Abstract
Structural degradation in liquid environments can hinder the applications of polymer composites as structural materials. In this work, we study the impacts of methanol on surface cracking and the propagation of pre-formed cracks in UV-irradiated poly(methyl methacrylate)/functionalized graphene (PMMA/FG) composites, followed by the uptake of three different crack-generated solvents, namely 1-butanol, cyclohexanol, and 2EA, respectively. The density of surface cracks increases with the increase in the uptake of the crack-generated solvent. The dependence of the nominal diffusivity for the surface cracking on temperature follows an Arrhenius-like law. The methanol in the composites enhances the uptake of the crack-generated solvent, accompanied by the desorption of methanol, and accelerates the initiation and propagation of surface cracks. The activation energy for the initiation of surface cracks shows an increasing dependence on the Hansen solubility distance from methanol. The progression of the pre-formed crack length with time follows a parabolic law. The nominal diffusivity of the crack-generated solvent for the propagation of the single-crack is greater in the healing zone than in the crack-free zone; the corresponding activation energies exhibit an opposite trend. Increasing the fraction of functionalized graphene and decreasing the UV-irradiation dose cause increases in the energy barriers that need to be overcome for the surface cracking and propagation of preexisting cracks.
1. Introduction
The progress in the fabrication of polymer-based composites has made it possible to manufacture polymer composites with the strengthening phases from graphene and carbon dots. Among them, poly(methyl methacrylate) (PMMA) reinforced with functionalized graphene (FG) has been successfully manufactured and employed as the components of artificial joints [1,2] and semiconductor sensors [3,4]. However, exposing polymer-based composites to liquid environments can cause liquid penetration, resulting in structural changes in the polymer, including swelling, cracking, healing, crystallization, voids, etc. The structural durability of polymer-based composites in liquid environments determines their applications in biomedical devices, wearable electronics, etc. [5].
Chau and Li [6] studied methanol transport in deformed PMMA. They found fast Case I transport in the shear band region and Case II transport in the undeformed region. Kwei and coworkers [7,8,9] proposed an analytical model of anomalous transport for the methanol–PMMA system. Harmon et al. [10] analyzed the methanol sorption in cross-linked PMMA, exhibiting anomalous transport and consisting of the characteristics of Case I and II transport mechanisms. Harmon et al. [11] also observed anomalous methanol transport in the deformed direction of cross-linked PMMA, which is faster than in an undeformed direction. Lin et al. [12] studied the methanol-induced opacity in PMMA and reported larger holes in the methanol-treated PMMA with furnace cooling than the corresponding one with air cooling. Lin et al. [13] also showed the reversal movement of a linear crack tip for anomalous transport of methanol in PMMA, which is controlled by Case I transport. Ouano and Carothers [14] studied the dissolution of PMMA and polystyrene in organic solvents and pointed out the dependence of the dissolution on the processing condition and tacticity. Kavda [15] reported the leaching of soluble components in PMMA after being immersed in ethanol and isopropanol for 30 days. Truong et al. [16] examined the cracking of low-molecular-weight PMMA caused by methanol and suggested the role of chain slippage in bond breakage. Recently, Yang et al. [17] examined how UV irradiation affects surface cracking in PMMA-FG composites in crack-generated solvents without methanol treatment. Currently, there are few studies on the kinetic analysis of the cracking of polymers under the sequential immersion of two different solvents. There is a great need to understand the combinational effects of solvents on the structural durability of polymer composites.
This work aims to investigate the roles of methanol in surface cracking and the propagation of the preexisting crack in PMMA/FG composites after being first exposed to UV light and then immersed in 1-butanol, cyclohexanol, or 2EA. The focus was on the kinetic analysis of the cracking behavior under the sequential immersion of two different solvents, which has not been reported in the literature. The propagations of the surface cracks and the preexisting crack were analyzed according to the Hansen solubility distance from methanol, and the corresponding activation energies were calculated.
2. Experimental Information
Detailed information on the materials and the sample preparation can be obtained in the study given by Yang et al. [17] Briefly, suspensions with the methyl methacrylate (MMA) monomer (Sigma-Aldrich, St. Louis, MO, US), FGs (Euflex Technology Corp., New Taipei City, Taiwan) of different ratios (0, 0.3, and 0.7 wt%), and Triton X-100 (Acros Organics, Janssen-Pharmaceuticalaan, Geel, Belgium) were first prepared. The PMMA/n-FG composites were polymerized in Pyrex plate containers during the heat treatment of the prepared suspension at 60 °C. Here, n (=0, 0.3, and 0.7) represents the weight percentage of FG in the PMMA matrix. The as-prepared PMMA/n-FG composites were cut into multiple samples, which underwent machining, grinding, and polishing to achieve dimensions of 10 × 10 × 1.5 mm3.
