Assessing the Impact of Additive Manufacturing on Dental Clinical Workflows: A Process-Oriented Approach
Abstract
1. Introduction
2. Literature Review
2.1. Materials (Input)
2.2. Design (Input)
2.3. Additive Manufacturing Techniques (Transformation)
2.4. Use of MA in Industry (Output)
2.5. Post-Processing (Output)
3. Materials and Methods
4. Results
4.1. Materials
| Resin | Biocompatibility | Viscosity | Flexural Modulus | Flexural Strength | References |
|---|---|---|---|---|---|
| New stetic—Portux SG | ISO 10993-5, 10 and 23 | 380 mPa.s | >1.5 GPa (ISO 10477) | >50 MPa (ISO 10477) | [106] |
| New stetic—Portux Temp | ISO 10993-5, 10 and 23 | 380 ± 80 mPa.s | >1.8 GPa | >90 MPa | [107,108] |
| PriZma 3D—Bio Crown (tint: Bleach) | In accordance with ISO 4049 standard | 255–500 mPa.s | 2.85 GPa | at 5%, ≥105.5 Mpa | [109,111] |
| PriZma 3D—Bio Crown (tint: A2) | In accordance with ISO 4049 standard | 255–500 mPa.s | 2.85 GPa | at 5%, ≥105.5 Mpa | [109,111] |
| PriZma 3D—Bio Splint | Anvisa Registration 80483740001 | 190–500 mPa.s | 2.97 GPa | at 5%, 102.8 MPa | [110,112] |
| Anycubic—Standard Resin, clear | Not applicable | 200–230 mPa.s | 1.4–1.6 GPa | 50–60 MPa | [113] |
| Antisky—Dental Guide Resin, clear | Not certified | 280–380 mPa.s | 2.3–2.4 GPa | 100–110 MPa | [114] |
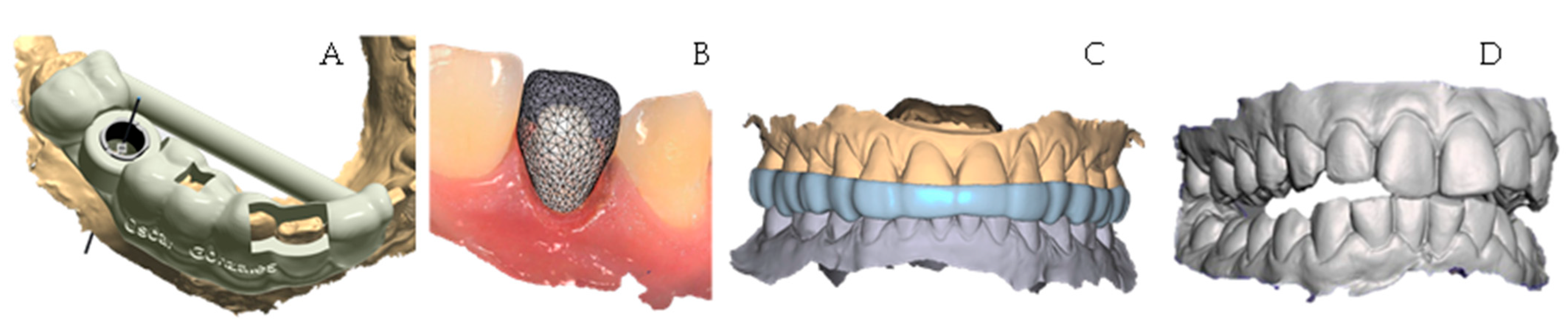
4.2. Design
4.3. Printing
4.4. Post Processing
4.5. Scanning Electron Microscope Analysis
4.6. Comparison Between Traditional and Additive Process
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, E.-H.; Ahn, J.-S.; Lim, Y.-J.; Kwon, H.-B.; Kim, M.-J. Effect of post-curing time on the color stability and related properties of a tooth-colored 3D-printed resin material. J. Mech. Behav. Biomed. Mater. 2022, 126, 104993. [Google Scholar] [CrossRef]
- Rubio, N.A. CAI: Computer-Assisted Imaging. In Digital Dental Implantology; Springer: Cham, Switzerland, 2021; pp. 3–18. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Qiang, Z.; Hu, Z.; Cui, X.; Wei, H.; Hu, J.; Xia, Y.; Huang, S.; Zhang, J.; et al. Cellulose nanocrystal enhanced, high dielectric 3D printing composite resin for energy applications. Compos. Sci. Technol. 2022, 227, 109601. [Google Scholar] [CrossRef]
- Chen, R.; Cai, J.; Chin, K.C.H.; Wang, S.; Boydston, A.J.; Thevamaran, R.; Gopalan, P. Block copolymer additives for toughening 3D printable epoxy resin. Giant 2023, 17, 100204. [Google Scholar] [CrossRef]
- Rivera, C.; Rodriguez, J.N.; Bas, A.O. Research on the Input-Transformation-Output Process of Additive Manufacturing: Comparing PLA/Polysmooth and Resin Printed Rings. Crystals 2023, 14, 7. [Google Scholar] [CrossRef]
- Chhabra, M.; Kumar, M.N.; RaghavendraSwamy, K.N.; Thippeswamy, H. Flexural strength and impact strength of heat-cured acrylic and 3D printed denture base resins- A comparative in vitro study. J. Oral Biol. Craniofacial Res. 2021, 12, 1–3. [Google Scholar] [CrossRef]
- Carreño, A.M.; Torres, M.E.; Rojas, M.S.R.; Rodriguez, J.N. Un estudio comparativo para evaluar el proceso sustractivo y aditivo en odontología: Una revisión sistemática. Rev. Ing. USBMed 2024, 15, 70–90. [Google Scholar] [CrossRef]
- Suganna, M.; Kausher, H.; Ahmed, S.T.; Alharbi, H.S.; Alsubaie, B.F.; Ds, A.; Haleem, S.; Ali, A.B.M.R. Contemporary Evidence of CAD-CAM in Dentistry: A Systematic Review. Cureus 2022, 14, e31687. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Sorrentino, R. The Role of Digital Devices in Dentistry: Clinical Trends and Scientific Evidences. J. Clin. Med. 2020, 9, 1692. [Google Scholar] [CrossRef]
- No-Cortes, J.; Ayres, A.P.; Lima, J.E.; Markarian, R.A.; Attard, N.J.; Cortes, A.R.G. Trueness, 3D Deviation, Time and Cost Comparisons Between Milled and 3D-Printed Resin Crowns. Eur. J. Prosthodont. Restor. Dent. 2022, 30, 107–112. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, H.-Y.; Kim, J.-H.; Kim, J.-H.; Kim, W.-C. Comparison of prosthetic models produced by traditional and additive manufacturing methods. J. Adv. Prosthodont. 2015, 7, 294–302. [Google Scholar] [CrossRef]
- El Samahy, M.M.; Abdelhamid, A.M.; El Shabrawy, S.M.; Hanno, K.I. Evaluation of physicomechanical properties of milled versus 3D-printed denture base resins: A comparative in vitro study. J. Prosthet. Dent. 2023, 129, 797.e1–797.e7. [Google Scholar] [CrossRef]
- Schweiger, J.; Edelhoff, D.; Güth, J.-F. 3D Printing in Digital Prosthetic Dentistry: An Overview of Recent Developments in Additive Manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef]
- Palavicini, J.; Quin, S.L.; Zakkour, W.; Zakkour, K.; Varkiani, S.M.; Xu, X.; Lawson, N.C.; Nejat, A.H. Bond Strength of Reline Materials to 3D-Printed Provisional Crown Resins. Polymers 2023, 15, 3745. [Google Scholar] [CrossRef]
- Farkas, A.Z.; Galatanu, S.-V.; Nagib, R. The Influence of Printing Layer Thickness and Orientation on the Mechanical Properties of DLP 3D-Printed Dental Resin. Polymers 2023, 15, 1113. [Google Scholar] [CrossRef]
- Zhang, P.; He, R. 3D-printed silicon nitride ceramic implants for clinical applications: The state of the art and prospects. RSC Adv. 2025, 15, 406–419. [Google Scholar] [CrossRef]
- Alghauli, M.A.; Alqutaibi, A.Y. 3D-printed intracoronal restorations, occlusal and laminate veneers: Clinical relevance, properties, and behavior compared to milled restorations; a systematic review and meta-analysis. J. Esthet. Restor. Dent. 2024, 36, 1153–1170. [Google Scholar] [CrossRef]
- Mandurino, M.; Cortili, S.; Coccoluto, L.; Greco, K.; Cantatore, G.; Gherlone, E.F.; Vichi, A.; Paolone, G. Mechanical Properties of 3D Printed vs. Subtractively Manufactured Composite Resins for Permanent Restorations: A Systematic Review. Materials 2025, 18, 985. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Radomski, K.; Lopez, D.; Liu, J.T.; Lee, J.D.; Lee, S.J. Materials and Applications of 3D Printing Technology in Dentistry: An Overview. Dent. J. 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Zhai, Z.; Nakano, T.; Ishigaki, S. Impact of internal design on the accuracy of 3-dimensionally printed casts fabricated by stereolithography and digital light processing technology. J. Prosthet. Dent. 2023, 130, 381.e1–381.e7. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of Printing Layer Thickness and Postprinting Conditions on the Flexural Strength and Hardness of a 3D-Printed Resin. BioMed Res. Int. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Ibrahim, A.M.; Tekerek, E.; Kontsos, A.; Palmese, G.R.; Alvarez, N.J. The effect of resin-rich layers on mechanical properties of 3D printed woven fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106339. [Google Scholar] [CrossRef]
- Kirby, S.; Pesun, I.; Nowakowski, A.; França, R. Effect of Different Post-Curing Methods on the Degree of Conversion of 3D-Printed Resin for Models in Dentistry. Polymers 2024, 16, 549. [Google Scholar] [CrossRef]
