Optical and Thermal Studies, Isothermal Crystallization Kinetics and Mechanical Properties of Poly(lactic acid) Nanocomposites Based on Hybrid Lignin/MWCNT Nanomaterial
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA Lignin-Based (Nano)Composites with Solution Casting Followed by Melt Mixing
Film Preparation
2.3. Characterization Techniques
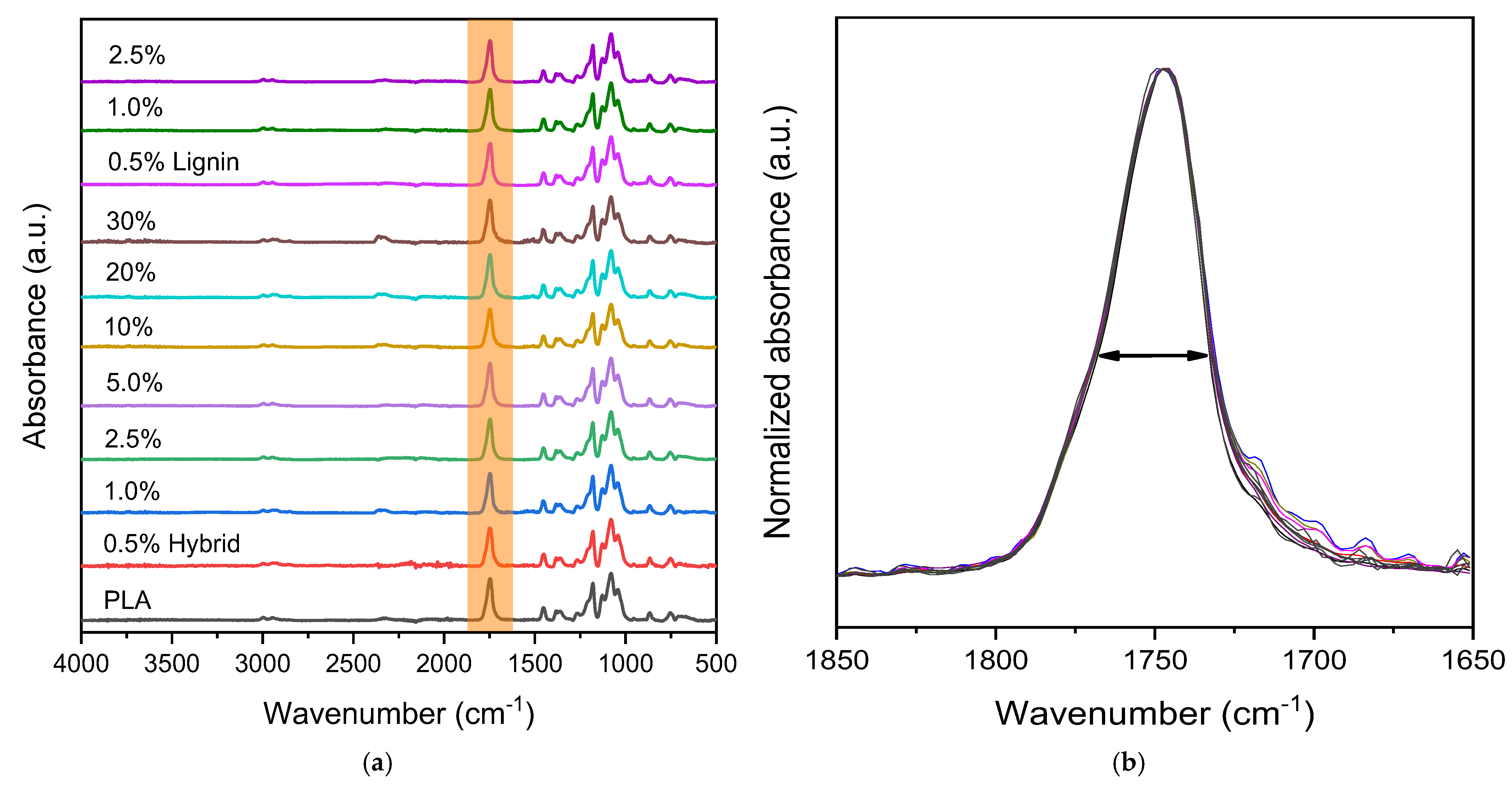
2.3.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
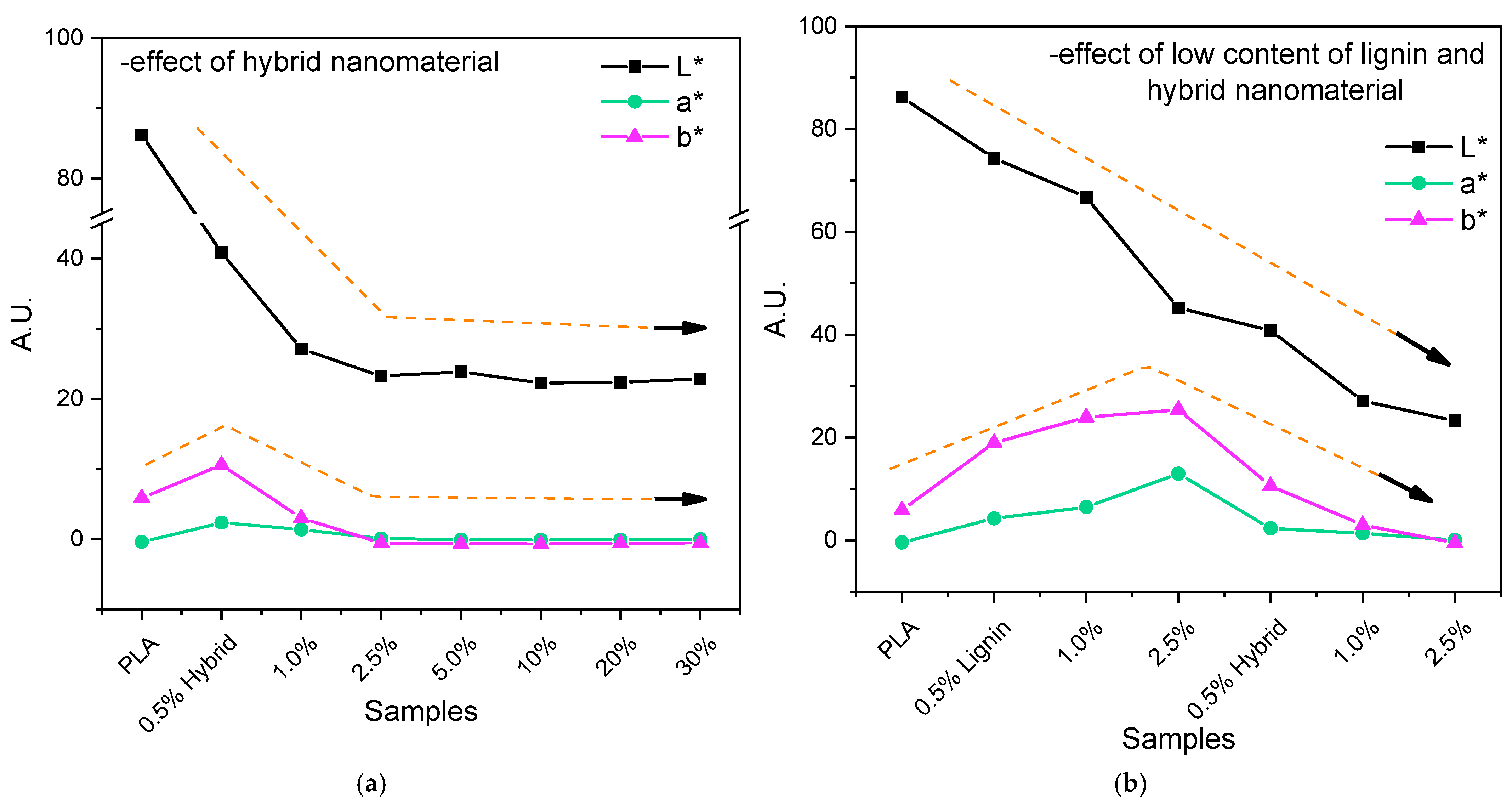
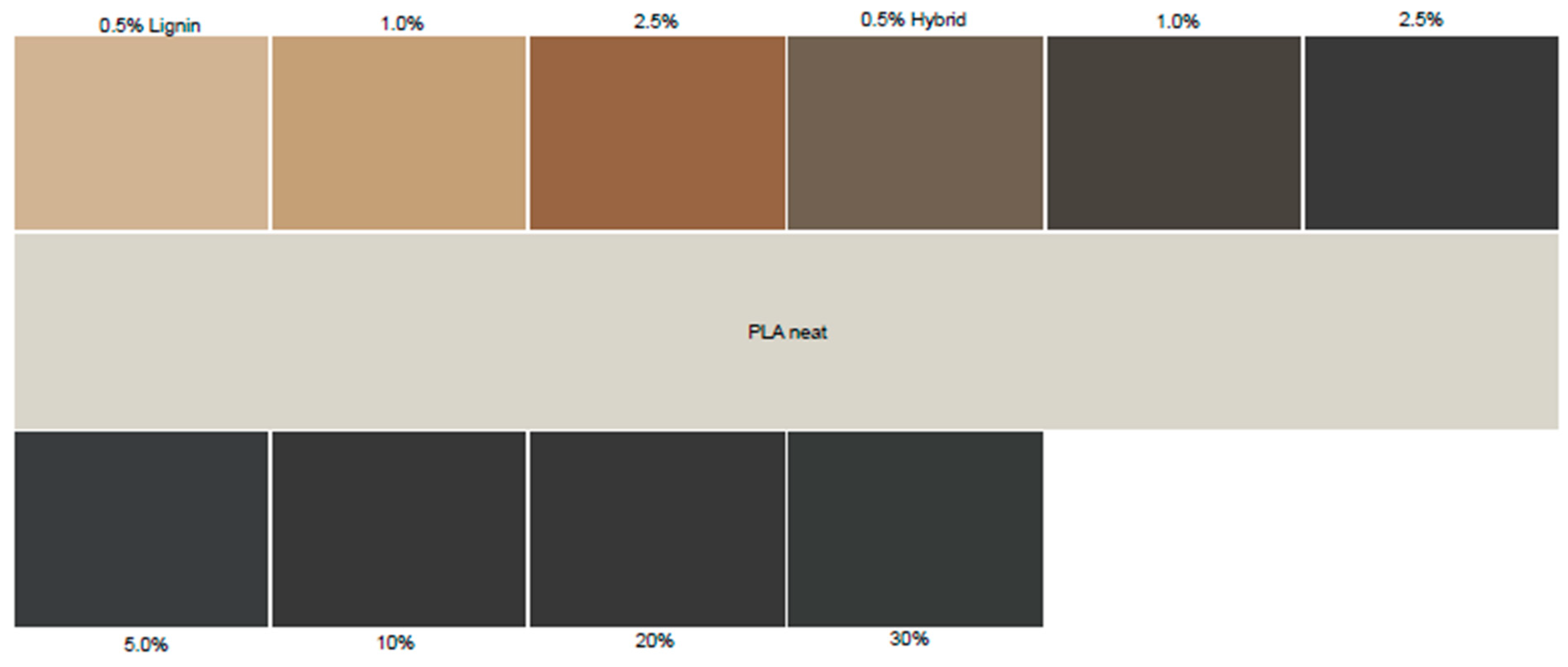
2.3.2. Color Measurements
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. X-Ray Diffraction (XRD)
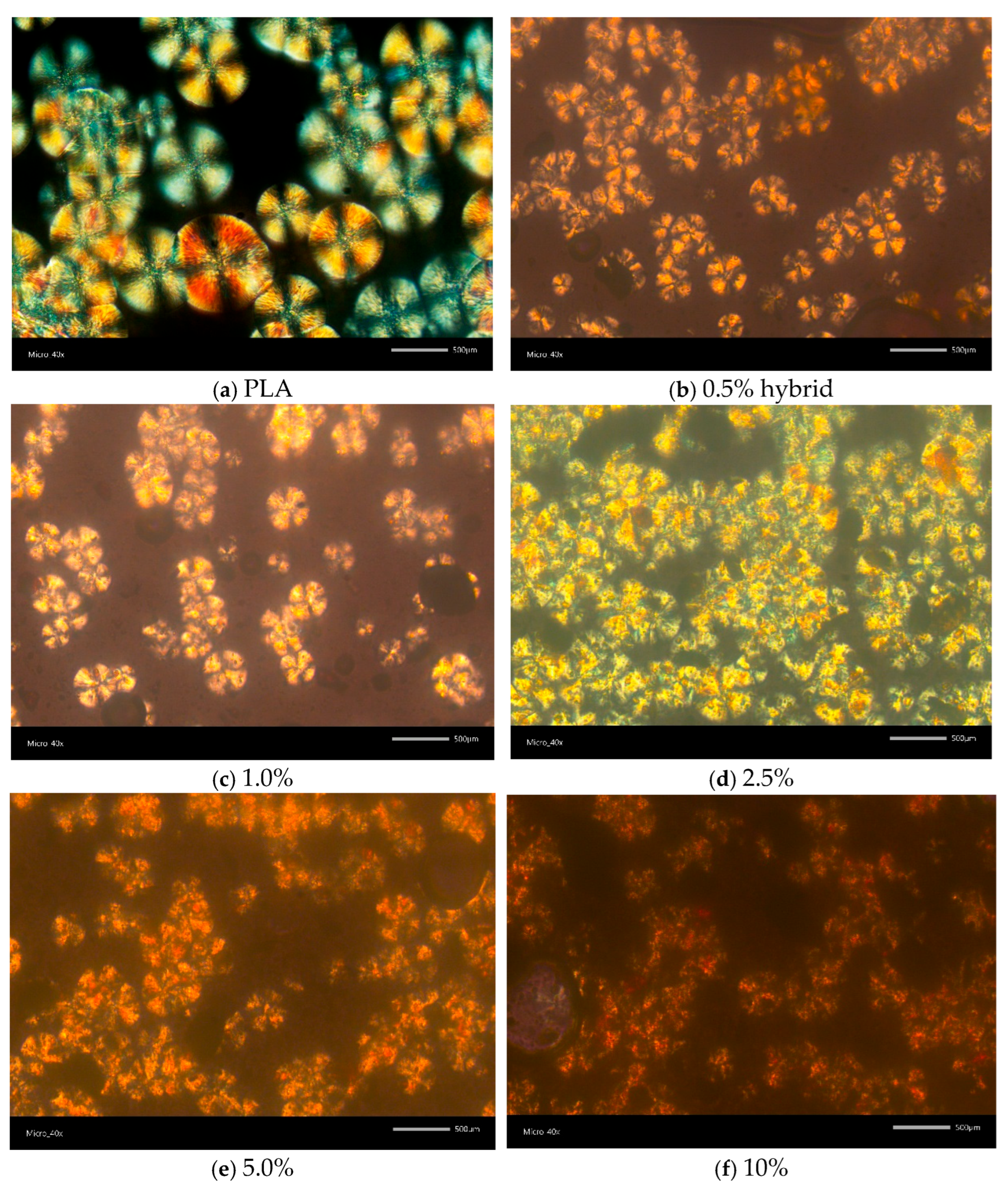
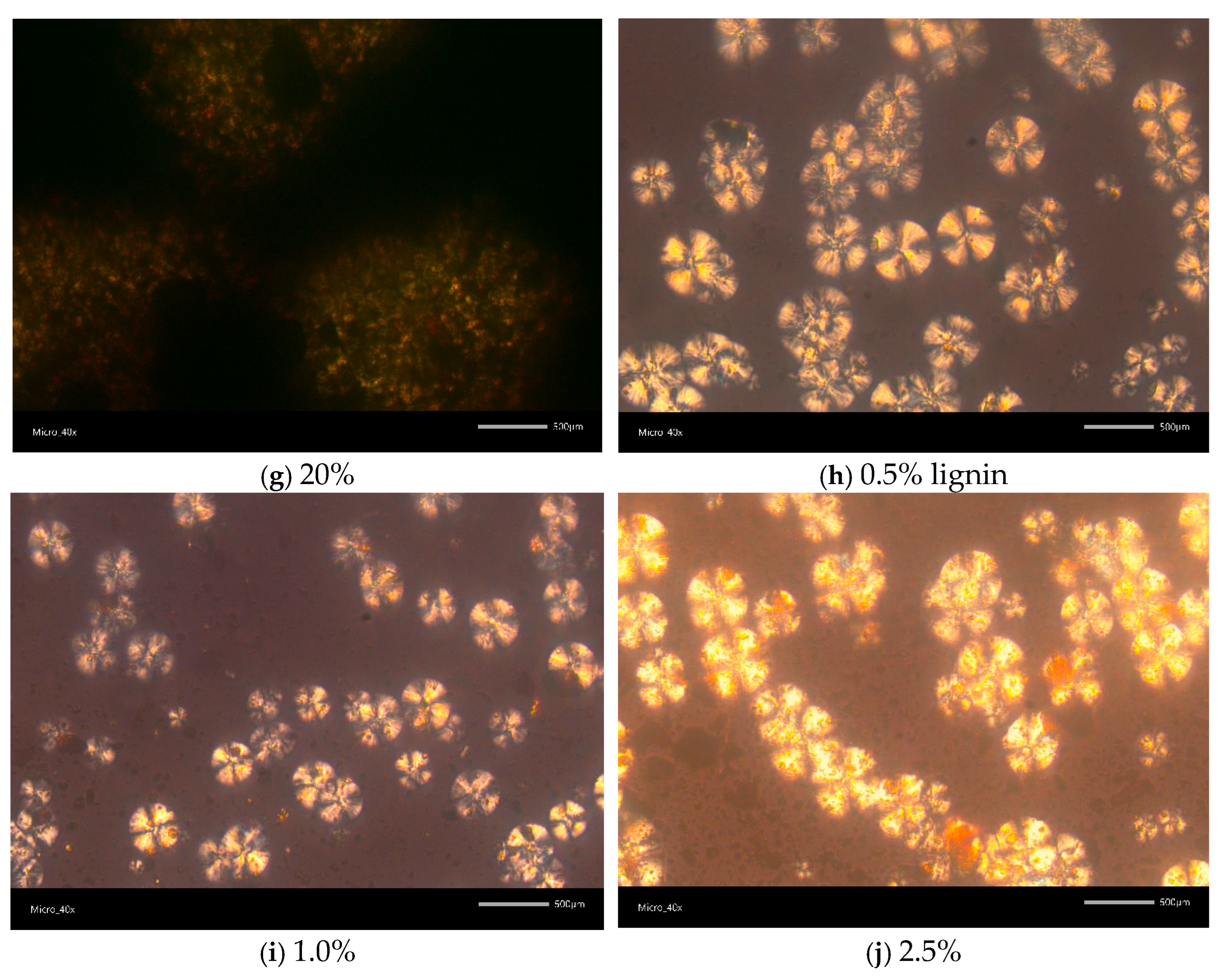
2.3.5. Polarized Light Microscopy (PLM)
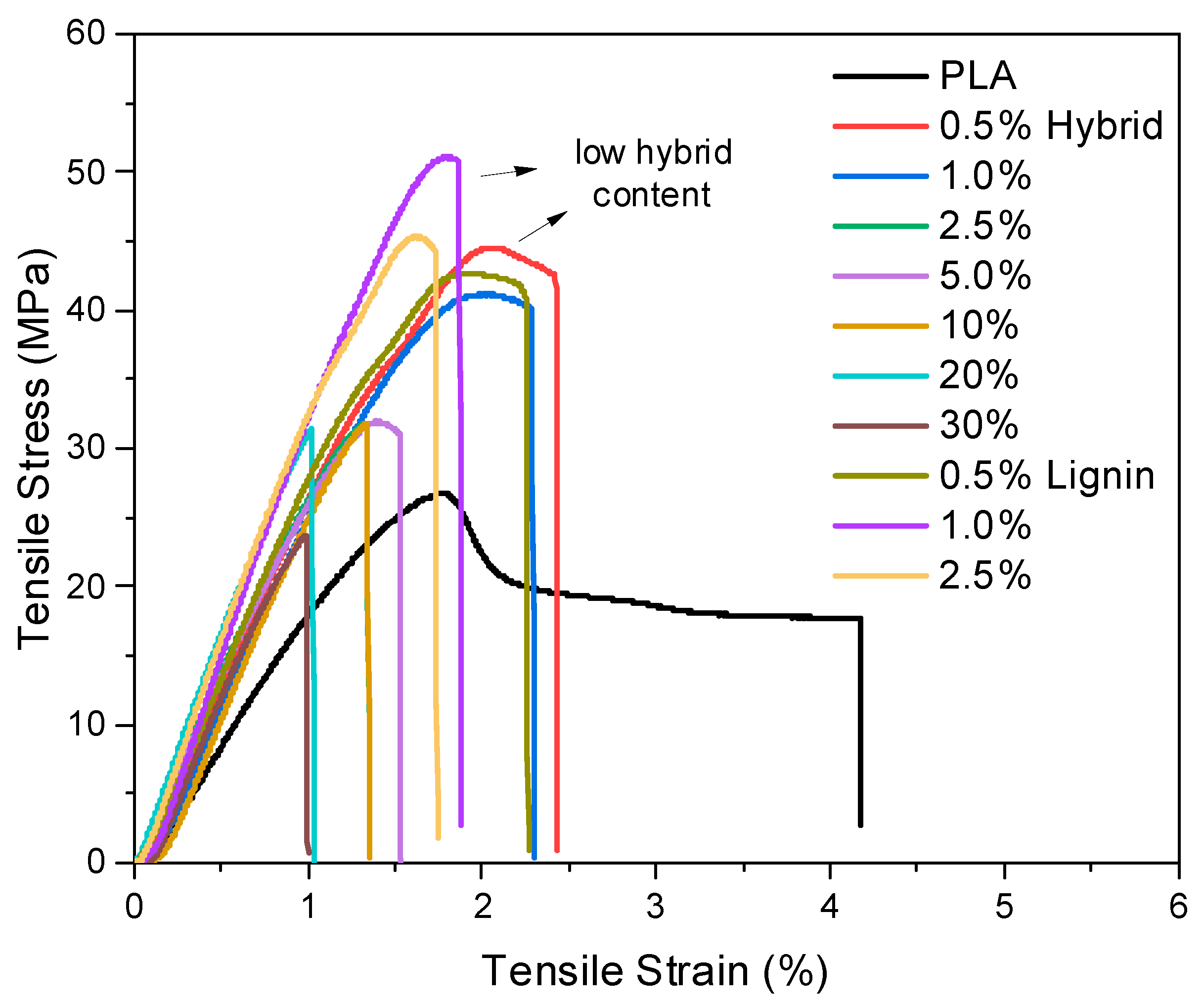
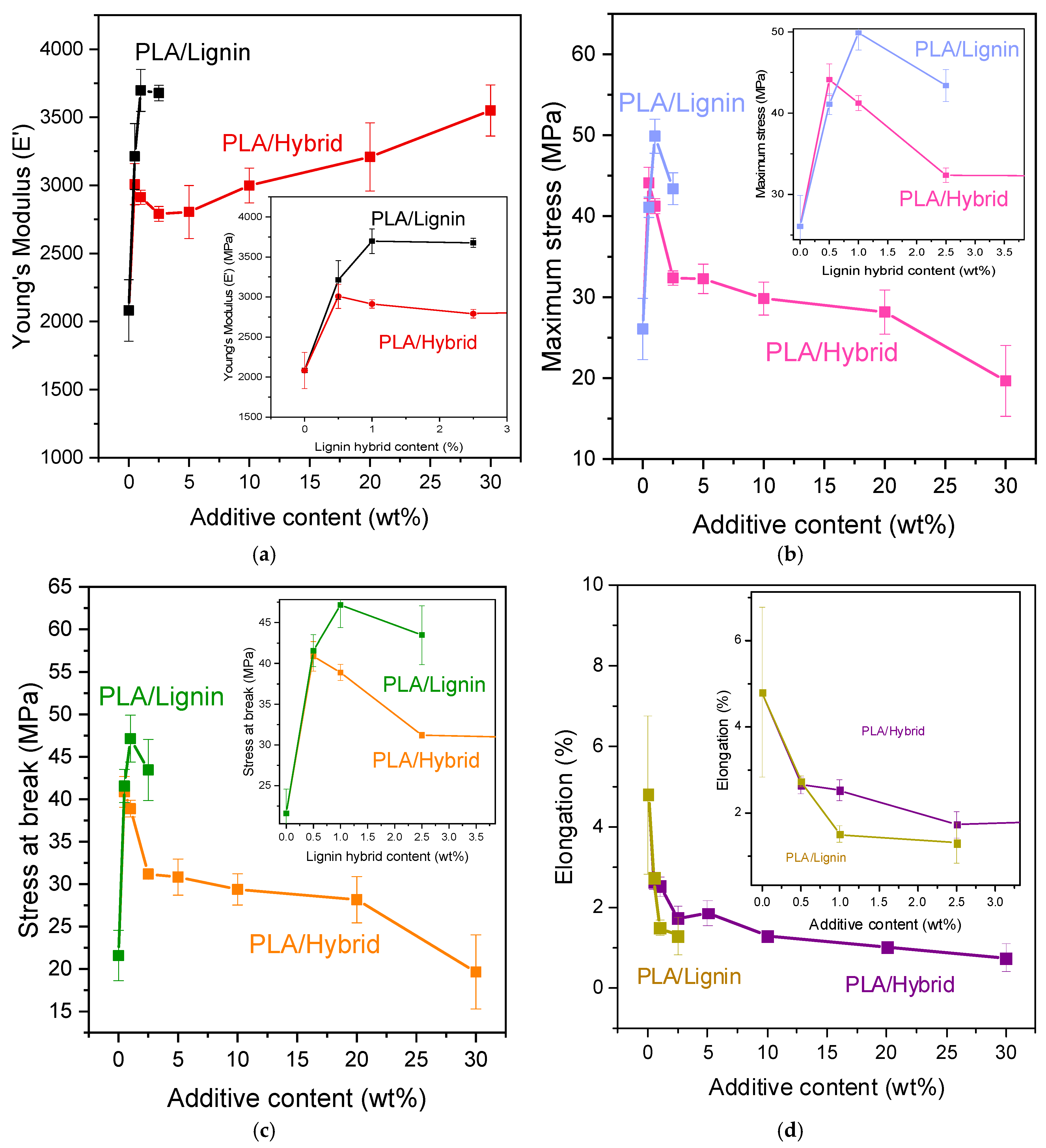
2.3.6. Mechanical Properties
2.3.7. Contact Angle
3. Results and Discussion
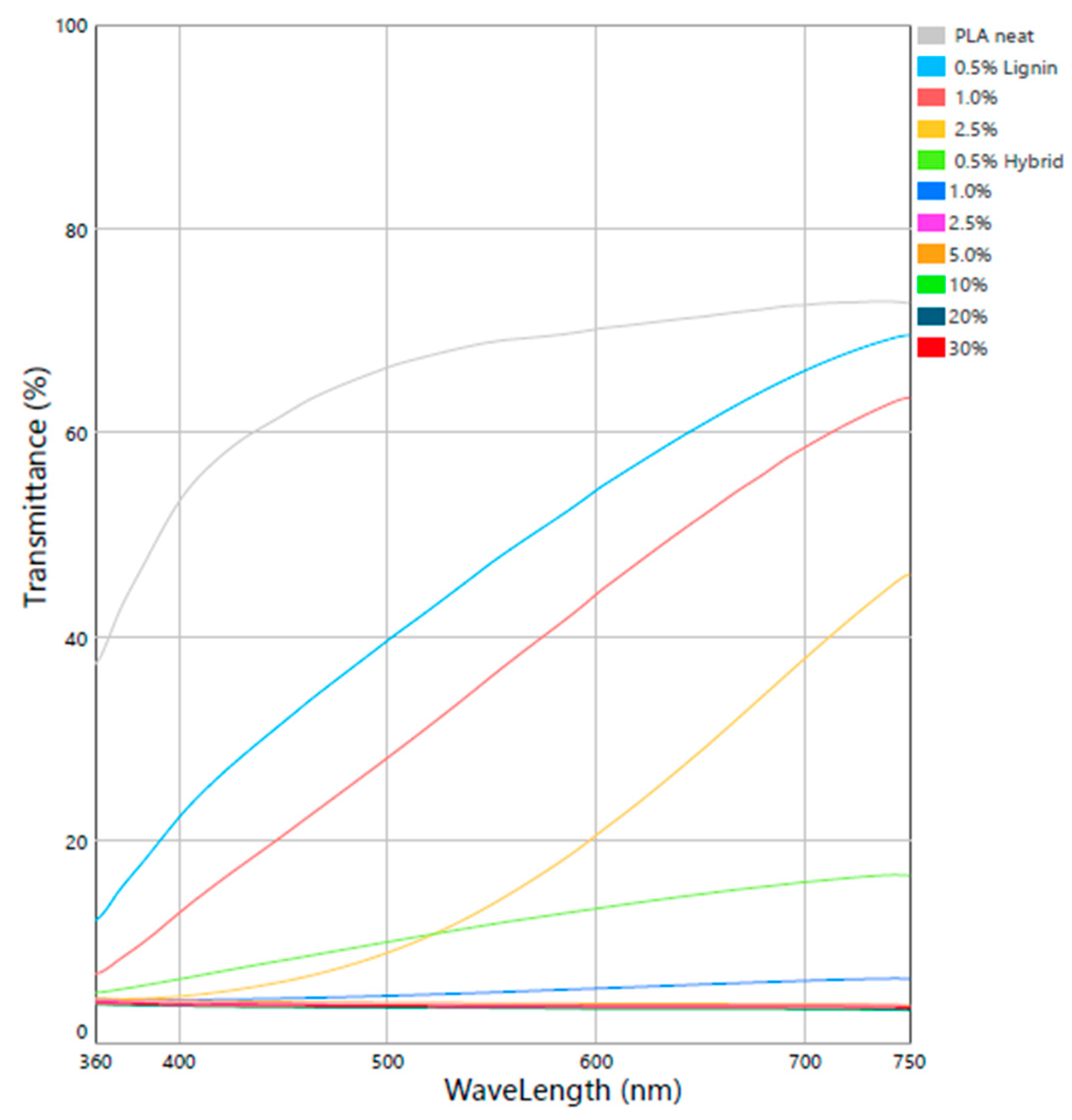
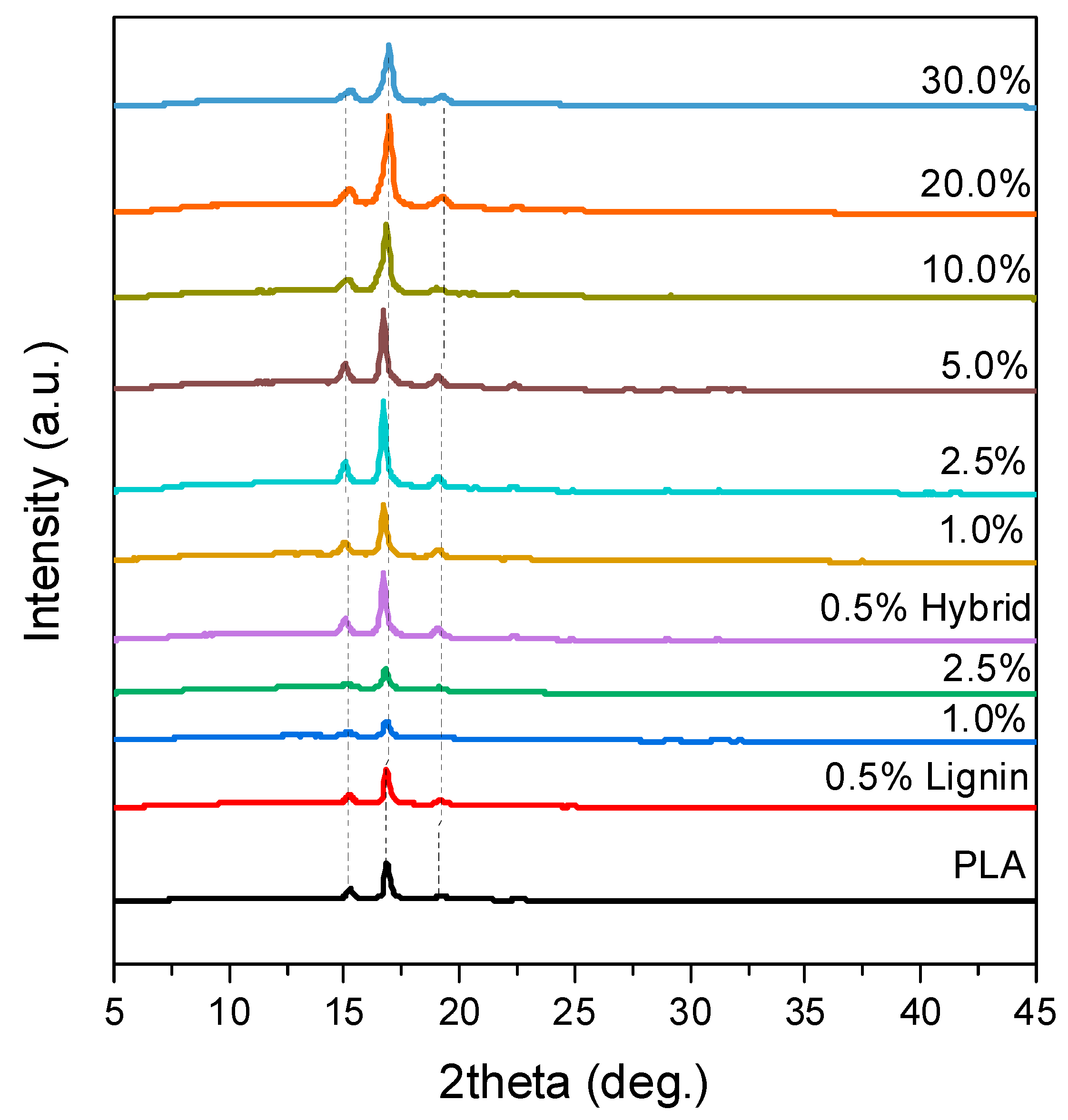
3.1. Structural and Optical Properties
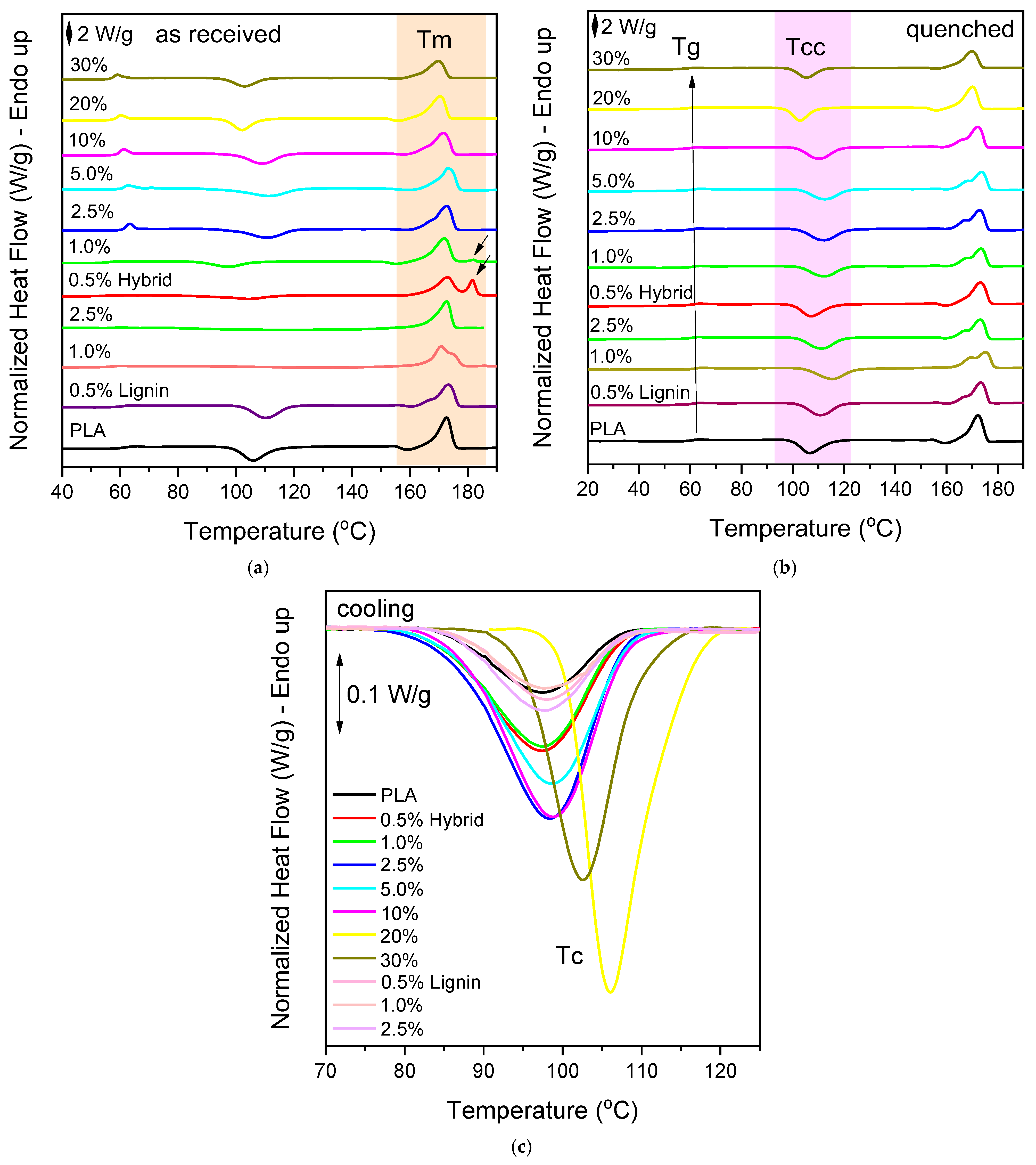
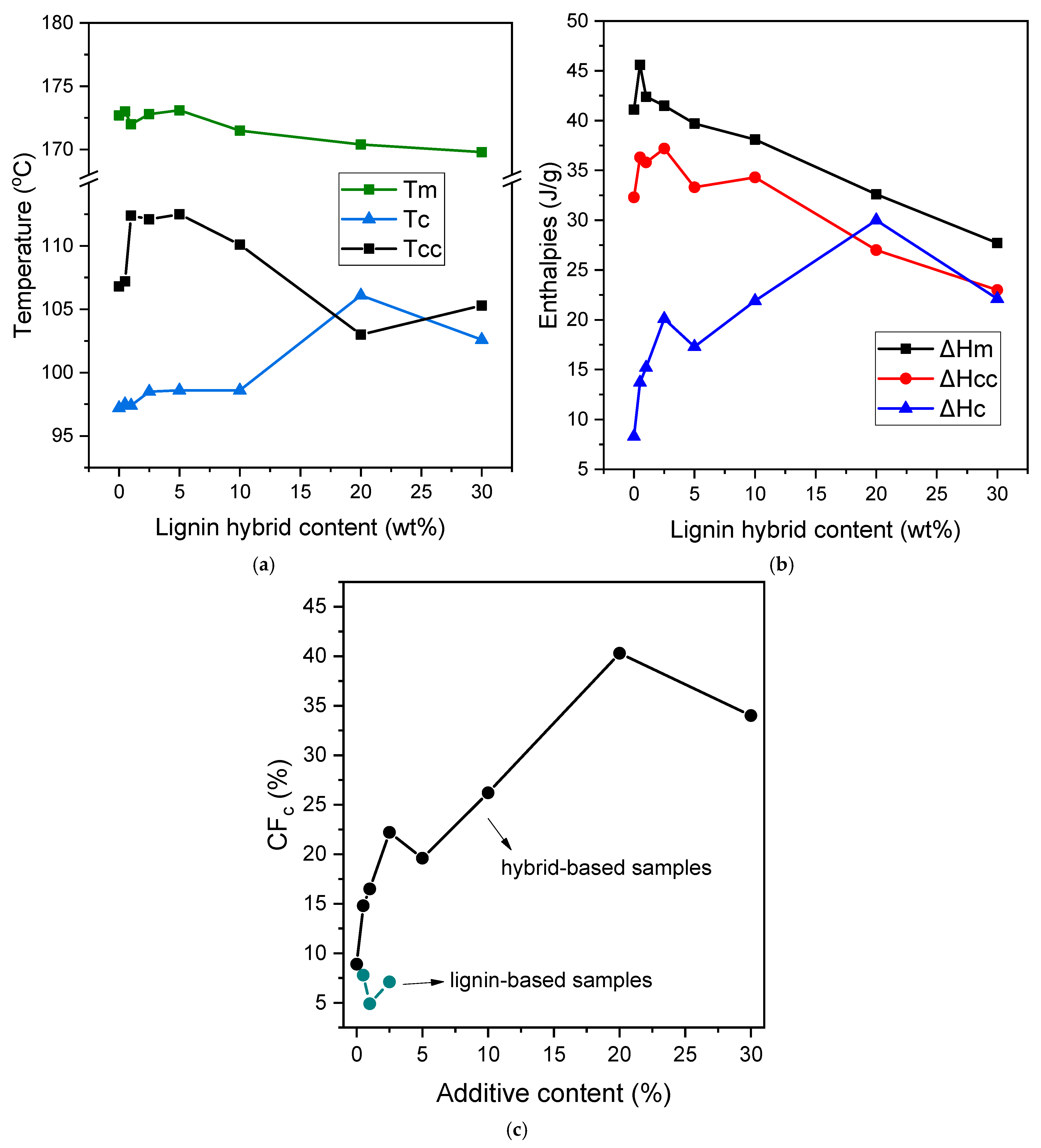
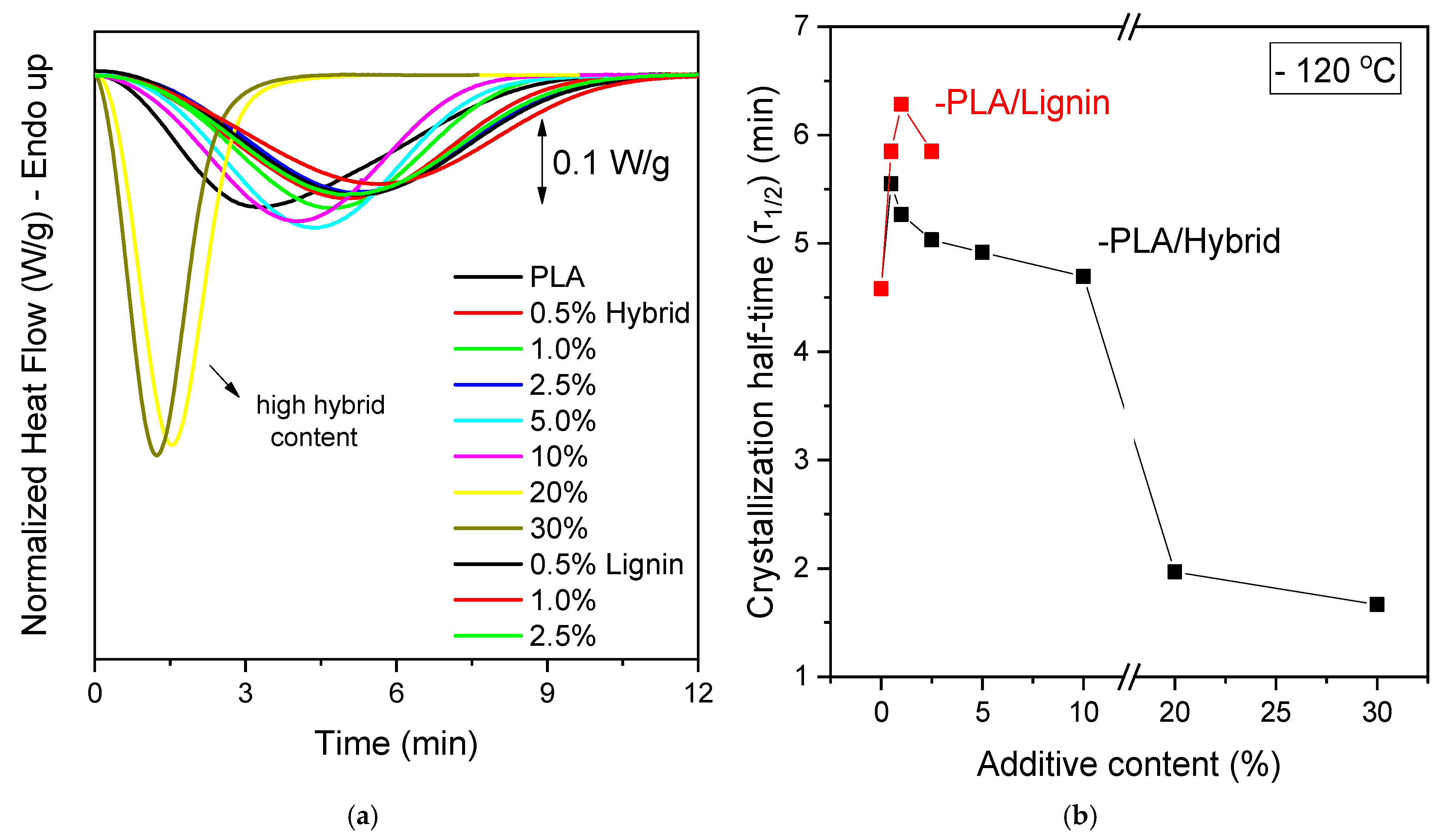
3.2. Thermal Properties, Crystallinine Behavior, and Isothermal Melt Crystallization
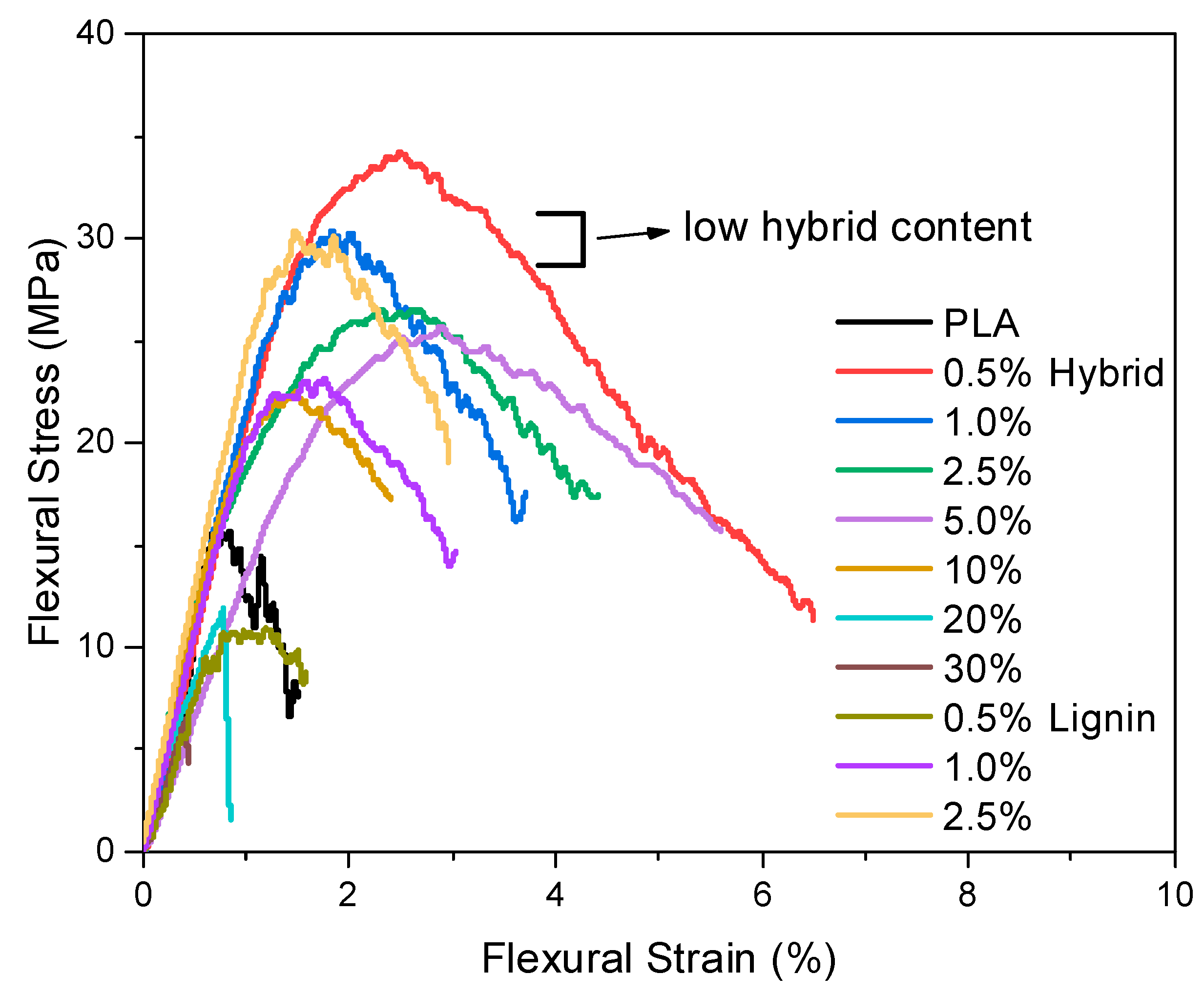
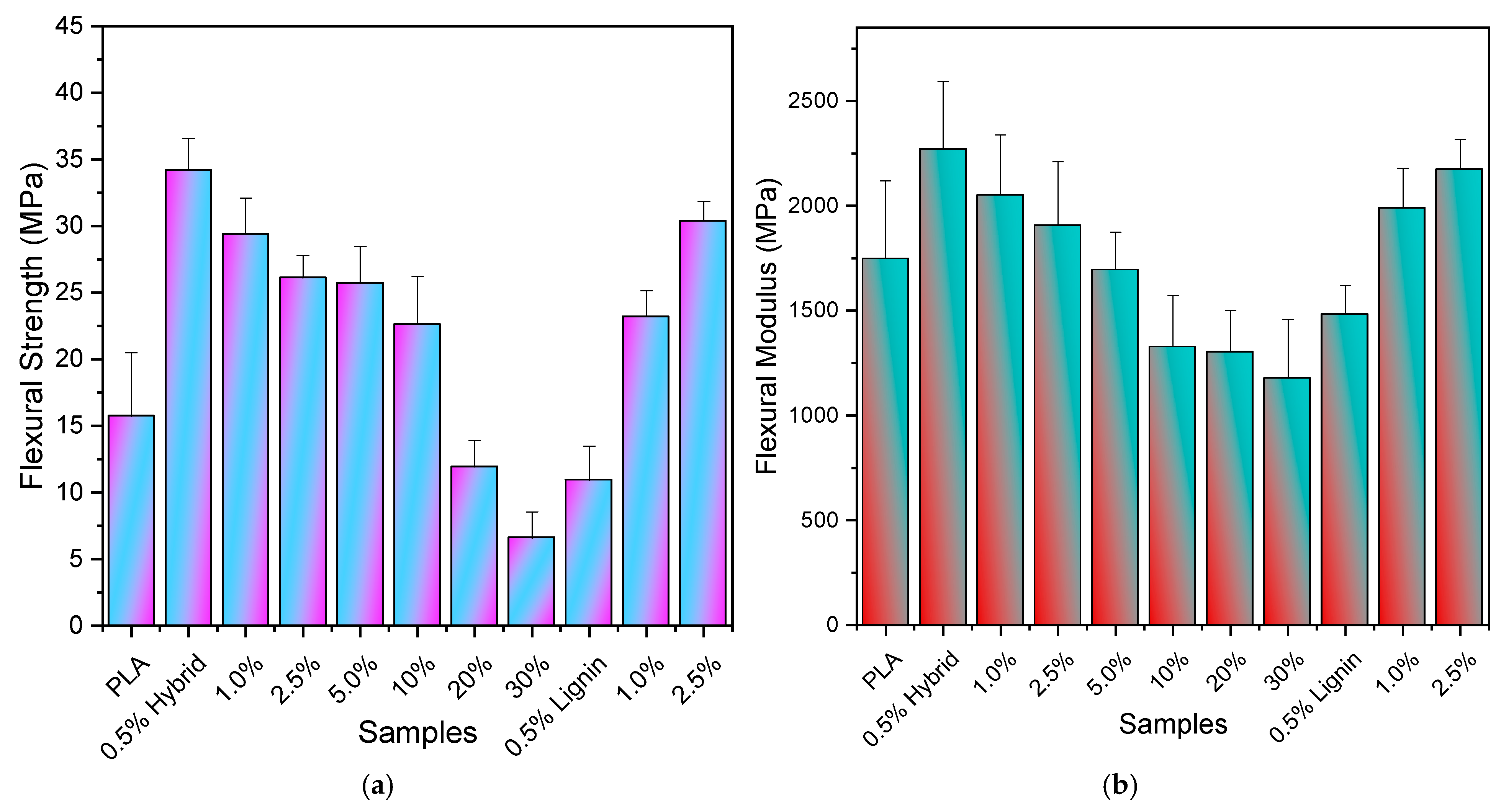
3.3. Mechanical Evaluation via Tensile and 3-Point Bending Tests
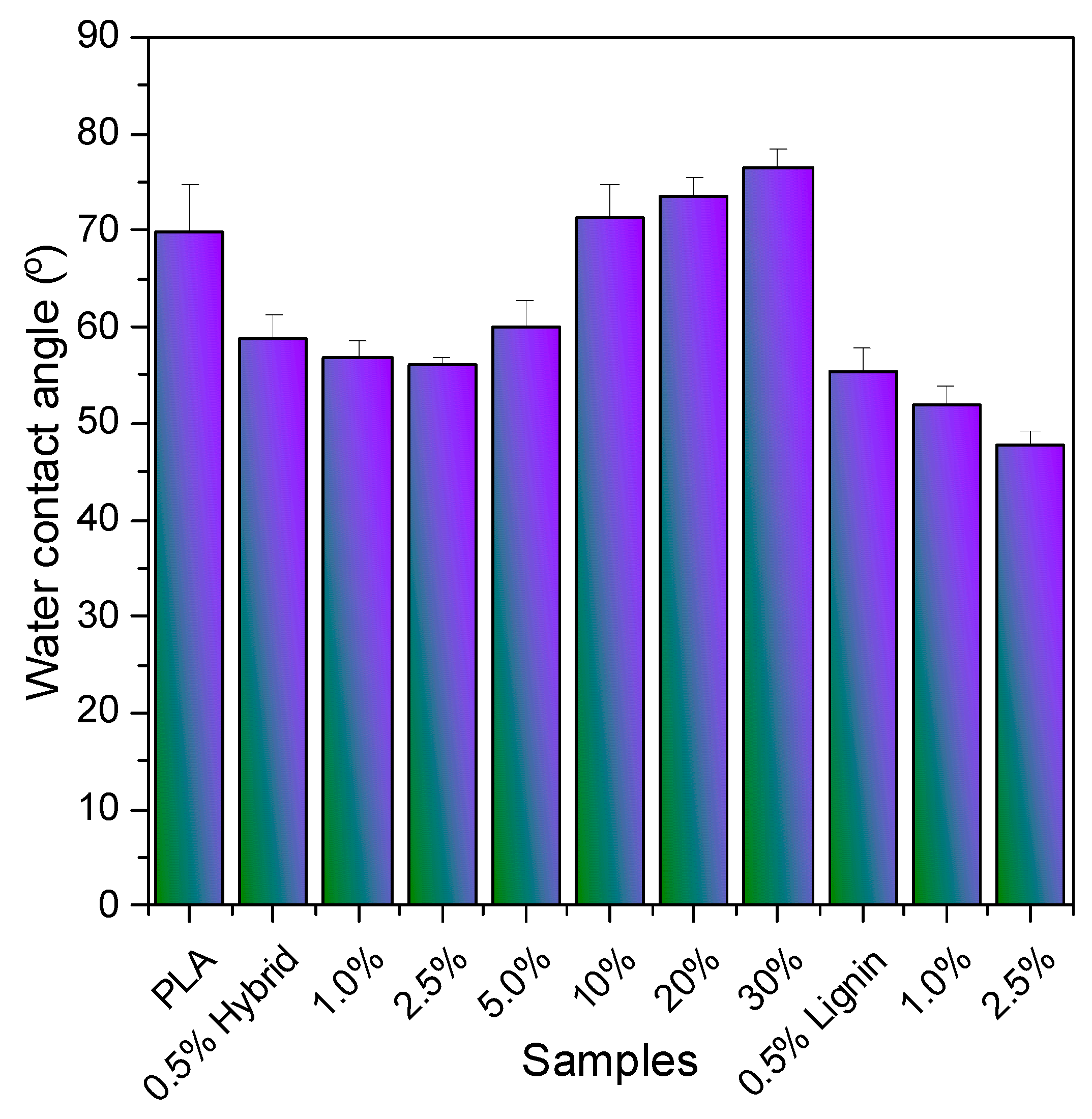
3.4. Surface Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Terzopoulou, Ζ.; Bikiaris, D.N. Biobased plastics for the transition to a circular economy. Mater. Lett. 2024, 362, 136174. [Google Scholar] [CrossRef]
- Mohammad, R.S.; Vivek Kumar, V. Impact of plastic pollution on ecosystems: A review of adverse effects and sustainable solutions. Environ. Monit. Assess. 2025, 197. [Google Scholar] [CrossRef]
