Graphene Oxide–Antibiotic Coatings with Improved Resistance to Microbial Colonization for Arthroplasty Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Conditions of the MAPLE Process
2.2.2. Physicochemical Investigation
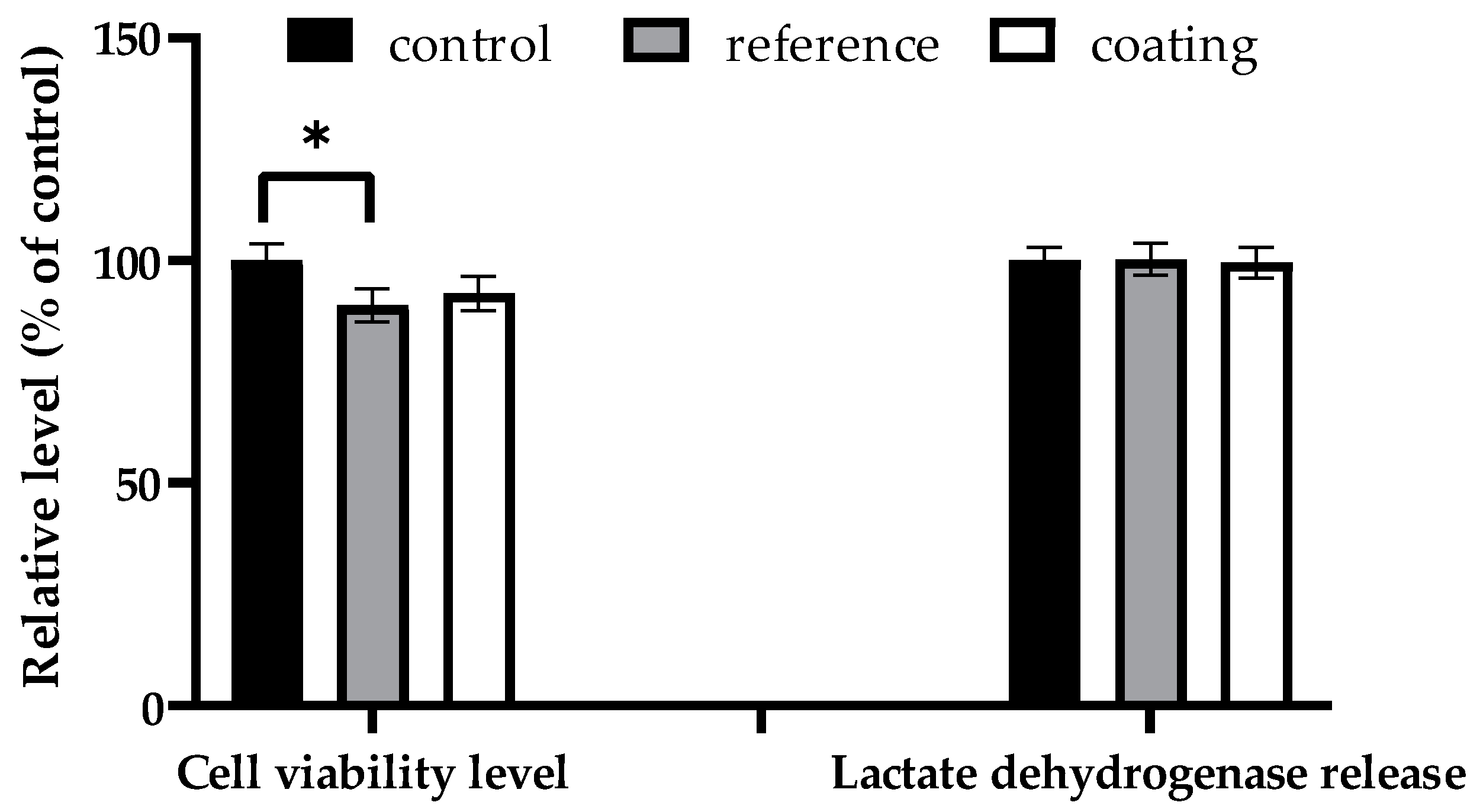
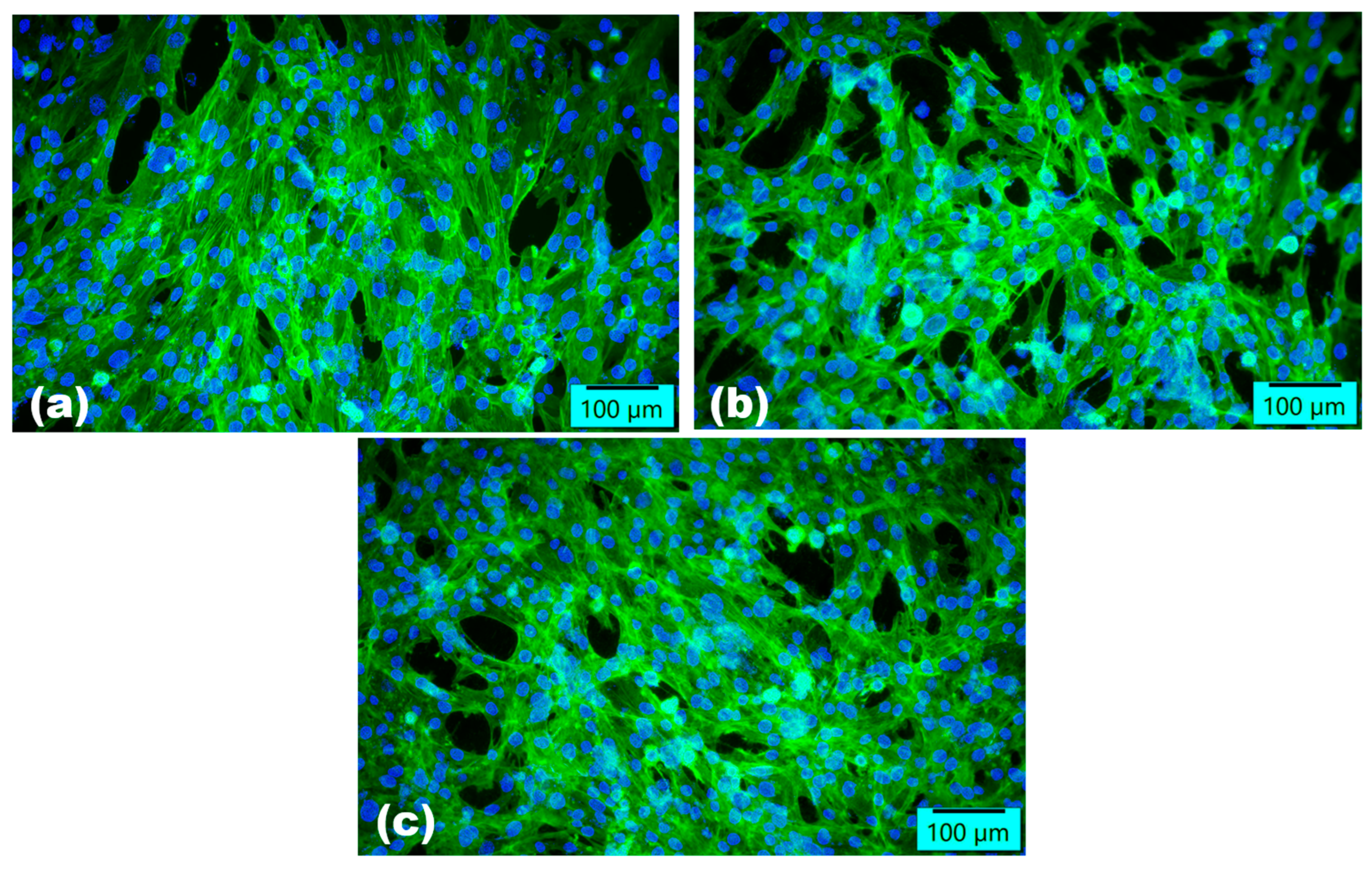
2.2.3. Assessment of In Vitro Biocompatibility
