Effect of Surface Treatment of Nano-Magnetite Particles on PLA/PBAT Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Pretreatment
2.2. Sample Preparations
2.2.1. Nano-Magnetite Particle Preparation
2.2.2. Surface Treatment of Magnetite Particle
- Surface treatment with KH560: An amount of 1 g of Fe3O4 particles was added to a mixed solution of 300 mL of water and 300 mL of anhydrous ethanol, followed by stirring and ultrasonication for 10 min under nitrogen protection. An amount of 3.5 mL of KH560 was added and the mixture was ultrasonicated for 30 min at 50 °C for 6.5 h to achieve surface modification. The pH of the solution was adjusted to an acidic condition by adding 60 mL of glacial acetic acid, and the subsequent steps (stirring, ultrasonication, reaction, and drying) were performed identically to the above procedure. The product was collected using a magnet, washed and dried under a vacuum at 60 °C for 2 h.
- Surface treatment with KH560 and dopamine: An amount of 1.0 g of Fe3O4 nanoparticles was dispersed in 250 mL of deionized water containing 10 mM Tris under stirring and ultrasonic treatment for 30 min. Subsequently, 1.0 g of dopamine was added to the suspension and the reaction was allowed to proceed for 4 h under stirring. The resulting dopamine-coated Fe3O4 nanoparticles were collected using a magnet, thoroughly washed and dried. Finally, the nanoparticles were treated with KH560, and this was repeated after the procedure.
- Surface treatment with KH560 and silica: We weighed 1 g of Fe3O4 nanoparticles and dispersed them in a mixture of 280 mL of anhydrous ethanol and 70 mL of deionized water. The mixture was subjected to ultrasonic agitation for 30 min to ensure homogeneous dispersion. The pH of the suspension was adjusted to 9 using an ammonia solution (NH4OH) under stirring. Then, 2 mL of tetraethyl silicate was added dropwise to the solution. The reaction was allowed to proceed with continuous stirring at room temperature for 12 h to facilitate silica deposition on the Fe3O4 surface. The resulting core–shell nanoparticles (Fe3O4@SiO2) were collected using a magnet, separated from the supernatant, and washed several times with ethanol and deionized water to remove unreacted species. The purified product is dried in an oven, resulting in a yellowish–brown powder. Finally, the nanoparticles were treated with KH560, and this was repeated after the procedure.
2.2.3. Mixing and Specimen Preparation
