1. Introduction
As modern production develops, along with its scale and growth rate, the problems of developing and implementing waste-free technologies become increasingly relevant. This trend is due to the fact that production activity, until very recently, was mainly based on one principle—the maximum exploitation of natural resources and ignoring the problem of the accumulation of industrial waste. At the same time, only a small part of the natural resources processed in the course of production activities are converted into target products, while most of them end up in waste. This path was possible until the scale of waste began to exceed the limits of the ability of ecological systems to self-repair and function without negative consequences for humanity on a regional and global scale. It should be noted that both solid and gaseous substances are meant as waste, including the increasing emissions of man-made CO
2, which increase the concentration of this greenhouse gas in the Earth’s atmosphere, which in turn negatively affects climate change [
1,
2].
Accordingly, there is an objective need to move to a fundamentally new model of socio-economic development—to closed production systems that assume the most limited impact of industrial production on the environment and social environment, taking into account the planned and purposeful growth of its volumes and environmental perfection. The key to such a transition is the requirement for the reasonable use of all components of the feedstock, the maximum reduction in production resources (energy, material, labor) and the search for new environmentally sound raw materials and energy technologies, which is largely associated with reducing the negative impact on the environment. The main aspect in achieving this goal is the development of new technological processes and productions and the improvement of existing ones in terms of energy technology and economic and environmental parameters.
Today, the metallurgical and construction industries are included in a number of the most significant sources of global CO
2 emissions. Thus, the production of Portland cement and other binders alone accounts for about 8% of the world’s man-made CO
2 emissions [
3] (each ton of Portland cement produced is accompanied by emissions of about 0.7–0.9 tons of CO
2 into the atmosphere on average), and about the same amount is accounted for by the steel industry [
4,
5]. The main reserve for the absorption of this CO
2 is various metallurgical slags accumulated at ferrous and non-ferrous metallurgy enterprises. The chemical and mineralogical compositions of most slags allow us to consider them as raw materials exhibiting astringent properties of the carbonate type of hardening and capable of active interaction with carbon dioxide, binding it into stable, insoluble and durable carbonate compounds. This aspect converts slags into efficient secondary material resources suitable for the production of various products. At the same time, these products should be in demand and of mass consumption, which will ensure the maximum CO
2 sequestration and a significant reduction in the carbon footprint.
In modern construction, one of the most widespread building materials is concrete. The most important component of concretes, which occupies the largest volume (mass) in their composition (up to 80%) and largely determines their construction and technical properties and purpose, is a variety of aggregates that can be ultralight to especially heavy. At the same time, natural aggregates (crushed stone, gravel) obtained from dense rocks are mainly used in the production of concrete, and the involvement of secondary material resources in the production cycle—waste from the production of the main product is extremely low. Of all the types of metallurgical slags, blast furnace slags that have undergone a wet granulation process and then been used in the production of Portland cement have received the greatest use in the production of building materials. Steelmaking and ferroalloy slags, due to their tendency to undergo various types of decomposition (silicate, lime, magnesia, ferruginous), are mainly of interest as raw materials for the production of crushed stone and sand for road construction on a limited scale due to the instability of their structure. One of the solutions to the problem of the resistance of steelmaking slags against these types of decay and an increase in their coefficient of use in the production of building materials may be their forced carbonation (hardening in an environment with an increased concentration of CO2).
The low utilization rate of steelmaking slags is mainly due to two reasons. Firstly, there is low or no hydraulic activity at all. Secondly, as mentioned above, the content of large amounts of free calcium and magnesium oxides (CaO, MgO) and thermodynamically unstable phases (β-C2S) can cause harmful expansion and instability of the volume [
6,
7]. According to [
8,
9], the forced carbonation of various steelmaking slags increases volume. The stability of the final product means that it is an effective method of overcoming these problems. References [
10,
11] also found that cements with a high content of belite (2CaO × SiO
2) bind more carbon dioxide and acquire better physical and mechanical characteristics as a result of this process, compared with alite cements (3CaO × SiO
2). This observation led scientists to search for alternative solutions to partially or completely replace the use of Portland cement in the production of building materials [
12]. The result of this search was the investigation of the possibility of the carbonate hardening of various metallurgical slags containing a significant amount of belite, represented by various polymorphic modifications. Studies have shown that these slags have a high reactivity to forced carbonation (carbonate hardening), and carbonation products (calcium/magnesium carbonates, calcium silicate hydrates) are the main phases with astringent properties and are responsible for the formation of the performance characteristics of the resulting artificial stone [
13,
14]. It has been established that after the process of the artificial carbonation of systems containing white, the total porosity decreases significantly, and a significant number of closed pores appear. It has also been revealed that the artificial carbonation of slags contributes to the formation of both cohesive porosity in experimental samples and closed porosity, as a result of which the properties of carbonized stone significantly change. The formation of different porosities depends mainly on the ratio of the polymorphic modifications of 2CaO×SiO
2 in the slag and the conditions of the forced carbonation process. Quantitatively, the total porosity decreases by 34–76% depending on the carbonation time. In this case, the number of macropores decreases, while the number of micro- and mesopores increases due to the formation of calcium and magnesium carbonate crystals at the nanoscale level [
15,
16,
17,
18,
19,
20,
21,
22]. Many researchers note that carbonation leads to a decrease in β-C2S and γ-C2S, as well as C3S with the simultaneous formation of various calcium carbonate polymorphs.