- Velasquez, S.T.R.; Hu, Q.; Kramm, J.; Santin, V.C.; Völker, C.; Wurm, F.R. Plastics of the Future? An Interdisciplinary Review on Biobased and Biodegradable Polymers: Progress in Chemistry, Societal Views, and Environmental Implications. Angew. Chem. Int. Ed. 2025, 64, e202423406. [Google Scholar] [CrossRef]
- Chanda, M.; Roy, S.K. Industrial Polymers, Specialty Polymers, and Their Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ali, W.; Ali, H.; Souissi, S.; Zinck, P. Are bioplastics an ecofriendly alternative to fossil fuel plastics? Environ. Chem. Lett. 2023, 21, 1991–2002. [Google Scholar] [CrossRef]
- García, R.A.; Vázquez, A.D.; Vázquez, B.F.; Francisco, L.A.; Bello, B.P.M. Sources, sinks and transformations of plastics in our oceans: Review, management strategies and modelling. Sci. Total Environ. 2023, 854, 158745. [Google Scholar] [CrossRef]
- Rowan, S.J. 100th Anniversary of macromolecular science viewpoints. ACS Macro Lett. 2021, 10, 466–468. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Norton, M. Green chemistry and the plastic pollution challenge: Towards a circular economy. Green Chem. 2020, 22, 6310–6322. [Google Scholar] [CrossRef]
- Kamran, F.; Afshar, H.; Shahi, F. Recent Advances and Applications of Sustainable and Recyclable Polymers. Polym. Eng. Sci. 2025, 65, 3845–3879. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid) a versatile biobased polymer of next decades with multifunctional properties. From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Comparing life cycle energy and GHG emissions of bio-based PET, recycled PET, PLA, and man-made cellulosics. Biofuels Bioprod. Biorefin. 2012, 6, 625–639. [Google Scholar] [CrossRef]