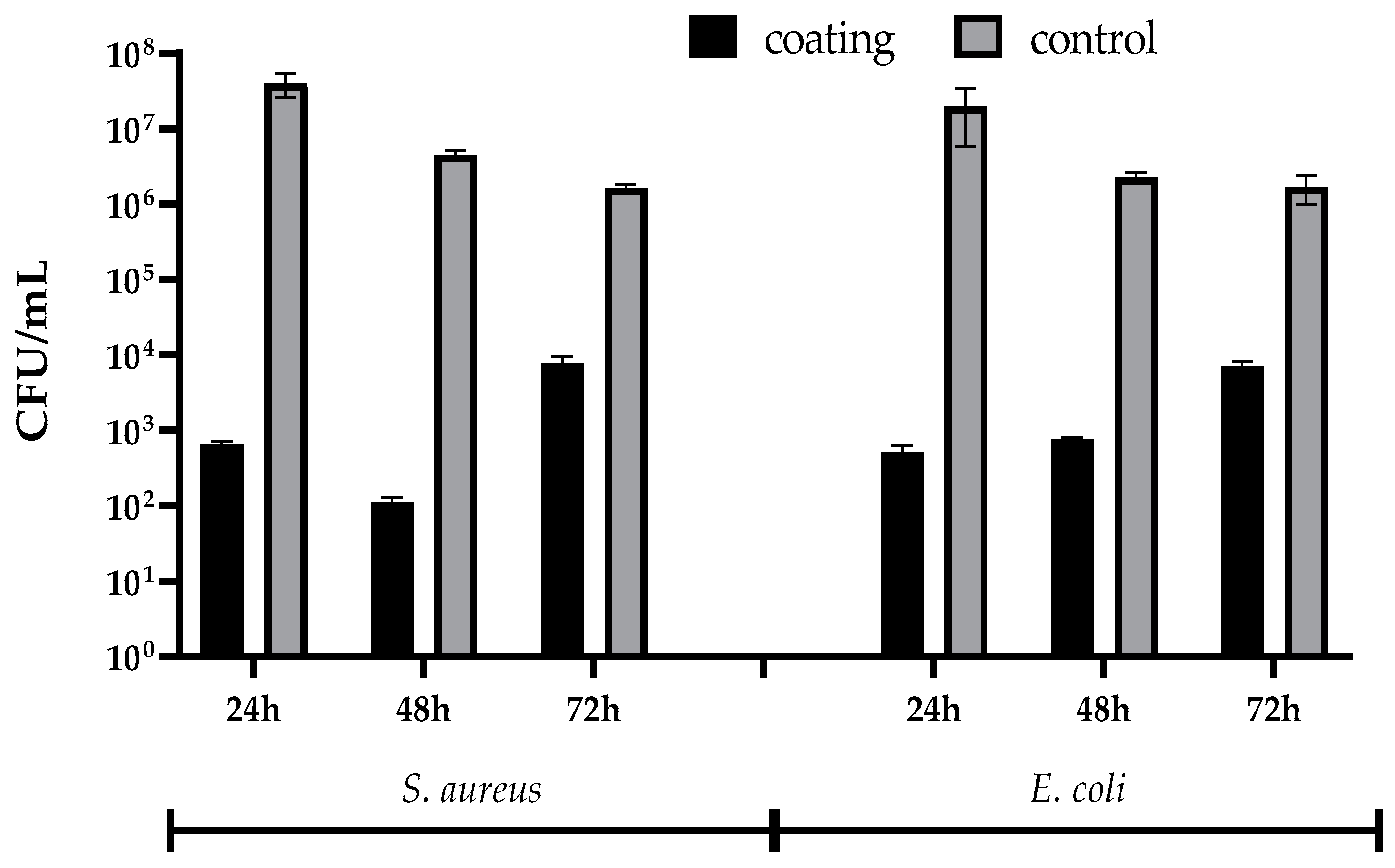
2.2.4. Microbiological Assay
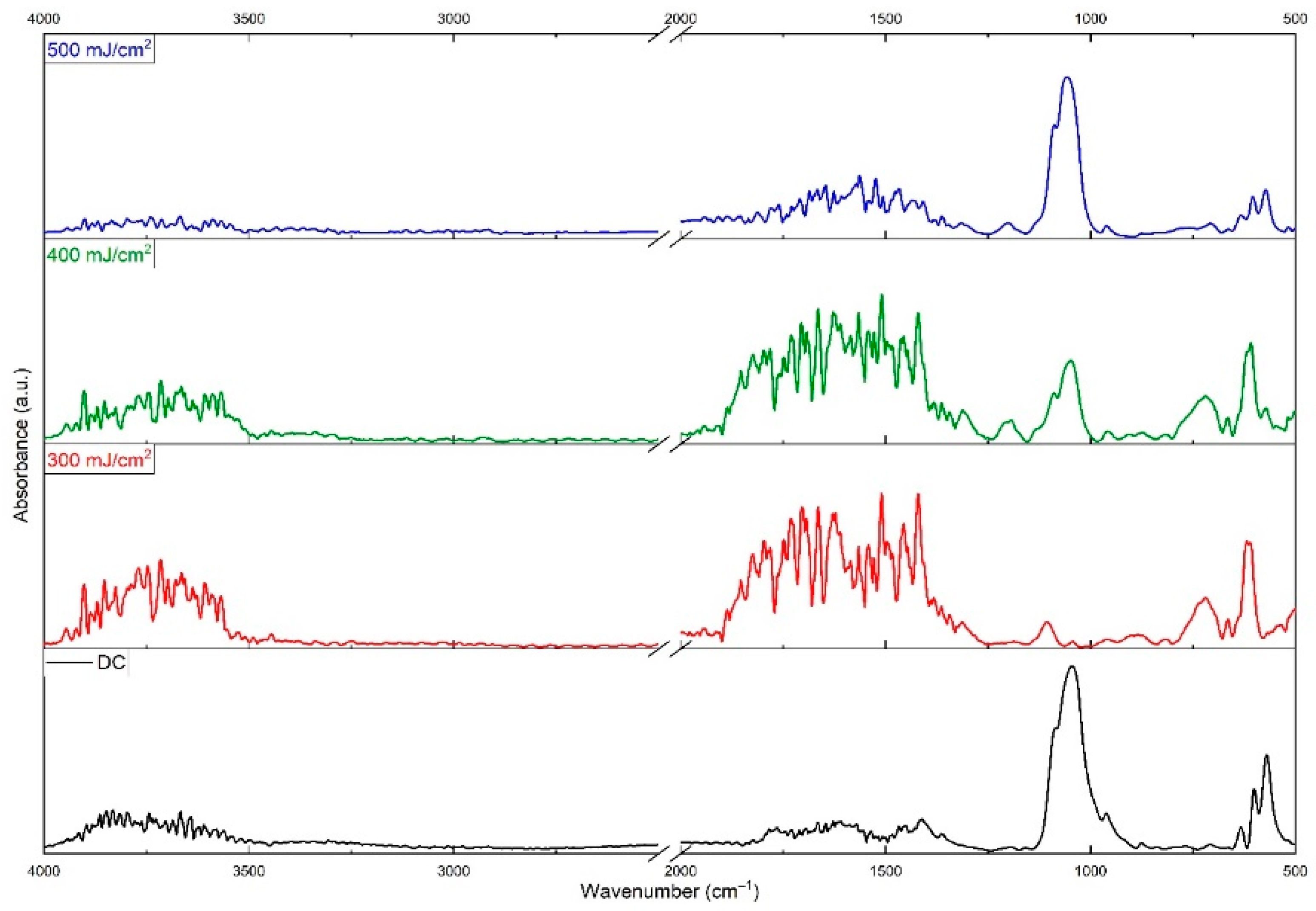
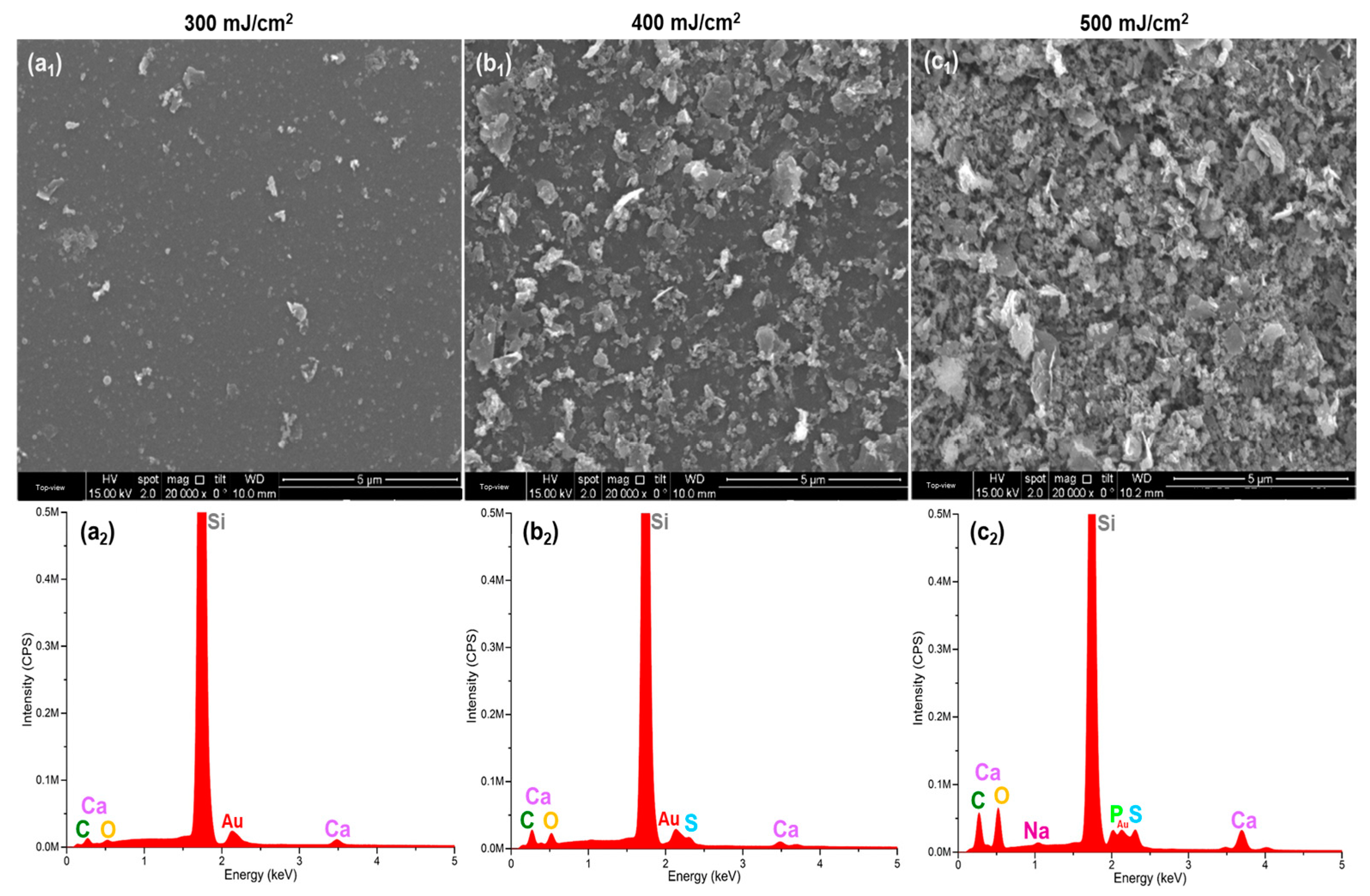
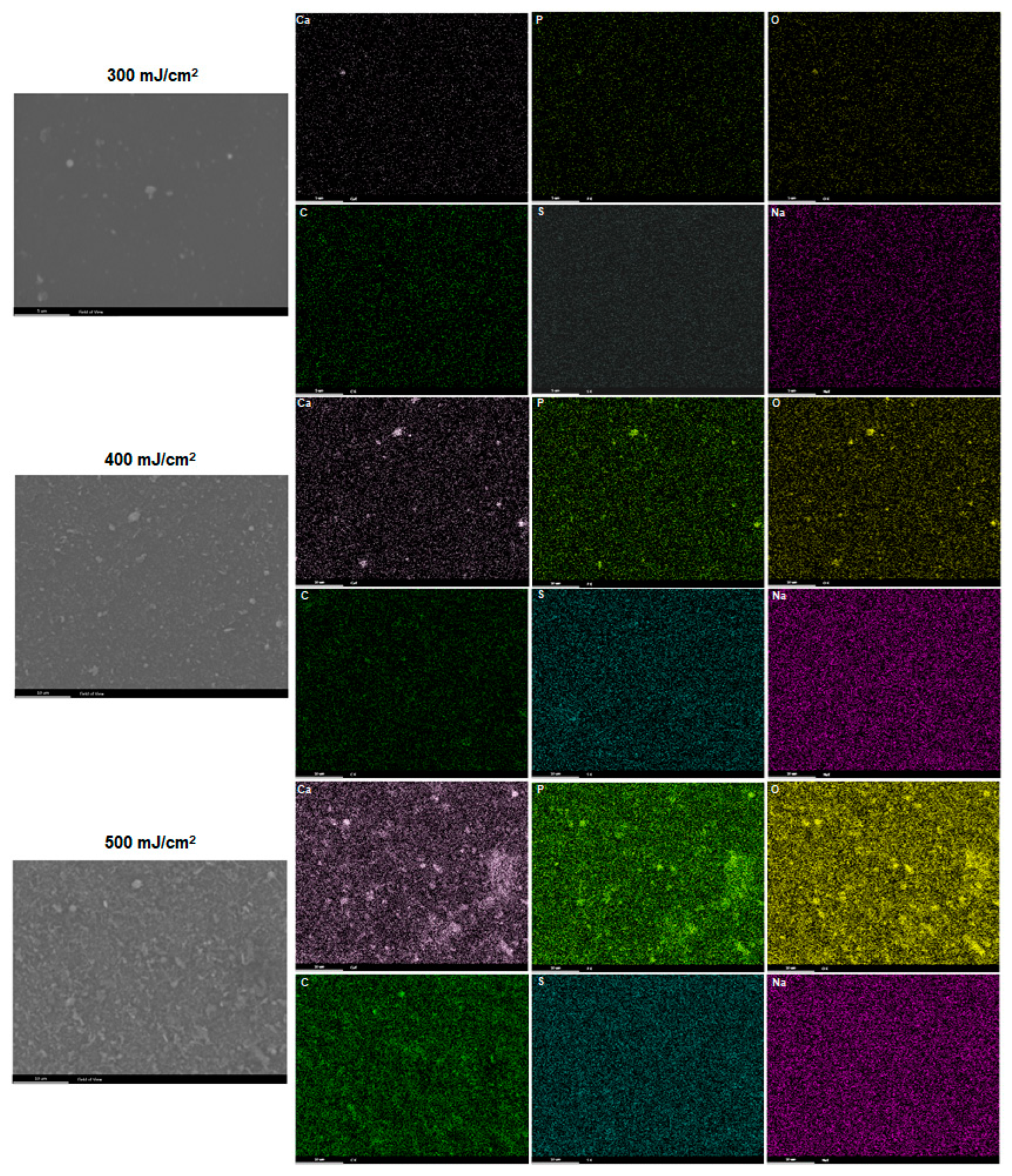
