater. 2017, 140, 374–384. [Google Scholar] [CrossRef]
- Shafiq, N.; Nuruddin, M.F.; Khan, S.U.; Ayub, T. Calcined kaolin as cement replacing material and its use in high strength concrete. Constr. Build. Mater. 2015, 81, 313–323. [Google Scholar]
- Msinjili, N.S.; Vogler, N.; Sturm, P.; Neubert, M.; Schröder, H.; Kühne, H.; Hünger, K.; Gluth, G.J.G. Calcined brick clays and mixed clays as supplementary cementitious materials: Effects on the performance of blended cement mortars. Constr. Build. Mater. 2021, 266, 120990. [Google Scholar] [CrossRef]
- Meddah, M.S.; Abdel-Gawwad, H.; Al Owaisi, M. The role of low-quality calcined clay in enhancing the performance of cement mortar exposed to normal and aggressive media. Sci. Rep. 2024, 14, 23855. [Google Scholar] [CrossRef] [PubMed]
- Tironi, A.; Cravero, F.; Scian, A.N.; Irassar, E.F. Pozzolanic activity of calcined halloysite-rich kaolinitic clays. Appl. Clay Sci. 2017, 147, 11–18. [Google Scholar]
- Solak, A.M.; Tenza-Abril, A.J.; Saval, J.M.; García-Vera, V.E. Effects of Multiple Supplementary Cementitious Materials on Workability and Segregation Resistance of Lightweight Aggregate Concrete. Sustainability 2018, 11, 4304. [Google Scholar] [CrossRef]
- Kocak, Y. Effects of metakaolin on the hydration development of Portland–composite cement. J. Build. Eng. 2020, 31, 101419. [Google Scholar]
- Cai, R.; He, Z.; Tang, S.; Wu, T.; Chen, E. The early hydration of metakaolin blended cements by non-contact impedance measurement. Cem. Concr. Compos. 2018, 92, 70–81. [Google Scholar]
- Jagtap, S.A.; Shirsath, M.N.; Karpe, S.L. Effect of metakaolin on the properties of concrete. Int. Res. J. Eng. Technol. 2017, 7, 643–645. [Google Scholar]
- Lenka, S.; Panda, K.C. Effect of metakaolin on the properties of conventional and self compacting concrete. Adv. Concr. Constr. 2017, 1, 31–48. [Google Scholar] [CrossRef]
- Akasha, A.M. Using of Libyan Calcined Clay in Concrete. In Calcined Clays for Sustainable Concrete; Springer: Dordrecht, The Netherlands, 2015; pp. 555–561. [Google Scholar]
- Gobinath, R.; Awoyera, P.O.; Praveen, N.; Babu, V.A.; Sai, P.S.; Prathibha, K. Effects of calcined clay on the engineering properties of cementitious mortars. Mater. Today Proc. 2021, 39, 110–113. [Google Scholar] [CrossRef]
- Gunjal, S.M.; Kondraivendhan, B. High temperature impact on calcined clay-limestone cement concrete (LC3). Mater. Today Proc. 2022, 61, 386–391. [Google Scholar] [CrossRef]
- Ng, S.; Jelle, B.P.; Stæhli, T. Calcined clays as binder for thermal insulating and structural aerogel incorporated mortar. Cem. Concr. Compos. 2016, 72, 213–221. [Google Scholar] [CrossRef]
- Parashar, A.K.; Gupta, N.; Kishore, K.; Nagar, P.A. An experimental investigation on mechanical properties of calcined clay concrete embedded with bacillus subtilis. Mater. Today Proc. 2021, 44, 129–134. [Google Scholar] [CrossRef]
- Atiemo, E.; Kankam, C.K.; Momade, F.; Boakye, K.A. Hydration properties of calcined clay pozzolan and limestone mineral admixtures in binary and ternary cements. J. Phys. Sci. Appl. 2014, 4, 323–327. [Google Scholar]
- Bheel, N.; Benjeddou, O.; Almujibah, H.R.; Abbasi, S.A.; Sohu, S.; Ahmad, M.; Sabri Sabri, M.M. Effect of calcined clay and marble dust powder as cementitious material on the mechanical properties and embodied carbon of high strength concrete by using RSM-based modelling. Heliyon 2023, 9, e15029. [Google Scholar] [CrossRef]