2.3. Characterizations
2.4. Statistical Analysis
3. Results
3.1. Surface Chemistry
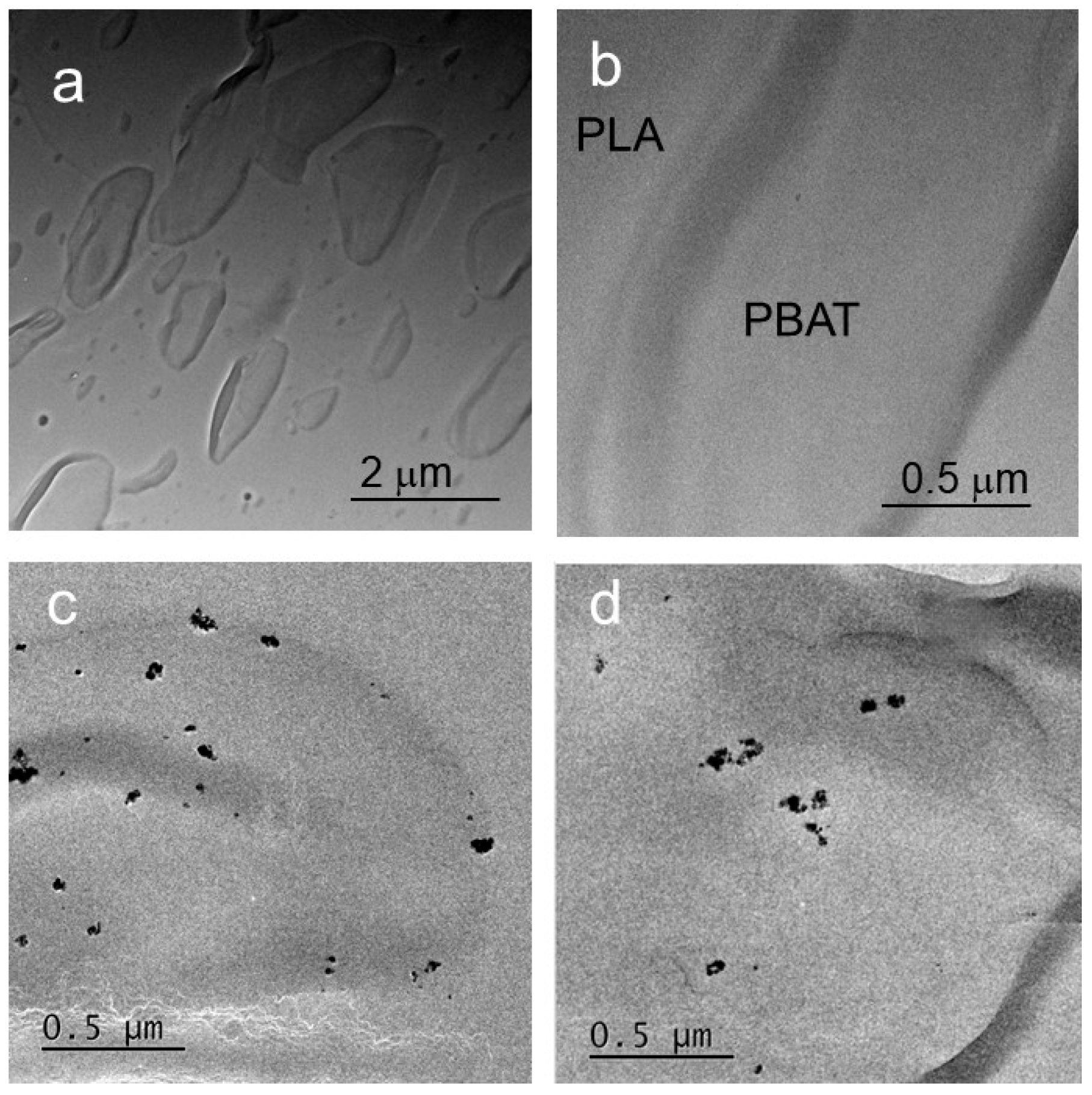
3.2. Morphologies and Fracture Surfaces
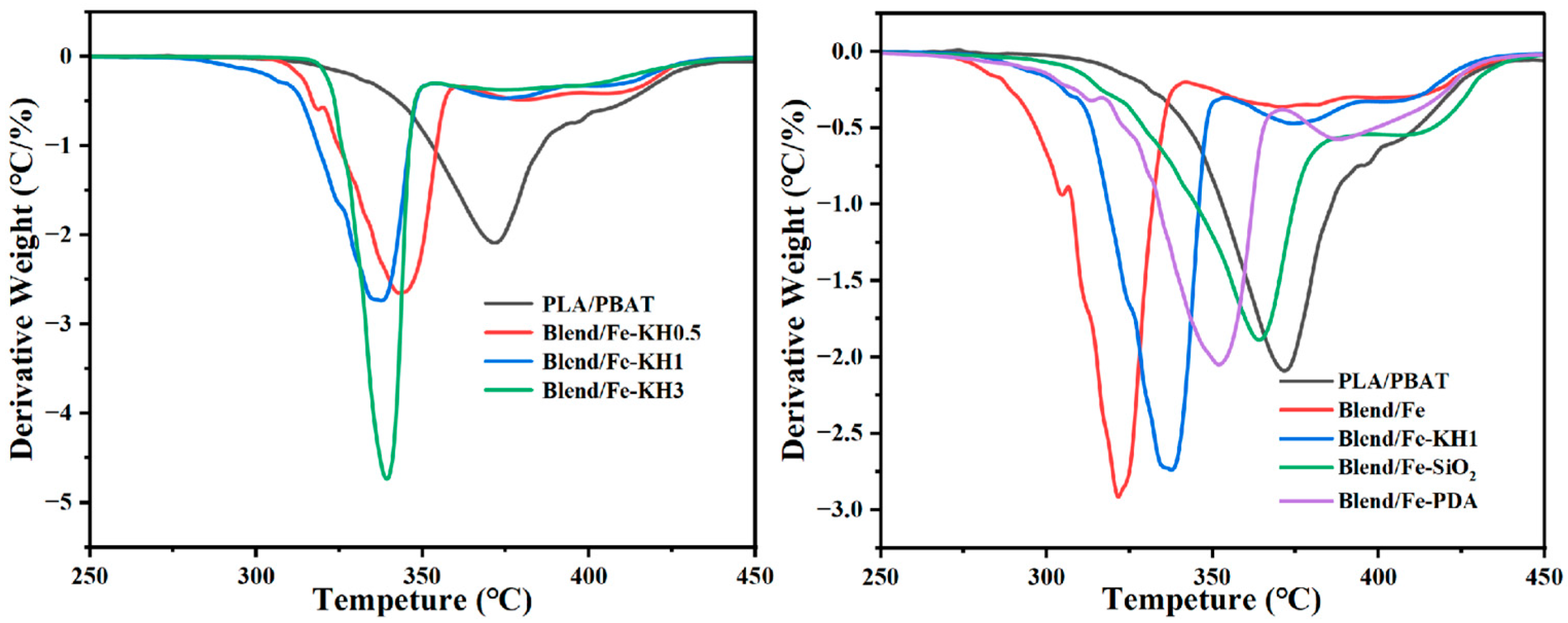
3.3. Thermal Properties
3.4. Mechanical Properties
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nofar, M.; Sacligil, D. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Pesaranhajiabbas, E.; Misra, M. Recent progress on biodegradable polylactic acid based blends and their biocomposites: A comprehensive review. Int. J. Biol. Macromol. 2023, 253, 126231. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Liu, X. Preparation of biodegradable PLA/PBAT blends with balanced toughness and strength by dynamic vulcanization process. Polymer 2024, 291, 126587. [Google Scholar] [CrossRef]
- Wen, W.; Li, C. Study on Compatibility of a Novel Cardanol-Based Compatibilizer in PLA/PBAT Blend. Ind. Eng. Chem. Res. 2024, 63, 8684–8696. [Google Scholar] [CrossRef]
- Nguyen, Q.-D.; Choi, C.-G. Recent advances in multifunctional electromagnetic interference shielding materials. Heliyon 2024, 10, e31118. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, B. Recent Advances in Asymmetric Structural Composites for Excellent Electromagnetic Interference Shielding: A Review. Chin. J. Polym. Sci. 2024, 42, 693–710. [Google Scholar] [CrossRef]
- Wu, N.; Hu, Q. Review on the electromagnetic interference shielding properties of carbon based materials and their novel composites: Recent progress, challenges and prospects. Carbon 2021, 176, 88–105. [Google Scholar] [CrossRef]
- Im, J.S.; Kim, J.G. Fluorination effects of carbon black additives for electrical properties and EMI shielding efficiency by improved dispersion and adhesion. Carbon 2009, 47, 2640–2647. [Google Scholar] [CrossRef]
- Goyal, R.K. Cost-efficient high performance polyetheretherketone/expanded graphite nanocomposites with high conductivity for EMI shielding application. Mater. Chem. Phys. 2013, 142, 195–198. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J. Reduced graphene oxide deposited carbon fiber reinforced polymer composites for electromagnetic interference shielding. Compos. Part A 2016, 82, 141–150. [Google Scholar] [CrossRef]
- Hwang, S.-S. Tensile, electrical conductivity and EMI shielding properties of solid and foamed PBT/carbon fiber composites. Compos. Part B 2016, 98, 1–8. [Google Scholar] [CrossRef]