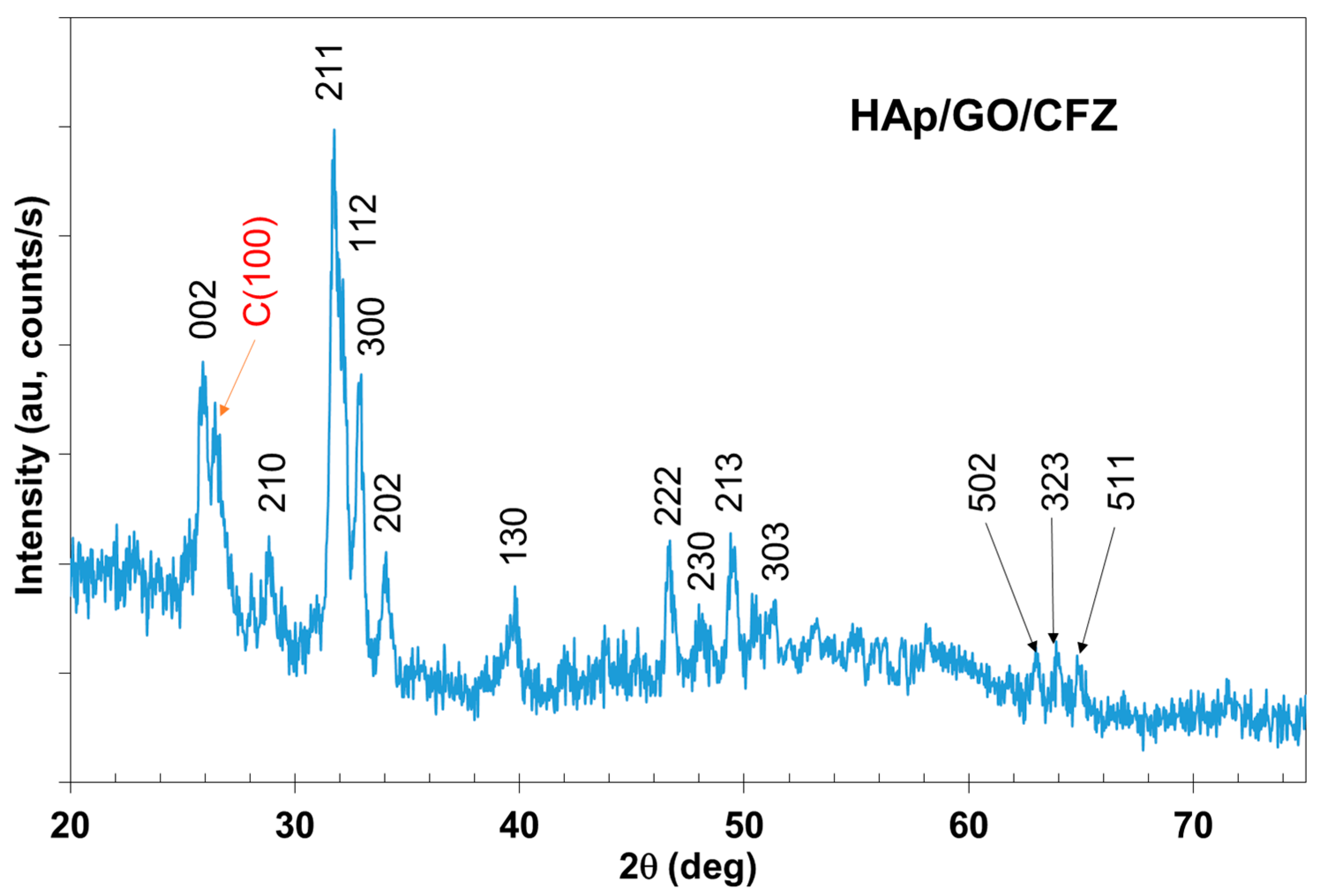
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Xing, D.; Dong, S.; Lin, J. The Primary Total Knee Arthroplasty: A Global Analysis. J. Orthop. Surg. Res. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Arthroplasty—Advanced Techniques and Future Perspectives; IntechOpen: Rijeka, Croatia, 2023.