The as-prepared samples were exposed to UV irradiation (KINGO Electrical Enterprise Co., Tainan, Taiwan) at room temperature. The UV doses amounted to 36.36 J/cm2 and 54.54 J/cm2, respectively. The UV-irradiated samples were immersed in the methanol solvent (Avantor Performance Materials, LLC, Radnor, PA, USA) at 50 °C for 25 min, followed by immersion in 2EA (Acros Organics, Geel, Belgium), 1-butanol (Sigma-Aldrich, St. Louis, MO, USA), or cyclohexanol (Alfa Aesar, Tewksbury, MA, USA) at different temperatures. Surface cracks were imaged on an Olympus BX51 microscope (Olympus Co., Shinjuku, Tokyo, Japan). The optical images were analyzed and used to calculate the surface density of the surface cracks. In addition, a pre-crack with a crack length of 0.8 times the sample width was constructed in individual samples. The samples with the pre-crack were solvent-treated; first, the samples were submerged in methanol for 3 min at 50 °C, followed by immersion in the solvent of 2EA, 1-butanol, or cyclohexanol at various temperatures. Note that the uptake of methanol basically causes the swelling of PMMA through diffusion without a chemical reaction; the diffusion of 1-butanol into PMMA mainly causes the dissolution of PMMA, and the diffusion of 1-butanol into PMMA majorly leads to the solvation of the PMMA radicals.
It is worth noting that the molecular weight of UV-irradiated PMMA was determined using a gel permeation chromatography (GPC) system (Waters Corporation, Milford, MA, USA) and was reported in our previous work [17]. The results reveal the decrease in the molecular weight of UV-irradiated PMMA with increasing UV-irradiation dose, suggesting the irradiation-induced scission of polymer chains. The results of the FTIR (Brucker Scientific LLC, Billerica, MA, USA) analysis of the UV-irradiated PMMA were presented in our previous work [18]. The UV-irradiation method eradicated the side groups of polymer chains and caused the broadening of the whole carbonyl group due to the formation of a new oxidized group.
3. Results
Figure 1a depicts the optical micrographs of a PMMA/0.3-FG composite, which was initially submerged in the methanol solvent at 50 °C for 25 min, followed by immersion in 1-butanol for different immersion times. Surface cracks are presented on the PMMA/0.3-FG composite. The size and density of surface cracks notably increase as the immersion time in 1-butanol increases. It is worth mentioning that without the pre-uptake of methanol, immersing PMMA in 1-butanol at temperatures of 40 °C or below does not cause surface cracking. This is because the tensile stress induced by 1-butanol was insufficient to lead to the initiation of surface cracks.
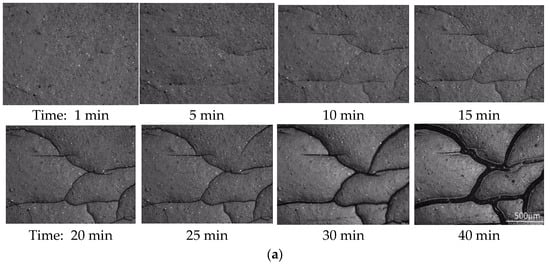
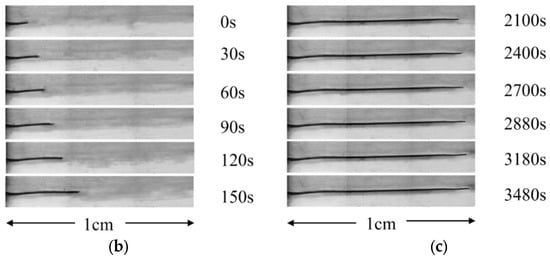
Figure 1.
(a) Optical images of a PMMA/0.3-FG composite that was initially submerged in methanol at 50 °C for 25 min, followed by immersion in 1-butanol for different times; optical images of the sizes of a crack grown from a pre-formed crack in an unirradiated, incompletely healed PMMA at different instances after being immersed in 2EA at 30 °C, which was immersed first in methanol at 30 °C for 3 min: (b) in a healed zone and (c) in the non-healed zone.
Figure 1b,c show the crack size of a pre-formed crack in the healed (see Figure 1b) and non-healed (see Figure 1c) regions at different moments in an un-irradiated PMMA after being placed in 2EA at a temperature of 30 °C. The PMMA was placed in the methanol solvent at 30 °C for 3 min to heal the pre-formed crack. The crack progressed gradually through the healed and the non-healed regions with increasing immersion time. The growth rate of the crack in the healed region is considerably higher than in the non-healed region. Figure S1 in Supporting Information shows an optical image of the cracked surface of a non-irradiated PMMA/0.7-FG composite. The PMMA/0.7-FG composite was placed first in the methanol solvent for 3 min at a temperature of 50 °C and then in 1-butanol for 40 min at 30 °C. The healing front and the crack front are represented, respectively, by a and b. The cracking zone generated by the tensile stress ahead of the crack front is denoted as c.
Figure 2 illustrates the time dependence of the crack density (defined as the ratio of total crack length to the corresponding surface area) for the PMMA/0.3-FG composites with and without UV irradiation. These composites were submerged first in the solvent of methanol at 50 °C for 25 min, followed by submersion in the solvent of 2EA at respective temperatures of 20, 30, 40, and 50 °C. Additional results are shown in Figures S2–S9 in Supporting Information. The crack density in the PMMA/n-FG composites submerged in cyclohexanol and 2EA exhibits an increasing trend with the increase in temperature and immersion time in contrast to the corresponding ones placed in 1-butanol, which is similar to the group without being immersed first in methanol [17]. At a given temperature, the longer the immersion time, the smaller the increase rate of the crack density. For the PMMA/n-FG composite placed in 1-butanol, the crack density increases to a peak value and then decreases as the immersion temperature rises at a given time, as presented in Figures S7–S9 in Supporting Information.