According to [
23], by 2030, the estimated market investment in CO
2 recovery in building materials will be USD 550 billion (USD 400 billion for concrete and USD 100 billion for aggregates), offering significant potential for reducing annual CO
2 emissions to 5 billion tons (USD 1.4 billion for concrete and 3.6 billion for aggregates). These estimates are highly attractive from both a scientific and commercial perspective, as currently, there is perhaps only one company, O.C.O Technology, that can be cited as a producer of a commercialized technology for using captured CO
2 in the production of carbonated aggregates. This company is the logical result of 20 years of scientific research in the field of carbon dioxide capture and is in the early stages of its development.
In connection with the above, it is possible to state the unique advantages of the method of the forced carbonization of steel-smelting slags. The uniqueness of the method lies in the fact that in the case of the traditional hydration mechanism of the hardening of inorganic binders, these slags are inert materials or exhibit weak hydraulic activity, which in turn does not allow these slags to be used as an independent binder, and the activation of their hardening requires excess pressure and elevated temperature. In turn, the hardening of building materials (for example, filler) or products (for example, brick) based on these slags in an environment of increased CO
2 concentration allows us to start a chemical reaction of CO
2 binding with certain initial slag minerals, with the formation of new compounds with binding properties. Accordingly, this method allows us to classify steelmaking slags as binders that harden in an environment with a high concentration of carbon dioxide, and the building materials and products obtained using this method will correspond to the concepts of combating climate change and the transition to a cyclical economy that are developing in the world [
1,
2]. In general, such an approach with the probable future implementation in industry determines the novelty of research in the field of forced carbonization, since the analysis of literary sources allows us to conclude that research in this area is at best at the stage of pilot industrial testing.
Thus, within the framework of current research, the aim of this work was to determine the fundamental possibility of processing steel-smelting slags into an artificial granulated filler for concrete. In this case, the formation of the physical and mechanical properties of raw granules is carried out due to their hardening in an environment of an increased concentration of carbon dioxide (forced carbonization).
2. Materials and Methods
Various steelmaking slags of metallurgical enterprises located in the Russian Federation were used as raw materials for scientific research, namely the following:
- ○
BOF slag from Cherepovets Steel Mill of Public Joint Stock Company “Severstal” (CherSM PJSC “Severstal”) with an initial particle size of 5–30 mm (hereinafter BOF slag);
- ○
Slag from the electric arc furnace of A.A. Ugarov Oskol Electrometallurgical Plant JSC. LLC MC Metalloinvest (Stary Oskol, Russia) with an initial particle size of 0.16–10 mm (hereinafter referred to as EAF slag).
A general view of the initial slags under study is presented in
Figure 1.
All slag samples were taken from the production shops of the above-mentioned enterprises, placed in hermetically sealed packaging and delivered to the laboratory for further studies. After the delivery to the laboratory, the slags were pre-dried to constant mass at a temperature of 95 °C (Memmert UF55, Büchenbach, Germany). The input control of the initial characteristics of the studied slags was carried out after drying and preliminary preparation (grinding). A chemical analysis of slags was conducted by X-ray fluorescence analysis (XRF) on an Epsilon 3XLE ED spectrometer (PANalytical, Eindhoven, The Netherlands).
To conduct research, all slag samples were pulverized in a drum ball mill with a capacity of 100 L (LBM 100, Saint Petersburg, Russia). The specific surface area of the obtained slag powders ranged from 280 to 310 m2/kg. The specific surface area was controlled by the air permeability method on an automatic Blaine apparatus model 1.0297E (Testing, Berlin, Germany). The granulometric composition of slag particles after pulverization was determined on a Partica LA-960 laser diffraction analyzer (Horiba, Kyoto, Japan).
A thermal analysis of the original slags, as well as the carbonized samples, was carried out using synchronous TG-DTA/DSC analysis on an STA 8000 high-temperature analyzer (Perkin Elmer, Waltham, MA, USA) in the temperature range of 30–1000 °C at a heating rate of 20 °C/min in a dynamic nitrogen environment. This analysis makes it possible to simultaneously record the change in heat flux and sample mass as a function of programmable temperature under a controlled atmosphere, which allows the temperature ranges of physicochemical transformations and phase transitions to be determined with high accuracy. The calculation of mass change on the TG curve was carried out in the Pyris (Version 11) program complex (Perkin Elmer).
An X-ray phase analysis of slags before and after carbonization was conducted on an “Ultima IV” high-resolution diffractometer (Rigaku, Tokyo, Japan) with a cobalt anode for the precise determination of the qualitative and quantitative phase composition of polycrystalline materials, including those with high iron content. Qualitative and semi-quantitative analyses were carried out using the PDXL program package (version 2) with the ICDD PDF2 database. The corundum number method (RIR) was used to determine the semi-quantitative ratio of each phase in the studied sample.
The scanning electron microscopy of the samples was performed on a Quattro ESEM scanning electron microscope (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an Octane Elite Plus (EDAX) energy-dispersive spectrometer (EDS). Images were captured using an Everhart–Thornley secondary electron detector (ETD) and a concentric semiconductor backscattered electron detector (CBS).