- Das, A.; Hayvaci, H.T. Superhydrophobic and conductive carbon nanofiber/PTFE composite coatings for EMI shielding. J. Colloid Interface Sci. 2011, 353, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, M.H. Electrical, EMI shielding and tensile properties of PP/PE blends filled with GNP:CNT hybrid nanofiller. Synth. Met. 2016, 217, 322–330. [Google Scholar] [CrossRef]
- Micheli, D.; Vricella, A. Ballistic and electromagnetic shielding behaviour of multifunctional Kevlar fiber reinforced epoxy composites modified by carbon nanotubes. Carbon 2016, 104, 141–156. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, M. Thin and flexible multi-walled carbon nanotube/waterborne polyurethane composites with high-performance electromagnetic interference shielding. Carbon 2016, 96, 768–777. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M. Designing, modeling and manufacturing of lightweight carbon nanotubes/polymer composite nanofibers for electromagnetic interference shielding application. Compos. Sci. Technol. 2017, 145, 46–54. [Google Scholar] [CrossRef]
- Gao, X.; Isayev, A.I. Ultrasonic treatment of polycarbonate/carbon nanotubes composites. Polymer 2016, 84, 209–222. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, M. Effect of Silane-Modified Ammonium Polyphosphate on the Mechanical, Thermal, and Flame-Retardant Properties of Rice Husk/Polylactic Acid Composites. J. Compos. Sci. 2025, 9, 251. [Google Scholar] [CrossRef]
- Di Matteo, P.; Barbero, F. Carbon Nanoparticle-Loaded PLA Nanofibers via Electrospinning for Food Packaging. J. Compos. Sci. 2025, 9, 25. [Google Scholar] [CrossRef]
- Bleija, M.; Platnieks, O. Comparison of Carbon-Nanoparticle-Filled Poly(Butylene Succinate-co-Adipate) Nanocomposites for Electromagnetic Applications. Nanomaterials 2022, 12, 3671. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K. Dielectric properties and electromagnetic interference shielding effectiveness of graphene-based biodegradable nanocomposites. Mater. Des. 2016, 109, 68–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.T. Conductivity of PLA/PBAT blends with carbon black as a conductive filler. Mater. Lett. 2025, 378, 137592. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J. Poly(lactide)/poly(butylene adipate-co-terephthalate)/carbon nanotubes composites with robust mechanical properties, fatigue-resistance and dielectric properties. Int. J. Biol. Macromol. 2025, 295, 139464. [Google Scholar] [CrossRef]
