Bio-Derived Porous Carbon/Nickel Oxide Composite for High-Performance Energy Storage Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porous Carbon from Orange Peel
2.3. NiO Deposition on Porous Carbon
2.4. Characterization
2.5. Electrochemical Measurements
2.5.1. Preparation of Electrodes
2.5.2. Calculation of Specific Capacitance
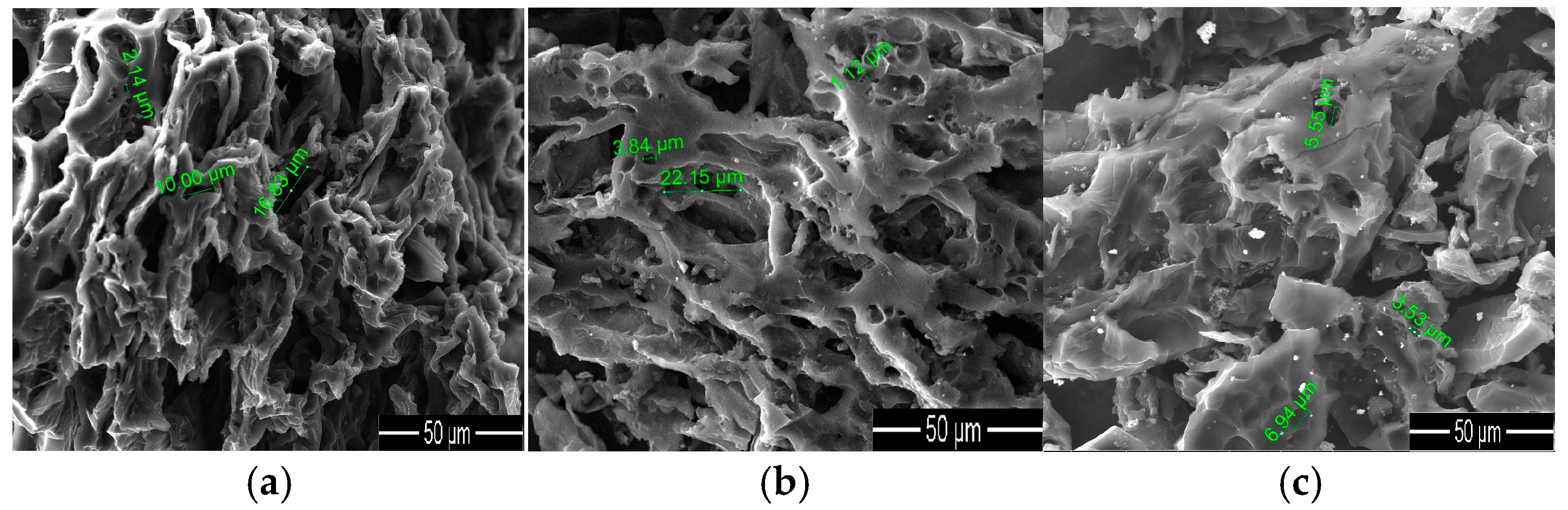
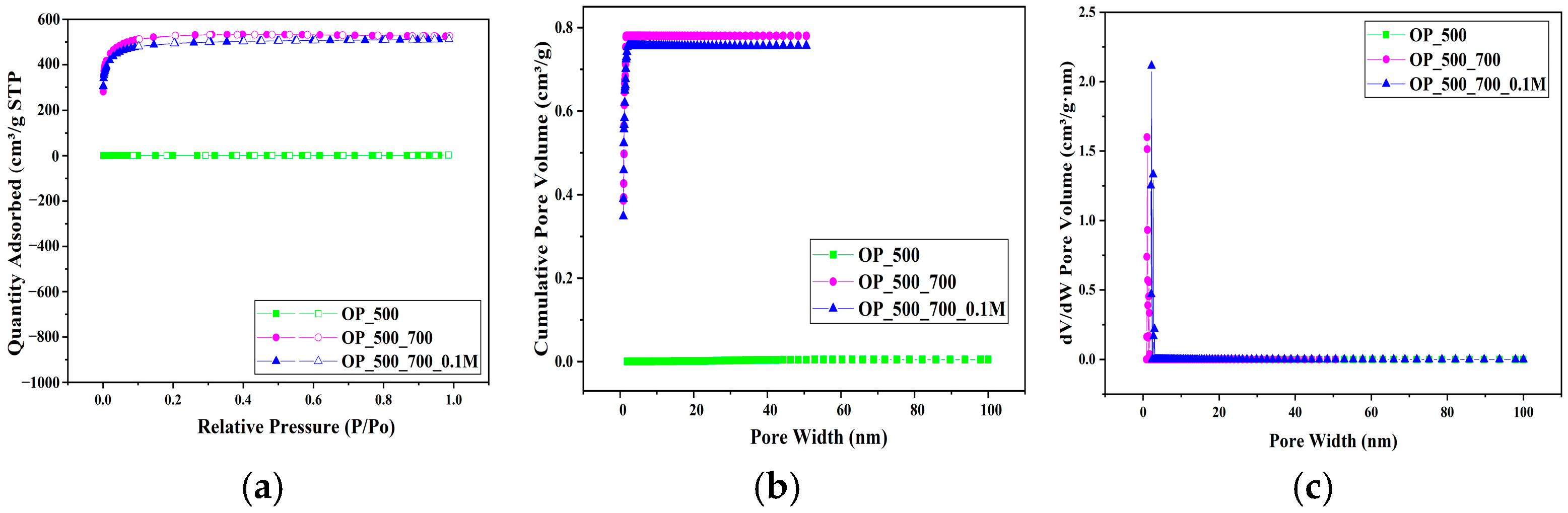
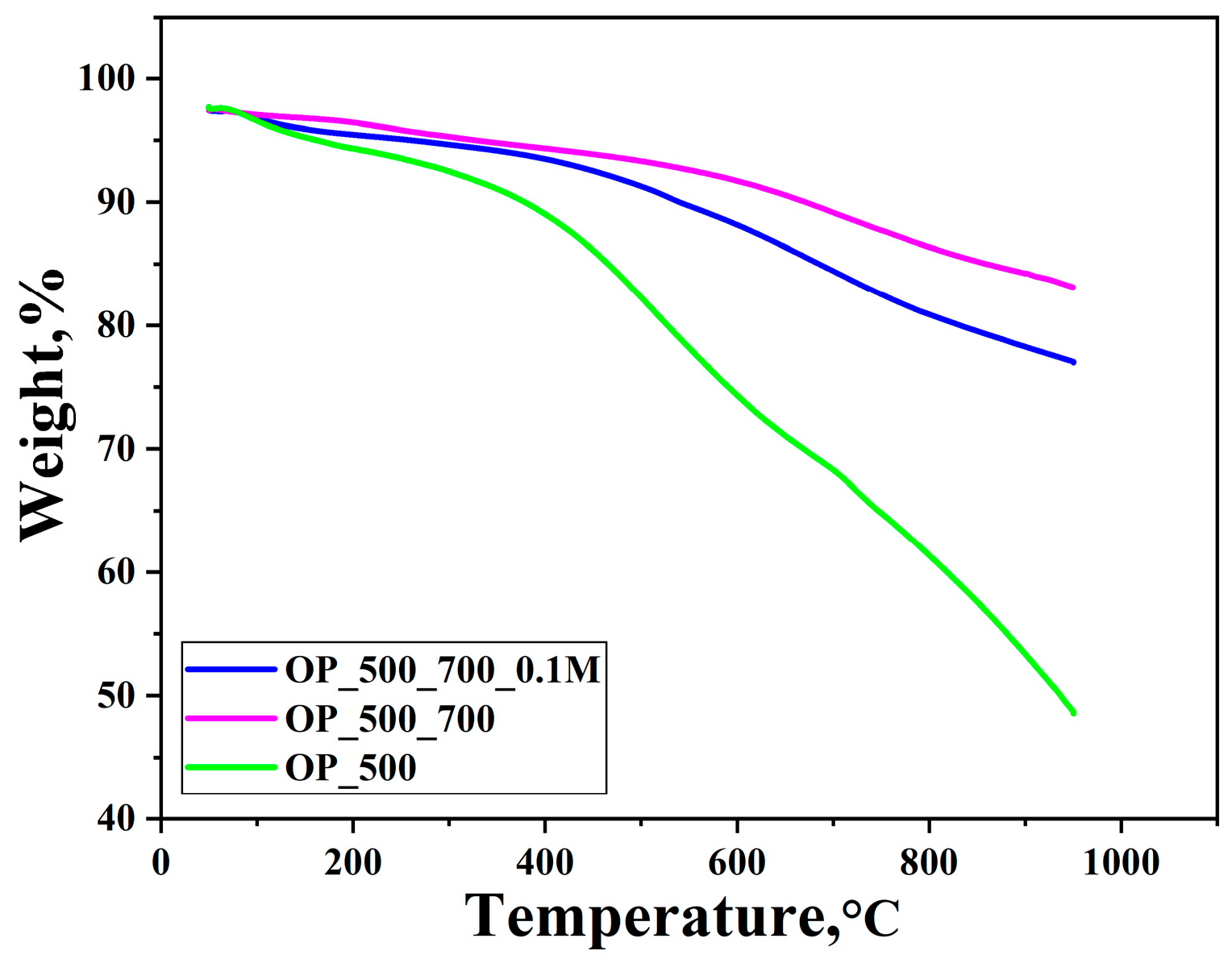
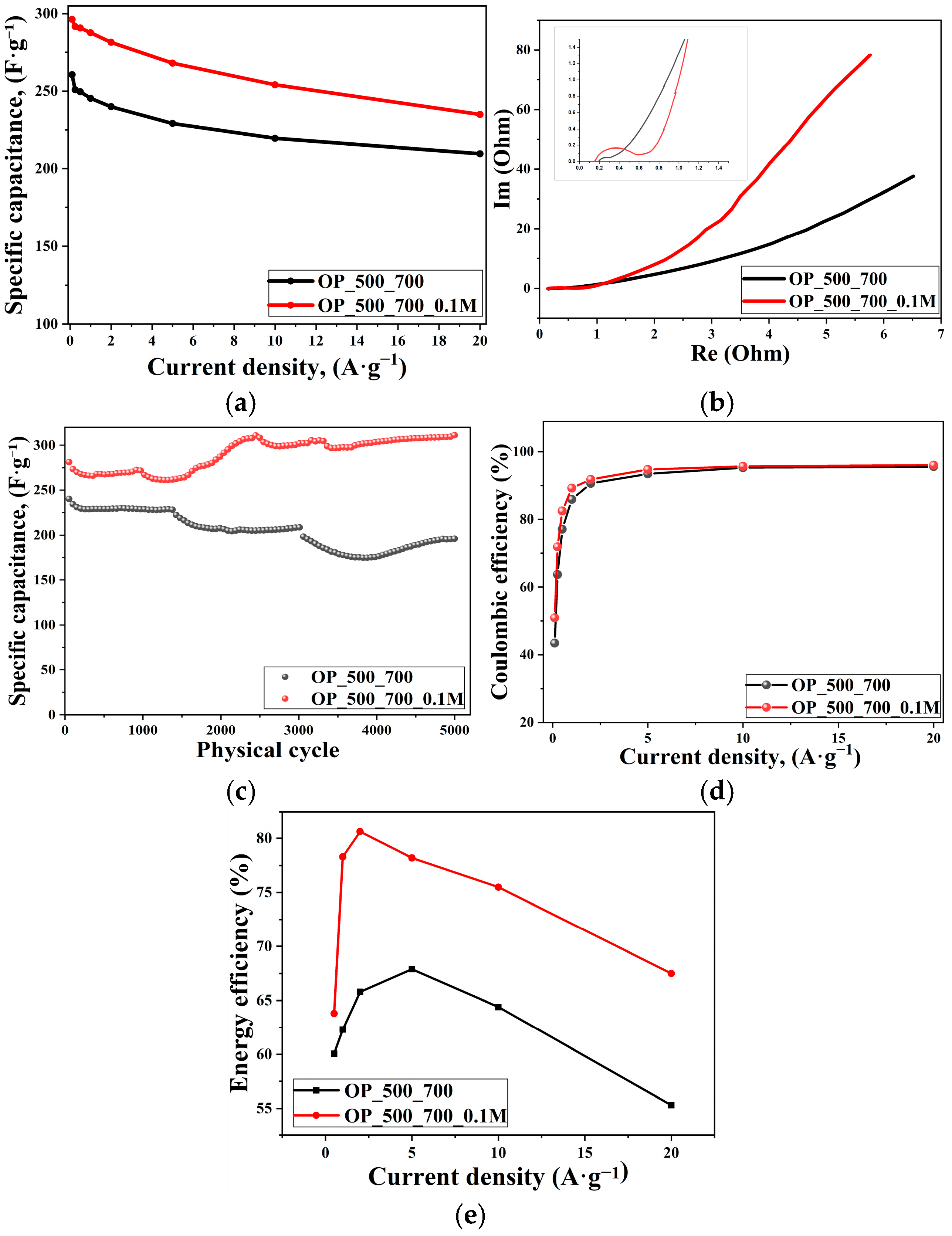
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909. [Google Scholar] [CrossRef]
- Mohsin, M.; Naseem, S.; Sarfraz, M.; Azam, T. Assessing the effects of fuel energy consumption, foreign direct investment and GDP on CO2 emission: New data science evidence from Europe & Central Asia. Fuel 2022, 314, 123098. [Google Scholar] [CrossRef]
- Brockway, P.E.; Owen, A.; Brand-Correa, L.I.; Hardt, L. Estimation of global final-stage energy-return-on-investment for fossil fuels with comparison to renewable energy sources. Nat. Energy 2019, 4, 612–621. [Google Scholar] [CrossRef]
- Maddukuri, S.; Malka, D.; Chae, M.S.; Elias, Y.; Luski, S.; Aurbach, D. On the challenge of large energy storage by electrochemical devices. Electrochim. Acta 2020, 354, 136771. [Google Scholar] [CrossRef]
- Dai, S.; Bai, Y.; Shen, W.; Zhang, S.; Hu, H.; Fu, J.; Wang, X.; Hu, C.; Liu, M. Core–shell structured Fe2O3@Fe3C@C nanochains and Ni–Co carbonate hydroxide hybridized microspheres for high-performance battery-type supercapacitor. J. Power Sources 2021, 482, 228915. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, K.; Wu, C. Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol. Rev. 2019, 8, 299–314. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass-derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Taer, E.; Taslim, R. Brief review: Preparation techniques of biomass-based activated carbon monolith electrode for supercapacitor applications. AIP Conf. Proc. 2018, 1927, 020004. [Google Scholar] [CrossRef]