To obtain raw granules of artificial aggregate, the method of the granulation of the moistened finely ground powder of the studied slags was applied. The granulation of the obtained slag powders was carried out in a laboratory plate granulator. The granulator has a diameter of 500 mm and a side height of 50 mm; in the area of the disk where the granules are formed, the side height was increased to 150 mm. The rotation speed for granulation was 33 revolutions per minute with a 50° tilt angle of the bowl. The production of gravel granules was carried out as follows. A strictly weighted amount of dry slag powder was fed to the rotating disk of the granulator, onto which a small amount of water was sprayed from the sprayer to wet the particles and form initial nuclei. Subsequently, the granules were enlarged as a result of the layering process, during which the formed granules collided with the granulator plates. Granulation was carried out to obtain granules of a maximum size up to 20 mm. The granulation process is shown in
Figure 2.
The forced carbonation of the obtained granules was carried out in a laboratory carbonation chamber for 6 h in a gas–air environment with a CO2 concentration of about 80% vol.
The forced carbonization of the obtained granules was carried out in the carbonization unit designed by the authors with the automatic control and maintenance of the required CO
2 concentration in it. The carbonization of samples was carried out under normal conditions (~293 K, atmospheric pressure) at ~80% vol. of CO
2 concentration. The granules were placed on a perforated shelf in a uniform layer up to 60 mm high. The carbonation time was 6 h. A scheme of the developed chamber and its general view are presented in
Figure 3. The carbonization chamber is a metal container made of stainless steel with a hydraulic jacket and hermetically sealed lid and is capable of taking overpressure up to 1.0 MPa. There is a fan inside the chamber for mixing and a uniform distribution of carbon dioxide in the internal space. A vacuum pump is connected to the chamber to pump out air from the internal volume and create the required concentration of CO
2 in the chamber. The system of pressure regulation and carbon dioxide supply to the carbonization chamber includes a cylinder with carbon dioxide and a gas pressure reducer.
High-pressure liquid carbon dioxide in tanks was used as a source of carbon dioxide in the laboratory studies.
After the end of the carbonation time, the granules were dried to a constant weight, and their physico-mechanical characteristics were determined in accordance with national standards [
24,
25]. Tests of filler fractions (5–10, 10–20) for crushability were carried out in steel cylinders with an internal diameter of 75 and 150 mm and a height of 75 and 150 mm (RNPO Ruspribor, Saint Petersburg, Russia). Mechanical tests were carried out on a servo-hydraulic test system based on the MCC8 (Controls Group, Milan, Italy) control console with a maximum force of 300 kN.
Samples of heavy concrete using the obtained artificial carbonized gravel from the studied slags as a filler were made on the basis of Portland cement CEM I 42.5 N. The concrete composition was selected in accordance with the national standard [
26]. The preliminary consumption of the concrete components was carried out by the calculation method of absolute volumes. During the preparation of concrete mixtures, the bulk dosing of bulk components (Portland cement, sand, carbonized gravel) and volumetric dosing of water were performed. The amount of water was adjusted depending on the moisture content of the sand. The dosage error did not exceed 1%. The mixing of the concrete mixture was carried out in a laboratory mixer for concrete mixtures (LM-CB-10, Moscow, Russia). The mobility of the concrete mix was determined by the draft of the cone in accordance with the national standard [
27]. The compressive strength of concrete was determined on 10 cm cube samples in accordance with the national standard [
28]. The testing of prototype cubes was carried out on a servo-hydraulic testing system based on the MCC8 control console (Controls Group, Milan, Italy). Concrete samples were molded into cubes with a rib size of 100 mm in metal molds. The compaction of the cube samples was performed on a laboratory vibrating pad with an oscillation frequency of 3000 revolutions per minute and an oscillation amplitude of 0.5 mm for 10 s. After pre-exposure (2 h), some of the samples were subjected to heat and moisture treatment, and some were sent to the normal hardening chamber of concrete samples (CNH-48, Moscow, Russia) (temperature 20 ± 3 °C, relative humidity 95 ± 5%). The mode of the heat and moisture treatment of concrete samples was as follows: pre-exposure—2 h; temperature rise to 80 °C—6 h; isothermal exposure of samples at a temperature of 80 °C—7 h; temperature decrease—free. After the completion of the heat and moisture treatment, the concrete samples were also placed in a normal curing chamber (CNH-48). The compressive strength values of concrete samples were determined both after heat and moisture treatment and after normal hardening at the age of 7 and 28 days of hardening.
3. Results
The results of chemical analysis are presented in
Table 1.
As can be seen from
Table 1, the slags are based on the oxides CaO, SiO
2, Fe
2O
3, MgO and Al
2O
3. The content of other oxides is insignificant.
The granulometric composition of slag samples in the form of graphs of the integral and differential particle size distribution is shown in
Figure 4.
The geometric dimensions of the pulverized slags were 19–21 μm, and the numerical values of the fractional distribution are presented in
Table 2.
As can be seen from
Table 2, there is no significant difference in the particle size distribution.