- Soares, B.G.; Barra, G.M.O. Conducting Polymeric Composites Based on Intrinsically Conducting Polymers as Electromagnetic Interference Shielding/Microwave Absorbing Materials—A Review. J. Compos. Sci. 2021, 5, 173. [Google Scholar] [CrossRef]
- Henriques, R.R.; Calheiros Souto, L.F. Bio-based PLA composites loaded with Fe3O4/graphene nanoplatelet hybrids for improved microwave absorbing properties. Mater. Sci. Eng. B 2025, 314, 117994. [Google Scholar] [CrossRef]
- Balakrishnan, K.P.; Senthilkumar, K.K. Surfactant assisted tuning of electrical conductivity, electromagnetic interference shielding effectiveness, wetting properties of poly(lactic acid)-expanded graphite-magnetite nanocube hybrid bio-nanocomposites. Eur. Polym. J. 2024, 214, 113135. [Google Scholar] [CrossRef]
- Yan, T.; Ye, X. GR-Fe3O4/PLA 3D printing composite materials with excellent microwave absorption properties. J. Alloys Compd. 2024, 972, 172799. [Google Scholar] [CrossRef]
- He, E.; Yan, T. Preparation of FeSiAl–Fe3O4 reinforced graphene/polylactic acid composites and their microwave absorption properties. J. Mater. Sci. 2023, 58, 11647–11665. [Google Scholar] [CrossRef]
- Faiyas, A.P.A.; Vinod, E.M. Dependence of pH and surfactant effect in the synthesis of magnetite (Fe3O4) nanoparticles and its properties. J. Magn. Magn. Mater. 2010, 322, 400–404. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, Q. Preparation of nanosized NaA zeolite and its surface modification by KH-550. Mater. Sci.-Pol. 2018, 36, 638–643. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.-Q.; Wang, Z.-M.; Shen, C.-J. Study on the preparation and characterization of high-dispersibility nanosilica. Sci. Eng. Compos. Mater 2016, 23, 401–406. [Google Scholar] [CrossRef]
- Ishii, M.; Nakahira, M. Infrared absorption spectra and cation distributions in (Mn, Fe)3O4. Solid State Commun. 1972, 11, 209–212. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Song, X.; Fang, C. Characterization of mechanical properties of jute/PLA composites containing nano SiO2 modified by coupling agents. Cellulose 2021, 29, 835–848. [Google Scholar] [CrossRef]
- Hu, L.; Fu, Z.; Gu, X.; Wang, H.; Li, Y. Strengthened interface as flame retarding belt: Compatibilized PLLA/PP blends by reactive boehmite nanorods. Polymer 2021, 228, 123879. [Google Scholar] [CrossRef]