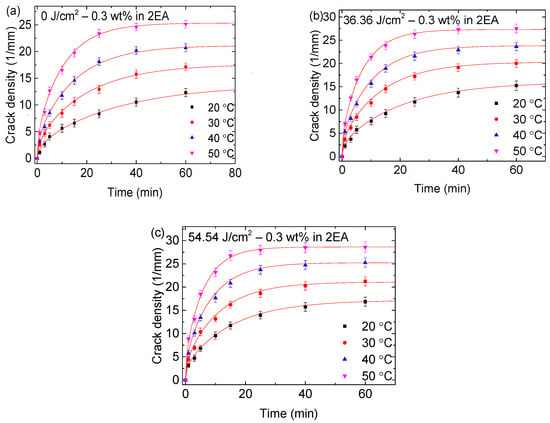
Figure 2.
Time dependence of the surface crack density in PMMA/0.3-FG composites with and without UV irradiation: (a) 0 J/cm2, (b) 36.36 J/cm2, and (c) 54.54 J/cm2. The PMMA/0.3-FG composites were placed first in methanol at 50 °C for 25 min and then in 2EA at respective temperatures of 20, 30, 40, and 50 °C.
Figure 3 depicts the time dependence of the square of the crack lengths of partially healed cracks in the PMMA/0.7-FG composites with and without UV irradiation, which were immersed in 1-butanol at various temperatures. The PMMA/0.7-FG composites were placed first in methanol to incompletely heal the preexisting crack. Additional results are available in Figures S10–S17 in Supporting Information. In contrast to the requirement of higher temperatures (50 °C or above), for the propagation of the preexisting cracks in the PMMA/0.7-FG composites with and without UV irradiation in the three organic solvents without being immersed first in methanol [7], the immersion in methanol allows for the propagation of the pre-formed cracks in the PMMA/n-FG composites in the three organic solvents at lower temperatures of 30, 35, and 40 °C, regardless of UV irradiation. The square of the crack lengths of the single cracks increases linearly with the immersion time in both the healed and non-healed regions. The slope of the ΔL2 versus time decreases with the FG weight fraction and increases with the UV-irradiation dose for the healed and non-healed regions. Such a result suggests that the FGs hinder the polymer chain movement and increase the energy barrier for crack propagation. The slope of the square of the crack length in relation to the immersion time is larger in the healed region compared to the non-healed region for the crack propagation at the same temperature. Note that the healing length increases linearly with the immersion time for methanol uptake, while the crack length increases parabolically with the immersion time for 1-butanol sorption.
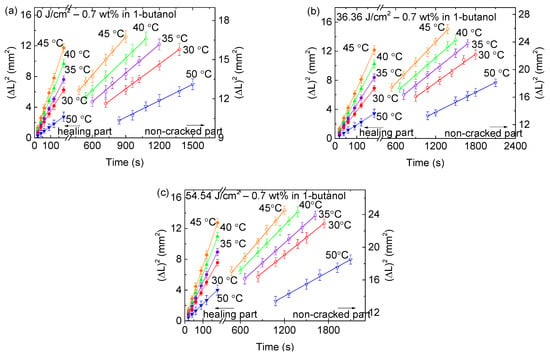
Figure 3.
Time dependence of the crack sizes of partially healed cracks in PMMA/0.7-FG composites subjected to (a) 0 J/cm2, (b) 36.36 J/cm2, and (c) 54.54 J/cm2 of UV irradiation. The PMMA/0.7-FG composites were submerged in the solvent of 1-butanol after being placed first in methanol to heal the preexisting cracks partially. Note that symbols orange diamond, green triangle, blue round, red round, and blue inverted triangle represent data for 45, 40, 35, 30, and 50 °C, respectively. Solid and hollow symbols stand for healing and non-cracked areas.
According to Figure 3, the crack length increases to a peak value and then decreases with increasing temperature for a given time, UV dose, and FG fraction for the UV-irradiated PMMA/n-FG composites immersed in 1-butanol. However, Figures S10–S17 in Supporting Information illustrate that the crack length increases monotonically with temperature for the UV-irradiated PMMA/n-FG composites immersed in cyclohexanol and 2EA at the same immersion time. The different crack propagation behaviors of the UV-irradiated PMMA/n-FG composites in cyclohexanol, 2EA, and 1-butanol are associated with the Hansen solubility parameter, as discussed later.