- AlRumaih, H.S.; Gad, M.M. The Effect of 3D Printing Layer Thickness and Post-Polymerization Time on the Flexural Strength and Hardness of Denture Base Resins. Prosthesis 2024, 6, 970–978. [Google Scholar] [CrossRef]
- Janssen, K.; Schnelting, G.H.; Waterink, M.; Guit, J.; Hul, J.; Ye, C.; Loos, K.; Voet, V.S. Renewable Methacrylate Resins for 3D Printing Containing Dynamic Hydroxyester Linkages for Reprocessability. Macromol. Mater. Eng. 2024, 309, 2400036. [Google Scholar] [CrossRef]
- Alamo, L.; Cassiano, F.B.; Bordini, E.A.F.; Stuani, V.T.; Pacheco, L.E.; Gallinari, M.d.O.; Costa, C.A.S.; Mondelli, R.F.L.; Soares, D.G. An organotypic model of oral mucosa cells for the biological assessment of 3D printed resins for interim restorations. J. Prosthet. Dent. 2024, 132, 251–259. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, J.H.; Kong, H.; Park, E.-J. Biofilm removal effect of diatom complex on 3D printed denture base resin. Sci. Rep. 2024, 14, 4034. [Google Scholar] [CrossRef]
- Gad, M.M.; Alzaki, F.A.; Abuwarwar, F.A.; Alhammad, A.; Al Hussain, M.; Khan, S.Q.; Nassar, E.A.; Ayad, N.M. Impact of printing layer thickness on the flexural strength of nanocomposite 3D printed resins: An in vitro comparative study. Saudi Dent. J. 2024, 36, 1307–1312. [Google Scholar] [CrossRef]
- Poker, B.d.C.; Oliveira, V.d.C.; Macedo, A.P.; Gonçalves, M.; Ramos, A.P.; Silva-Lovato, C.H. Evaluation of surface roughness, wettability and adhesion of multispecies biofilm on 3D-printed resins for the base and teeth of complete dentures. J. Appl. Oral Sci. 2024, 32, e20230326. [Google Scholar] [CrossRef]
- Coelho, S.R.G.; da Silva, M.D.D.; Nunes, T.S.B.S.; Viotto, H.E.C.; Marin, D.O.M.; Pero, A.C. Effect of immersion in disinfectants on the color stability of denture base resins and artificial teeth obtained by 3D printing. J. Prosthodont. 2023, 33, 157–163. [Google Scholar] [CrossRef]
- Gad, M.M.; Al Hamad, H.M.; Almohsin, F.M.; Fouda, S.M.; Akhtar, S.; Khan, S.Q.; Rahoma, A.; Al-Qarni, F.D.; Baba, N.Z.; Al-Harbi, F.A. Repair strength of 3D-printed denture base resins: Effect of surface treatment and repair material type. J. Prosthodont. 2024. [Google Scholar] [CrossRef] [PubMed]
- Aydin, N.; Kavrama, F.U.; Yosuncigir, H.; Ucar, Y. A comparison of the shear bond strength between denture teeth and denture base resins manufactured either conventionally or with a 3D printer. J. Prosthet. Dent. 2023, 130, 742.e1–742.e6. [Google Scholar] [CrossRef] [PubMed]
- Al-Qarni, F.D.; Gad, M.M. Printing Accuracy and Flexural Properties of Different 3D-Printed Denture Base Resins. Materials 2022, 15, 2410. [Google Scholar] [CrossRef]
- Di Fiore, A.; Meneghello, R.; Brun, P.; Rosso, S.; Gattazzo, A.; Stellini, E.; Yilmaz, B. Comparison of the flexural and surface properties of milled, 3D-printed, and heat polymerized PMMA resins for denture bases: An in vitro study. J. Prosthodont. Res. 2022, 66, 502–508. [Google Scholar] [CrossRef]
- Zattera, A.C.A.; Morganti, F.A.; Balbinot, G.d.S.; Della Bona, A.; Collares, F.M. The influence of filler load in 3D printing resin-based composites. Dent. Mater. 2024, 40, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Lassila, L.; Mangoush, E.; He, J.; Vallittu, P.K.; Garoushi, S. Effect of Post-Printing Conditions on the Mechanical and Optical Properties of 3D-Printed Dental Resin. Polymers 2024, 16, 1713. [Google Scholar] [CrossRef]
- Kim, J.-E.; Mangal, U.; Yu, J.-H.; Kim, G.-T.; Kim, H.; Seo, J.-Y.; Cha, J.-Y.; Lee, K.-J.; Kwon, J.-S.; Choi, S.-H. Evaluation of the effects of temperature and centrifugation time on elimination of uncured resin from 3D-printed dental aligners. Sci. Rep. 2024, 14, 15206. [Google Scholar] [CrossRef]
- Korkmaz, Y.N.; Buyuk, S.K.; Simsek, H.; Abay, F. Comparison of the Flexural Strength of Three Different Aged and Nonaged 3D-Printed Permanent Crown Resins. Int. J. Prosthodont. 2024, 37, S203–S207. [Google Scholar] [CrossRef]
- Celikel, P.; Sengul, F. Investigating the impact of post-curing cycles on surface hardness and color stability in 3D printed resin crowns. Odontology 2024, 113, 156–162. [Google Scholar] [CrossRef]
- Aktas, N.; Güngör, M.B. Effects of 3D-Printing Technology and Cement Type on the Fracture Resistance of Permanent Resin Crowns for Primary Teeth. Int. J. Prosthodont. 2024, 37, S195–S202. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.S.; da Cruz, M.B.; Marques, J.F.; Roque, J.C.; Martins, J.P.; Malheiro, R.C.; da Mata, A.D. Cellular responses to 3D printed dental resins produced using a manufacturer recommended printer versus a third party printer. J. Adv. Prosthodont. 2024, 16, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Baytur, S.; Turksayar, A.A.D. Effects of post-polymerization conditions on color properties, surface roughness, and flexural strength of 3D-printed permanent resin material after thermal aging. J. Prosthodont. 2024, 34, 298–307. [Google Scholar] [CrossRef]
- Lask, M.; Stawarczyk, B.; Reymus, M.; Meinen, J.; Mayinger, F. Impact of varnishing, coating, and polishing on the chemical and mechanical properties of a 3D printed resin and two veneering composite resins. J. Prosthet. Dent. 2024, 132, 466.e1–466.e9. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Stellini, E.; Alageel, O.; Alhotan, A. Comparison of mechanical and surface properties of two 3D printed composite resins for definitive restoration. J. Prosthet. Dent. 2024, 132, 839.e1–839.e7. [Google Scholar] [CrossRef]
- Prause, E.; Malgaj, T.; Kocjan, A.; Beuer, F.; Hey, J.; Jevnikar, P.; Schmidt, F. Mechanical properties of 3D-printed and milled composite resins for definitive restorations: An in vitro comparison of initial strength and fatigue behavior. J. Esthet. Restor. Dent. 2023, 36, 391–401. [Google Scholar] [CrossRef]
- Sasany, R.; Jamjoon, F.Z.; Kendirci, M.Y.; Yilmaz, B. Effect of Printing Layer Thickness on Optical Properties and Surface Roughness of 3D-Printed Resins: An In Vitro Study. Int. J. Prosthodont. 2024, 37, S165–S173. [Google Scholar] [CrossRef]
- Espinar, C.; Della Bona, A.; Tejada-Casado, M.; Pulgar, R.; Pérez, M.M. Optical behavior of 3D-printed dental restorative resins: Influence of thickness and printing angle. Dent. Mater. 2023, 39, 894–902. [Google Scholar] [CrossRef]
- Berli, C.; Thieringer, F.M.; Sharma, N.; Müller, J.A.; Dedem, P.; Fischer, J.; Rohr, N. Comparing the mechanical properties of pressed, milled, and 3D-printed resins for occlusal devices. J. Prosthet. Dent. 2020, 124, 780–786. [Google Scholar] [CrossRef]