- Ali, W.; Ali, H.; Gillani, S.; Zinck, P.; Souissi, S. Polylactic acid synthesis, biodegradability, conversion to microplastics and toxicity: A review. Environ. Chem. Lett. 2023, 21, 1761–1786. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nquyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Klonos, P.A.; Bikiaris, N.D.; Barmpalexis, P.; Kyritsis, A. Segmental mobility in linear polylactides of various molecular weights. Polymer 2024, 305, 127177. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers—A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Li, J.; Liang, X.; Zhou, W.; Peng, S. Strategies and techniques for improving heat resistance and mechanical performances of poly(lactic acid) (PLA) biodegradable materials. Int. J. Biol. Macromol. 2022, 218, 115–134. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic acid based nanocomposites: Promising safe and biodegradable materials in biomedical field. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Pappa, C.; Triantafyllidis, K.; Bikiaris, D.N. Unravelling the impact of lignin particle size and content on enhanced value in plastic composites. Sustain. Chem. Environ. 2025, 9, 100194. [Google Scholar] [CrossRef]
- Makri, S.P.; Xanthopoulou, E.; Klonos, P.A.; Grigoropoulos, A.; Kyritsis, A.; Deligkiozi, I.; Zoikis-Karathanasis, A.; Nikolaidis, N.; Bikiaris, D.N.; Terzopoulou, Z. Lignin Particle Size Affects the Properties of PLA Composites Prepared by In Situ Ring-Opening Polymerization. Polymers 2024, 16, 3542. [Google Scholar] [CrossRef]
- Makri, S.P.; Xanthopoulou, E.; Valera, M.A.; Mangas, A.; Marra, G.; Ruiz, V.; Koltsakidis, S.; Tzetzis, D.; Zoikis-Karathanasis, A.; Deligkiozi, I.; et al. Poly(Lactic Acid) Composites with Lignin and Nanolignin Synthesized by In Situ Reactive Processing. Polymers 2023, 15, 2386. [Google Scholar] [CrossRef]
- Kapuge Dona, N.L.; Smith, R.C. Tailoring Polymer Properties Through Lignin Addition: A Recent Perspective on Lignin-Derived Polymer Modifications. Molecules 2025, 30, 2455. [Google Scholar] [CrossRef]