4. Discussion
Yang et al. [17] showed that the tensile stress from the penetration of an organic solvent into PMMA/n-FG composites can cause the nucleation and propagation of surface cracks. They suggested that the time dependence of the crack density (Δℓc) associated with the penetration of an organic solvent into a PMMA/n-FG composite can be formulated as [17]
Here, Δℓ∞ represents the ultimate crack density of surface cracks, expressed as t → ∞; D stands for the diffusivity controlling the crack growth, and 2ℓ denotes the thickness of the PMMA/n-FG composite. Using Equation (1) to curve-fit the results in Figure 2 and Figures S2–S9 in Supporting Information, we determine the nominal diffusivity controlling the crack propagation in the PMMA/n-FG composites and include the fitting results in the corresponding figures. It is evident that the results in Figure 2 and Figures S2–S9 can be well described by Equation (1). Note that Equation (1) is based on the uptake of organic solvent, which is the dominant mechanism controlling the cracking of PMMA in the organic solvent. There are two processes likely playing important roles in the solvent-induced cracking: one is the solvent-induced swelling of PMMA, as widely reported in the literature, and the other is the solvent-induced changes in the FG functionality, as reported by Politano and Versace [19] and Pendolino et al. [20]. The solvent-induced swelling of PMMA leads to the weak bonding between polymer chains and the easy migration of polymer chains in a stress field. The solvent-induced changes in the FG functionality might reduce the interaction between FGs and the PMMA matrix, resulting in a decrease in the resistance to the migration of polymer chains.
The total amount of solvent during the uptake as a function of the immersion time can be written as
where DS represents the diffusivity of the solvent and is equal to D in numerical value. It is worth mentioning that no surface cracks due to the solvent transport were observed at temperatures below 40 °C. Without methanol, the uptake of the crack-generated solvent in PMMA/n-FG composites was undertaken at temperatures above 50 °C. At temperatures in the range of 20–55 °C, we pre-treated the samples with methanol before taking the measurement of the solvent-induced cracking. Figure 4 shows the temporal evolution of the solvent residing in the PMMA/0.7-FG composites after first being UV-irradiated at the dose of 36.36 J/cm2 and then immersed in three respective solvents at different temperatures. Figures S18–S25 in Supporting Information present the additional results of the time dependence of the amount of solvent residing in the PMMA/n-FG composites, consisting of different FG weight fractions and experiencing UV irradiation of different doses. Note that Equation (2) cannot be used to describe the results in Figure 4 and Figures S18–S25 because the solvent uptake and the methanol desorption co-occur. It is impossible to separate the solvents during the uptake.
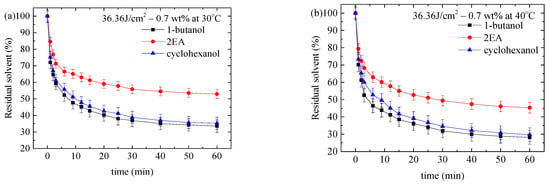
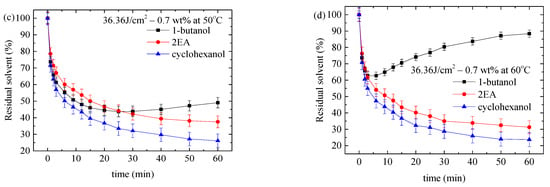
Figure 4.
Time dependence of the solvent residing in the PMMA/0.7-FG composites, which were UV-irradiated at the dose of 36.36 J/cm2 and immersed in different desorption liquids at temperatures of (a) 30 °C, (b) 40 °C, (c) 50 °C, and (d) 60 °C.
According to Figure 4 and Figures S18–S25, the amount of the solvent residing in the PMMA/n-FG composites for a given temperature, time, FG weight fraction, and UV-irradiation dose varies with the solvent in an increasing sequence of 1-butanol, cyclohexanol, and 2EA, except for the uptake of 1-butanol at 50 and 60 °C. The amount of 1-butanol residing in the methanol-treated PMMA/n-FG composites decreases first and then increases with increasing immersion time at 50 and 60 °C, implying the rapid desorption of methanol and the large uptake of 1-butanol. Also, after the long immersion time, the solvent exceeds the initial amount of methanol in the samples.
There are two regions: One is that the residual solvent decreases monotonically with time for 2EA and cyclohexanol at all the temperatures and 1-butanol at 30 and 40 °C, in which the methanol in the sample is greater than the three solvents. Methanol increases the nucleation and propagation of surface cracks. The other is that the residual solvent of 1-butanol decreases first and then increases over time at 50 and 60 °C. Methanol desorbs much faster, so 1-butanol also contributes significantly to the formation and propagation of surface cracks.
Figure 5 shows the temperature dependence of the nominal diffusivity for the migration of solvent in the PMMA/0.7-FG composites, which were treated first in methanol over 25 min at 50 °C and then in respective solvents of cyclohexanol, 2EA, and 1-butanol. Additional results are illustrated in Figures S26–S28 in Supporting Information. According to Figure 5, the nominal diffusivity exhibits an increasing trend in the sequence of 1-butanol, cyclohexanol, and 2EA for a given temperature, UV-irradiation dose, and FG weight fraction. Such behavior can be associated with the Hansen solubility parameter. From Figure 5c, we note that there are two distinct regions for the temperature dependence of the nominal diffusivity of 1-butanol in the PMMA/0.7-FG composites with and without UV irradiation, consistent with the time dependence of the crack density shown in Figures S7–S9. Such a result is in good accord with the two regions shown in Figure 4c,d and Figures S18–S25.
