Hot-Dip PVC-Based Polymeric Composite Coating for Advanced Electrical Insulation of Electric Vehicle Battery Systems
Abstract
1. Introduction
- 1.
- Ease of application: plastisols can be dip, spray, or screen-coated and even room temperature cast because solidification occurs only on subsequent heating.
- 2.
- Mechanical robustness: cured films resist impact, abrasion, and deformation, and withstand many chemicals, solvents, and weathering agents.
- 3.
- Tunability: formulation adjustments allow hardness from soft and flexible to rigid, in both thin and thick builds.
- 4.
- Aesthetic versatility: available in virtually any colour, with matte, glossy or textured finishes and high UV stability that minimises fading.
- 5.
- Cost-effectiveness: raw materials are inexpensive, and the process is energy-efficient, requiring only a heat-curing step.
- 6.
- Moisture resistance: excellent barrier performance makes plastisols suitable for outdoor or wet service environments.
- 7.
- Electrical insulation: high volume resistivity enables long-term dielectric protection.
- 8.
2. Materials and Methods
2.1. Materials
2.2. Characterisation
3. Results and Discussion
3.1. Withdrawing Rate and Thickness Relation Results
3.2. Mechanical Results
3.3. Morphological Results
3.4. Fourier Transform Infrared Spectroscopy (FTIR) Results

| Vibrational Mode a | Wavenumber (cm−1) |
|---|---|
| –CH stretching | 2911 |
| –CH2 deformation | 1330 |
| CH rocking | 1253 |
| trans CH wagging | 961 |
| C–Cl stretching | 835 |
| cis CH wagging | 613 |
3.5. Contact Angle Results
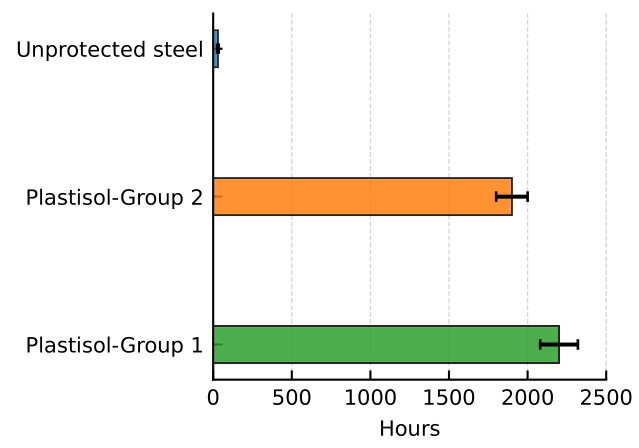
3.6. Corrosion Performance Results of Coatings
3.7. Dielectric Strength Results of Coatings
3.8. Thermogravimetric Analysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSE | Back-scattered electron |
| DEHP | Di(2-ethylhexyl) phthalate |
| DOA | Dioctyl adipate |
| DOP | Dioctyl phthalate |
| DOTP | Dioctyl terephthalate |
| EDX | Energy-dispersive X-ray spectroscopy |
| EV | Electric vehicle |
| FTIR | Fourier-transform infrared spectroscopy |
| NIR | Near-infrared |
| PC | Polycarbonate |
| PP | Polypropylene |
| PPS | Polyphenylene sulfide |
| PTFE | Polytetrafluoroethylene |
| PVDF | Poly(vinylidene fluoride) |
| PVC | Poly(vinyl chloride) |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| UV | Ultraviolet radiation |
| VLF | Very-low-frequency |
References
- Najafi, V.; Abdollahi, H. Internally plasticized PVC by four different green plasticizer compounds. Eur. Polym. J. 2020, 128, 109620. [Google Scholar] [CrossRef]
- Gomez Caturla, J.; Miranda Pinzon, M.; Arrieta, M.P.; Quiles Carrillo, L.; Garcia Garcia, D. Optimization of the curing conditions of PVC plastisols plasticized with ethyl cinnamate. Polymer 2024, 311, 127526. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, C.; Li, F.; Liu, H.; Yang, J. Stocks and flows of polyvinyl chloride (PVC) in China: 1980–2050. Resour. Conserv. Recycl. 2020, 154, 104584. [Google Scholar] [CrossRef]
- Yu, B.Y.; Lee, A.R.; Kwak, S.Y. Gelation/fusion behaviour of PVC plastisol with a cyclodextrin derivative and an anti-migration plasticiser in flexible PVC. Eur. Polym. J. 2012, 48, 885–895. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Zhu, H.; Ma, Y.; Lian, C.; Shi, X. Influences of plasticiser molecular structure and content on flexural fatigue of soft poly(vinyl chloride). J. Vinyl Addit. Technol. 2022, 28, 706–718. [Google Scholar] [CrossRef]
- Oota, K. Fuji Pavilion, Osaka Expo ’70 (Photograph). Wikimedia Commons. License: CC BY 2.0. Available online: https://commons.wikimedia.org/wiki/File:Fuji_Pavilion_Expo70.jpg (accessed on 4 September 2025).