- Majid, S.; Ali, A.S.G.; Cao, W.Q.; Reza, R.; Ge, Q. Biomass-derived porous carbons as supercapacitor electrodes—A review. New Carbon Mater. 2021, 36, 546–572. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Muthusamy, A.; Cho, I.; Kim, H.J.; Senthil, K.; Prabakar, K. Ultrahigh surface area biomass-derived 3D hierarchical porous carbon nanosheet electrodes for high energy density supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; Ferreira de Barros, A.L. Biomass-derived porous activated carbon from Syzygium cumini fruit shells and Chrysopogon zizanioides roots for high-energy density symmetric supercapacitors. Biomass Bioenergy 2020, 143, 105838. [Google Scholar] [CrossRef]
- Apriwandi, A.; Taer, E.; Farma, R.; Setiadi, R.N.; Amiruddin, E. A facile approach of micro-mesopores structure binder-free coin/monolith solid design activated carbon for electrode supercapacitor. J. Energy Storage 2021, 40, 102823. [Google Scholar] [CrossRef]
- Luo, X.Y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Kurmanbayeva, G.; Seitkazinova, A.; Zhaparova, A.A.; Assylkhanova, D.D.; Yeleuov, M.; Nurgain, A. Activated carbon derived from cucumber peel for use as a supercapacitor electrode material. Nanomaterials 2024, 14, 686. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Yeleuov, M.; Sultakhan, S.T.; Maltay, A.B.; Zhaparova, A.A.; Assylkhanova, D.D.; Nemkayeva, R.R. Electrochemical performance of chemically activated carbons from sawdust as supercapacitor electrodes. Nanomaterials 2022, 12, 3391. [Google Scholar] [CrossRef]
- Khajonrit, J.; Sichumsaeng, T.; Kalawa, O.; Chaisit, S.; Chinnakorn, A.; Chanlek, N.; Maensiri, S. Mangosteen peel-derived activated carbon for supercapacitors. Prog. Nat. Sci. Mater. Int. 2022, 32, 570–578. [Google Scholar] [CrossRef]