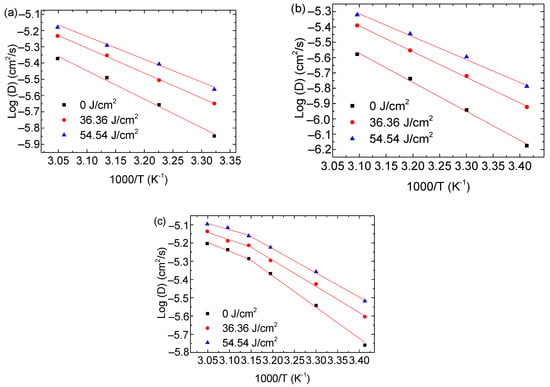
Figure 5.
Temperature dependence of nominal diffusivities for the surface cracking in PMMA/0.7-FG composites with and without UV irradiation. The PMMA/0.7-FG composites were immersed first in methanol at the temperature of 50 °C for 25 min and then in (a) cyclohexanol, (b) 2EA, and (c) 1-butanol.
Table 1 shows the Hansen solubility parameters of the four organic solvents used in this work and the corresponding Hansen solubility distances from methanol. The Hansen solubility distance (RH) between solvent and methanol is calculated from the following formulation:
where the subscripts H, D, and P denote, respectively, the hydrogen bond, dispersive, and polar components of the solubility parameter. The subscripts 1 and 2 represent solvent and methanol, respectively. Table 1 reveals that the Hansen solubility distance from methanol increases with the solvents in the 1-butanol, cyclohexanol, and 2EA sequences. The larger the Hansen solubility distance from methanol, the smaller the miscibility between the two solvents. When the methanol-treated PMMA/n-FG composite is immersed in one of the three solvents, the solvent penetrates the sample and then the methanol escapes from the sample to the solvent reservoir.

Table 1.
List of Hansen solubility parameters of PMMA [21], methanol [22], 2EA [22], 1-butanol [22], and Cyclohexanol [22], as well as Hansen solubility distance (RM) from methanol shown as molar volume (V). The units of Hansen solubility parameter and distance are (MPa)1/2.
According to Figure 5 and Table 1, the larger the Hansen solubility distance from methanol, the smaller the nominal diffusivity for all the temperatures, UV doses, and FG fractions used in this work. Note that the value of the nominal diffusivity is equal to that of solvent diffusivity. Comparing Figure 5 and Figures S26–S28 in Supporting Information with Table 1, the nominal diffusivity decreases with increasing molar volume of the solvent due to the easy movement of small molecules if the effect of chemical bonding is negligible.
We use an Arrhenius-type relationship to determine the activation energies associated with the nominal diffusivities. The variation in the activation energy with the UV-irradiation dose is depicted in Figure 6a–d for the PMMA/n-FG composites, which were submerged first in methanol over a period of 25 min at 50 °C and then in three individual organic solvents. For the PMMA/n-FG composites of the same FG weight fraction, the activation energy decreases linearly with the UV-irradiation dose, attributing to the increase in the structural defects of free volume during the UV irradiation, which causes a decrease in the energy barrier for the migration of polymer chains. The activation energy increases with the solvents in the sequence of 1-butanol, cyclohexanol, and 2EA for the diffusion of organic solvents in PMMA/n-FG composites of the same FG weight fraction and UV-irradiation dose. This is because the energy barrier for the solvent diffusion increases with the Hansen solubility distance from methanol. Note that no surface cracks were observed when methanol-free PMMA/n-FG composites were submerged in the three individual solvents below 40 °C. Immersing the PMMA/n-FG composites in methanol at 50 °C for 25 min causes a decrease in the energy barrier for the rate process controlling the crack propagation. Such behavior is likely attributed to the crack formation and propagation caused by the tensile stress near the free surface, which is introduced by the methanol desorption, as well as the decrease in the resistance to the migration of the individual organic solvents. Again, the activation energy increases with the molar volume of the solvent. According to Figure 6a–d, the higher the FG weight fraction, the larger the activation energy due to the hindrance of FGs to the motion of PMMA chains.
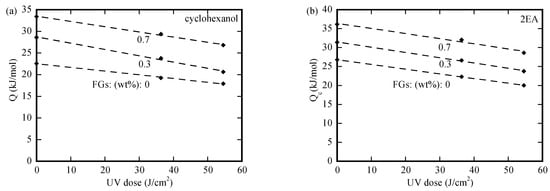
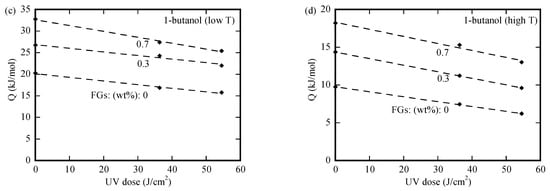
Figure 6.
Variation in the activation energy with UV-irradiation dose for the PMMA/n-FG composites placed first in the solvent of methanol over a period of 25 min at the temperature of 50 °C, followed by respective immersions in (a) cyclohexanol, (b) 2EA, (c) 1-butanol (at low temperatures), and (d) 1-butanol (at high temperatures).
Linear regression was used to determine the slopes for the data shown in Figure 6a–d. The slopes are (−0.087, −0.144, −0.120), (−0.124, −0.139, −0.135), (−0.084, −0.084, −0.136), and (−0.065, −0.087, −0.093) in the unit of 103 cm2/mol for the crack propagation in the PMMA/n-FG composites, which were submerged in the individual solvents of cyclohexanol, 2EA, and 1-butanol at low temperatures and 1-butanol at high temperatures, respectively. The three numerical values in each parenthesis correspond to the FG weight fractions of 0, 0.3%, and 0.7%. The slope is always negative, and there is no definite trend for the slope variation with the FG weight fraction. Such behavior suggests the presence of a complex interaction between the FGs and the UV-induced breakage.