- Kumar Singla, R.; Maiti, S.N.; Ghosh, A.K. Crystallization, Morphological, and Mechanical Response of Poly(Lactic Acid)/Lignin-Based Biodegradable Composites. Polym.-Plast. Technol. Eng. 2016, 55, 475–485. [Google Scholar] [CrossRef]
- Tao, Z.; Zhao, Y.; Wang, Y.; Zhang, G. Recent Advances in Carbon Nanotube Technology: Bridging the Gap from Fundamental Science to Wide Applications. J. Carb. Res. 2024, 10, 69. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Yim, Y.-J.; Yoon, Y.-H.; Kim, S.-H.; Lee, J.-H.; Chung, D.-C.; Kim, B.-J. Carbon Nanotube/Polymer Composites for Functional Applications. Polymers 2025, 17, 119. [Google Scholar] [CrossRef]
- Klonos, P.A.; Bikiaris, R.D.; Terzopoulou, Z.; Mouchlianiti, K.; Tsachouridis, K.; Anastasiou, A.D.; Kyritsis, A.; Kyzas, G.Z. Structure-properties relationships in new polymer nanocomposites based on the renewable poly(butylene succinate) filled with low amounts of nanoparticles of 1-3D geometries. Polymer 2024, 296, 126841. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Kaminskii, V.V.; Iturralde, P.; Chumak, M.A. Advances in Electrically and Thermally Conductive Functional Nanocomposites Based on Carbon Nanotubes. Polymers 2024, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Sarma, S.; Rao, V.R. Emerging synthesis and characterization techniques for hybrid polymer nanocomposites. Nanotechnology 2023, 35, 012002. [Google Scholar] [CrossRef]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rohas, O.J.; Puglia, D. Multifunctional lignin-based nanocomposites and nanohybrids. R. Soc. Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, Y.O.; Ha, Y.M.; Lim, D.; Hwang, J.Y.; Kim, J.; Park, M.; Cho, J.W.; Jung, C.Y. Antimicrobial properties of lignin-decorated thin multi-walled carbon nanotubes in poly(vinyl alcohol) nanocomposites. Eur. Polym. J. 2018, 105, 79–84. [Google Scholar] [CrossRef]