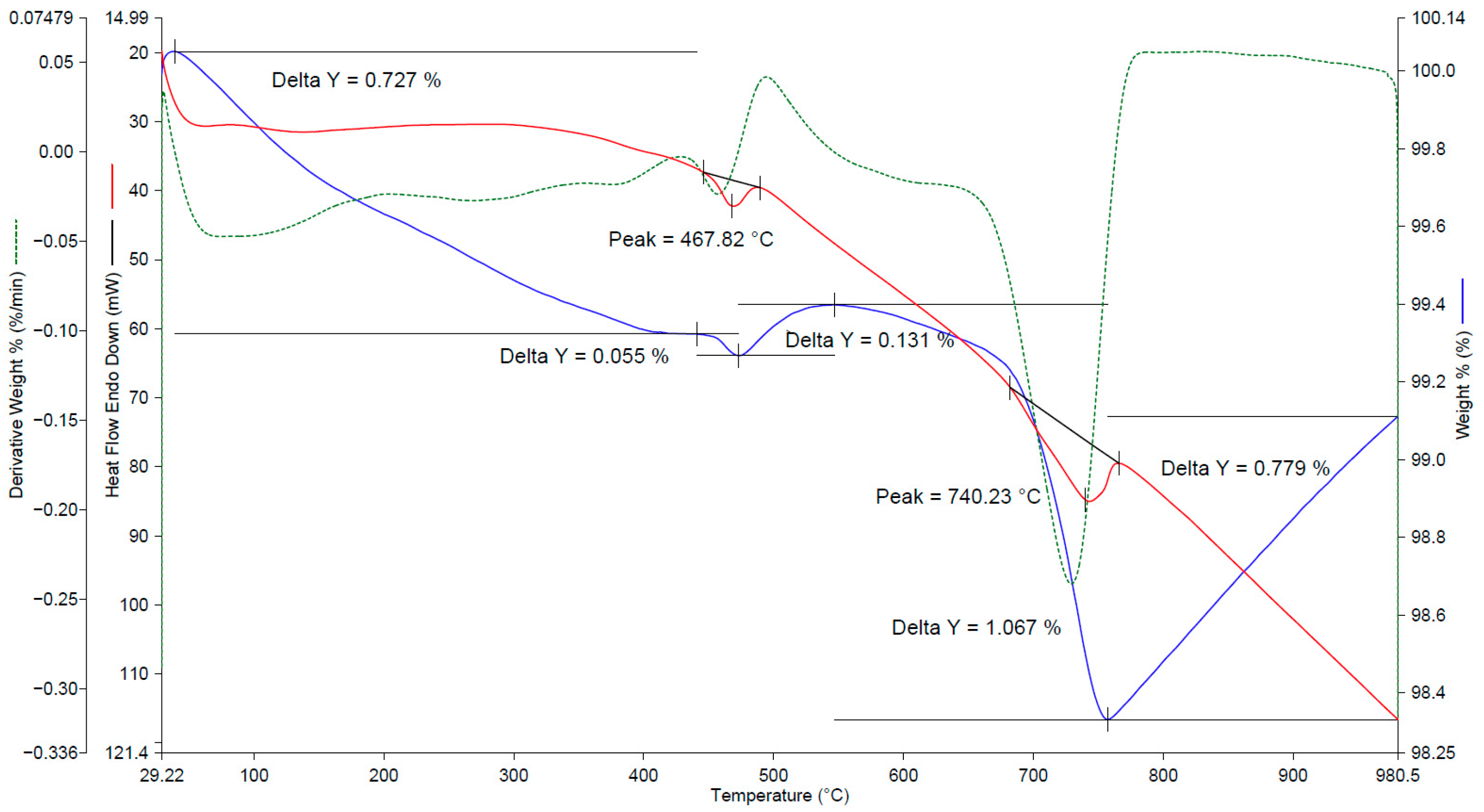
The results of the thermal analysis of the initial slags (non-carbonization) of BOF and EAF are presented in
Figure 5 and
Figure 6.
The thermogram of BOF slag (see
Figure 5) has two minor endothermic effects at temperatures of 468 and 740 °C. The endothermic effect in the temperature range of 450 to 560 °C, with a maximum at 467.8 °C, probably corresponds to the removal of chemically bound water, and the endothermic effect with a maximum at 740 °C with a mass loss of about 1.1% corresponds to the decomposition of calcium carbonate. It is notable that the mass of the sample increases above 450 °C and 750 °C. Apparently, under the influence of temperature, phase transformations occur in the sample, accompanied by a change in the crystal lattice and a possible change in mass. The total mass loss of the sample in the range of 30–780 °C was 1.7%.