As discussed above, the uptake of organic solvents depends on the migration of the organic solvents and can cause the formation and propagation of cracks in the PMMA/n-FG composites. The classical Fick law is applicable to analyze the diffusion of the organic solvent, and the scaling law yields that the mean square displacement of molecules is directly proportional to the diffusion time. The time dependence of the crack propagation can be formulated as
where ΔL is the crack length, α is the proportionality constants, Dc represents the nominal diffusivity for the rate process controlling the crack propagation in both the healed and crack-free areas, and tc represents the incubation time for the crack propagation in the healed area. ΔLh is the longest crack length in the healed region at the finished time, th.
We analyze the results in Figure 3 and Figures S10–S17 for the propagation of the pre-formed cracks in the crack-free and incompletely healed regions. Here, the samples were submerged first in methanol for 3 min at the temperature of 50 °C and then in cyclohexanol, 2EA, or 1-butanol. Equation (3) is used to curve-fit the results in Figure 3 and Figures S10–S17 to obtain the numerical values of α2Dc and tc. The temperature dependence of the numerical values of α2Dc is depicted in Figures S29–S31 in Supporting Information, which correlated with the propagation of the cracks in the partially healed region, and Figures S32–S34 in Supporting Information, which correlated with the propagation of the cracks in the crack-free region. There exists an inverse proportion between log(α2Dc) and T−1 (reciprocal of temperature), indicating that the propagation of the preexisting cracks in both the crack-free and partially healed regions is a thermally activated process and correlates with the diffusion of the solvents.
The dependence of log(1/tc) on T−1 is presented in Figures S35–S37 in Supporting Information. Increasing the UV-irradiation dose and/or decreasing the FG fraction leads to the increase in log(1/tc). The log(1/tc) increases with the solvents, following the sequence of 2EA, cyclohexanol, and 1-butanol. Such a trend implies that the larger the Hansen solubility distance from methanol, the smaller the surface wettability.
Figure 7a–c depict the variation in the activation energy with the UV-irradiation dose for the propagation of the preexisting cracks in the PMMA/n-FG composites in both the crack-free and partially healed regions. The activation energy for the propagation of the preexisting cracks in the partially healed region is larger than the corresponding one in the crack-free region under the same conditions. The faster propagation of the preexisting cracks in the healed region can be attributed to the lower cohesive strength of the healed crack by the methanol due to the limited travel distance of polymer chains through the crack surfaces. It should be pointed out that increasing the desorption time of methanol increases the cohesive strength of a methanol-healed crack. In contrast, the activation energy for the crack propagation has the opposite trend. This implies that the pre-factor of the nominal diffusivity plays a role in the solvent-induced initiation and propagation of surface cracks in the PMMA/n-FG composites pre-treated with methanol and UV irradiation.
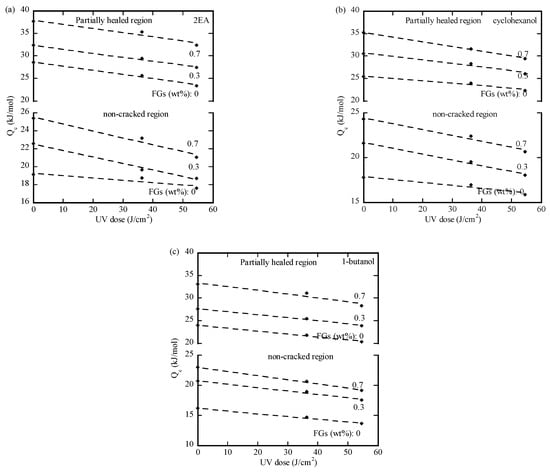
Figure 7.
Variation in the activation energy with the UV dose for the propagation of the preexisting cracks in the methanol-treated PMMA/n-FG composites in (a) 2EA, (b) cyclohexanol, and (c) 1-butanol.
Increasing the UV-irradiation dose causes a decrease in the activation energy for the propagation of the pre-formed cracks, attributed to the UV-induced breakage of polymer chains and the presence of more free volume. This reduces the resistance to the migration of polymer chains and the associated energy barrier. For the PMMA/n-FG composites irradiated with the same irradiation dose, the higher the weight fraction of FGs, the larger the activation energy. Such a trend suggests that FGs hinder the migration of polymer chains in the PMMA/n-FG composites. Increasing the weight fraction of FGs increases the resistance to the motion of polymer chains. The propagation of the preexisting cracks in the methanol-treated PMMA/n-FG composites is similar to that in the methanol-free PMMA/n-FG composites. Comparing the data in Figure 7 with the data in Table 1, the activation energy for the propagation of the preexisting crack increases with the solvents in the sequence of 1-butanol, cyclohexanol, and 2EA, implying that the propagation of the preexisting crack under the same conditions is correlated with the Hansen solubility distance from methanol.