- Klonos, P.A.; Ioannidis, R.O.; Pitsavas, A.; Bikiaris, N.D.; Makri, S.P.; Koutsourea, S.; Grigoropoulos, A.; Deligkiozi, I.; Zoikis-Karathanasis, A.; Kyritsis, A.; et al. Segmental mobility, interfacial polymer, crystallization and conductivity study in polylactides filled with hybrid lignin-CNT particles. Nanomaterials 2025, 15, 660. [Google Scholar] [CrossRef]
- Gorman, A.M.; Clayton, A.; O’Connell, T.; Johnson, D. A recyclable screen ink with state-of-the-art performance developed using a bottom-up, safety and sustainability-driven approach. MRS Adv. 2023, 8, 311–316. [Google Scholar] [CrossRef]
- Vogel, K.; Carniello, S.; Beni, V.; Sudheshwar, A.; Malinverno, N.; Alesanco, Y.; Torrellas, M.; Harkema, S.; De Kok, M.; Rentrop, C.; et al. Defining and achieving next-generation green electronics: A perspective on best practices through the lens of hybrid printed electronics. IEEE Access 2025, 13, 117135–117161. [Google Scholar] [CrossRef]
- Qian, F.; Jia, R.; Cheng, M.; Chaudhary, A.; Melhi, S.; Mekkey, S.D.; Zhu, N.; Wang, C.; Razak, F.; Xu, X.; et al. An overview of polylactic acid (PLA) nanocomposites for sensors. Adv. Comp. Hybrid Mat. 2024, 7, 75. [Google Scholar] [CrossRef]
- Silva, M.M.; Lopes, P.E.; Li, Y.; Pötschke, P.; Ferreira, F.N.; Paiva, M.C. Polylactic acid/carbon nanoparticle composite filaments for sensing. Appl. Sci. 2021, 11, 2580. [Google Scholar] [CrossRef]
- Stopforth, R. Conductive polylactic acid filaments for 3D printed sensors: Experimental electrical and thermal characterization. Sci. Afr. 2021, 14, e01040. [Google Scholar] [CrossRef]
- Makri, S.; Grigoropoulos, A.; Bikiaris, D.; Deligkiozi, I.; Zoikis-Karathanasis, A. Assignee Creative Nano P.C. Ultrasound-Assisted Process for the Production of Lignin Nanoparticles, p. European Patent Office EP4471093A1, 4 December 2024. [Google Scholar]