- Dimitrova, M.; Corsalini, M.; Kazakova, R.; Vlahova, A.; Chuchulska, B.; Barile, G.; Capodiferro, S.; Kazakov, S. Comparison between Conventional PMMA and 3D Printed Resins for Denture Bases: A Narrative Review. J. Compos. Sci. 2022, 6, 87. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Meng, X.; Ye, Y.; Feng, D.; Xue, J.; Wang, H.; Huang, H.; Wang, M.; Wang, J. Rheological and Mechanical Properties of Resin-Based Materials Applied in Dental Restorations. Polymers 2021, 13, 2975. [Google Scholar] [CrossRef]
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A Comparison of the Flexural and Impact Strengths and Flexural Modulus of CAD/CAM and Conventional Heat-Cured Polymethyl Methacrylate (PMMA). J. Prosthodont. 2018, 29, 341–349. [Google Scholar] [CrossRef]
- Sartori, N.; Sanchez, S.A.; Oliveira, D.; Hosney, S.; Zoidis, P.; Martin, W.; Gonzaga, L.; Rocha, M.G. Flexural properties and fatigue limit of 3D-printed and milled resin-based materials. J. Prosthodont. 2024, 34, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.; Xiao, N.; Zhu, Y.; Gao, Z.; Qin, Q.; Shan, S.; Li, W.; Sun, Y.; Wang, Y.; Zhao, Y. Spatial Trueness Evaluation of 3D-Printed Dental Model Made of Photopolymer Resin: Use of Special Structurized Dental Model. Polymers 2024, 16, 1083. [Google Scholar] [CrossRef] [PubMed]
- Alghauli, M.A.; Aljohani, W.; Almutairi, S.; Aljohani, R.; Alqutaibi, A.Y. Advancements in digital data acquisition and CAD technology in Dentistry: Innovation, clinical Impact, and promising integration of artificial intelligence. Clin. eHealth 2025, 8, 32–35. [Google Scholar] [CrossRef]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef]
- Kim, J.; Lin, Y.; Danielak, M.; Van, M.; Lee, D.; Kim, H.; Arany, P.R. Virtual Planning and Rapid 3D Prototyping Surgical Guide for Anterior Crown Lengthening Surgery: A Clinical Case Report. J. Prosthodont. 2021, 31, 275–281. [Google Scholar] [CrossRef]
- Caussin, E.; Moussally, C.; Le Goff, S.; Fasham, T.; Troizier-Cheyne, M.; Tapie, L.; Dursun, E.; Attal, J.-P.; François, P. Vat Photopolymerization 3D Printing in Dentistry: A Comprehensive Review of Actual Popular Technologies. Materials 2024, 17, 950. [Google Scholar] [CrossRef]
- Güth, J.-F.; Runkel, C.; Beuer, F.; Stimmelmayr, M.; Edelhoff, D.; Keul, C. Accuracy of five intraoral scanners compared to indirect digitalization. Clin. Oral Investig. 2016, 21, 1445–1455. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; AlFulaij, F.; Bokhary, A.; Sallustio, A.; Chochlidakis, K. Complete Digital Workflow for Prosthesis Prototype Fabrication with Double Digital Scanning: Accuracy of Fit Assessment. J. Prosthodont. 2022, 32, 49–53. [Google Scholar] [CrossRef]
- Da Son, K.; Lee, W.-S.; Lee, K.-B. Prediction of the learning curves of 2 dental CAD software programs. J. Prosthet. Dent. 2019, 121, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Hegedus, T.; Kreuter, P.; Kismarczi-Antalffy, A.A.; Demeter, T.; Banyai, D.; Vegh, A.; Geczi, Z.; Hermann, P.; Payer, M.; Zsembery, A.; et al. User Experience and Sustainability of 3D Printing in Dentistry. Int. J. Environ. Res. Public Heal. 2022, 19, 1921. [Google Scholar] [CrossRef] [PubMed]
- Escáneres Intraorales 3Shape TRIOS—Comparar Todos los Modelos. Available online: https://www.3shape.com/es/scanners/trios (accessed on 26 May 2025).
- 3M True Definition Scanner. Available online: https://www.3m.com.ni/3M/es_NI/dental-la/productos/true-definition-scanner/ (accessed on 26 May 2025).
- iTeroEd—How to Export a File. Available online: https://www.iteroed.com/en-SG/faqs/6Ha7BtPCuv9zeJz1gIyyzB (accessed on 26 May 2025).
- Planmeca PlanScan Lab—MedicalVet. Available online: https://medicalvet.com.uy/product/planmeca-planscan-lab/ (accessed on 26 May 2025).
- Digital, Flexible y Abierto—CEREC, un Sistema Completo Para las Restauraciones, la Implantología y la Ortodoncia. Available online: https://news.dentsplysirona.com/es/business-units/cad-cam/2017/digital--flexible-y-abierto--cerec--un-sistema-completo-para-las.html (accessed on 26 May 2025).
- CS3500—MEDISER. Available online: https://www.medisercontrol.com/es/carestream/85-cs3500.html (accessed on 27 May 2025).
- ISO/ASTM 52900:2021(en); Additive Manufacturing—General Principles—Fundamentals and Vocabulary. International Standard Organization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso-astm:52900:ed-2:v1:en (accessed on 18 September 2025).
- Miyanaji, H.; Zhang, S.; Lassell, A.; Zandinejad, A.; Yang, L. Process Development of Porcelain Ceramic Material with Binder Jetting Process for Dental Applications. JOM 2016, 68, 831–841. [Google Scholar] [CrossRef]
- Mostafaei, A.; Stevens, E.L.; Ference, J.J.; Schmidt, D.E.; Chmielus, M. Binder jetting of a complex-shaped metal partial denture framework. Addit. Manuf. 2018, 21, 63–68. [Google Scholar] [CrossRef]
- Kamio, T.; Iwata, H.; Kawai, T. Challenges and Solutions for Cost-Effective and Practical Dental Cast Fabrication With a Desktop Fused Deposition Modeling (FDM) 3D Printer. Cureus 2024, 16, e73354. [Google Scholar] [CrossRef]
- Gupta, A.; Alifui-Segbaya, F.; Hasanov, S.; White, A.R.; Ahmed, K.E.; Love, R.M.; Fidan, I. Material extrusion of thermoplastic acrylic for intraoral devices: Technical feasibility and evaluation. J. Mech. Behav. Biomed. Mater. 2023, 143, 105950. [Google Scholar] [CrossRef]
- Erdemli, B.Y.; Kalyoncuoğlu, Ü.T.; Ayyıldız, S.; Tahmasebifar, A.; Baran, E.T.; Akça, G. Plasma treatment of dental zirconia produced by nano particle jetting additive manufacturing and conventional milling. J. Aust. Ceram. Soc. 2025, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J. Application of 3D Printing Technology in Dentistry: A Review. Polymers 2025, 17, 886. [Google Scholar] [CrossRef]
- Arora, O.; Ahmed, N.; Maiti, S. Comparison of the marginal accuracy of metal copings fabricated by 3D-printed resin and milled polymethyl methacrylate—An in vitro study. J. Adv. Pharm. Technol. Res. 2022, 13, S238–S242. [Google Scholar] [CrossRef]
- Ciocan, L.T.; Vasilescu, V.G.; Pantea, M.; Pițuru, S.M.; Imre, M.; Totan, A.R.; Froimovici, F.O. The Evaluation of the Trueness of Dental Mastercasts Obtained through Different 3D Printing Technologies. J. Funct. Biomater. 2024, 15, 210. [Google Scholar] [CrossRef]
- Ahmed, I.; Sullivan, K.; Priye, A. Multi-Resin Masked Stereolithography (MSLA) 3D Printing for Rapid and Inexpensive Prototyping of Microfluidic Chips with Integrated Functional Components. Biosensors 2022, 12, 652. [Google Scholar] [CrossRef]
- Parrales, K.G.M.; Lino, E.A.M.; Hernández, M.M.O. Impresión 3d como eje de desarrollo en la industria 4.0. In Serie Científica de la Universidad de las Ciencias Informáticas; Universidad de las ciencias informáticas: Havana, Cuba, 2021; Volume 14, pp. 151–160, ISSN-e 2306-2495; Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=8590504&info=resumen&idioma=SPA (accessed on 26 May 2025).