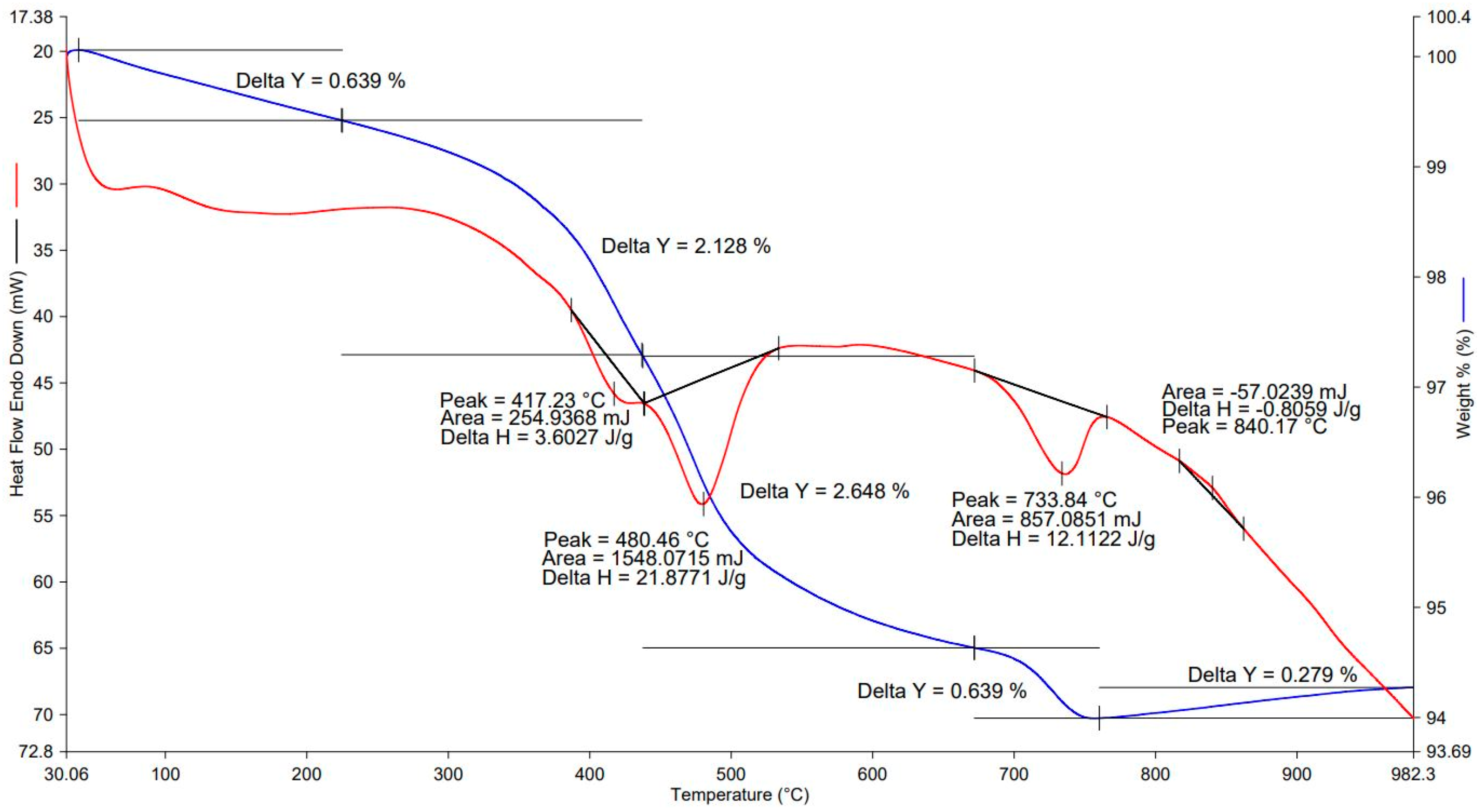
The thermogram of EAF slag (see
Figure 6) identifies endothermic mass loss effects at the following temperatures: 417, 480 and 734 °C. The endothermic effects at 417 and 480 °C are caused by the removal of chemically bound water. The endothermic effect with a maximum at 734 °C corresponds to the decomposition of calcium carbonate. The slight exothermic effect at 840 °C is probably related to the phase transition of dehydrated calcium silicates into wollastonite. The total mass loss of the sample in the range of 30–780 °C was 6.1%.
Table 3 presents the results of the X-ray phase analysis in the form of the minerals that make up the basis of the studied slags.
According to the XRD analysis data, the examined slags are represented by different minerals, which are formed in the melt depending on the technology of steel production. Thus, BOF slag is mainly represented by okermanite and belite of γ modification (shannonite). EAF slag is mainly represented by mervinite, belite γ modification (shannonite) and gedenbergite and fayalite.
The average granulation time of slags on a plate granulator ranged from 1 to 1.5 min until the moment of the spontaneous collision of the formed granules from the rotating disk of the disk granulator. The granules removed from the granulator had sufficient strength to allow the granules to be poured and classified on sieves. The moisture content of raw pellets from EAF and BOF slag before carbonation was 11.8 and 5.9% by weight, respectively.
The moisture content of granules after forced carbonization based on EAF and BOF slag, respectively, was 7.4 and 3.3% by weight. A general view of carbonized gravel from BOF and EAF sample slags is shown in
Figure 7.
Gravel is a granular material made of well-formed spherical granules. The grain compositions of gravel are similar and consist mainly of grains of fractions 5–10 and 10–20 mm, the total content of which is 90.3% by weight in gravel from EAF slag and 89% by weight in gravel from BOF slag.
For each type of slag, three batches of forced carbonization were carried out, and specific tests were carried out for each batch of granules. The arithmetic mean test results obtained from three batches of gravel samples from EAF and BOF steelmaking slags are presented in
Table 4. The standard deviations of the indicators in the tests conducted did not exceed 5%.
The key indicator characterizing the qualitative course of the carbonization reaction in granules is the loss of mass during the testing of carbonized granules for crushing. This indicator indirectly characterizes the mechanical strength of the granules. Accordingly, the lower the mass loss rate during the test, the stronger the granules.