- Kang, H.; Jung, S.; Jeong, S.; Kim, G.; Lee, Κ. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 2015, 6, 6503. [Google Scholar] [CrossRef]
- Jepiti, P.; Kim, J.; Bark, S.; Lim, S. Transparent and printed RF electronics: A comprehensive review of materials, and applications. Mater. Des. 2025, 258, 114583. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2020, 26, 126. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.; Siginer, D.A. PLA crystallization kinetics and morphology development. Int. Polym. Process. 2018, 3, 336–344. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Müller, A.J.; Michell, R.M.; Lorenzo, A.T. Isothermal Crystallization Kinetics of Polymers. In Polymer Morphology: Principles, Characterization, and Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to void common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Klonos, P.A.; Terzopoulou, Z.; Psochia, E.; Sanusi, O.M.; Hocine, N.A.; Benelfellah, A.; Giliopoulos, D.; Triantafyllidis, K.; Kyritsis, A.; et al. Comparative study of crystallization, semicrystalline morphology, and molecular mobility in nanocomposites based on polylactide and various inclusions at low filler loadings. Polymer 2021, 217, 123457. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Iura, K.; Dan, Y. Melting behavior of poly(l-lactic acid): Effects of crystallization temperature and time. Polymer 2007, 48, 5398–5407. [Google Scholar] [CrossRef]
- Zhu, A.J.; Sternstein, S.S. Nonlinear viscoelasticity of nanofilled polymers: Interfaces, chain statistics and properties recovery kinetics. Compos. Sci. Technol. 2003, 63, 1113–1126. [Google Scholar] [CrossRef]