- Gao, C.; Wang, F.; Hu, X.; Zhang, M. Research on the analysis and application of polymer materials in contemporary sculpture art creation. Polymers 2023, 15, 2727. [Google Scholar] [CrossRef]
- Shashoua, Y.; Ward, C. Plastics: Modern resins with ageing problems. In Resins, Ancient and Modern; Wright, M.M., Townsend, J.H., Eds.; Scottish Society for Conservation and Restoration: Aberdeen, UK, 1995; pp. 33–37. [Google Scholar]
- Bodaghi, A. An overview on the recent developments in reactive plasticizers in polymers. Polym. Adv. Technol. 2020, 31, 355–367. [Google Scholar] [CrossRef]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Godwin, A.D. Plasticizers. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; Kutz, M., Ed.; William Andrew/Elsevier: Oxford, UK, 2016; pp. 595–618. [Google Scholar]
- Wang, M.Y.; Zhou, N.Q.; Hu, J. Effect of plasticiser (DOP) on cell structure and mechanical properties of extrusion-foamed PVC sheet. Mater. Sci. Forum 2015, 815, 601–606. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Xia, J.; Ding, C.; Wang, M.; Xu, L.; Huang, K. Tung-oil-based plasticiser and auxiliary stabiliser for poly(vinyl chloride). Mater. Des. 2017, 122, 366–375. [Google Scholar] [CrossRef]
- Tüzüm Demir, A.P.; Ulutan, S. Migration of phthalate and non-phthalate plasticisers out of plasticised PVC films into air. J. Appl. Polym. Sci. 2013, 128, 1948–1961. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, G.; Yang, Z.; Luo, H.; Tan, H. Study on viscosity and ageing process of CaCO3-filled poly(vinyl chloride) plastisols. J. Vinyl Addit. Technol. 2018, 24, E53–E61. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the application of synthetic polymer materials in contemporary public art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Jaoua Bahloul, H.; Varieras, D.; Beyou, E. Solar spectral properties of PVC plastisol-based films filled with various fillers. J. Vinyl Addit. Technol. 2019, 25, E188–E194. [Google Scholar] [CrossRef]
- Tüzüm Demir, A.P.; Ergun, Y. Assessment of the effect of inorganic fillers on plasticiser diffusion in plasticised poly(vinyl chloride) composite films for cable-sheath applications. J. Appl. Polym. Sci. 2023, 140, e53996. [Google Scholar] [CrossRef]
- Yang, R.; Sun, Y.; Wu, H.; Zhang, X. Insulation resistance measurement of voltage-leakage system for EV battery pack. Int. J. Perform. Eng. 2020, 16, 560–568. [Google Scholar] [CrossRef]
- Moghadam, D.E.; Herold, C.; Zbinden, R. Electrical Insulation at 800 V Electric Vehicle. In Proceedings of the 2020 International Symposium on Electrical Insulating Materials (ISEIM), Tokyo, Japan, 13–17 September 2020; Institute of Electrical Engineers of Japan: Tokyo, Japan, 2020; pp. 115–119. [Google Scholar]
- Nyamathulla, S.; Dhanamjayulu, C. A review of battery energy-storage systems and advanced battery-management systems for different applications: Challenges and recommendations. J. Energy Storage 2024, 86, 111179. [Google Scholar] [CrossRef]
- Balasingam, B.; Ahmed, M.; Pattipati, K. Battery-management systems—Challenges and some solutions. Energies 2020, 13, 2825. [Google Scholar] [CrossRef]