As can be seen from the data in
Table 4, the strength of grains of fraction 5–10 is higher than the strength of grains of fraction 10–20, which is associated with a higher degree of carbonation of small grains. At the same time, the mass loss values obtained when testing fractions 5–10 correspond to similar indicators when testing natural igneous rocks. For example, comparative tests of the crushability of granulated filler of fraction 5–10 with natural diabases of the Crimean deposit (also fraction 5–10) showed that the mass loss of natural diabase filler was 10.7% by weight, comparable with the indicator for carbonized gravel based on BOF slag (13.6% by weight). The mass loss during crushability testing for fraction 10–20 is significantly higher, which indicates the lower mechanical strength of larger granules and, accordingly, a worse carbonization process.
Figure 8 shows a cross-section of a concrete sample with the resulting carbonized aggregate. The cut shows that some large granules have a central zone where the carbonation reaction did not take place, and consequently the strength of such granules will be lower.
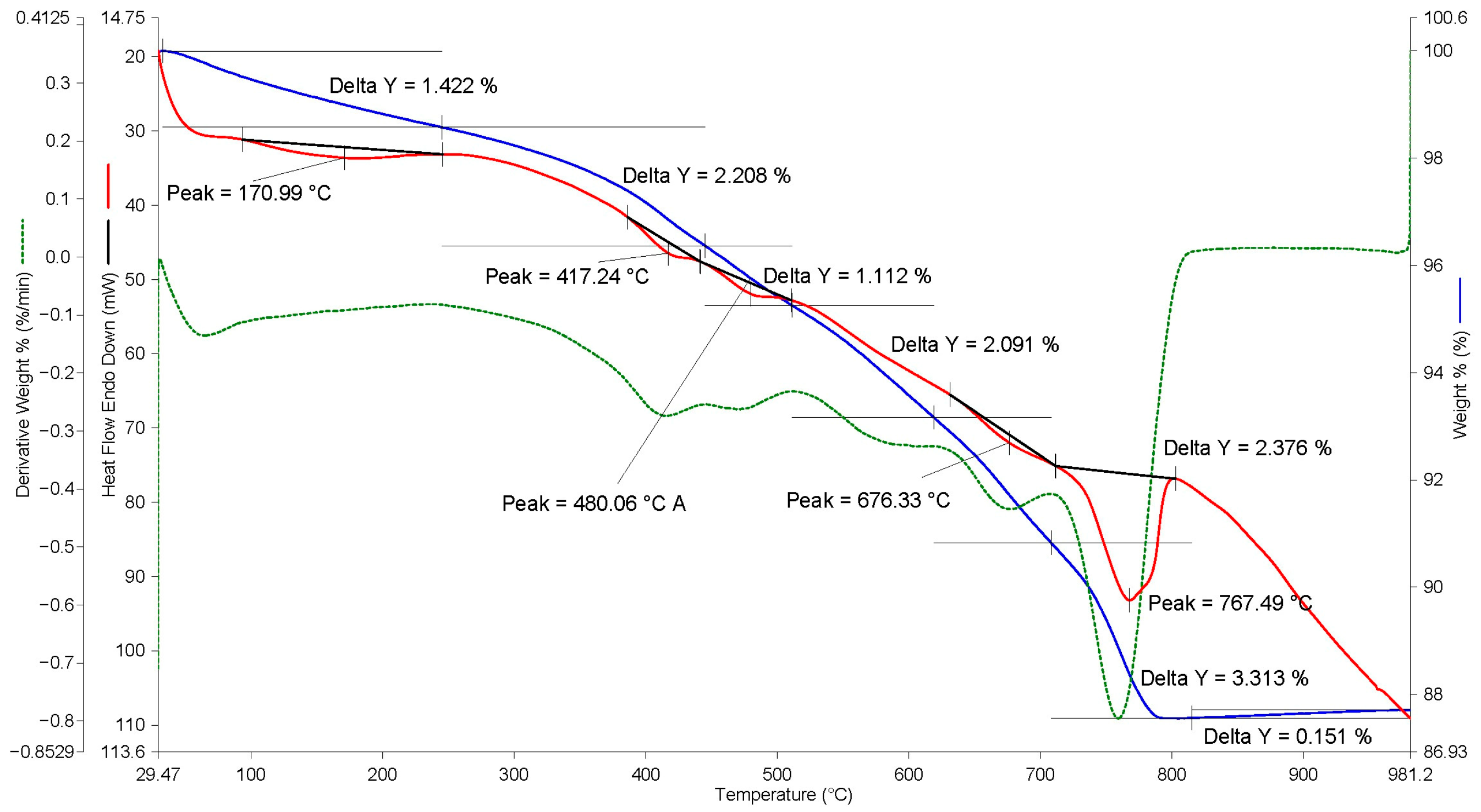
A thermal analysis of the obtained carbonized granules was performed for a quantitative assessment of the bound CO
2. In this case, a granule with a diameter of 20 mm was taken for analysis, and samples were taken from the outer layer up to 4 mm deep, as well as from the center of the granule. In this way, the depth of carbonization of the granules, noticeable on the granules cut presented in
Figure 8, was compared. The results of the analysis are presented in
Figure 9,
Figure 10,
Figure 11 and
Figure 12.