- Colibazzi, V.; Coladonato, A.; Zanazzo, M.; Romanini, E. Evidence Based Rehabilitation After Hip Arthroplasty. HIP Int. 2020, 30, 20–29. [Google Scholar] [CrossRef]
- Konnyu, K.J.B.; Thoma, L.M.; Cao, W.; Aaron, R.K.; Panagiotou, O.A.; Bhuma, M.R.B.; Adam, G.P.M.; Balk, E.M.; Pinto, D. Rehabilitation for Total Knee Arthroplasty: A Systematic Review. Am. J. Phys. Med. Rehabil. 2022, 102, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Samdanis, V.; Manoharan, G.; Jordan, R.W.; Watts, A.C.; Jenkins, P.; Kulkarni, R.; Thomas, M.; Rangan, A.; Hay, S.M. Indications and Outcome in Total Elbow Arthroplasty: A Systematic Review. Shoulder Elb. 2019, 12, 353–361. [Google Scholar] [CrossRef]
- McKenna, B.J.; Cook, J.; Cook, E.A.; Crafton, J.; Knabel, M.; Swenson, E.; Miner, S.; Manning, E.; Basile, P. Total Ankle Arthroplasty Survivorship: A Meta-Analysis. J. Foot Ankle Surg. 2020, 59, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Boeckstyns, M.E.H.; Herzberg, G. Complications After Total Wrist Arthroplasty. J. Hand Surg. 2024, 49, 177–187. [Google Scholar] [CrossRef]
- Leafblad, N.; Asghar, E.; Tashjian, R.Z. Innovations in Shoulder Arthroplasty. J. Clin. Med. 2022, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Docter, S.; Philpott, H.T.; Godkin, L.; Bryant, D.; Somerville, L.; Jennings, M.; Marsh, J.; Lanting, B. Comparison of Intra and Post-Operative Complication Rates Among Surgical Approaches in Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Orthop. 2020, 20, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, B.T.; Samuelsen, B.T.; Spratt, J.D.; Dornan, G.J.; Millett, P.J. Complications and Implant Survivorship Following Primary Reverse Total Shoulder Arthroplasty in Patients Younger than 65 years: A Systematic Review. J. Shoulder Elb. Surg. 2020, 29, 1703–1711. [Google Scholar] [CrossRef]
- Hermus, J.; Voesenek, J.; van Gansewinkel, E.; Witlox, M.; Poeze, M.; Arts, J. Complications Following Total Ankle Arthroplasty: A Systematic Literature Review and Meta-Analysis. Foot Ankle Surg. 2022, 28, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Júnior, D.J.E.; e Castro, A.D.A.; Fonseca, E.K.U.N.; Baptista, E.; Padial, M.B.; Rosemberg, L.A. Main Complications of Hip Arthroplasty: Pictorial Essay. Radiol. Bras. 2020, 53, 56–62. [Google Scholar] [CrossRef]
- Porrino, J.; Wang, A.; Moats, A.; Mulcahy, H.; Kani, K. Prosthetic Joint Infections: Diagnosis, Management, and Complications of the Two-Stage Replacement Arthroplasty. Skelet. Radiol. 2020, 49, 847–859. [Google Scholar] [CrossRef]
- Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Iosif, A.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Bioactive Hydroxyapatite-Magnesium Phosphate Coatings Deposited by MAPLE for Preventing Infection and Promoting Orthopedic Implants Osteointegration. Materials 2022, 15, 7337. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, M.; Ahmad, M.; Ansari, M.A.; Ahmad, S.; Kumar, A. Tools and Techniques Used for the Development of Scaffold for Bone Tissue Regeneration: A Detailed Review. Bioint. Res. App. Chem. 2024, 14, 123. [Google Scholar] [CrossRef]
- Burduşel, A.-C.; Sarchizian, D.; Niculescu, A.-G.; Holban, A.M.; Popescu, R.-C.; Truşcă, R.; Andronescu, E. Synthesis and Characterization of Hydroxyapatite–Zinc Oxide Nanocomposites Incorporating Rosemary and Thyme Essential Oils for Enhanced Bone Regeneration and Antimicrobial Activity. Rom. J. Morphol. Embryol. 2024, 65, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Es-Saddik, M.; Laasri, S.; Bensemlali, M.; Hariti, N.; Laghzizil, A.; Taha, M. Experimental and Finite Element Study of the Mechanical Behavior of Hydroxyapatite/Tricalcium Phosphate/Alumina Biocomposite. Bioint. Res. Appl. Chem. 2024, 14, 37. [Google Scholar] [CrossRef]
- Buradagunta, R.S.; Madiga, J.; Dumpala, R. Producing Calcium Deficient Nano-hydroxyapatite from Silver pomfret Fish Bones for Biomedical Applications. Lett. Appl. NanoBioSci. 2024, 13, 130. [Google Scholar] [CrossRef]
- Fu, X.-K.; Cao, H.-B.; An, Y.-L.; Zhou, H.-D.; Shi, Y.-P.; Hou, G.-L.; Ha, W. Bioinspired Hydroxyapatite Coating Infiltrated with a Graphene Oxide Hybrid Supramolecular Hydrogel Orchestrates Antibacterial and Self-Lubricating Performance. ACS Appl. Mater. Interfaces 2022, 14, 31702–31714. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Dinischiotu, A.; Grumezescu, V.; Stoica, A.E.; Holban, A.M.; Grumezescu, A. Novel Coatings Based on Nanostructured Cefepime-Functionalized Magnetite for Implantable Devices. Mater. Proc. 2020, 2, 19. [Google Scholar] [CrossRef]
- Al-Wafi, R.; Mansour, S.; AlHammad, M.; Ahmed, M. Biological Response, Antibacterial Properties of ZrO2/Hydroxyapatite/Graphene Oxide Encapsulated into Nanofibrous Scaffolds of Polylactic Acid for Wound Healing Applications. Int. J. Pharm. 2021, 601, 120517. [Google Scholar] [CrossRef]