- Hu, L.; Han, Y.; Rong, C.; Wang, X.; Wang, H.; Li, Y. Interfacial Engineering with Rigid Nanoplatelets in Immiscible Polymer Blends: Interface Strengthening and Interfacial Curvature Controlling. ACS Appl. Mater. Interfaces 2022, 14, 11016–11027. [Google Scholar] [CrossRef]
- Aksimentyeva, O.I.; Opaynych, I.Y. Structure and Thermal Stability of the Polymer-Magnet Nanocomposites. Phys. Chem. Solid stat 2012, 13, 438–442. [Google Scholar]
- De Melo Barbosa, M.M.; de França, J.O.C. Tailoring the Properties of Magnetite/PLA Nanocomposites: A Composition-Dependent Study. Polymers 2025, 17, 1713. [Google Scholar] [CrossRef]







| Sample Code | PLA/PBAT (Ratio) | Fe3O4 (Adding %) | KH560 (Ratio) * | GA Acid (pH Value) | SiO2 (Ratio) * | DA (Ratio) * |
|---|---|---|---|---|---|---|
| PLA/PBAT | 70/30 | 0 | 0 | 0 | 0 | 0 |
| Blend/Fe-KH3 | 70/30 | 3 | 3.5 | 4.5 | 0 | 0 |
| Blend/Fe-KHSiO2 | 70/30 | 1 | 3.5 | 0 | 2 | 0 |
| Blend/Fe-KHPDA | 70/30 | 1 | 3.5 | 0 | 0 | 1 |
| Sample Code | Tg °C | Tcc °C | Tm °C | Ton °C | Residue % |
|---|---|---|---|---|---|
| PLA/PBAT | 63 | 111.55 | 168.91 | 317.41 | 2.12 |
| Blend/Fe | 62.55 | 112.55 | 169.57 | 272.46 | 3.42 |
| Blend/Fe-KH | 62.75 | 112.59 | 169.22 | - | 3.52 |
| Blend/Fe-KH0.5 | 62.25 | 112.83 | 169.04 | 311.52 | 3.40 |
| Blend/Fe-KH1 | 62.11 | 110.98 | 168.38 | 262.43 | 5.64 |
| Blend/Fe-KH3 | 62.25 | 110.52 | 168.72 | 325.17 | 3.44 |
| Blend/Fe-KHSiO2 | 62.85 | 111.18 | 168.61 | 270.53 | 6.21 |
| Blend/Fe-KHPDA | 62.85 | 110.49 | 168.51 | 268.83 | 3.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, W.; Li, K.; Chen, J.; Xu, Y.; Zhao, Z.; Li, Y.; Yu, L. Effect of Surface Treatment of Nano-Magnetite Particles on PLA/PBAT Composites. J. Compos. Sci. 2025, 9, 592. https://doi.org/10.3390/jcs9110592
Zhang L, Wang W, Li K, Chen J, Xu Y, Zhao Z, Li Y, Yu L. Effect of Surface Treatment of Nano-Magnetite Particles on PLA/PBAT Composites. Journal of Composites Science. 2025; 9(11):592. https://doi.org/10.3390/jcs9110592
Chicago/Turabian StyleZhang, Le, Wenbo Wang, Kun Li, Jingbo Chen, Yunlong Xu, Zhibo Zhao, Yanan Li, and Long Yu. 2025. "Effect of Surface Treatment of Nano-Magnetite Particles on PLA/PBAT Composites" Journal of Composites Science 9, no. 11: 592. https://doi.org/10.3390/jcs9110592
APA StyleZhang, L., Wang, W., Li, K., Chen, J., Xu, Y., Zhao, Z., Li, Y., & Yu, L. (2025). Effect of Surface Treatment of Nano-Magnetite Particles on PLA/PBAT Composites. Journal of Composites Science, 9(11), 592. https://doi.org/10.3390/jcs9110592