- Mercado de Impresión 3D—Tamaño, Tendencias de la Industria, Crecimiento y Pronóstico. Available online: https://www.mordorintelligence.com/es/industry-reports/3d-printing-market (accessed on 26 May 2025).
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Mercado de Impresión Dental 3D—Tendencias, Análisis y Tamaño. Available online: https://www.mordorintelligence.com/es/industry-reports/dental-3d-printing-market (accessed on 26 May 2025).
- Da Silva, M.D.D.; Nunes, T.S.B.S.; Viotto, H.E.D.C.; de Souza, R.F.; Pero, A.C. Effects of brushing on denture resins for 3D printing. J. Prosthodont. Res. 2023, 68, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, C. A combined 3D printed metal and resin digital denture: 5-year follow-up data and a creative design concept. J. Prosthet. Dent. 2024, 134, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.M.; Immich, F.; De Araujo, T.S.; Lund, R.G.; Da Silva, A.F.; Piva, E.; Rosa, W.L.D.O.D. Photosensitive resins used in additive manufacturing for oral application in dentistry: A scoping review from lab to clinic. J. Mech. Behav. Biomed. Mater. 2023, 141, 105732. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.-E.; Hwangbo, N.-K.; Kim, J.-E. Effects of surface glazing on the mechanical and biological properties of 3D printed permanent dental resin materials. J. Prosthodont. Res. 2024, 68, 273–282. [Google Scholar] [CrossRef]
- Russo, L.L.; Guida, L.; Mariani, P.; Ronsivalle, V.; Gallo, C.; Cicciù, M.; Laino, L. Effect of Fabrication Technology on the Accuracy of Surgical Guides for Dental-Implant Surgery. Bioengineering 2023, 10, 875. [Google Scholar] [CrossRef]
- Riccio, C.; Civera, M.; Ruiz, O.G.; Pedullà, P.; Reinoso, M.R.; Tommasi, G.; Vollaro, M.; Burgio, V.; Surace, C. Effects of Curing on Photosensitive Resins in SLA Additive Manufacturing. Appl. Mech. 2021, 2, 942–955. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed; Springer: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Kuo, C.Y. Safety Data Sheet Aqua-Gray 8K. January 2024. Available online: https://cdn.shopify.com/s/files/1/0436/6965/1618/files/SDS_AquaGray_8K.pdf?v=1720766366 (accessed on 31 May 2025).
- Beyond 3D Ltd. Resin Aqua 8K Gray—High Resolution And Low Shrinkage Percentage. Available online: https://beyond3d.co.il/en/product/phrozen-aqua-8k-resin-gray/ (accessed on 31 May 2025).
- Sunlu. SUNLU Water-Wash-ABS-LikeResin Technical Data Sheet. Available online: https://cdn.shopify.com/s/files/1/0909/3450/9859/files/Water-Wash_ABS-Like_Resin.pdf?v=1735900797 (accessed on 31 May 2025).
- eSUN. Hard-Tough Resin Technical Data Sheet. November 2021. Available online: https://www.esun3d.com/uploads/eSUN_Hard-Tough-Resin_TDS_V4.0.pdf (accessed on 31 May 2025).
- Newbest Testing Service. Safety Data Sheet for Chemical Products: EResin-Elastic; Newbest Testing Service: Guangdong, China, 23 June 2022. [Google Scholar]
- Siraya Tech. Siraya Tech Technical Data Sheet- Castable Resin. Available online: https://drive.google.com/file/d/1WeMzB3VqyfW5yzjWkbSNQDnE6J_KbQLP/view (accessed on 8 July 2025).
- Makertech Labs. Resina priZma 3D Wide. Available online: https://www.makertechlabs.com.br/produto/resina-prizma-3d-wide-70424 (accessed on 31 May 2025).
- Jamg, H. Technical Data Sheet 2024. Shenzhen. 2024. Available online: www.jamghe.com (accessed on 8 July 2025).
- New Stetic S.A. Ficha Técnica Portux 3D Model Dpftpt-128. January 2024. Available online: https://www.newstetic.com/documentos/ft_portux3d_model.pdf (accessed on 10 July 2025).
- New Stetic S.A. Ficha de Seguridad Portux 3D Model Dpddfs-095. 2025. Available online: https://www.bing.com/ck/a?!&&p=09dfb67ec1d5f37f933d5d26e1bdd6227644d0f9473ba2d6c586f3fca3928fc7JmltdHM9MTc1OTQ0OTYwMA&ptn=3&ver=2&hsh=4&fclid=18225737-4b9d-6f03-2c3b-41454ac86e4d&u=a1aHR0cHM6Ly93d3cubmV3c3RldGljLmNvbS9kb2N1bWVudG9zL2ZzX3BvcnR1eDNkX21vZGVsLnBkZg (accessed on 22 June 2025).
- Anycubic. User Guide for Water-Wash Resin+. 2022. Available online: https://cdn.shopify.com/s/files/1/0245/5519/2380/files/Anycubic_Water-Wash_Resin_User_Manual_V1.0-EN_1.pdf?v=1663574590&ref=loox-pr. (accessed on 10 July 2025).
- Anycubic. User Guide for Standard Resin+. 2022. Available online: https://cdn.shopify.com/s/files/1/0245/5519/2380/files/Anycubic_Standard_Resin_User_Manual_V1.0-EN_1_df2a47fb-f6aa-477c-8c56-faaa2b1293ef.pdf?v=1663574587&ref=loox-pr (accessed on 10 July 2025).
- Anycubic. Plant-based UV Eco-Resin: Sustainable Printing with Exceptional Detail. Available online: https://store.anycubic.com/products/plant-based-uv-resin?_pos=1&_sid=514fbda10&_ss=r&variant=40701612097698&_sasdk=dMTk3OGEzZGE1YWE4MTgtMGYxMGI3MmIwZDhlMTUtMjYwMTFlNTEtOTIxNjAwLTE5NzhhM2RhNWFiOWM5 (accessed on 22 June 2025).
- New Stetic S.A. Ficha Técnica Portux 3D SG Dpftpt-142. October 2024. Available online: https://www.bing.com/ck/a?!&&p=0442a79c76c7de51ec6ac9f944f5a4bce8af03a4fec9472dad472f73888ce0c7JmltdHM9MTc1MjEwNTYwMA&ptn=3&ver=2&hsh=4&fclid=092de0e6-ebd3-6ecb-3fb1-ef0defd36802&u=a1aHR0cHM6Ly93d3cubmV3c3RldGljLmNvbS9kb2N1bWVudG9zL2Z0X3BvcnR1eF8zZF9zZy5wZGY&ntb=1 (accessed on 9 July 2025).
- New Stetic S.A. Ficha Técnica Portux 3D Temp Dpftpt-153. Available online: https://www.bing.com/ck/a?!&&p=26538badeb12d4c2be17029d26c4598e8178a1e59c744f0b5c19cd8fd425b545JmltdHM9MTc1MjEwNTYwMA&ptn=3&ver=2&hsh=4&fclid=092de0e6-ebd3-6ecb-3fb1-ef0defd36802&u=a1aHR0cHM6Ly93d3cubmV3c3RldGljLmNvbS9kb2N1bWVudG9zL2Z0X3BvcnR1eF8zZF90ZW1wLnBkZg&ntb=1 (accessed on 10 July 2025).