- Zulkarnain, N.A.; Ramli, A.; Kadir, N.H.A. Optimization on Characterization and Physicochemical of Non-Calcined and Calcined Nanocrystallite of Cuttlefish (Sepia officinalis) Bones Powder for Sustainable Green Technology Applications. Biointerface Res. Appl. Chem. 2024, 14, 58. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a Green Graphene Oxide Reduction with Reusable Potassium Carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Constantinescu, S.; Gherasim, O.; Dorcioman, G.; Grumezescu, V.; Iosub, G.; Niculescu, A.-G.; Moldoveanu, E.-T.; Rădulescu, D.M.; Grumezescu, A.M. MAPLE-Prepared Graphene Oxide-Based Coatings for Improved Orthopedic Screws used in Knee Interventions. Rom. J. Morphol. Embryol. 2024, 65, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, I.; Wannasen, L.; Pinitsoontorn, S. Synthesis, Characterization, and Electrochemical Properties of Reduced Graphene Oxide Produced from Sugarcane Bagasse for Supercapacitor Applications. Biointerface Res. Appl. Chem. 2024, 14, 109. [Google Scholar] [CrossRef]
- Debbarma, J.; Debnath, R.; Saha, M. Graphene from Sugarcane Bagasse for Nonenzymatic Electrochemical Determination of Glucose. Lett. Appl. NanoBioSci. 2023, 12, 115. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Luo, J.; Li, C.; Luan, S. A Novel Hydrogen Peroxide Biosensor based on Au–Graphene–HRP–Chitosan Biocomposites. Electrochim. Acta 2010, 55, 3055–3060. [Google Scholar] [CrossRef]
- Feng, P.; Niu, M.; Gao, C.; Peng, S.; Shuai, C. A Novel Two-Step Sintering for Nano-Hydroxyapatite Scaffolds for Bone Tissue Engineering. Sci. Rep. 2014, 4, 5599. [Google Scholar] [CrossRef]
- Basavarajappa, N.S.; Niranjan, K.; Shilpa, K.C. Computation of Degree-Based Numerical Descriptors of Porous Graphene. Lett. Appl. NanoBioSci. 2023, 12, 117. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef]
- Kalil, H.; Maher, S.; Bose, T.; Bayachou, M. Manganese Oxide/Hemin-Functionalized Graphene as a Platform for Peroxynitrite Sensing. J. Electrochem. Soc. 2018, 165, G3133–G3140. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P. Fabrication of Reduced Graphene Oxide Encapsulated MnO2/MnS2 Nanocomposite for High Performance Electrochemical Devices. J. Porous Mater. 2023, 30, 1897–1910. [Google Scholar] [CrossRef]
- Horst, D.J.; Vieira, R.A.; García-García, A.; Moreno-Barcenas, A. X-MoS2/GO/Graphene Heterostructures and Doping Effects: KPFM Based Study of Surface Potential. Bioint. Res. App. Chem. 2024, 14, 118. [Google Scholar] [CrossRef]
- Alfe, M.; Minopoli, G.; Tartaglia, M.; Gargiulo, V.; Ausanio, G. Biocompatible Hybrid Graphenic Thin Coatings on Flexible Substrates through Matrix-Assisted Pulsed Laser Evaporation (MAPLE). ACS Appl. Mater. Interfaces 2024, 16, 38956–38967. [Google Scholar] [CrossRef]
- Ciocîlteu, M.V.; Mocanu, A.G.; Biță, A.; Manda, C.V.; Nicolicescu, C.; Rău, G.; Belu, I.; Pîrvu, A.S.; Balasoiu, M.; Nănescu, V.; et al. Development of Hybrid Implantable Local Release Systems Based on PLGA Nanoparticles with Applications in Bone Diseases. Polymers 2024, 16, 3064. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Henao, J.; Mandujano-González, V.; Giraldo-Betancur, A.; Forero-Sossa, P.A.; Corona-Castuera, J.; Rivera-Gil, M.A.; Poblano-Salas, C. Exploring Hydroxyapatite/Graphene Oxide/Zinc Oxide Composite Powders for the Preparation of Bioactive-Antibacterial Coatings by HVOF. Appl. Phys. A 2022, 128, 813. [Google Scholar] [CrossRef]
- Khosalim, I.P.; Zhang, Y.Y.; Yiu, C.K.Y.; Wong, H.M. Synthesis of a Graphene Oxide/Agarose/Hydroxyapatite Biomaterial with the Evaluation of Antibacterial Activity and Initial Cell Attachment. Sci. Rep. 2022, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Daulbayev, C.; Sultanov, F.; Korobeinyk, A.V.; Yeleuov, M.; Taurbekov, A.; Bakbolat, B.; Umirzakov, A.; Baimenov, A.; Daulbayev, O. Effect of Graphene Oxide/Hydroxyapatite Nanocomposite on Osteogenic Differentiation and Antimicrobial Activity. Surf. Inter. 2021, 28, 101683. [Google Scholar] [CrossRef]
- Ryu, D.J.; Ban, H.Y.; Jung, E.Y.; Sonn, C.-H.; Hong, D.H.; Ahmad, S.; Gweon, B.; Lim, D.; Wang, J.H. Osteo-Compatibility of 3D Titanium Porous Coating Applied by Direct Energy Deposition (DED) for a Cementless Total Knee Arthroplasty Implant: In Vitro and In Vivo Study. J. Clin. Med. 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.J.; Jung, A.; Ban, H.Y.; Kwak, T.Y.; Shin, E.J.; Gweon, B.; Lim, D.; Wang, J.H. Enhanced Osseointegration Through Direct Energy Deposition Porous Coating for Cementless Orthopedic Implant Fixation. Sci. Rep. 2021, 11, 22317. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Niculescu, A.-G.; Slave, Ș.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.-C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef]