- Zievie, P.; Paa-Kofi Yalley, P.; Danso, H.; Antwi, K. Enhancing the Strength and Durability Behaviour of Concrete Produced with Brown-Loamy Kaolin Clay Polymer. J. Build. Mater. Struct. 2024, 11, 34–46. [Google Scholar] [CrossRef]
- Muduli, R.; Mukharjee, B.B. Effect of incorporation of metakaolin and recycled coarse aggregate on properties of concrete. J. Clean. Prod. 2019, 209, 398–414. [Google Scholar] [CrossRef]
- Yaba, H.K.; Naji, H.S.; Younis, K.H.; Ibrahim, T.K. Compressive and flexural strengths of recycled aggregate concrete: Effect of different contents of metakaolin. Mater. Today Proc. 2021, 45, 4719–4723. [Google Scholar] [CrossRef]
- Salimi, J.; Ramezanianpour, A.M.; Moradi, M.J. Studying the effect of low reactivity metakaolin on free and restrained shrinkage of high performance concrete. J. Build. Eng. 2020, 28, 101053. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Comparison of different methods for enhancing reactivity of pozzolans. Cem. Concr. Res. 2001, 5, 813–818. [Google Scholar]
- Yadak Yaraghi, A.H.; Ramezanianpour, A.M.; Ramezanianpour, A.A.; Bahman-Zadeh, F.; Zolfagharnasab, A. Evaluation of test procedures for durability and permeability assessment of concretes containing calcined clay. J. Build. Eng. 2022, 58, 105016. [Google Scholar]
- Ann, K.Y.; Moon, H.Y.; Kim, Y.B.; Ryou, J. Durability of recycled aggregate concrete using pozzolanic materials. Waste Manag. 2008, 6, 993–999. [Google Scholar]
- Hossain, M.M.; Karim, M.R.; Hasan, M.; Hossain, M.K.; Zain, M.F.M. Durability of mortar and concrete made up of pozzolans as a partial replacement of cement: A review. Constr. Build. Mater. 2016, 116, 128–140. [Google Scholar] [CrossRef]
- Kaid, N.; Cyr, M.; Julien, S.; Khelafi, H. Durability of concrete containing a natural pozzolan as defined by a performance-based approach. Constr. Build. Mater. 2009, 12, 3457–3467. [Google Scholar]
- Al Menhosh, A.; Wang, Y.; Wang, Y.; Augusthus-Nelson, L. Long term durability properties of concrete modified with metakaolin and polymer admixture. Constr. Build. Mater. 2018, 172, 41–51. [Google Scholar]
- Bermúdez Odriozola, M.Á.; Alaejos Gutiérrez, P. Comparative study of different test methods for reinforced concrete durability assessment in marine environment. Mater. Struct. 2008, 41, 527–541. [Google Scholar]
- Ramezanianpour, A.A.; Pilvar, A.; Mahdikhani, M.; Moodi, F. Practical evaluation of relationship between concrete resistivity, water penetration, rapid chloride penetration and compressive strength. Constr. Build. Mater. 2011, 5, 2472–2479. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Bernal, S.A. A Review on Durability Performance of Calcined Clay Binders for Adoption in the Construction Industry. In Proceedings of the 75th RILEM Annual Week 2021, Mérida, Mexico, August 2021; pp. 269–279. [Google Scholar]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar]
- Dhandapani, Y.; Santhanam, M. Investigation on the microstructure-related characteristics to elucidate performance of composite cement with limestone-calcined clay combination. Cem. Concr. Res. 2020, 129, 105959. [Google Scholar]