- Tan, D.Q. The search for enhanced dielectric strength of polymer-based dielectrics: A focused review on polymer nanocomposites. J. Appl. Polym. Sci. 2020, 137, 49379. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.H.; Kim, H.T.; Kim, J.D.; Uluduz, C.; Kim, M.; Lee, J.M. Evaluation of PVC-type insulation foam material for cryogenic applications. Polymers 2023, 15, 1401. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Li, N.; Tang, T.; Xie, X.; Zhang, C.; Zuo, Y. The Flame Retardancy and Smoke Suppression Performance of Polyvinyl Chloride Composites with an Efficient Flame Retardant System. Coatings 2023, 13, 1814. [Google Scholar] [CrossRef]
- Siekierka, P.; Makarewicz, E.; Wilczewski, S.; Lewandowski, K.; Skórczewska, K.; Mirowski, J.; Osial, M. Composite of poly(vinyl chloride) plastisol and wood flour as a potential coating material. Coatings 2023, 13, 1892. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-film coating methods: A successful marriage of high-quality and cost-effectiveness—A brief exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Perito, E.D.; Guerra, N.B.; Giovanela, M.; Machado, G.; da Silva Crespo, J. Chemical, thermal and mechanical evaluation of poly(vinyl chloride) plastisol with different plasticisers. J. Elastomers Plast. 2022, 54, 1277–1294. [Google Scholar] [CrossRef]
- Badi, N.S.A.; Makmud, M.Z.H.; Jamain, Z.; Mohd Amin, K.N.; Illias, H.A. Enhancement of structural and dielectric properties of PVC/CNC nanocomposites as electrical insulation materials. Plast. Rubber Compos. 2024. Advance online publication. [Google Scholar] [CrossRef]
- Kowalik, J.; Tworek, M.; Ligocka, A.; Osial, M. Modification of PVC plastisol with silver nanoparticles to create protective coatings with improved properties. Adv. Sci. Technol. Res. J. 2025, 19, 353–368. [Google Scholar] [CrossRef]
- Zoller, A.; Marcilla, A. Soft PVC foams: Study of the gelation, fusion and foaming processes. I. Phthalate-ester plasticizers. J. Appl. Polym. Sci. 2011, 121, 1495–1505. [Google Scholar] [CrossRef]
- Zoller, A.; Marcilla, A. Soft PVC foams: Study of the gelation, fusion and foaming processes. II. Adipate, citrate and other types of plasticizers. J. Appl. Polym. Sci. 2011, 122, 2981–2991. [Google Scholar] [CrossRef]
- Crawford, R.J.; Throne, J.L. Rotational Molding Polymers. In Rotational Molding Technology; Throne, J.L., Ed.; William Andrew: Norwich, NY, USA, 2001; pp. 19–68. [Google Scholar] [CrossRef]
- Carvalho, Í.M.D.; Mei, L.H.I.; Rodolfo Junior, A. Crosslinking of PVC plastisols using a polyfunctional epoxy resin: Synthesis and characterisation. Polímeros 2013, 23, 501–508. [Google Scholar] [CrossRef]
- Elsad, R.A.; Habashy, M.M.; Izzularab, M.A.; Abd-Elhady, A.M. Evaluation of dielectric properties for PVC/SiO2 nanocomposites under the effect of water absorption. J. Mater. Sci. Mater. Electron. 2023, 34, 786. [Google Scholar] [CrossRef]
- Jaiyen, S.; Phunpueok, A.; Thongpool, V. Determination of radiation attenuation coefficients of BaSO4/PVC and BaSO4/PS for X-ray shielding. J. Phys. Conf. Ser. 2019, 1380, 012133. [Google Scholar] [CrossRef]
- Baltacioğlu, H.; Balköse, D. Effect of zinc stearate and/or epoxidized soybean oil on gelation and thermal stability of PVC–DOP plastigels. J. Appl. Polym. Sci. 1999, 74, 2488–2498. [Google Scholar] [CrossRef]
- ASTM D1045-92(2018); Standard Test Methods for Plastics Viscosity and Flow. ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 48-4:2018; Rubber, Vulcanized or Thermoplastic—Determination of Hardness—Part 4: Indentation Hardness by Durometer Method (Shore Hardness). ISO: Geneva, Switzerland, 2018.