- New Stetic S.A. ICHA DE SEGURIDAD PORTUX 3D TEMP DPDDFS-115. June 2024. Available online: https://www.bing.com/ck/a?!&&p=87b9f3c46cbb53c5ebaa3c5bc030bd67dba5ef895b88bca373254aceb59c2429JmltdHM9MTc1MjEwNTYwMA&ptn=3&ver=2&hsh=4&fclid=092de0e6-ebd3-6ecb-3fb1-ef0defd36802&u=a1aHR0cHM6Ly93d3cubmV3c3RldGljLmNvbS9kb2N1bWVudG9zL2ZzX3BvcnR1eF8zZF90ZW1wLnBkZg&ntb=1 (accessed on 10 July 2025).
- Makertech Labs Ltda. Guia do Produto: Resina Bio Crown. Available online: https://imagens.makertechlabs.com.br/guia/bio-crown.pdf?utm_source=site&utm_medium=webpage&utm_campaign=ebook-bio-crown&utm_id=guia-produtos&_gl=1*1uowadd*_gcl_au*ODg0NzcyNDQwLjE3NTIxMTYxNjU (accessed on 9 July 2025).
- Makertech Labs Ltda. Guia do Produto: Resina Prizma bio-splint. Available online: https://imagens.makertechlabs.com.br/guia/bio-splint.pdf?utm_source=site&utm_medium=webpage&utm_campaign=ebook-bio-splint&utm_id=guia-produtos&_gl=1*tu9pov*_gcl_au*ODg0NzcyNDQwLjE3NTIxMTYxNjU (accessed on 10 July 2025).
- Krear 3D. Resina Prizma 3D Bio Crown A1 250g|Krear 3D Dental. Available online: https://www.krear3dental.com/producto/resina-prizma-3d-bio-crown-a1-250g-2/ (accessed on 1 June 2025).
- Makertech Labs Ltda. Ficha de Dados de Segurança—Resinas PriZma 3D Bio Splint; Makertech Labs Ltda: Tatuí, Brazil, 2019. [Google Scholar]
- Anycubic. Standard Resin. Available online: https://store.anycubic.com/products/colored-uv-resin?variant=34622234362018&_sasdk=dMTk3OGEzZGE1YWE4MTgtMGYxMGI3MmIwZDhlMTUtMjYwMTFlNTEtOTIxNjAwLTE5NzhhM2RhNWFiOWM5 (accessed on 22 June 2025).
- Antinsky. Dental Guide Resin. Available online: https://antinsky.com/products/antinsky-dental-guide-resin-for-dlp-lcd-resin-3d-printer-405nm-1kg-high-precision-and-low-shrinkage (accessed on 22 June 2025).
- Janjić, K.; Valentova, A.; Arellano, S.; Unterhuber, A.; Krause, A.; Oberoi, G.; Unger, E.; Tabrizi, H.A.S.; Schedle, A. The impact of print orientation and graphene nanoplatelets on biaxial flexural strength and cytotoxicity of a 3D printable resin for occlusal splints. Dent. Mater. 2024, 40, 1742–1752. [Google Scholar] [CrossRef]
- Bolat, Ç.; Salmaz, S. An investigation on the wear properties of the photocurable components produced by additive manufacturing for dentistry applications: Combined influences of UV exposure time, building direction, and sliding loads. Polym. Eng. Sci. 2024, 64, 5940–5958. [Google Scholar] [CrossRef]
- Aati, S.; Shrestha, B.; Fawzy, A. Cytotoxicity and antimicrobial efficiency of ZrO2 nanoparticles reinforced 3D printed resins. Dent. Mater. 2022, 38, 1432–1442. [Google Scholar] [CrossRef]
- Henao, J.; Ramos, J.S.; Valencia, C.H.; Adamms, I.; Rico, C.A.; Escandón, J.M.; Echeverri-Cárdenas, D. Elaboración de un nuevo tipo de guías quirúrgicas para implantes dentales mediante impresión 3D. Inf. Técnico 2018, 82, 78. [Google Scholar] [CrossRef]
- Juneja, M.; Thakur, N.; Kumar, D.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Adrián, L.; Juan, M.; Costa, L.D.; Ignacio, M.; Ibanez, J. Protocolo T.A.C para la confección de guías quirúrgicas de precisión en Implantología. 2020. Available online: https://doi.org/10.13140/RG.2.2.35324.56963 (accessed on 28 May 2025).
- Nassif, M.; Haddad, C.; Habli, L.; Zoghby, A. Materials and manufacturing techniques for occlusal splints: A literature review. J. Oral Rehabil. 2023, 50, 1348–1354. [Google Scholar] [CrossRef]
- Grymak, A.; Waddell, J.N.; Aarts, J.M.; Ma, S.; Choi, J.J.E. Evaluation of wear behaviour of various occlusal splint materials and manufacturing processes. J. Mech. Behav. Biomed. Mater. 2022, 126, 105053. [Google Scholar] [CrossRef] [PubMed]
- Morón-Conejo, B.; Berrendero, S.; Bai, S.; Martínez-Rus, F.; Pradies, G. Fit comparison of interim crowns manufactured with open and proprietary 3D printing modes versus milling technology: An in vitro study. J. Esthet. Restor. Dent. 2024, 36, 1693–1703. [Google Scholar] [CrossRef]
- Kim, E.-K.; Park, E.Y.; Kang, S. Three-dimensional printing of temporary crowns with polylactic acid polymer using the fused deposition modeling technique: A case series. Yeungnam Univ. J. Med. 2022, 40, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, N.; Bani, M.; Ocak, M.; Güngör, M.B. Effects of design software program and manufacturing method on the marginal and internal adaptation of esthetic crowns for primary teeth: A microcomputed tomography evaluation. J. Prosthet. Dent. 2024, 131, 519.e1–519.e9. [Google Scholar] [CrossRef] [PubMed]
- Shahin, S.Y.; Nassar, E.A.; Gad, M.M. A 3D-Printed Crown Integrated with 3D-Printed Orthodontic Brackets: A Novel One-Unit Printing Technique. Materials 2025, 18, 2727. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Meng, Z.; Sun, Q.; Peng, L.; Zhang, L.; Lu, W.; Liang, W.; Chen, G.; Wei, Y. Accuracy of additive manufacturing in stomatology. Front. Bioeng. Biotechnol. 2022, 10, 964651. [Google Scholar] [CrossRef] [PubMed]
- Alghauli, M.A.; Almuzaini, S.A.; Aljohani, R.; Alqutaibi, A.Y. Impact of 3D printing orientation on accuracy, properties, cost, and time efficiency of additively manufactured dental models: A systematic review. BMC Oral Health 2024, 24, 1550. [Google Scholar] [CrossRef] [PubMed]