Figure 8a–c illustrates the dependence of the activation energy associated with the incubation time on the UV-irradiation dose for the propagation of the cracks pre-formed in the PMMA/n-FG composites in partially healed regions. Increasing the UV-irradiation dose increases the activation energy, which can be attributed to the decrease in surface wettability with increasing incubation time. Increasing the FG weight fraction causes the activation energy to decrease due to the hindrance of FGs to the surface wetting of the solvents. The activation energy associated with the incubation time increases with the solvents in the sequence of 2EA, cyclohexanol, and 1-butanol, opposite to the trend of the Hansen solubility distance from the methanol.
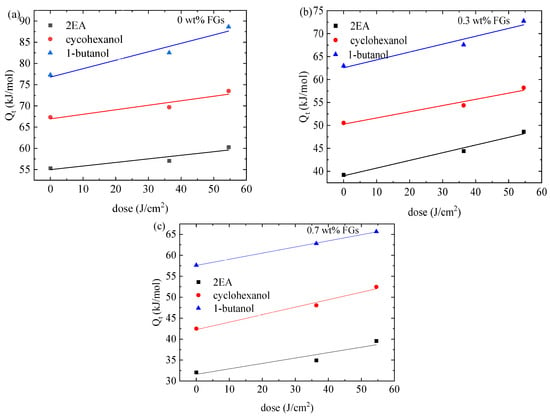
Figure 8.
Dependence of the activation energy associated with the incubation time on the UV-irradiation dose for the propagation of the pre-formed cracks in the partially healed region of methanol-treated PMMA/n-FG composites in (a) 2EA, (b) cyclohexanol, and (c) 1-butanol.
5. Summary
In summary, we have shown the important role of pre-methanol treatment in surface cracking and the propagation of the preexisting cracks in the UV-irradiated PMMA/n-FG composites in three different crack-generated solvents, namely 2EA, cyclohexanol, and 1-butanol. The uptake of the respective crack-generated solvents produces tensile stress near the free surface which causes the initiation and propagation of surface cracks, as well as near the crack tip in the UV-irradiated PMMA/n-FG composites which causes crack propagation. The surface crack density was linearly proportional to the uptake of the organic solvent. The nominal diffusivity associated with the increment of the surface crack density follows the Arrhenius-type relation. Methanol desorption produces tensile stress to accelerate the initiation and propagation of surface cracks and the propagation of the preexisting crack. The methanol treatment lowers the temperature for the initiation of surface cracks, as well as the propagation of the preexisting cracks in the UV-irradiated PMMA/n-FG composites. The solvents with increasing order of the Hansen solubility distance from methanol are 1-butanol, cyclohexanol, and 2EA. The activation energy for the surface cracking increases with the solvents in the sequence of 1-butanol, cyclohexanol, and 2EA, implying that the activation energy increases with the Hansen solubility distance from methanol. There are two activation energies for surface cracking in 1-butanol. At low temperatures, the longer the immersion time, the less the total residual solvent in samples; at high temperatures, increasing the immersion time causes the decrease in the total residual solvent first and then the increase.
The propagation of the preexisting crack follows the parabolic law in two regions: one region is the healed zone by methanol, and the other is the crack-free zone. The activation energy for the solvent diffusion is greater in the healed zone than in the crack-free one, and the nominal diffusivity is greater in the healed zone than in the crack-free one. This behavior is attributed to the weaker bonding of the healed zone than that of the crack-free one. The activation energy for the crack propagation in individual zones increases with the Hansen solubility distance from methanol.
The results obtained in this work illustrate the role of the interactions between organic solvents in the structural integrity of polymer, which can be used to understand drug release kinetics in organic solvents. It can lead to the development of analytical practices to evaluate the in vivo performance of a drug release and provide a mechanism to control the drug release for clinic applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcs9020084/s1. Figure S1 shows an optical image of the cracked surface of a non-irradiated PMMA/0.7-FG composite. Figures S2–S9 show the time dependence of the crack density for the PMMA/FG composites of different FG fractions with and without UV irradiation. Figures S10–S17 depict the propagation of the preformed crack in the crack-free region. Figures S18–S25 describe the amount of the solvent residing in the PMMA/nFG composites. Figures S26–S28 show temperature dependence of nominal diffusivities for the surface cracking in PMMA/-FG composites of different FG fractions with and without UV irradiation. Figures S29–S31 and S32–S34 correspond the temperature dependence of the α2Dc with the propagation of the cracks in the partially healed and crack-free regions. Figures S35–S37 show the dependence of log(1/tc) on T−1.
Author Contributions
B.-H.Y.: Data curation, methodology, and writing—original draft. S.-Y.C.: Methodology, project administration, supervision, and validation. Y.Z.: Formal analysis, methodology, and validation. F.Y.: Conceptualization, methodology, validation, and writing—review and editing. S.L.: Conceptualization, funding acquisition, project administration, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
S.Y.C. and S.L. thank the National Science and Technology Council, Taiwan, for the financial support through the grant number NSTC 113-2221-E-007-049.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare the following financial interests/personal relationships, which may be considered potential competing interests: Sanboh Lee reports financial support was provided by the National Science and Technology Council of Taiwan.