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- ASTM D1004-13; Standard Test Method for Tear Resistance (Graves Tear) of Plastic Film and Sheeting. ASTM International: West Conshohocken, PA, USA, 2013.
- ISO 4649:2017; Rubber, Vulcanized or Thermoplastic—Determination of Abrasion Resistance Using a Rotating Cylindrical Drum Device. ISO: Geneva, Switzerland, 2017.
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D149-20; Standard Test Method for Dielectric Breakdown Voltage and Dielectric Strength of Solid Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2020.
- IEC 60243-1:2013; Electric Strength of Insulating Materials—Test Methods—Part 1: Tests at Power Frequencies. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of solutions applied to fruits and vegetables by dipping. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Grosso, D. How to exploit the full potential of the dip-coating process to better control film formation. J. Mater. Chem. 2011, 21, 17033–17038. [Google Scholar] [CrossRef]
- Crespo, J.E.; Sanchez, L.; Parres, F.; López, J. Mechanical and morphological characterization of PVC plastisol composites with almond-husk fillers. Polym. Compos. 2007, 28, 71–77. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; La Mantia, F.L. Recycling of the ‘‘light fraction’’ from municipal post-consumer plastics: Effect of adding wood fibres. Polym. Adv. Technol. 1999, 10, 607–614. [Google Scholar] [CrossRef]
- Bendjaouahdou, C.; Aidaoui, K. Synthesis and characterization of poly(vinyl chloride)/wood flour/organoclay ternary composites. Polym. Polym. Compos. 2021, 29, S949–S958. [Google Scholar] [CrossRef]
- Lallement, M.; Chabert, F.; Evon, P.; Mérian, T.; Delbé, K. Taber test characterization of PLA-based bio-composites reinforced with oleaginous flax shives. Wear 2025, 570, 205889. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Bourbigot, S.; Zhang, J.; Guo, K.; Baolati, J. Solid-phase reaction mechanism and chlorine migration in co-pyrolysing PVC–CaCO3 composites. Polym. Degrad. Stab. 2021, 193, 109741. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.; Zhang, T.; Jia, Y.; Hou, Y.; Chen, J.; Li, W. Comparative study on pyrolysis behaviours and chlorine release of pure PVC and commercial PVC plastics. Fuel 2023, 340, 127555. [Google Scholar] [CrossRef]
- Soman, V.V.; Kelkar, D.S. FTIR studies of doped PMMA–PVC blend system. Macromol. Symp. 2009, 277, 152–161. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of surface roughness on contact-angle hysteresis and spreading work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Bao, L.; Yang, S.; Luo, X.; Lei, J.; Cao, Q.; Wang, J. Fabrication of hydrophobic CaCO3 grafted by hydroxylated PVC chains. Appl. Surf. Sci. 2015, 357, 564–572. [Google Scholar] [CrossRef]
- Bicy, K.; Kalarikkal, N.; Stephen, A.M.; Rouxel, D.; Thomas, S. Effects of nanofillers on morphology and surface wetting of microporous polypropylene composite membranes. Mater. Chem. Phys. 2021, 257, 123742. [Google Scholar] [CrossRef]