- Katheng, A.; Prawatvatchara, W.; Chaiamornsup, P.; Sornsuwan, T.; Lekatana, H.; Palasuk, J. Comparison of mechanical properties of different 3D printing technologies. Sci. Rep. 2025, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Cianci, D.; Pranno, N.; Lerner, H.; Zarone, F.; Admakin, O. Trueness, precision, time-efficiency and cost analysis of chairside additive and subtractive versus lab-based workflows for manufacturing single crowns: An in vitro study. J. Dent. 2023, 141, 104792. [Google Scholar] [CrossRef]
- Chauhan, D.; Singh, A.P.; Chauhan, A.; Arora, R. Sustainable supply chain: An optimization and resource efficiency in additive manufacturing for automotive spare part. Sustain. Futures 2025, 9, 100563. [Google Scholar] [CrossRef]












| Resin Types | Articles Where Mentioned |
|---|---|
| Acrylic | [6,12,25,26,27,28,29,30,31,32,33,34,35,36] |
| Biocompatible | [12,15,26,28,37,38,39,40,41,42,43,44] |
| Resin composites | [15,37,40,45,46,47,48,49] |
| Process Categories | Processes | Applications in Dentistry |
|---|---|---|
| Binder jetting | Binder jetting (BJ) | Frameworks for removable partial dentures and ceramic restorations [73,74] |
| Directed energy deposition | Laser engineering net shape (LENS) Electron beam additive manufacturing (EBAM) | - |
| Material extrusion | Fused deposition modeling (FDM) Fused filament fabrication (FFF) | Scaffolds, denture bases, implant surgical guides, orthodontic splints, impression trays, record bases and obturators for cleft palates or other maxillary defects [75,76]. |
| Material jetting | Nano particle jetting (NPJ) Drop on demand (DOD) | Dental models, temporary crowns, and experimental ceramic (zirconia) NPJ parts for fixed dental restorations [17,77]. |
| Powder bed fusion | Selective laser sintering (SLS) Direct metal laser sintering (DMLS) Electron beam melting (EBM) Multi jet fusion (MJF) | Metallic prosthetic frameworks and implantology (CoCr crowns and bridges) [13] |
| Sheet lamination | Laminate object manufacturing (LOM) | - |
| Vat photopolymerization | Stereolithography (SLA) Direct light processing (DLP) Continuous direct light processing (CDLP) Direct UV printing (DUP) | Crowns, splints, surgical guides, and study models [13,17] |
| Category | Product Type | Articles Where Mentioned |
|---|---|---|
| Prosthodontics | Denture bases | [6,12,26,29,30,33,34,35,36] |
| Dentures (teeth only) | [32,55,83,86,87] | |
| Complete dentures | [31,88] | |
| Dental restorations | Crowns | [10,15,40,41,42,43,49,55] |
| Temporary restorations | [48,88,89] | |
| Permanent restorations | [46,47,48,89] | |
| Orthodontics | Aligners | [39] |
| Rehabilitation | Occlusal splints | [88] |
| Other applications | Surgical guides | [88,90] |
| Dental models or molds | [22,25,56] |
| Resin | Viscosity | Elastic Modulus | Impact Strength | Flexural Strength | Volumetric Shrinkage | References |
|---|---|---|---|---|---|---|
| Phrozen—Aqua 8K, grey | 280–380 mPa.s | Not reported, flexural modulus: 1551 Mpa | 9.6 J/m | 54 Mpa | Low (exact value not reported) | [93,94] |
| Sunlu—Water-wash ABS Like | 400–800 mPa.s | Not reported, flexural modulus: 952 Mpa | 120 J/m (ISO 179) | 34 Mpa (ISO 178) | 8.8% | [95] |
| eSUN—Hard-Tough resin | 200–300 mPa.s at 25 °C | Not reported | 40–110 J/m (ASTM D638) | 30–75 Mpa (ASTM D790) | Not reported | [96] |
| eSUN-eLastic | 2200–3500 mPa.s at 25° | Not reported | Not reported | Not reported | Not reported | [97] |
| Siraya Tech—Castable resin, purple | 300 mPa.s | 600 Mpa (Young’s modulus) | Not reported | Not reported | 6% | [98] |
| PriZma 3D—Wide | Not reported | 2080 MPa | Not reported | 65.4 MPa | Not reported | [99] |
| Jamg He—Standard plus, grey | 250–400 mPa.s | Not reported, flexural modulus: 1699.7 ± 10% Mpa | 72 ± 10% J/m | 57.9 ± 10% MPa | 0.2–0.7% | [100] |
| Portux—Model, bone | 420–580 mPa.s | Not reported, flexural modulus: >1900 MPa | Not reported | >70 MPa | Not reported | [101,102] |
| Anycubic—Water-wash+, white | 150–250 mPa.s at 25 °C | Not reported, flexural modulus: 1500–1600 MPa | 21 J/m | 50–60 MPa | 3.7–4.2% | [103] |
| Anycubic—Standard+, translucent green | 200 mPa.s at 25 °C | Not reported, flexural modulus: 950 Mpa | 28 J/m | 32 MPa | 3.7–4.2% | [104] |
| Anycubic—Plant-based resin, clear | 300–350 mPa.s | Not reported, flexural modulus: 1400–1600 MPa | Not reported | 42–48 MPa | 3.8–4.5% | [105] |
| Material | Technique/Technology Used |
|---|---|
| Phrozen—Aqua 8K, grey | SLA/Phrozen Mini 4K |
| Sunlu—Water-Wash ABS Like | SLA/Phrozen Mini 4K |
| eSUN—Hard-Tough resin | SLA/Phrozen Mini 4K |
| eSUN-eLastic | SLA/Phrozen Mini 4K |
| Siraya Tech—Castable resin, purple | SLA/Phrozen Mini 4K |
| New stetic—Portux SG | MSLA/Elegoo Mars 3 |
| New stetic—Portux Temp | MSLA/Anycubic Photon Mono 4 Ultra 10K |
| PriZma 3D—Bio Crown (tint: Bleach) | MSLA/Elegoo Mars 3 |
| PriZma 3D—Bio Crown (tint: A2) | MSLA/Anycubic Photon Mono X |
| PriZma 3D—Bio Splint | MSLA/Anycubic Photon Mono 2 |
| PriZma—Wide | SLA/Uniz IBEE |
| Jamg He—Standard plus, grey | MSLA/Anycubic Photon Mono 4 Ultra 10K |