- Ismanto, A.E.; Wang, S.; Soetaredjo, F.E.; Ismadji, S. Preparation of the capacitor’s electrode from cassava peel waste. Bioresour. Technol. 2010, 101, 3534–3540. [Google Scholar] [CrossRef]
- Subramani, K.; Sudhan, N.; Karnan, M.; Sathish, M. Orange peel derived activated carbon for fabrication of high-energy and high-rate supercapacitors. ChemistrySelect 2017, 2, 11384–11392. [Google Scholar] [CrossRef]
- Gou, H.; He, J.; Zhao, G.; Zhang, L.; Yang, C.; Rao, H. Porous nitrogen-doped carbon networks derived from orange peel for high-performance supercapacitors. Ionics 2019, 25, 4371–4380. [Google Scholar] [CrossRef]
- Wan, L.; Chen, D.; Liu, J.; Zhang, Y.; Chen, J.; Du, C.; Xie, M. Facile preparation of porous carbons derived from orange peel via basic copper carbonate activation for supercapacitors. J. Alloys Compd. 2020, 823, 153747. [Google Scholar] [CrossRef]
- Taer, E.; Apriwandi, A.; Mustika, W.S.; Mardiah, M.A.; Taslim, R. An environmental approach in production of activated carbon monolithic derived from garlic peels for supercapacitor application. Trends Sci. 2023, 20, 6396. [Google Scholar] [CrossRef]
- Fasakin, O.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Oyedotun, K.O.; Eleruja, M.A.; Dangbegnon, J.B.; Manyala, N. Synthesis and characterization of porous carbon derived from activated banana peels with hierarchical porosity for improved electrochemical performance. Electrochim. Acta 2018, 262, 187–196. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, R.S.; Atchudan, R.; Kim, H.-J.; Yi, M.; Samyn, L.M.; De Barros, A.L.F. Fabrication of high-performance asymmetric supercapacitor consists of nickel oxide and activated carbon (NiO//AC). Catalysts 2022, 12, 375. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Hussain, S.; Ikram, M.; Park, S.J. Supercapacitor performance based on nitrogen and sulfur co-doped hierarchically porous carbons: Superior rate capability and cycle stability. Int. J. Energy Res. 2022, 46, 15602–15616. [Google Scholar] [CrossRef]