- Sobotova, L.; Brezinova, J.; Badida, M.; Badidova, M.; Ciecinska, B.; Maslejova, A. Effect of artificial ageing on selected properties of organic-coated sheets. Materials 2024, 17, 3891. [Google Scholar] [CrossRef]
- Romanenko, K.; Jerschow, A. Numerical modelling of surface-scan MRI for diagnostics of commercial battery cells. J. Magn. Reson. Open 2022, 10, 100061. [Google Scholar] [CrossRef]
- Kausar, A. Corrosion prevention prospects of polymeric nanocomposites: A review. J. Plast. Film Sheeting 2019, 35, 181–202. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/inorganic nanocomposite coatings with superior corrosion protection performance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Tuncer, E.; Sauers, I.; James, D.R.; Ellis, A.R.; Paranthaman, M.P.; Goyal, A.; More, K.L. Enhancement of dielectric strength in nanocomposites. Nanotechnology 2007, 18, 325704. [Google Scholar] [CrossRef]
- Clauß, S.; Dijkstra, D.J.; Gabrie, J.; Karbach, A.; Matner, M.; Meckel, W.; Niemz, P. Influence of filler material on the thermal stability of one-component moisture-curing polyurethane adhesives. J. Appl. Polym. Sci. 2012, 124, 3641–3649. [Google Scholar] [CrossRef]
- Poyraz, B.; Eren, Ş.; Subaşı, S. Filler type and particle distribution effect on compact properties of polymer composites. Celal Bayar Univ. J. Sci. 2020, 17, 79–89. [Google Scholar] [CrossRef]
- Banat, R.; Fares, M.M. Thermo-gravimetric stability of high-density polyethylene composite filled with olive-shell flour. Am. J. Polym. Sci. 2015, 5, 65–74. [Google Scholar]
- ASTM D2863-17; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method). ISO: Geneva, Switzerland, 2015.











| Study (Ref.) | System & Process | Target Properties | Key Variables | Main Outcome (One Line) |
|---|---|---|---|---|
| Ji et al. (2018) [15] | PVC plastisols with CaCO3; films (thickness not stated) | Viscosity/aging behaviour | CaCO3 loading; resin morphology | Rheology governed by plasticiser–particle electrostatics + resin morphology. |
| Bahloul et al. (2019) [17] | PVC plastisol films with NIR-barrier fillers; thin films (thickness not stated) | Optical (NIR/visible) | Nacre; mica/TiO2; glass beads; Al2O3; boehmite; ZnO; MgO; rutile TiO2 | Geometry/loading tunes NIR; TiO2: decreased visible and increased NIR reflectance. |
| Perito et al. (2022) [28] | PVC plastisol (shoes/toys); films (thickness not stated) | Mechanical (pre/post aging), DSC, SAXS | DOP, DOA, polymeric (Lestarflex), etc. | DOP → DOA/Lestarflex feasible without loss. |
| Siekierka et al. (2023) [26] | Emulsion PVC plastisols; hydraulic-pressed films (numeric thickness not stated) | Density, hardness, thermal, mech/thermomech | Wood flour (fine/coarse); gel 150 °C | Best balance at 20wt% fine flour (SEM-supported). |
| Tüzüm & Ergin (2023) [18] | PVC plastigel for cable sheaths; thin applicator films (thickness not stated) | Plasticiser migration; property retention | Boric acid, boron clay, sintered boron waste, talc, ZrO2 | Migration decreases: boric-acid > unfilled > talc ≈ sintered-boron clay > zircon > boron clay. |