References
- Al-Waily, M.; Al Saffar, I.Q.; Hussein, S.G.; Al-Shammari, M.A. Life enhancement of partial removable denture made by biomaterials reinforced by graphene nanoplates and hydroxyapatite with the aid of artificial neural network. J. Mech. Eng. Res. Dev. 2020, 43, 269–285. [Google Scholar]
- Han, B.; Zhang, Y.L.; Zhu, L.; Li, Y.; Ma, Z.C.; Liu, Y.Q.; Zhang, X.L.; Cao, X.W.; Chen, Q.D.; Qiu, C.W.; et al. Plasmonic-Assisted Graphene Oxide Artificial Muscles. Adv. Mater. 2019, 31, 1806386. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, B.; Jia, N.; Yu, Y.; Xu, X.; Wang, Y.; Wu, B.; Qian, J.; Xia, R.; Wang, C. Enhanced thermally conductive and thermomechanical properties of polymethyl methacrylate (PMMA)/graphene nanoplatelets (GNPs) nanocomposites for radiator of electronic components. Polym. Test. 2021, 101, 107237. [Google Scholar] [CrossRef]
- Pawar, D.; Kanawade, R.; Kumar, A.; Rao, C.N.; Cao, P.; Gaware, S.; Late, D.; Kale, S.N.; Navale, S.; Liu, W. High-performance dual cavity-interferometric volatile gas sensor utilizing Graphene/PMMA nanocomposite. Sens. Actuators B Chem. 2020, 312, 127921. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Hu, F.; Liu, D.; Zhang, X.; Ren, J.; Guo, H.; Shalash, M.; He, M.; Hou, H.; et al. An overview of polymer-based thermally conductive functional materials. J. Mater. Sci. Technol. 2025, 218, 191–210. [Google Scholar] [CrossRef]
- Chau, C.C.; Li, J.C.M. Diffusion of methanol in PMMA shear bands. Philos. Mag. A 1981, 44, 493–498. [Google Scholar] [CrossRef]
- Wang, T.T.; Kwei, T.K. Diffusion in glassy polymers. Reexamination of vapor sorption data. Macromolecules 1973, 6, 919–921. [Google Scholar] [CrossRef]
- Kwei, T.K.; Wang, T.T.; Zupko, H. Diffusion in glassy polymers. V. Combination of Fickian and Case II mechanisms. Macromolecules 1972, 5, 645–646. [Google Scholar] [CrossRef]
- Wang, T.T.; Kwei, T.K.; Frisch, H. Diffusion in glassy polymers. III. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 2019–2028. [Google Scholar] [CrossRef]
- Harmon, J.P.; Lee, S.; Li, J.C.M. Methanol transport in PMMA: The effect of mechanical deformation. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 3215–3229. [Google Scholar] [CrossRef]
- Harmon, J.P.; Lee, S.; Li, C.M.J. Anisotropic methanol transport in PMMA after mechanical deformation. Polymer 1988, 29, 1221–1226. [Google Scholar] [CrossRef]
- Lin, C.B.; Liu, K.S.; Lee, S. Methanol-induced opacity in poly (methyl methacrylate). J. Polym. Sci. Part B Polym. Phys. 1991, 29, 1457–1466. [Google Scholar] [CrossRef]
- Lin, C.B.; Lee, S.; Liu, K.S. Methanol-Induced crack healing in poly (methyl methacrylate). Polym. Eng. Sci. 1990, 30, 1399–1406. [Google Scholar] [CrossRef]
- Ouano, A.; Carothers, J. Dissolution dynamics of some polymers: Solvent-polymer boundaries. Polym. Eng. Sci. 1980, 20, 160–166. [Google Scholar] [CrossRef]
- Kavda, S.; Golfomitsou, S.; Richardson, E. Effects of selected solvents on PMMA after prolonged exposure: Unilateral NMR and ATR-FTIR investigations. Herit. Sci. 2023, 11, 63. [Google Scholar] [CrossRef]
- Truong, V.T.; Williams, D.; Allen, P. The effect of molecular properties on the mechanical behaviour of PMMA—II. Environmental stress cracking in methanol. Eur. Polym. J. 1987, 23, 41–50. [Google Scholar] [CrossRef]
- Yang, B.-H.; Chang, S.-Y.; Zhang, Y.; Yang, F.; Lee, S. Cracking in UV-irradiated poly (methyl methacrylate)/functionalized graphene composites: Solvent effect. J. Polym. Res. 2024, 31, 274. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Zhang, Y.; Ouyang, H.; Yang, F.; Lee, S. Photo-response of polylactide-poly (methyl methacrylate) blends: Effect of ultraviolet irradiation. Mater. Chem. Phys. 2022, 277, 125528. [Google Scholar] [CrossRef]
- Politano, G.G.; Versace, C. Variable Angle Spectroscopic Ellipsometry Characterization of Graphene Oxide in Methanol Films. Crystals 2022, 12, 696. [Google Scholar] [CrossRef]
- Pendolino, F.; Capurso, G.; Maddalena, A.; Russo, S.L. The structural change of graphene oxide in a methanol dispersion. RSC Adv. 2014, 4, 32914–32917. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- ACCU DYNE TEST. Available online: https://www.accudynetest.com/solubility_table.html (accessed on 15 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).