A comprehensive analysis of the presented thermograms shows that, regardless of the depth of sample extraction from the granule (outer layer, center), the TG and DSC curves are similar and have two zones of mass reduction, accompanied by certain endothermic effects. The first zone is in the temperature range of 30–500 °C and corresponds to the removal of chemically bound water from hydrate compounds. The second zone of mass reduction is, on average, in the temperature range of 500–850 °C and corresponds to the decomposition of newly formed CaCO
3. Thus, for BOF slag, the total mass loss in the temperature range of 500–850 °C was 5.2% by weight (see
Figure 9), in contrast to 1.07% by weight for this slag without carbonization (see
Figure 5). This indicator characterizes the quantitative content of bound CO
2. Moreover, in the center of the BOF slag granule, the total mass loss in the temperature range of 500–850 °C was 3.6% by weight (see
Figure 10), which confirms the worse progress of the carbonization reaction in the center of granules larger than 10 mm.
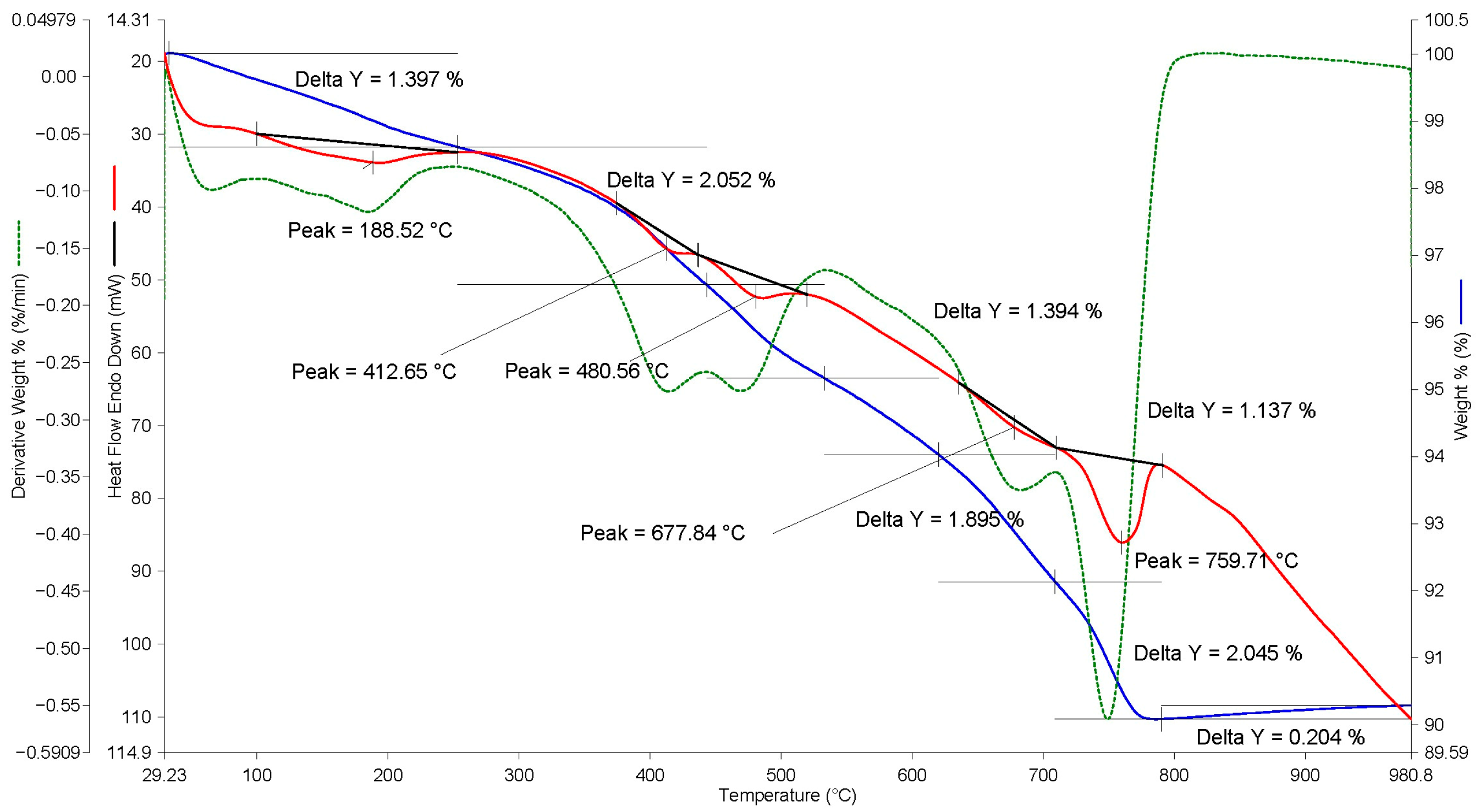
Similar results are shown by the analysis of thermograms based on EAF slag (see
Figure 11 and
Figure 12). Thus, the total mass loss in the temperature range of 500–850 °C was 7.8% by weight (see
Figure 11), in contrast to 0.64% by weight for this slag without carbonization (see
Figure 6). At the same time, in the center of the EAF slag granule, the total mass loss in the temperature range of 500–850 °C was 5.1% by weight (see
Figure 12), which also confirms the worse course of the carbonization reaction in the center of granules larger than 10 mm.