- Diop, M.; Ndiaye, B.M.; Dieng, S.; Ngom, B.D.; Chaker, M. High Electrochemical Performance of Nickel Cobaltite@Biomass Carbon Composite (NiCoO@BC) Derived from the Bark of Anacardium occidentale for Supercapacitor Application. RSC Adv. 2024, 14, 5782–5796. [Google Scholar] [CrossRef]
- Mirzazadeh Khomambazari, S.; Lokhande, P.; Padervand, S.; Zaulkiflee, N.D.; Irandoost, M.; Dubal, S.; Sharifan, H. A Review of Recent Progresses on Nickel Oxide/Carbonous Material Composites as Supercapacitor Electrodes. J. Compos. Compd. 2022, 4, 195–208. [Google Scholar] [CrossRef]
- Shukeri, M.Z.A.; Yahaya, N.Z.; Zainuddin, E.A.; Mahmud, N.; Zahid, L.; Rahim, H.A. Microwave Absorption Properties of Porous Activated Carbon/Nickel Oxide Composites Derived from Coconut Husks. J. Met. Mater. Miner. 2025, 35, e2336. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, H.; Li, X. NiO nanoparticles embedded in porous carbon matrix for high-performance supercapacitors. J. Power Sources 2015, 281, 432–439. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Coaxial NiO@carbon nanotube core–shell nanowires for high-performance supercapacitors. Electrochim. Acta. 2013, 93, 195–202. [Google Scholar] [CrossRef]
- Pai, S.H.; Pandey, S.K.; Samuel, E.J.J.; Jang, J.U.; Nayak, A.K.; Han, H.S. Recent advances in NiO-based nanostructures for energy storage device applications. J. Energy Storage 2024, 76, 109731. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.-C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando studies redirect spatiotemporal restructuration of model coordinated oxides in electrochemical oxidation. Adv. Mater. 2024, 37, 2413073. [Google Scholar] [CrossRef]