- Maraghechi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC3) with various kaolinite contents with respect to chloride transport. Mater. Struct. 2018, 51, 125. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service life and life cycle assessment of reinforced concrete systems with limestone calcined clay cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- Wilson, W.; Georget, F.; Scrivener, K. Unravelling chloride transport/microstructure relationships for blended-cement pastes with the mini-migration method. Cem. Concr. Res. 2021, 140, 106264. [Google Scholar] [CrossRef]
- Nas, M.; Kurbetci, S. Durability properties of concrete containing metakaolin. Adv. Concr. Construction. 2018, 2, 159–175. [Google Scholar]
- Badogiannis, E.G.; Sfikas, I.P.; Voukia, D.V.; Trezos, K.G.; Tsivilis, S.G. Durability of metakaolin Self-Compacting Concrete. Constr. Build. Mater. 2015, 82, 133–141. [Google Scholar] [CrossRef]
- Jafari, K.; Rajabipour, F. Performance of Impure Calcined Clay as a Pozzolan in Concrete. Transp. Res. Rec. 2020, 2, 643–645. [Google Scholar] [CrossRef]
- Sui, S.; Georget, F.; Maraghechi, H.; Sun, W.; Scrivener, K. Towards a generic approach to durability: Factors affecting chloride transport in binary and ternary cementitious materials. Cem. Concr. Res. 2019, 124, 105783. [Google Scholar] [CrossRef]
- Monteiro PJ, M.; Kurtis, K.E. Time to Failure for Concrete Exposed to Severe Sulfate Attack. Cem. Concr. Res. 2003, 33, 987–993. [Google Scholar] [CrossRef]
- Stark, J.; Freyburg, E.; Seyfarth, K.; Giebson, C.; Erfurt, D. 70 Years of ASR with no End in Sight? (Part 1). Zkg International 2010, 63, 86–95. [Google Scholar]
- Aramburo, C.H.; Pedrajas, C.; Talero, R. Portland Cements with High Content of Calcined Clay: Mechanical Strength Behaviour and Sulfate Durability. Materials 2020, 18, 4206. [Google Scholar] [CrossRef]
- Hu, L.; He, Z. A fresh perspective on effect of metakaolin and limestone powder on sulfate resistance of cement-based materials. Constr. Build. Mater. 2020, 262, 119847. [Google Scholar] [CrossRef]
- Cordoba, G.; Irassar, E.F. Sulfate performance of calcined illitic shales. Constr. Build. Mater. 2021, 291, 123215. [Google Scholar] [CrossRef]
- Ding, W.; Wang, P.; Zhao, C.; He, Y.; Lu, L.; Wang, F.; Hu, S.; Zhan, Q. Study on the microstructure and impermeability of calcium aluminate cement containing metakaolin for development of high-performance marine engineering materials. Sustain. Chem. Pharm. 2024, 42, 101746. [Google Scholar]
- Khan, M.S.H.; Nguyen, Q.D.; Castel, A. Carbonation of Limestone Calcined Clay Cement Concrete. In Calcined Clays for Sustainable Concrete; RILEM Bookseries; Springer: Dordrecht, The Netherland, 2018; Volume 16, pp. 238–243. ISBN 9789402412062. [Google Scholar]
- Trümer, A.; Ludwig, H.-; Schellhorn, M.; Diedel, R. Effect of a calcined Westerwald bentonite as supplementary cementitious material on the long-term performance of concrete. Appl. Clay Sci. 2019, 168, 36–42. [Google Scholar] [CrossRef]
- Bakera, A.T.; Alexander, M.G. Use of Metakaolin As Supplementary Cementitious Material in Concrete, With Focus on Durability Properties. RILEM Tech. Lett. 2019, 4, 89–102. [Google Scholar]
- Wei, J.; Gencturk, B.; Jain, A.; Hanifehzadeh, M. Mitigating alkali-silica reaction induced concrete degradation through cement substitution by metakaolin and bentonite. Appl. Clay Sci. 2019, 182, 105257. [Google Scholar]
- Sarfo-Ansah, J.; Atiemo, E.; Bediako, M.; Tagbor, T.A.; Boakye, K.A.; Adjei, D. The influence of calcined clay pozzolan, low-CaO steel slag, and granite dust on alkali silica reaction in concrete. Int. J. Eng. Res. Appl. 2015, 5, 19–27. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).