- Chen, Q.; Gong, S.; Moll, J.; Zhao, D.; Kumar, S.K.; Colby, R.H. Mechanical reinforcement of polymer nanocomposites from percolation of a nanoparticle network. ACS Macro Lett. 2015, 4, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A.C. A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mater. 2020, 32, 1905279. [Google Scholar] [CrossRef]

| Abbreviation | Content wt% | ||
|---|---|---|---|
| PLA | Lignin | Hybrid (Lignin/10%MWCNTs) | |
| PLA | 100 | - | - |
| PLA/lignin | 99.5 | 0.5 | - |
| PLA/lignin | 99.0 | 1.0 | - |
| PLA/lignin | 97.5 | 2.5 | - |
| PLA/hybrid | 99.5 | - | 0.5 (0.05 wt% of CNTs) |
| PLA/hybrid | 99.0 | - | 1.0 (0.1 wt% of CNTs) |
| PLA/hybrid | 97.5 | - | 2.5 (0.25 wt% of CNTs) |
| PLA/hybrid | 95 | - | 5.0 (0.5 wt% of CNTs) |
| PLA/hybrid | 90 | - | 10 (1.0 wt% of CNTs) |
| PLA/hybrid | 80 | - | 20 (2.0 wt% of CNTs) |
| PLA/hybrid | 70 | - | 30 (3.0 wt% of CNTs) |
| Sample | As Received | Quenched | Cooling | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tg (°C) | ΔCp [J/(g·°C)] | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | CFc (%) | |
| PLA | 172.7 | 41.1 | 60.9 | 0.409 | 106.8 | 32.3 | 172.2 | 38.3 | 97.2 | 8.3 | 8.9 |
| 0.5% Hybrid | 173.0 + 181.7 | 45.6 + 8.4 | 60.7 | 0.403 | 107.2 | 36.3 | 173.3 | 37.1 | 97.5 | 13.7 | 14.8 |
| 1.0% | 172.1 + 182.1 | 42.4 + 3.7 | 60.5 | 0.393 | 112.4 | 35.8 | 173.5 | 33.1 | 97.4 | 15.2 | 16.5 |
| 2.5% | 172.8 | 41.5 | 60.4 | 0.391 | 112.1 | 37.2 | 172.8 | 39.8 | 98.5 | 20.1 | 22.2 |
| 5.0% | 173.1 | 39.7 | 60.3 | 0.390 | 112.5 | 33.3 | 173.8 | 34.8 | 98.6 | 17.3 | 19.6 |
| 10% | 171.5 | 38.1 | 59.9 | 0.380 | 110.1 | 34.3 | 172.1 | 37.2 | 98.6 | 21.9 | 26.2 |
| 20% | 170.4 | 32.6 | 59.1 | 0.325 | 103.2 | 27 | 170.1 | 31.9 | 106.1 | 30.0 | 40.3 |
| 30% | 169.8 | 27.7 | 58.4 | 0.285 | 105.3 | 23 | 170.1 | 26.5 | 102.6 | 22.1 | 34.0 |
| 0.5% Lignin | 173.4 | 37.2 | 60.4 | 0.392 | 110.7 | 36.4 | 174.3 | 36.8 | 98.2 | 7.2 | 7.8 |
| 1.0% | 170.7 | 38.9 | 61.1 | 0.390 | 115.1 | 35.8 | 175.1 | 36.0 | 98.4 | 4.5 | 4.9 |