- Alshuiael, S.M.; Al-Ghouti, M.A. Multivariate Analysis for FTIR in Understanding Treatment of Used Cooking Oil Using Activated Carbon Prepared from Olive Stone. PLoS ONE 2020, 15, e0232997. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Nurgain, A.; Zhaparova, A.A.; Seitkazinova, A.R.; Prikhodko, N.G. Raman characteristics of multiwall carbon nanotubes on diatomite. Eurasian Chem.-Technol. J. 2018, 20, 123–128. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Orange-peel-derived carbon: Designing sustainable and high-performance supercapacitor electrodes. C-J. Carbon Res. 2017, 3, 25. [Google Scholar] [CrossRef]
- Li, G.; Iakunkov, A.; Boulanger, N.; Lazar, O.A.; Enachescu, M.; Grimm, A.; Talyzin, A.V. Activated carbons with extremely high surface area produced from cones, bark and wood using the same procedure. RSC Adv. 2023, 13, 14543–14553. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Satpati, B.; Nandi, M.; Das, S. NiO-CNT composite for high-performance supercapacitor electrode and oxygen evolution reaction. Electrochim. Acta 2018, 283, 327–337. [Google Scholar] [CrossRef]
- Fayed, M.G.; Aman, D.; Mohamed, S.G. Synergetic electrochemical behavior of NiO and activated carbon composites for advanced supercapacitors. J. Clust. Sci. 2025, 36, 94. [Google Scholar] [CrossRef]
- Lesbayev, B.; Auyelkhankyzy, M.; Ustayeva, G.; Yeleuov, M.; Rakhymzhan, N.; Maral, Y.; Tolynbekov, A. Modification of biomass-derived nanoporous carbon with nickel oxide nanoparticles for supercapacitor application. J. Compos. Sci. 2023, 7, 20. [Google Scholar] [CrossRef]








| № | Material | SSA (m2 g−1) | Specific Capacitance (F g−1) | Retention (Cycles) | Reference |
|---|---|---|---|---|---|
| 1 | Orange-peel-derived activated carbon (KOH, optimized) | up to 2521 | 407 at 0.5 A g−1; 217 at 1 A·g−1 | >100% after 5000 | [36] |