| Portux—Model, bone | MSLA/Anycubic Photon Mono 4 Ultra 10K |
| Anycubic—Water-wash+, white | MSLA/Anycubic Photon Mono 4 Ultra 10K |
| Anycubic—Standard+, translucent green | MSLA/Elegoo Mars 5 ultra |
| Anycubic—Plant-based resin, clear | MSLA/Elegoo Mars 5 ultra |
| Anycubic—Standard resin, clear | MSLA/Elegoo Mars 5 ultra |
| Antisky—Dental Guide Resin, clear | MSLA/Elegoo Mars 5 ultra |
| Material | Lifting Distance | Lifting Speed | Exposure Time | Layer Height | Bottom Layer Count | Bottom Exposure Time |
| Anycubic—Standard+, translucent green Anycubic—Plant-based resin, clear Anycubic—Standard resin, clear Antisky—Dental guide resin, clear | - | - | 2.5 s | 0.05 mm | 2 | 32 s |
| Jamg He—Standard plus, grey Portux—Model, bone Anycubic—Water-wash+, white | 3 + 5 mm | 120 + 240 mm/min | 5.5 s | 0.05 mm | 5 | 50 s |
| New stetic—Portux Temp | 2 + 2 mm | 65 + 180 mm/min | 4 s | 0.04 mm | 2 | 45 s |
| Material | Application | 200× | 1000× | 2000× |
|---|---|---|---|---|
| Phrozen Aqua 8K grey | Dental models/impressions (generic resin) | 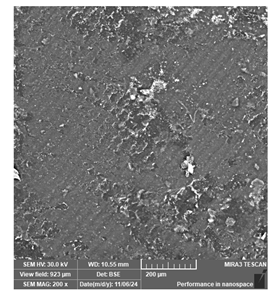 |  | 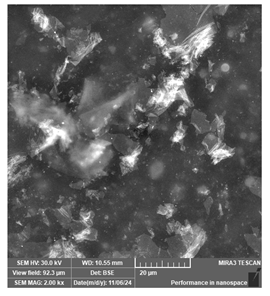 |
| Clear layer lines but irregular spots, suggesting potential deficiencies in detailed modeling. | ||||
| Sunlu Water—Wash ABS Like | Dental models/impressions (generic resin) |  | 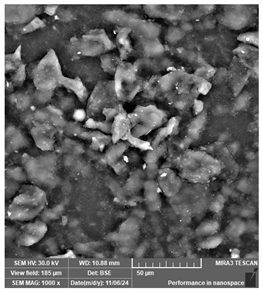 |  |
| Rough and uneven surface, suggesting potential deficiencies in detailed modeling. | ||||
| eSUN Hard—Tough resin | Dental models/impressions (generic resin) |  | 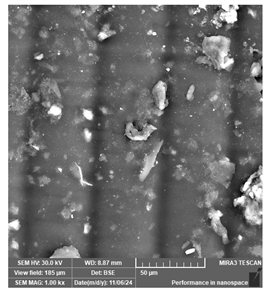 |  |
| Distinct parallel lines pattern. Embedded defects suggest incomplete polymerization or presence of impurities that may affect fine detail reproduction. | ||||
| eSUN-eLastic | Dental models/impressions (generic resin) |  | 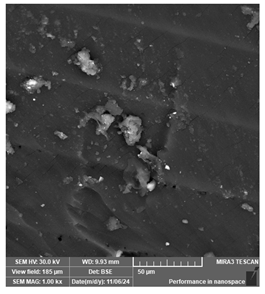 |  |
| Distinct parallel lines pattern and visible particles, suggesting incomplete polymerization or presence of impurities. | ||||
| Siraya Tech—Castable resin, purple | Dental models/impressions (generic resin) | 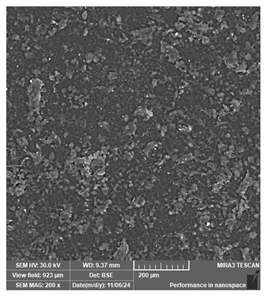 | 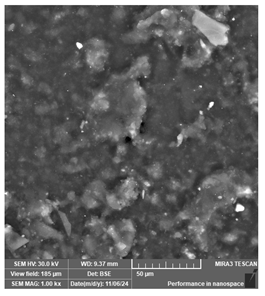 | 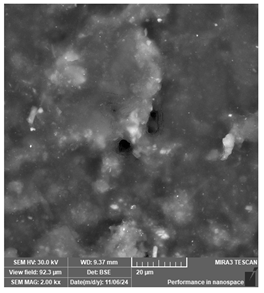 |
| Uniform but coarse surface texture. May hinder fine detail reproduction during casting. | ||||
| PriZma 3D—Bio Crown (tint: Bleach) | Permanent restorations |  |  | 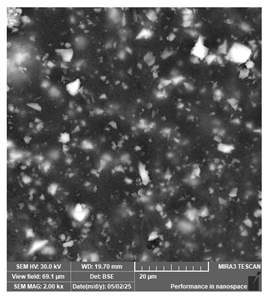 |
| Homogeneous surface. Multiple irregular particles suggest presence of ceramic or zirconia (not impactful for clinical viability). | ||||
| PriZma 3D—Bio Crown (tint: A2) | Permanent restorations |  | 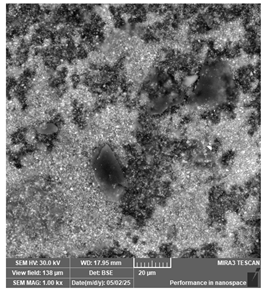 | 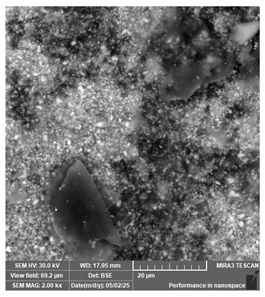 |
| Rougher surface and non-uniform particle distribution, likely linked to filler or pigment incorporation. | ||||
| PriZma 3D—Bio Splint | Occlusal splints |  | 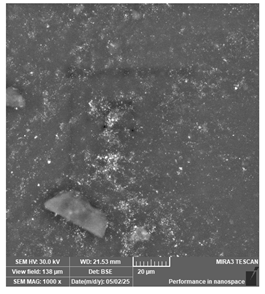 | 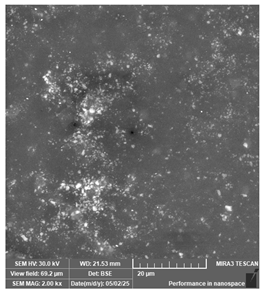 |
| Unevenly distributed filler may comrpomise structural homogeneity. | ||||
| PriZma 3D—Wide | Dental models/impressions |  | 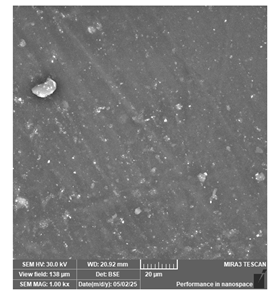 |  |
| The occasional large embedded particles are possibly impurities (not impactful for clinical viability). | ||||
| Material | Application | 500× | 1000× | 5000× | 10,000× |
|---|---|---|---|---|---|
| New stetic—Portux Temp | Temporary restorations | 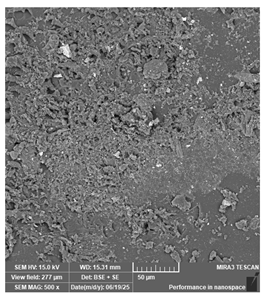 |  |  | 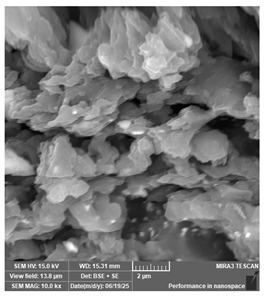 |
| New stetic—Portux SG | Surgical guides |  |  |  | 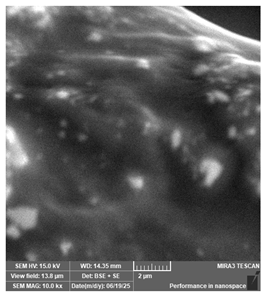 |
| Jamg He—Standard plus, grey | Dental models/impressions | 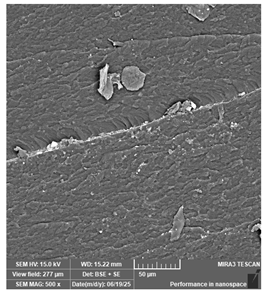 |  |  |  |
| Portux—Model, bone | Dental models/impressions | 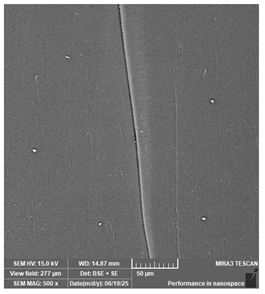 | 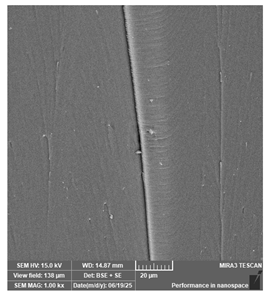 |  | 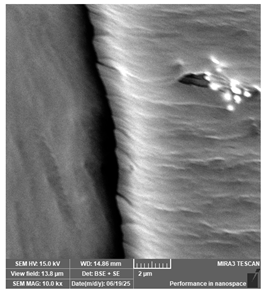 |
| Anycubic—Water-wash+, white | Dental models/impressions |  |  |  |  |
| Anycubic—Standard+, translucent green | Dental models/impressions |  | 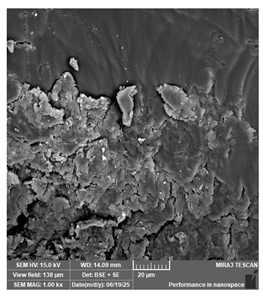 |  |  |
| Anycubic—Plant-based resin, clear | Dental models/impressions |  |  |  |  |
| Anycubic—Standard, clear | Surgical guides |  |  |  | 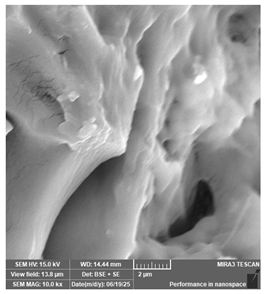 |
| Antisky—Dental Guide Resin, clear | Surgical guides | 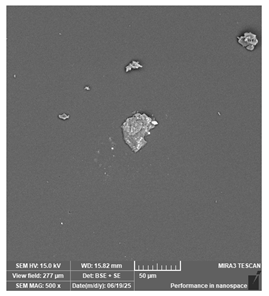 | 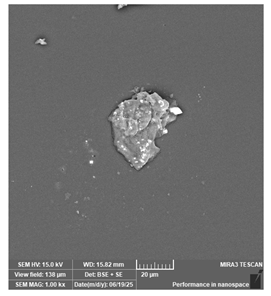 | 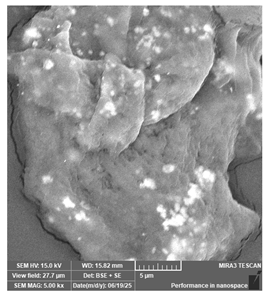 | 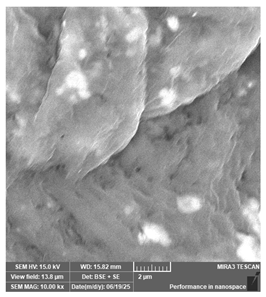 |
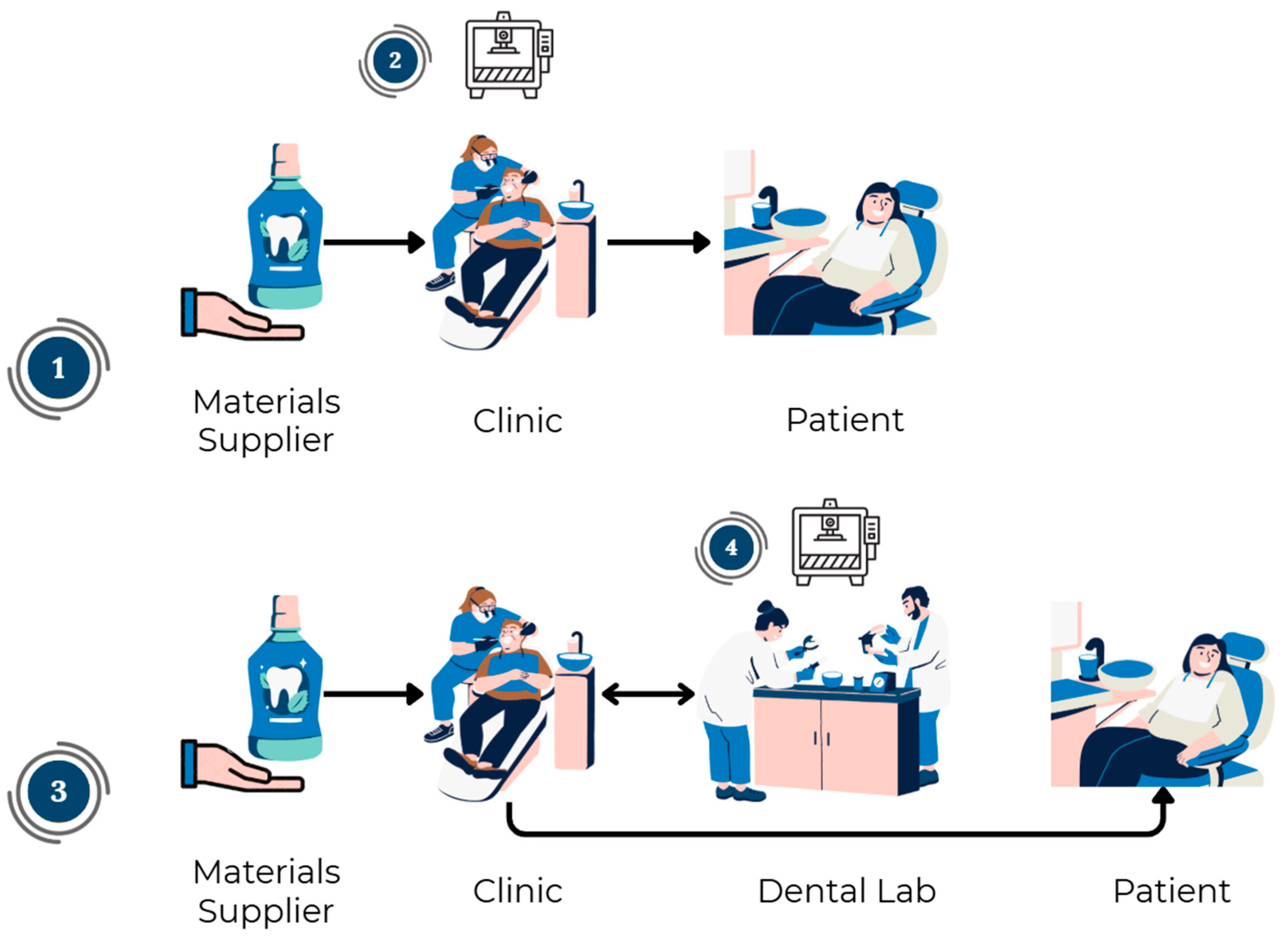
| Dental Application | Traditional Manufacturing | Subtractive Manufacturing | Additive Manufacturing | References |
|---|---|---|---|---|
| Dental models/impressions | Creation of plaster models using physical impressions with alginate. | Not applicable. | Scanning of the mouth or dental model assisted by CAI technology. Fabrication of the piece assisted by CAM technology and 3D printing. | [14] |