| 2.5% | 172.8 | 41.0 | 59.8 | 0.316 | 111.1 | 33.7 | 173.2 | 33.7 | 98.0 | 6.4 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitsavas, A.; Ioannidis, R.O.; Makri, S.; Koutsourea, S.; Grigoropoulos, A.; Deligkiozi, I.; Zoikis-Karathanasis, A.; Xanthopoulou, E.; Bikiaris, D.N. Optical and Thermal Studies, Isothermal Crystallization Kinetics and Mechanical Properties of Poly(lactic acid) Nanocomposites Based on Hybrid Lignin/MWCNT Nanomaterial. J. Compos. Sci. 2025, 9, 560. https://doi.org/10.3390/jcs9100560
Pitsavas A, Ioannidis RO, Makri S, Koutsourea S, Grigoropoulos A, Deligkiozi I, Zoikis-Karathanasis A, Xanthopoulou E, Bikiaris DN. Optical and Thermal Studies, Isothermal Crystallization Kinetics and Mechanical Properties of Poly(lactic acid) Nanocomposites Based on Hybrid Lignin/MWCNT Nanomaterial. Journal of Composites Science. 2025; 9(10):560. https://doi.org/10.3390/jcs9100560
Chicago/Turabian StylePitsavas, Andreas, Rafail O. Ioannidis, Sofia Makri, Stefania Koutsourea, Alexios Grigoropoulos, Ioanna Deligkiozi, Alexandros Zoikis-Karathanasis, Eleftheria Xanthopoulou, and Dimitrios N. Bikiaris. 2025. "Optical and Thermal Studies, Isothermal Crystallization Kinetics and Mechanical Properties of Poly(lactic acid) Nanocomposites Based on Hybrid Lignin/MWCNT Nanomaterial" Journal of Composites Science 9, no. 10: 560. https://doi.org/10.3390/jcs9100560
APA StylePitsavas, A., Ioannidis, R. O., Makri, S., Koutsourea, S., Grigoropoulos, A., Deligkiozi, I., Zoikis-Karathanasis, A., Xanthopoulou, E., & Bikiaris, D. N. (2025). Optical and Thermal Studies, Isothermal Crystallization Kinetics and Mechanical Properties of Poly(lactic acid) Nanocomposites Based on Hybrid Lignin/MWCNT Nanomaterial. Journal of Composites Science, 9(10), 560. https://doi.org/10.3390/jcs9100560