| 2 | KOH-activated wood/pinecone carbons | up to 3500 | 178–187 (depending on sample) at 0.5 A g−1 | ~93% after 10,000 at 3 A·g−1 | [37] |
| 3 | NiO–CNT composites | — | up to ~800 (depending on synthesis conditions) | High stability | [38] |
| 4 | NiO/AC 50/50% (commercial AC) | 1167 | 325 at 1 A·g−1 | ~90% after 4000 at 5 mA·g−1 | [39] |
| 5 | WS-dAC/NiO-0.005 | 2200 | 279 at 0.5 A g−1 | not reported | [40] |
| 6 | OP_500_700_0.1M | 1968 | 291 at 0.5 A g−1; 306 at 1 A g−1 | ~111% after 5000 at 20 A·g−1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitkazinova, A.R.; Nazhipkyzy, M.; Kudaibergenov, K.; Issanbekova, A.; Bergeneva, N.S.; Abdisattar, A.; Kyzgarina, M. Bio-Derived Porous Carbon/Nickel Oxide Composite for High-Performance Energy Storage Applications. J. Compos. Sci. 2025, 9, 561. https://doi.org/10.3390/jcs9100561
Seitkazinova AR, Nazhipkyzy M, Kudaibergenov K, Issanbekova A, Bergeneva NS, Abdisattar A, Kyzgarina M. Bio-Derived Porous Carbon/Nickel Oxide Composite for High-Performance Energy Storage Applications. Journal of Composites Science. 2025; 9(10):561. https://doi.org/10.3390/jcs9100561
Chicago/Turabian StyleSeitkazinova, Aigerim R., Meruyert Nazhipkyzy, Kenes Kudaibergenov, Almagul Issanbekova, Nurgul S. Bergeneva, Alisher Abdisattar, and Meiramgul Kyzgarina. 2025. "Bio-Derived Porous Carbon/Nickel Oxide Composite for High-Performance Energy Storage Applications" Journal of Composites Science 9, no. 10: 561. https://doi.org/10.3390/jcs9100561
APA StyleSeitkazinova, A. R., Nazhipkyzy, M., Kudaibergenov, K., Issanbekova, A., Bergeneva, N. S., Abdisattar, A., & Kyzgarina, M. (2025). Bio-Derived Porous Carbon/Nickel Oxide Composite for High-Performance Energy Storage Applications. Journal of Composites Science, 9(10), 561. https://doi.org/10.3390/jcs9100561