| Surgical guides | Molding with plaster of the prosthetic pretermination. Fabrication employing vacuum thermoforming of a thermoplastic acetate sheet on the plaster model. Reinforcement of the piece with PMMA or light-curing resin. Drilling and incorporation of radiopaque markers (pellets, metallic tubes, gutta-percha, barium sulfate, among others) where the implants will be placed. Clinical validation of the guide. | Scanning of the mouth or dental model assisted by CAI technology and matching them to tomographies of the bones (obtaining and matching DICOM and STL files). Surgery planning and surgical guide design using CAD technology. Machining processes (milling, turning, etc.) performed on a block of material (resin). | Scanning of the mouth or dental model assisted by CAI technology and matching them to tomographies of the bones (obtaining and matching DICOM and STL files). Surgery planning and surgical guide design using CAD technology. Fabrication of the piece assisted by CAM technology and 3D printing. * DLP is not suitable for this device due to the level of precision required | [14,90,118,119,120] |
| Restorations (crowns, bridges, etc.) | Molding with plaster and alginate. Design and fabrication using the lost wax technique. Manual finishing | Molding with plaster and alginate and/or CAI assisted. Device design in CAD software. Based upon the scan, a digital model is created in STL format. It is sent to the CAD software for treatment planning and designing of the device. Machining processes (milling, turning, etc.) performed on a block of material (resin). Finishing includes cleaning and sanding, but less manual adjustment. | Molding with plaster and alginate and/or CAI assisted. Device design in CAD software. Fabrication of the piece assisted by CAM technology and 3D printing. Finishing includes resin curing, cleaning and sanding, but less manual adjustment. | [14,25,79,84] |
| Occlusal splints | Molding with plaster and alginate. Occlusal (bite) registration using wax to determine centric relation. Design and fabrication with techniques such as lost wax, thermoforming, dusting, etc. Manual finishing (sanding). | Molding with plaster and/or CAI assisted. Device design in CAD software. Machining processes (milling, turning, etc.) performed on a block of material (resin). Finishing includes cleaning and sanding, but less manual adjustment. | Molding with plaster and/or CAI assisted. Device design in CAD software. Fabrication of the piece assisted by CAM technology and 3D printing. Finishing includes resin curing, cleaning and sanding, but less manual adjustment. | [14,121,122] |
| Traditional Manufacturing | Subtractive Manufacturing | Additive Manufacturing |
|---|---|---|
| Manual and artistic skills for the handling of specialized materials (wax, plaster, resin) and manufacturing complex designs. | Good computing skills, specialized knowledge of CAD/CAM Software. | Immaculate planning, good computing skills, specialized knowledge of CAD/CAM Software, slicers and post processing equipment. |
| Technical knowledge on control of manufacturing variables such as quantity and type of material, temperature, setting and curing times. | Technical knowledge on the control of manufacturing variables such as material properties, cutting tools and their functional limits. | Technical knowledge on control of manufacturing variables such as material properties, polymerization times, print orientation, support geometry. |
| Accuracy, efficiency and attention to detail. | Monitoring the operations performed (milling or printing, curing and finishing), knowledge and control of the digital workflow, dimensional validation of the final piece. | |
| Ability to interpret traditional imaging and physical models. | Proficiency in reading digital images such as intraoral scans, tomography (DICOM), and STL, and accuracy in image data fusion. | |
| - | Knowledge of proper calibration and preventive maintenance of equipment, and management of common technical failures | |
| Neatness and tidiness in both the workplace and in the temporary storage of pieces (finished and semi-finished goods). | Systematizing and traceability of digital files and temporary storage of finished goods. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutis Gómez, M.; Guerrero Torres, M.; Villarreal-Archila, S.M.; Núñez Rodríguez, J. Assessing the Impact of Additive Manufacturing on Dental Clinical Workflows: A Process-Oriented Approach. J. Compos. Sci. 2025, 9, 579. https://doi.org/10.3390/jcs9110579
Mutis Gómez M, Guerrero Torres M, Villarreal-Archila SM, Núñez Rodríguez J. Assessing the Impact of Additive Manufacturing on Dental Clinical Workflows: A Process-Oriented Approach. Journal of Composites Science. 2025; 9(11):579. https://doi.org/10.3390/jcs9110579
Chicago/Turabian StyleMutis Gómez, Mariana, Mario Guerrero Torres, Sylvia María Villarreal-Archila, and Jairo Núñez Rodríguez. 2025. "Assessing the Impact of Additive Manufacturing on Dental Clinical Workflows: A Process-Oriented Approach" Journal of Composites Science 9, no. 11: 579. https://doi.org/10.3390/jcs9110579
APA StyleMutis Gómez, M., Guerrero Torres, M., Villarreal-Archila, S. M., & Núñez Rodríguez, J. (2025). Assessing the Impact of Additive Manufacturing on Dental Clinical Workflows: A Process-Oriented Approach. Journal of Composites Science, 9(11), 579. https://doi.org/10.3390/jcs9110579