- Pirușcă, I.A.; Balaure, P.C.; Grumezescu, V.; Irimiciuc, S.-A.; Oprea, O.-C.; Bîrcă, A.C.; Vasile, B.; Holban, A.M.; Voinea, I.C.; Stan, M.S.; et al. New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics 2024, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Manoratne, C.H.; Rosa, S.R.D.; Kottegoda, I.R.M. XRD-HTA, UV Visible, FTIR and SEM Interpretation of Reduced Graphene Oxide Synthesized from High Purity Vein Graphite. Mater. Sci. Res. India 2017, 14, 19–30. [Google Scholar] [CrossRef]
- Sudesh; Kumar, N.; Das, S.; Bernhard, C.; Varma, G.D. Effect of Graphene Oxide Doping on Superconducting Properties of Bulk MgB2. Supercond. Sci. Technol. 2013, 26, 95008. [Google Scholar] [CrossRef]
- Vidhya, G.; Kumar, G.S.; Kattimani, V.S.; Girija, E. Comparative Study of Hydroxyapatite Prepared from Eggshells and Synthetic Precursors by Microwave Irradiation Method for Medical Applications. Mater. Today Proc. 2019, 15, 344–352. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Hu, R.; Yang, Y.; Li, P.; Wu, Q. Bifunctional Nano-Ag3PO4 with Capabilities of Enhancing Ceftazidime for Sterilization and Removing Residues. RSC Adv. 2019, 9, 17913–17920. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.A.d.A.; Neto, A.F.S.; Scavuzzi, A.M.L.; Lopes, A.C.D.S.; Santos-Magalhães, N.S.; Cavalcanti, I.M.F. Ceftazidime/Tobramycin Co-Loaded Chitosan-Coated Zein Nanoparticles against Antibiotic-Resistant and Biofilm-Producing Pseudomonas aeruginosa and Klebsiella pneumoniae. Pharmaceuticals 2024, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Bonciu, A.; Vasilescu, A.; Dinca, V.; Peteu, S.F. Interfaces obtained by MAPLE for chemical and biosensors applications. Sens. Actuators Rep. 2021, 3, 100040. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, V.; Socol, G.; Ficai, A. Chapter 2—Nanoarchitectonics prepared by laser processing and their biomedicinal applications. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 23–53. [Google Scholar]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Negut, I.; Dumitrescu, M.F.; Stan, M.S.; Nica, I.C.; Holban, A.M.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef]
- Patz, T.M.; Doraiswamy, A.; Narayan, R.J.; Menegazzo, N.; Kranz, C.; Mizaikoff, B.; Zhong, Y.; Bellamkonda, R.; Bumgardner, J.D.; Elder, S.H.; et al. Matrix assisted pulsed laser evaporation of biomaterial thin films. Mater. Sci. Eng. C 2007, 27, 514–522. [Google Scholar] [CrossRef]
- Tan, J.; Li, L.; Li, B.; Tian, X.; Song, P.; Wang, X. Titanium Surfaces Modified with Graphene Oxide/Gelatin Composite Coatings for Enhanced Antibacterial Properties and Biological Activities. ACS Omega 2022, 7, 27359–27368. [Google Scholar] [CrossRef] [PubMed]
- Liauw, C.M.; Vaidya, M.; Slate, A.J.; Hickey, N.A.; Ryder, S.; Martínez-Periñán, E.; McBain, A.J.; Banks, C.E.; Whitehead, K.A. Analysis of Cellular Damage Resulting from Exposure of Bacteria to Graphene Oxide and Hybrids Using Fourier Transform Infrared Spectroscopy. Antibiotics 2023, 12, 776. [Google Scholar] [CrossRef]
- Gabor, R.; Cvrček, L.; Doubková, M.; Nehasil, V.; Hlinka, J.; Unucka, P.; Buřil, M.; Podepřelová, A.; Seidlerová, J.; Bačáková, L. Hybrid Coatings for Orthopaedic Implants Formed by Physical Vapour Deposition and Microarc Oxidation. Mater. Des. 2022, 219, 110811. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iosub, G.; Niculescu, A.-G.; Grumezescu, V.; Dorcioman, G.; Gherasim, O.; Crăciun, V.; Rădulescu, D.M.; Grumezescu, A.M.; Stan, M.S.; Constantinescu, S.; et al. Graphene Oxide–Antibiotic Coatings with Improved Resistance to Microbial Colonization for Arthroplasty Implants. J. Compos. Sci. 2025, 9, 82. https://doi.org/10.3390/jcs9020082
Iosub G, Niculescu A-G, Grumezescu V, Dorcioman G, Gherasim O, Crăciun V, Rădulescu DM, Grumezescu AM, Stan MS, Constantinescu S, et al. Graphene Oxide–Antibiotic Coatings with Improved Resistance to Microbial Colonization for Arthroplasty Implants. Journal of Composites Science. 2025; 9(2):82. https://doi.org/10.3390/jcs9020082
Chicago/Turabian StyleIosub, Gheorghe, Adelina-Gabriela Niculescu, Valentina Grumezescu, Gabriela Dorcioman, Oana Gherasim, Valentin Crăciun, Dragoș Mihai Rădulescu, Alexandru Mihai Grumezescu, Miruna Silvia Stan, Sorin Constantinescu, and et al. 2025. "Graphene Oxide–Antibiotic Coatings with Improved Resistance to Microbial Colonization for Arthroplasty Implants" Journal of Composites Science 9, no. 2: 82. https://doi.org/10.3390/jcs9020082
APA StyleIosub, G., Niculescu, A.-G., Grumezescu, V., Dorcioman, G., Gherasim, O., Crăciun, V., Rădulescu, D. M., Grumezescu, A. M., Stan, M. S., Constantinescu, S., Holban, A. M., & Rădulescu, A.-R. (2025). Graphene Oxide–Antibiotic Coatings with Improved Resistance to Microbial Colonization for Arthroplasty Implants. Journal of Composites Science, 9(2), 82. https://doi.org/10.3390/jcs9020082