| Caturla et al. (2024) [2] | PVC plastisols plasticised with ethyl cinnamate (70 phr); films (thickness not stated) | Curing window; mechanical | Cure 190 °C/11.5 min | Tensile 6.4 N mm−2; elongation ∼570%. |
| Badi et al. (2024) [29] | PVC/CNC nanocomposite films (thickness not stated) | Dielectric response; conductivity (HV insulation potential) | CNC content (casting method) | Bio-based CNC can enhance dielectric performance (complementary route). |
| Kowalik et al. (2025) [30] | PVC plastisol coatings; AgNP@silica; gelation + pressing; films (thickness not stated) | Mechanical, thermal, antibacterial (Shore, TMA, TGA) | AgNP (1–2wt%), DEHA plasticiser | 1–1.5wt% AgNP gives balanced mech/thermal + antibacterial. |
| This work | Hot-dip PVC plastisol (DOTP); mm-scale builds (0.9–2.1 mm) | Mechanical, dielectric, corrosion (EV housing) | BaSO4 + CaCO3 loading; withdrawal rate | Tensile 11.9 N mm−2; dielectric 22.1 kV mm−1; salt-spray ≥2000 h. |
| Component | Group 1 | Group 2 Amount (phr) a | Group 3 |
|---|---|---|---|
| PVC emulsion | 100 | 100 | 100 |
| Dioctyl terephthalate (DOTP) | 50 | 50 | 50 |
| Antimony tin oxide (ATO) | 5 | 5 | 5 |
| Paraffinic oil | 2 | 2 | 2 |
| Silicon dioxide (SiO2) | 5 | 5 | 5 |
| Barium sulfate (Ba2SO4) | 10 | 10 | 15 |
| Calcium carbonate (CaCO3) | 10 | 25 | 30 |
| Pigment (red/blue) | 0.5 | 0.5 | 0.5 |
| Total | 182.5 | 196.5 | 207.5 |
| Parameter | Unit | Group 1 | Group 2 | Group 3 |
|---|---|---|---|---|
| Hardness | Shore A | |||
| Tensile strength | N mm−2 | |||
| Elongation at break | % | |||
| Tear strength | N mm−1 | |||
| Taber abrasion a | % | |||
| Acceptance b | — | OK | OK | NOK |
| Sample | (°C) | (°C) | Residual Mass (%) |
|---|---|---|---|
| Group 1 | 261.3 | 299.1 | 23.5 |
| Group 2 | 239.2 | 263.8 | 19.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altuncu, E.; Altuncu, A.P.; Kılıç, N.T.; Uçanok, Z.; Yilmaz, H. Hot-Dip PVC-Based Polymeric Composite Coating for Advanced Electrical Insulation of Electric Vehicle Battery Systems. J. Compos. Sci. 2025, 9, 629. https://doi.org/10.3390/jcs9110629
Altuncu E, Altuncu AP, Kılıç NT, Uçanok Z, Yilmaz H. Hot-Dip PVC-Based Polymeric Composite Coating for Advanced Electrical Insulation of Electric Vehicle Battery Systems. Journal of Composites Science. 2025; 9(11):629. https://doi.org/10.3390/jcs9110629
Chicago/Turabian StyleAltuncu, Ekrem, Arzu Parten Altuncu, Nilay Tüccar Kılıç, Zeynep Uçanok, and Handan Yilmaz. 2025. "Hot-Dip PVC-Based Polymeric Composite Coating for Advanced Electrical Insulation of Electric Vehicle Battery Systems" Journal of Composites Science 9, no. 11: 629. https://doi.org/10.3390/jcs9110629
APA StyleAltuncu, E., Altuncu, A. P., Kılıç, N. T., Uçanok, Z., & Yilmaz, H. (2025). Hot-Dip PVC-Based Polymeric Composite Coating for Advanced Electrical Insulation of Electric Vehicle Battery Systems. Journal of Composites Science, 9(11), 629. https://doi.org/10.3390/jcs9110629