The results of thermal analysis are in full agreement with the mass loss data during crushability testing (see
Table 4) and explain the greater mass loss by the 10–20 mm fraction compared to the 5–10 mm fraction. In filler grains smaller than 10 mm, more uniform carbonization is observed over the entire cross-section of the granule, which is accompanied by the greater mechanical strength of the carbonized granule material and, accordingly, less mass loss.
The scanning electron microscopy results of the carbonized granules from BOF and EAF slags, as well as the point elemental analysis, are shown in
Figure 13 and
Figure 14. The SEM analysis of samples taken from the granules based on different slags showed differences in the size, morphology and density of the new formations. The size of the new formations (CaCO
3) in the BOF slag granules is smaller than in the EAF slag granules (see
Figure 13a and
Figure 14a). The newly formed calcium carbonate in the BOF slag granules is a uniformly distributed mass of submicron crystals (no more than 1 μm) that do not have clear morphological features. An analysis of the energy spectra confirms the presence of calcium carbonate among the larger original BOF slag particles.
A comparison of microstructures showed that, in contrast to the new formations in the granule based on BOF slag, the new formations in the granule based on EAF slag are more ordered (see
Figure 14a), with a clearer morphology in the form of needle-shaped calcium carbonate crystals of larger sizes (2–4 μm in length) being distinguished. The data presented in
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13 and
Figure 14 are in complete agreement with the results of [
29], in terms of the chemical reaction of carbonization and the formation of carbonate formations with binding properties.
In general, the test results in
Table 4 show that gravel samples of fractions 5–10 and 10–20 obtained from the studied EAF and BOF slags are suitable for use as aggregates for heavy concrete and other types of construction work according to their basic physical and mechanical characteristics.
To test the possibility of using the obtained gravel as a filler for heavy concrete, two series of samples were made—cubes with a rib size of 100 mm from heavy concrete on Portland cement and gravel samples from steelmaking slag samples EAF and BOF. The compositions of concrete mixtures were selected, taking into account the production of concrete of compressive strength class B25.
The consumption of components calculated for the production of 1 m3 of concrete mixture is as follows: Portland cement—290 kg; carbonized gravel from the studied slags—580 kg; quartz sand—320 kg; screening diorite fractions 1–4 mm—580 kg; water—230 L. The mobility of the concrete mixture according to the slump of the cone for the specified concrete composition was 14 cm. For testing, 24 concrete cube samples were prepared. A total of 12 samples were placed in a normal hardening chamber, and the remaining 12 samples were subjected to heat and moisture treatment, after which they were also placed in a normal hardening chamber. At 7 and 28 days of hardening, six cube samples from each batch were tested. The standard deviation in the determined indicators did not exceed 5%.
The results of the tests of experimental concrete cube samples based on artificial carbonized gravel are presented in
Table 5.
As can be seen from the test results of the cube samples, it is possible to obtain class B25 concretes based on artificial carbonized gravel from the studied slags. Gravel-based concrete from BOF slag has higher density and compressive strength compared to gravel-based concrete samples from EAF slag, which is explained by the better physical and mechanical properties of gravel from BOF slag (see
Table 4 and
Table 5 together). An increase in the compressive strength of concrete is also observed depending on the curing time, regardless of the type of slag used to make the aggregates. Therefore, the use of carbonated aggregate does not disrupt the cement hydration mechanism or the interaction of carbonated granules with the cement matrix, resulting in the achievement of the design grade of concrete (in this case, B25) at the design age (28 days). Accordingly, the use of this aggregate does not interfere with the calculation methodology for designing heavy-duty concrete compositions with specified properties.
The structure of concrete samples based on carbonized gravel (see
Figure 7) is homogeneous, and the gravel grains are evenly distributed in the mortar part of the concrete. A carbonized layer in gravel grains is clearly visible on a cross-section of concrete with gravel from BOF slag (see
Figure 7a). It can be seen that some grains have a minimal layer, and some grains are uniformly carbonized throughout, while both large and small gravel grains have a minimal layer; similarly, both large and small granules are completely carbonized. That is, the granules are carbonized unevenly, most likely due to the way they are carbonized in a fixed multi-tiered layer. The granules deposited on the surface of the layer are carbonized to a greater extent, while the granules in the lower layers are waterlogged due to the water released during the carbonization reaction, and they carbonize to a lesser extent. Thus, the forced carbonation of artificial gravel should be carried out in movable layers, ensuring the uniform contact of granules with a CO
2 gas medium, for example, in rotating drums or fluidized bed apparatuses. It can also be noted that the disintegration processes of granules (silicate, calcareous, magnesian, ferruginous) inherent in the studied steelmaking slags are not observed on sawn cube samples at the age of 28 days. Accordingly, at this stage of research, it can be indirectly argued that forced carbonation is a way to stabilize these slags against various types of decomposition characteristic of this secondary raw material, especially silicate.
The obtained preliminary results of experimental studies confirm the possibility of obtaining high-quality artificial gravel from steelmaking slags, suitable for use as aggregates for heavy concrete, road construction and other types of construction work.